Intensification of Electrocoagulation in Compost-Derived Wastewater
Abstract
1. Introduction
2. Materials and Methods
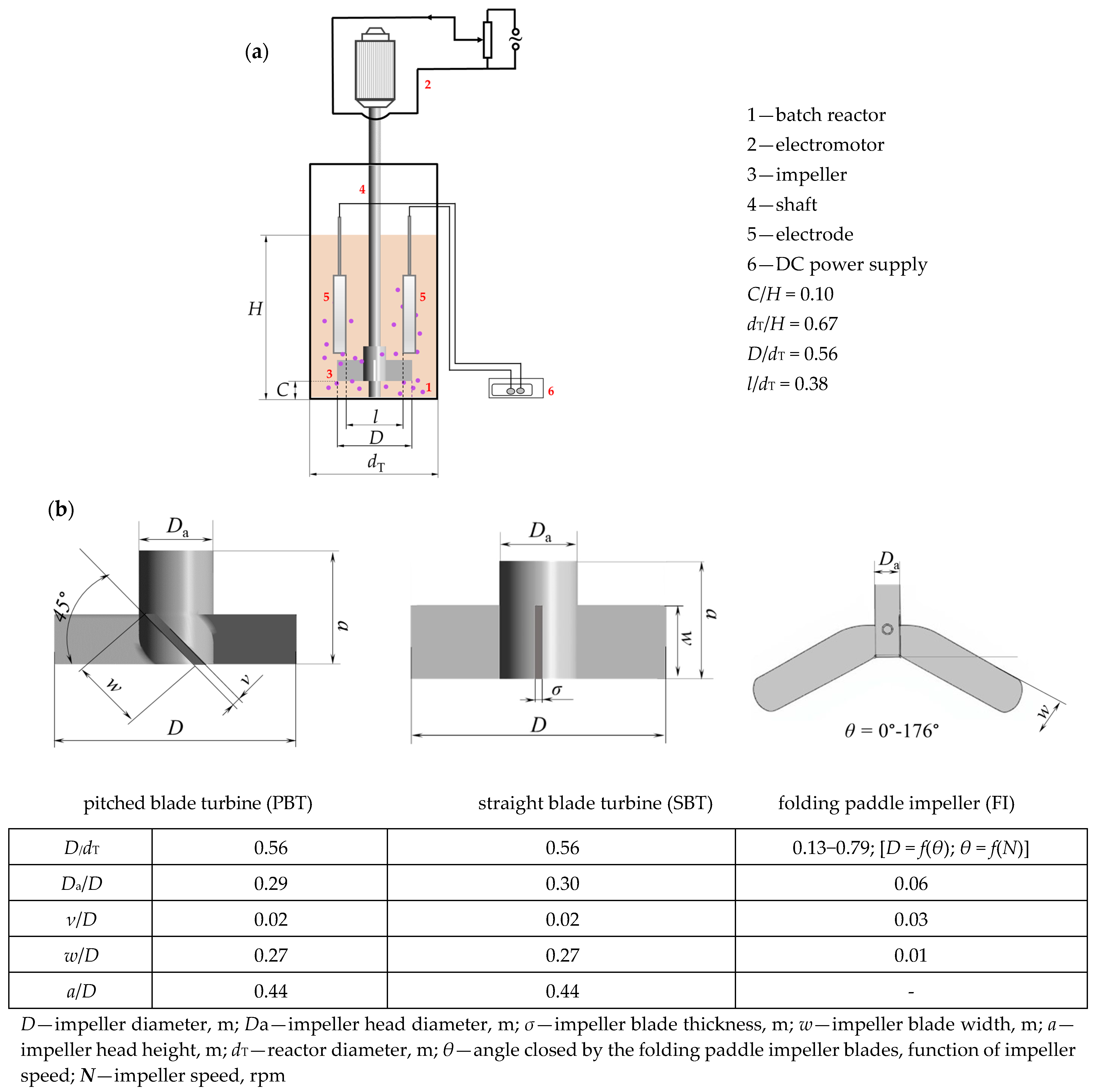
2.1. Materials
2.2. Experimental Performance
2.3. Hybrid Process Efficiency Calculation
2.4. Electrode and Energy Consumption, Faradaic Efficiency Calculation
2.5. Electrodes Surface Morphology Analysis
3. Results and Discussion
3.1. Analysis of Process Parameters
3.1.1. Analysis of pH Variation
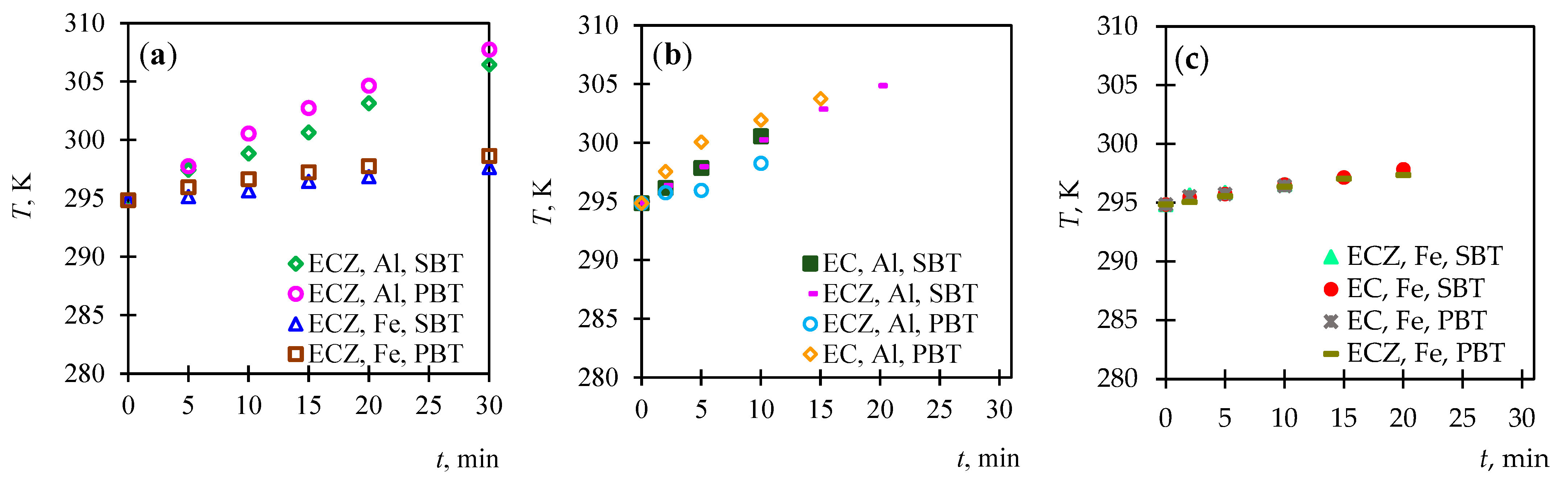
3.1.2. Analysis of Temperature Variation
3.1.3. Analysis of Electrical Conductivity
3.1.4. Analysis of Chemical Oxygen Demand
3.1.5. Analysis of Turbidity
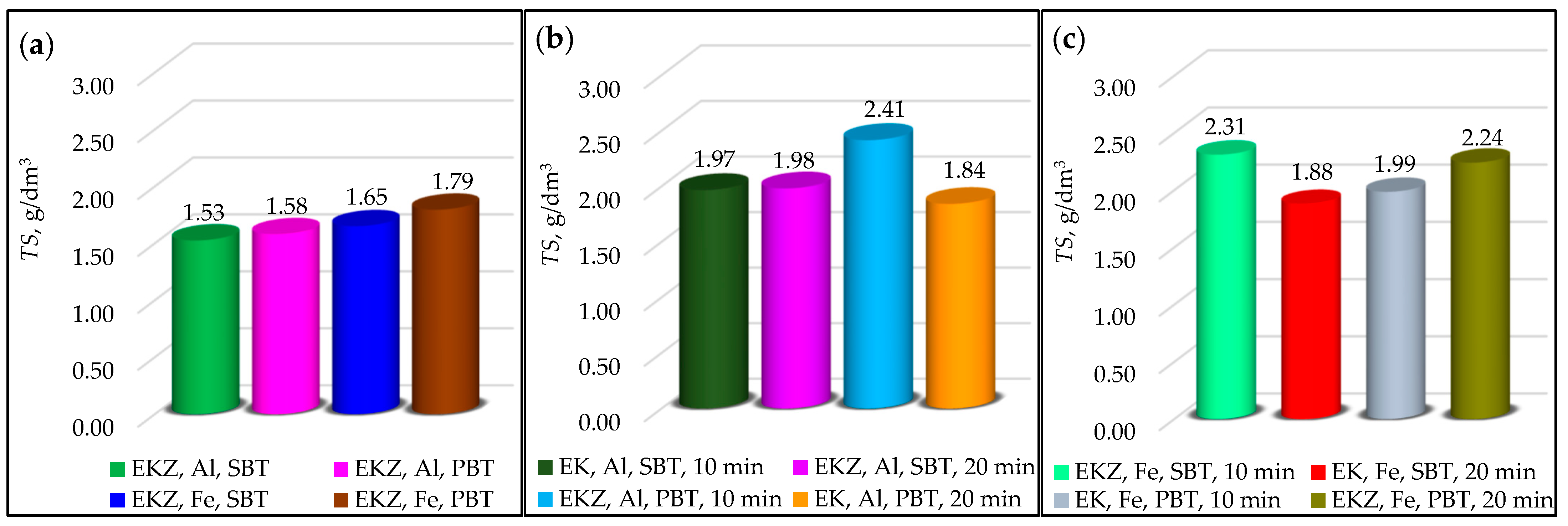
3.1.6. Analysis of Total Solids
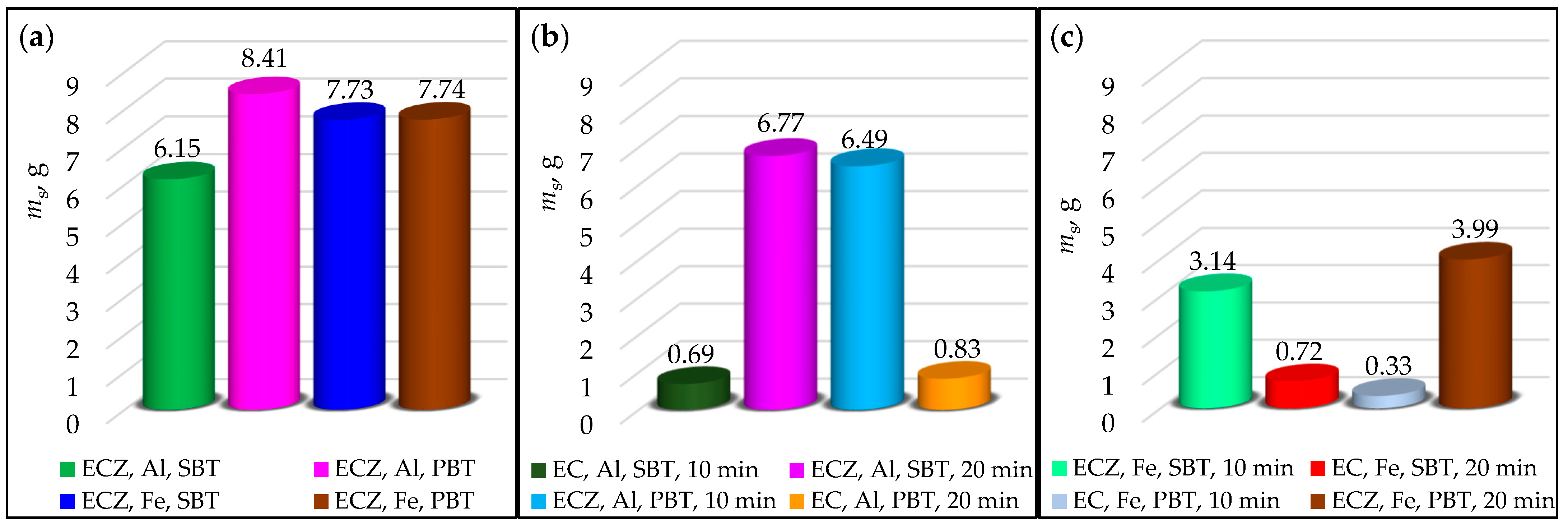
3.2. Analysis of Settling Test Results and Sludge Mass
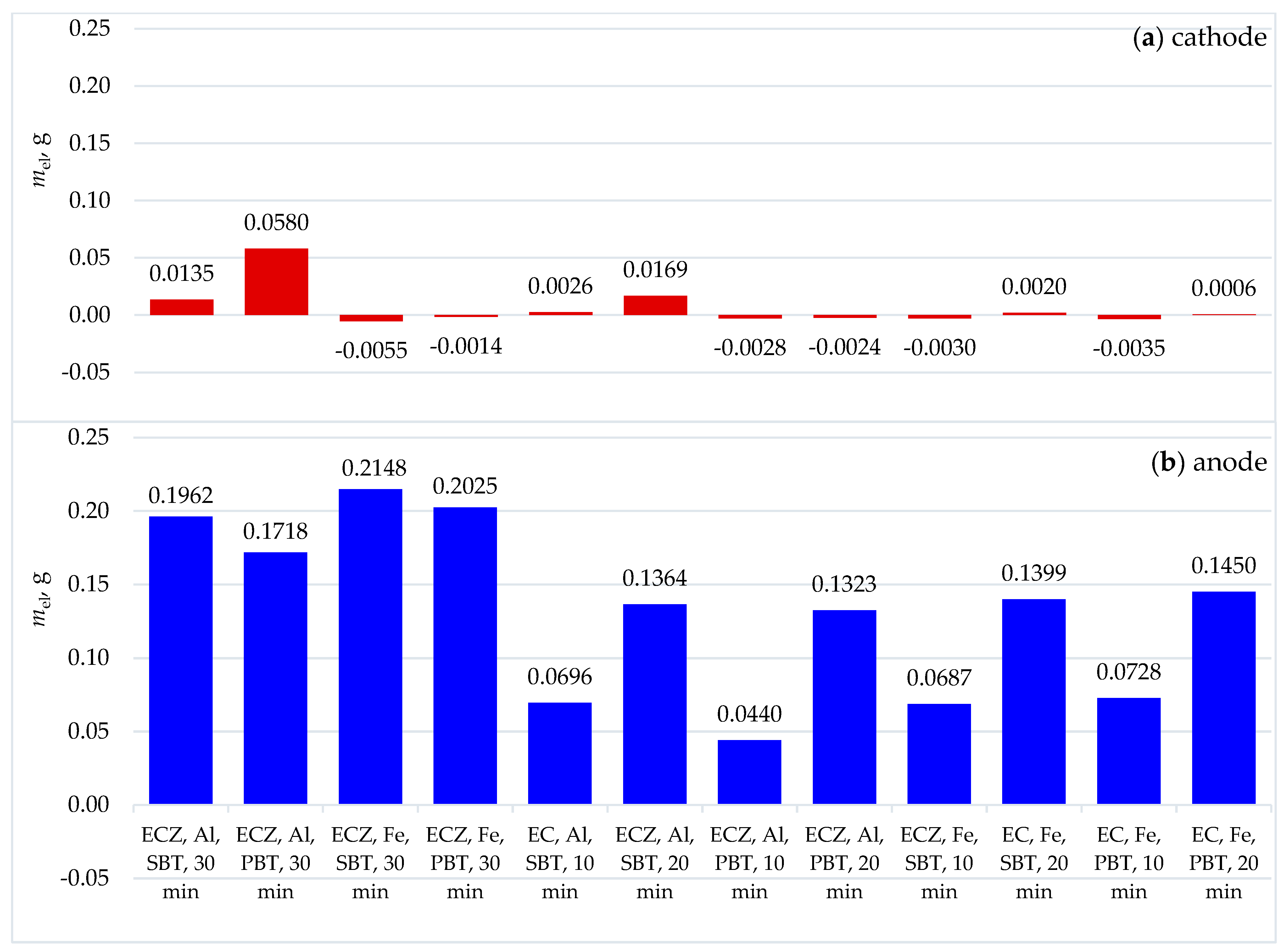
3.3. Analysis of Electrode Mass Loss and Surface Changes
3.4. Analysis of Energy and Electrode Consumption and Faraday Efficiency
3.5. Taguchi Approach for Identifying Critical Parameters for Optimising EC Technologies with Different Impellers
4. Conclusions
Challenges and Further Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| a | impeller head height, m |
| A/V | the electrode-to-volume ratio |
| ac | electricity price, € |
| b | approximate cost of electrode materials, € |
| C | distance from the bottom of the reactor to the impeller, m |
| C/H | off-bottom clearance |
| Cactual_electrode | actual electrode consumption per cubic metre of wastewater, kg/m3 |
| Cactual_electrode | theoretical electrode consumption per cubic metre of wastewater, kg/m3 |
| Celectrode | electrode mass consumed per cubic metre of wastewater, kg/m3 |
| Cenergy | electrical energy consumption per cubic metre of wastewater, kWh/m3 |
| cf | final concentration, mg/dm3 or NTU |
| ci | initial concentration, mg/dm3 or NTU |
| COD | chemical oxygen demand, mg O2/dm3 |
| D | impeller diameter, m |
| Da | impeller head diameter, m |
| DF | degrees of freedom |
| DOE | design of experiments |
| dT | reactor diameter, m |
| EC | electrocoagulation |
| ECZ | electrocoagulation in combination with zeolite |
| F | Faraday’s constant, 96,487 C/mol |
| FE | Faraday efficiency |
| FI | folding paddle impeller |
| h | height of the solid–liquid interface, cm |
| H | liquid height, m |
| I | correspond to impeller type (SBT or PBT) |
| Ii | current intensity, A |
| L1 and L2 | condition in level 1 and 2 |
| M | electrodes material (Al or Fe) |
| mactual | experimentally measured electrodes mass loss, g |
| mel | electrode mass loss results, g |
| MS | mean squares |
| ms | sludge mass, g |
| Mw | metal molar mass (g/mol) |
| N | impeller speed, rpm |
| n | number of repetitions under the same experimental conditions |
| OC | operating cost, €/m3 |
| PBT | Pitched Blade Turbine |
| pC | percentage of contribution |
| Q | heat generated, J |
| R | electrical resistance of the system, Ω |
| S/N | signal-to-noise ratio |
| S/NSB | Signal-to-Noise Smaller the Better |
| SB | smaller the better function |
| SBT | Straight Blade Turbine |
| SS | sum of squares |
| t | time, min |
| TS | total solids, g/dm3 |
| U | voltage applied, V |
| Ve | effective reactor volume, m3 |
| w | impeller blade width, m |
| y | measured response value (COD, turbidity and difference in electrodes mass) |
| z | the number of electrons transferred per aluminium or iron ion |
| Z | zeolite |
| θ | angle closed by the folding impeller blades, function of impeller speed |
| ν | impeller blade thickness, m |
| σ | electrical conductivity, mS/cm |
References
- Cravotto, G. Reshaping Chemical Manufacturing Towards Green Process Intensification: Recent Findings and Perspectives. Processes 2025, 13, 459. [Google Scholar] [CrossRef]
- Sitter, S.; Chen, Q.; Grossmann, I. An overview of process intensification methods. Curr. Opin. Chem. Eng. 2019, 25, 87–94. [Google Scholar] [CrossRef]
- Xia, C.; Yuan, Y.; Mathimani, T.; Rene, E.; Brindhadevi, K.; Le, Q.; Pugazhendhi, A. Process intensification approaches in wastewater and sludge treatment for the removal of pollutants. J. Environ. Manag. 2023, 345, 118837. [Google Scholar] [CrossRef]
- AlJaberi, F.Y.; Alardhi, S.M.; Ahmed, S.A.; Salman, A.D.; Juzsakova, T.; Cretescu, I.; Le, P.-C.; Chung, W.J.; Chang, S.W.; Nguyen, D.D. Can electrocoagulation technology be integrated with wastewater treatment systems to improve treatment efficiency? Environ. Res. 2022, 214, 113890. [Google Scholar] [CrossRef]
- Medina Collana, J.T.; Ayllon Ormeño, M.; Julca Meza, C.; Moreyra Cuadros, G.; Carrasco Venegas, L.A.; Ancieta Dextre, C.A.; Rodríguez Taranco, O.J.; Avelino Carhuaricra, C.; Diaz Bravo, P.; Montaño Pisfil, J.A. Processes Coupled to Electrocoagulation for the Treatment of Distillery Wastewaters. Sustainability 2024, 16, 6383. [Google Scholar] [CrossRef]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Tegladza, I.-D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Y.; Cotterill, S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water 2023, 15, 1455. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Niaragh, E.K.; Usman, M.; Khan, S.U.; Sandoval, M.A.; Al-Qodah, Z.; Khalid, Z.B.; Gihotra, V.; Emamjimeh, M.M. A critical review of state-of-the-art electrocoagulation technique applied to COD-rich industrial wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 43143–43172. [Google Scholar] [CrossRef]
- Das, P.P.; Sharma, M.; Purkait, M.K. Recent progress on electrocoagulation process for wastewater treatment: A review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Mousa, D.T.; El-Naas, M.H.; Nasser, M.; AL-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Bharath, M.; Krishna, B.M.; Manoj Kumar, B. A Review of Electrocoagulation Process for Wastewater Treatment. Int. J. Chem Tech Res. 2018, 11, 289–302. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Tuomikoski, S.; Lassi, U. Electrocoagulation Sludge Valorization—A Review. Resources 2021, 10, 127. [Google Scholar] [CrossRef]
- Ammar, M.; Yousef, E.; Mahmoud, M.A.; Ashraf, S.; Baltrusaitis, J. A Comprehensive Review of the Developments in Electrocoagulation for the Removal of Contaminants from Wastewater. Separations 2023, 10, 337. [Google Scholar] [CrossRef]
- Millán, M.; Fernández-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Management of solar energy to power electrochemical wastewater treatments. J. Water Process Eng. 2021, 41, 102056. [Google Scholar] [CrossRef]
- Twizerimana, P.; Wu, Y. Overview of integrated electrocoagulation-adsorption strategies for the removal of heavy metal pollutants from wastewater. Discov. Chem. Eng. 2024, 4, 14. [Google Scholar] [CrossRef]
- Tang, G. A literature review of electrocoagulation integrated systems on wastewater treatment. Theor. Nat. Sci. 2024, 47, 102–109. [Google Scholar] [CrossRef]
- Mirkhalafi, S.; Hashim, K.S.; Al-Hashimi, O.; Majdi, A. Hybrid Electrocoagulation–Adsorption Process for Montelukast Sodium Removal from Water. Clean Technol. 2024, 6, 1537–1564. [Google Scholar] [CrossRef]
- Perez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Vukojević Medvidović, N.; Vrsalović, L.; Svilović, S.; Magaš, K.; Jozić, D.; Čović, A. Electrocoagulation Combined with Synthetic Zeolite—Does the Size of Zeolite Particles Matter? Minerals 2023, 13, 1141. [Google Scholar] [CrossRef]
- Cui, H.; Yin, L.; Huang, X.; Yu, Z.; Zhang, Z.; Dai, Z. Zeolite fly ash-enhanced coagulation treatment of oil recovery wastewater from polymer flooding. Environ. Sci. Pollut. Res. Int. 2022, 29, 90318–90327. [Google Scholar] [CrossRef]
- Kumari, S.; Chowdhry, J.; Kumar, M.; Garg, M.C. Zeolites in wastewater treatment: A comprehensive review on scientometric analysis, adsorption mechanisms, and future prospects. Environ. Res. 2024, 260, 119782. [Google Scholar] [CrossRef] [PubMed]
- Svilović, S.; Vukojević Medvidović, N.; Vrsalović, L.; Kulić, A. Combining natural zeolite and electrocoagulation with different electrode materials–electrode surface analysis and Taguchi optimization. Appl. Surf. Sci. Adv. 2022, 12, 100330. [Google Scholar] [CrossRef]
- Vukojević Medvidović, N.; Vrsalović, L.; Svilović, S.; Bobanović, A. Electrocoagulation vs. Integrate Electrocoagulation-Natural Zeolite for Treatment of Biowaste Compost Leachate—Whether the Optimum Is Truly Optimal. Minerals 2022, 12, 442. [Google Scholar] [CrossRef]
- Vrsalović, L.; Vukojević Medvidović, N.; Svilović, S.; Pavlinović, A. Taguchi method in the optimisation of municipal wastewater treatment by electrocoagulation integrated with zeolite. Energy Rep. 2023, 9, 59–76. [Google Scholar] [CrossRef]
- Bašić, A.; Penga, Ž.; Mužek, M.N.; Svilović, S. Impact of turbine impeller blade inclination on the batch sorption process. Results Eng. 2022, 16, 100554. [Google Scholar] [CrossRef]
- Ducoste, J.J.; Clark, M.M. The Influence of Tank Size and Impeller Geometry on Turbulent Flocculation: I. Experimental. Environ. Eng. Sci. 1998, 15, 215–224. [Google Scholar] [CrossRef]
- Song, P.; Yang, Z.; Zeng, G.; Yang, X.; Xu, H.; Wang, L.; Xu, R.; Xiong, W.; Ahmad, K. Electrocoagulation treatment of arsenic in wastewaters: A comprehensive review. Chem. Eng. J. 2017, 317, 707–725. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, Y.; He, X.; Liu, H.; Ullah, Y.; Bilal, H.; Lu, J. Mass transfer and multi-phase fluid flow in electro-coagulation: A review. Water Res. 2025, 282, 123715. [Google Scholar] [CrossRef]
- Huerta-Chavez, O.; Rodriguez-Arias, Y.; Mendoza-Escamilla, V.X.; Mollinedo, H.; Morales-Mora, M.A.; Martinez-Delgadillo, S.A. The effect of internal impellers on mixing in an electrochemical reactor with rotating rings electrodes. Chem. Eng. Process. Process Intensif. 2015, 88, 37–46. [Google Scholar] [CrossRef]
- Nugroho, F.A.; Aryanti, P.T.P.; Dania, A.A.; Sari, F.F. The influence of mixing on electrocoagulation performance during soy sauce wastewater treatment. In Proceedings of the 7th International Conference on Industrial, Mechanical, Electrical and Chemical Engineering, Surakarta, Indonesia, 5 October 2021. [Google Scholar] [CrossRef]
- Nugroho, F.A.; Lestari, S.K.; Pebriani, W.; Aryanti, P.T.P. Hotel Wastewater Treatment by Integrating Mixing and Electrocoagulation Process. J. Phys. Conf. Ser. 2021, 1764, 012154. [Google Scholar] [CrossRef]
- Vukojević Medvidović, N.; Vrsalović, L.; Svilović, S.; Gudić, S.; Čule, I. Sono- and Zeolite-Assisted Electrocoagulation for Compost Wastewater Treatment: Does Ultrasound Power Make a Difference? Minerals 2024, 14, 1190. [Google Scholar] [CrossRef]
- Vukojević Medvidović, N.; Vrsalović, L.; Svilović, S.; Gudić, S.; Peran, L. Hybrid Electrocoagulation with Al Electrodes Assisted by Magnet and Zeolite: How Effective Is It for Compost Wastewater Treatment? Appl. Sci. 2025, 15, 8194. [Google Scholar] [CrossRef]
- Hashem, S.A.M.; Gaber, G.A.; Hussein, W.A.; Ahmed, A.S.I. Electrocoagulation process with Fe/Al electrodes to eliminate pollutants from real and synthetic wastewater. Results Mater. 2024, 23, 100606. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF): Washington, DC, USA, 2005. [Google Scholar]
- Kyziol-Komosinska, J.; Pająk, M.; Dzieniszewska, A.; Panek, R. Comparative study on cadmium ion removal from acidic solutions using zeolites and bentonite. Sci. Rep. 2025, 15, 10125. [Google Scholar] [CrossRef]
- Fan, X.; Liu, H.; Anang, E.; Ren, D. Effects of Electronegativity and Hydration Energy on the Selective Adsorption of Heavy Metal Ions by Synthetic NaX Zeolite. Materials 2021, 14, 4066. [Google Scholar] [CrossRef]
- Vukojević Medvidović, N.; Vrsalović, L.; Svilović, S.; Bilušić, A.; Jozić, D. Electrocoagulation treatment of compost leachate using aluminium alloy, carbon steel and zinc anode. Appl. Surf. Sci. Adv. 2023, 15, 100404. [Google Scholar] [CrossRef]
- Reátegui-Romero, W.; Morales-Quevedo, S.E.; Huanca-Colos, K.W.; Figueroa-Gómez, N.M.; King-Santos, M.E.; Zaldivar-Alvarez, W.F.; Flores-Del Pino, L.V.; Yuli-Posadas, R.A.; Bulege-Gutiérrez, B. Effect of current density on COD removal efficiency for wastewater using the electrocoagulation process. Desalin. Water Treat. 2020, 184, 15–29. [Google Scholar] [CrossRef]
- Tanatti, N.P.; Sezer, M. Optimizing electrocoagulation for poultry slaughterhouse wastewater treatment: A fuzzy axiomatic design approach. Environ. Sci. Pollut. Res. 2024, 31, 31159–31173. [Google Scholar] [CrossRef]
- Croatian Regulation on Emission Limits Values in Wastewaeter, NN 26/2020. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2020_03_26_622.html (accessed on 2 September 2025). (In Croatian).
- Syafalni, S.; Johari, N.A.; Satrio, S. Pre Treatment of River Water by Using Bentonite and Modified Zeolite. Int. J. Appl. Eng. Res. 2015, 10, 14515–14528. [Google Scholar]
- Arbabi, M.; Samaneh, S.; Sadeghi, M.; Abbas, K.; Ashkan, A.; Arman, A. Electrocoagulation Process Using Aluminum Electrodes for Treatment of Baker’s Yeast Industry Wastewater. Int. J. Environ. Health Eng. 2022, 11, 3. [Google Scholar] [CrossRef]
- Jallouli, S.; Wali, W.; Buonerba, A.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Ksibi, M. Efficient and sustainable treatment of tannery wastewater by a sequential electrocoagulation-UV photolytic process. J. Water Process Eng. 2020, 38, 101642. [Google Scholar] [CrossRef]
- Sridhar, R.; Sivakumar, V.; Prince Immanuel, V.; Prakash Maran, J. Treatment of pulp and paper industry bleaching effluent by electrocoagulant process. J. Hazard. Mater. 2011, 186, 1495–1502. [Google Scholar] [CrossRef]
- Mechelhoff, M.; Kelsall, G.H.; Graham, N.J.D. Super-faradaic charge yields for aluminium dissolution in neutral aqueous solutions. Chem. Eng. Sci. 2013, 95, 353–359. [Google Scholar] [CrossRef]
- Ozyonar, F.; Karagozoglu, B. Operating cost analysis and treatment of domestic wastewater by electrocoagulation using aluminium electrodes. Pol. J. Environ. Stud. 2011, 20, 173–179. [Google Scholar]
- Kobya, M.; Hiz, H.; Senturk, E.; Aydiner, C.; Demirbas, E. Treatment of Potato Chips Manufacturing Wastewater by Electrocoagulation. Desalination 2006, 190, 201–211. [Google Scholar] [CrossRef]
- Eskibalci, M.F.; Ozkan, M.F. Comparison of conventional coagulation and electrocoagulation methods for dewatering of coal preparation plant. Miner. Eng. 2018, 122, 106–112. [Google Scholar] [CrossRef]
- Chow, H.; Ingelsson, M.; Roberts, E.P.L.; Pham, A.L. How does periodic polarity reversal affect the faradaic efficiency and electrode fouling during iron electrocoagulation? Water Res. 2021, 203, 117497. [Google Scholar] [CrossRef]
- Kobya, M.; Ciftci, C.; Bayramoglu, M.; Sensoy, M.T. Study on the treatment of waste metal cutting fluids using electrocoagulation. Sep. Purif. Technol. 2008, 60, 285–291. [Google Scholar] [CrossRef]
- Hellal, M.S.; Doma, H.S.; Abou-Taleb, E.M. Techno-economic evaluation of electrocoagulation for cattle slaughterhouse wastewater treatment using aluminum electrodes in batch and continuous experiment. Sustain. Environ. Res. 2023, 33, 2. [Google Scholar] [CrossRef]
- Özdemir, U.; Özbay, B.; Özbay, I.; Veli, S. Application of Taguchi L32 orthogonal array design to optimize copper biosorption by using Spaghnum moss. Ecotoxicol. Environ. Saf. 2014, 17, 229–235. [Google Scholar] [CrossRef]
- Ozyonar, F. Optimization of operational parameters of electrocoagulation process for real textile wastewater treatment using Taguchi experimental design method. Desalin. Water Treat. 2016, 57, 2389–2399. [Google Scholar] [CrossRef]
- Chen, W.-H.; Carrera Uribe, M.; Luo, D.; Jin, L.; Saw, L.H.; Lamba, R. Taguchi optimization and analysis of variance for thermoelectric generators with forced convection air cooling. Appl. Therm. Eng. 2023, 231, 120878. [Google Scholar] [CrossRef]
- Asgari, A.; Kamalabadi, M.; Farzinia, H. Electrochemical Removal of Methylene Blue from Aqueous Solutions Using Taguchi Experimental Design. Chem. Biochem. Eng. Q. 2012, 26, 145–154. [Google Scholar]
- Praful, N.K.; Pattnaik, B.K.; Das, S. Comparative evaluation between Taguchi method and response surface method for optimization of electrocoagulation process in the context of treatment of dairy industry wastewater. Environ. Monit. Assess. 2024, 196, 663. [Google Scholar] [CrossRef] [PubMed]
- Chanwala, J.; Kaushik, G.; Dar, M.A.; Upadhyay, S.; Agrawal, A. Process optimization and enhanced decolorization of textile effluent by Planococcus sp. isolated from textile sludge. Environ. Technol. Innov. 2019, 13, 122–129. [Google Scholar] [CrossRef]
- Lin, H.; Huang, X.; Chang, J.; Li, B.; Bai, Y.; Su, B.; Shi, L.; Dong, Y. Improving sludge settling performance of secondary settling tank and simultaneously adsorbing nitrate and phosphate with surfactant modified zeolite (SMZ) ballasted flocculation. J. Environ. Chem. Eng. 2023, 11, 109650. [Google Scholar] [CrossRef]
- Akarsu, C.; Deniz, F. Electrocoagulation/Electroflotation Process for Removal of Organics and Microplastics in Laundry Wastewater. Clean Soil Air Water 2020, 49, 2000146. [Google Scholar] [CrossRef]







| Exp. Conditions | Zeolit | Electrode Type | Contact Time, min | Impeller Type |
|---|---|---|---|---|
| First experiment series | ||||
| ECZ, Al, SBT | Yes | Al | 30 | SBT |
| ECZ, Al, PBT | Yes | Al | 30 | PBT |
| ECZ, Fe, SBT | Yes | Fe | 30 | SBT |
| ECZ, Fe, PBT | Yes | Fe | 30 | PBT |
| Note: Constant conditions: synthetic zeolite addition (NaX, <40 µm, 15.00 g/dm3), fixed inter-electrode distance of 3 cm, constant current density of 0.0182 A/cm2, solution volume 500 cm3, contact time of 30 min. | ||||
| Second experiment series | ||||
| EC, Al, SBT, 10 min | No | Al | 10 | SBT |
| ECZ, Al, SBT, 20 min | Yes | Al | 20 | SBT |
| ECZ, Al, PBT, 10 min | Yes | Al | 10 | PBT |
| EC, Al, PBT, 20 min | No | Al | 20 | PBT |
| ECZ, Fe, SBT, 10 min | Yes | Fe | 10 | SBT |
| EC, Fe, SBT, 20 min | No | Fe | 20 | SBT |
| EC, Fe, PBT, 10 min | No | Fe | 10 | PBT |
| ECZ, Fe, PBT, 20 min | Yes | Fe | 20 | PBT |
| Note: Constant condition: fixed inter-electrode distance of 3 cm, constant current density of 0.0182 A/cm2, solution volume 500 cm3. | ||||
| Experiment Mark | Current A | Voltage V | Cenergy kWh/m3 | Theoretical Mass Loss | Actual Electrode Mass Loss | FE, % | Operating Cost €/m3 | |
|---|---|---|---|---|---|---|---|---|
| Celectrode kg/m3 | Canode kg/m3 | Ccathode kg/m3 | ||||||
| First experiment series | ||||||||
| ECZ, Al, SBT, 30 min | 0.868 | 19.120 | 16.600 | 0.291 | 0.390 | 0.027 | 134.02 | 2.60 |
| ECZ, Al, PBT, 30 min | 0.868 | 20.760 | 18.020 | 0.291 | 0.340 | 0.116 | 116.84 | 2.63 |
| ECZ, Fe, SBT, 30 min | 0.382 | 11.300 | 4.320 | 0.398 | 0.430 | −0.010 | 108.04 | 0.83 |
| ECZ, Fe, PBT, 30 min | 0.382 | 15.050 | 5.750 | 0.398 | 0.410 | −0.003 | 103.02 | 0.96 |
| Second experiment series | ||||||||
| EC, Al, SBT, 10 min | 0.862 | 20.850 | 6.110 | 0.096 | 0.140 | 0.005 | 145.83 | 0.95 |
| ECZ, Al, SBT, 20 min | 0.878 | 10.110 | 6.040 | 0.196 | 0.270 | 0.034 | 137.76 | 1.26 |
| ECZ, Al, PBT, 10 min | 0.878 | 19.060 | 5.690 | 0.098 | 0.090 | −0.006 | 91.84 | 0.89 |
| EC, Al, PBT, 20 min | 0.862 | 17.120 | 10.040 | 0.193 | 0.260 | −0.005 | 134.72 | 1.56 |
| ECZ, Fe, SBT, 10 min | 0.382 | 11.250 | 1.460 | 0.133 | 0.140 | −0.006 | 105.26 | 0.27 |
| EC, Fe, SBT, 20 min | 0.382 | 13.990 | 3.630 | 0.265 | 0.280 | 0.004 | 105.66 | 0.64 |
| EC, Fe, PBT, 10 min | 0.382 | 11.750 | 1.530 | 0.133 | 0.150 | −0.007 | 112.78 | 0.30 |
| ECZ, Fe, PBT, 20 min | 0.382 | 14.460 | 3.760 | 0.265 | 0.290 | 0.001 | 109.43 | 0.68 |
| Factors | L1 | L2 |
|---|---|---|
| M: electrodes material | Al | Fe |
| I: impeller type | SBT | PBT |
| t: process time | 10 min | 20 min |
| Z: zeolite addition | no | yes |
| Experimental Mark | Experimental Conditions | COD, mg O2/dm3 | Electrodes Consumption | S/NSB Ratio COD | S/NSB Ratio Electrode Consumption |
|---|---|---|---|---|---|
| F1 | Al, SBT, 10 min, EC | 159.20 | 0.0722 | −44.039 | 22.829 |
| F2 | Al, SBT, 20 min, ECZ | 248.75 | 0.1533 | −47.915 | 16.289 |
| F3 | Al, PBT, 10 min, ECZ | 256.71 | 0.0412 | −48.189 | 27.702 |
| F4 | Al, PBT, 20 min, EC | 135.32 | 0.1299 | −42.627 | 17.728 |
| F5 | Fe, SBT, 10 min, ECZ | 328.35 | 0.0657 | −50.327 | 23.649 |
| F6 | Fe, SBT, 20 min, EC | 149.25 | 0.1419 | −43.478 | 16.96 |
| F7 | Fe, PBT, 10 min, EC | 220.89 | 0.0693 | −46.884 | 23.185 |
| F8 | Fe, PBT, 20 min, ECZ | 276.61 | 0.1456 | −48.837 | 16.737 |
| COD—Means | COD—S/N | Electrode Consumption—Means | Electrode Consumption—S/N | |||||
|---|---|---|---|---|---|---|---|---|
| Factor | Delta | Rank | Delta | Rank | Delta | Rank | Delta | Rank |
| M | 43.8 | 2 | 1.69 | 2 | 0.0065 | 3 | 1.00 | 3 |
| I | 1.0 | 4 | 0.19 | 4 | 0.0118 | 2 | 1.41 | 2 |
| T | 38.8 | 3 | 1.64 | 3 | 0.0806 | 1 | 7.41 | 1 |
| Z | 111.4 | 1 | 4.56 | 1 | 0.0019 | 4 | 0.92 | 4 |
| Optimum | M1, I1, t2, Z1 | M1, I1, t2, Z1 | M1, I2, t1, Z2 | M1, I2, t1, Z2 | ||||
| Factor | DF | pC, % | SS | MS | p Value | Significance | |
|---|---|---|---|---|---|---|---|
| COD—Means | M | 1 | 11.63 | 3833.4 | 3833.4 | 0.058 | marginally significant |
| I | 1 | 0.01 | 2.0 | 2.0 | 0.950 | not significant | |
| t | 1 | 9.14 | 3011.7 | 3011.7 | 0.076 | marginally significant | |
| Z | 1 | 75.35 | 24,837 | 24,837 | 0.005 | highly significant | |
| Error | 3 | 3.88 | 1279.1 | 436.4 | |||
| Total | 7 | 100.00 | |||||
| COD—S/N | M | 1 | 10.32 | 5.705 | 5.705 | 0.079 | marginally significant |
| I | 1 | 0.14 | 0.076 | 0.076 | 0.783 | not significant | |
| t | 1 | 9.79 | 5.412 | 5.412 | 0.084 | marginally significant | |
| Z | 1 | 75.23 | 41.589 | 41.589 | 0.006 | highly significant | |
| Error | 3 | 4.53 | 2.504 | 0.835 | |||
| Total | 7 | 100.00 | |||||
| Electrode consumption- Means | M | 1 | 0.60 | 0.00008 | 0.00008 | 0.537 | not significant |
| I | 1 | 2.00 | 0.00028 | 0.00028 | 0.296 | not significant | |
| t | 1 | 93.59 | 0.01299 | 0.01299 | 0.003 | highly significant | |
| Z | 1 | 0.05 | 0.00001 | 0.00001 | 0.853 | not significant | |
| Error | 3 | 3.75 | 0.00052 | 0.00017 | |||
| Total | 7 | 100.00 | |||||
| Electrode consumption—S/N | M | 1 | 1.60 | 2.017 | 2.017 | 0.468 | not significant |
| I | 1 | 3.13 | 3.954 | 3.954 | 0.330 | not significant | |
| t | 1 | 86.96 | 109.9 | 109,9 | 0.009 | highly significant | |
| Z | 1 | 1.34 | 1.687 | 1.687 | 0.504 | not significant | |
| Error | 3 | 6.98 | 8.824 | 2.941 | |||
| Total | 7 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svilović, S.; Vukojević Medvidović, N.; Vrsalović, L.; Gudić, S.; Bašić, A.; Dujmović, K. Intensification of Electrocoagulation in Compost-Derived Wastewater. Processes 2025, 13, 3207. https://doi.org/10.3390/pr13103207
Svilović S, Vukojević Medvidović N, Vrsalović L, Gudić S, Bašić A, Dujmović K. Intensification of Electrocoagulation in Compost-Derived Wastewater. Processes. 2025; 13(10):3207. https://doi.org/10.3390/pr13103207
Chicago/Turabian StyleSvilović, Sandra, Nediljka Vukojević Medvidović, Ladislav Vrsalović, Senka Gudić, Anita Bašić, and Klara Dujmović. 2025. "Intensification of Electrocoagulation in Compost-Derived Wastewater" Processes 13, no. 10: 3207. https://doi.org/10.3390/pr13103207
APA StyleSvilović, S., Vukojević Medvidović, N., Vrsalović, L., Gudić, S., Bašić, A., & Dujmović, K. (2025). Intensification of Electrocoagulation in Compost-Derived Wastewater. Processes, 13(10), 3207. https://doi.org/10.3390/pr13103207








