Abstract
Chlorpyrifos (CHP) is a persistent organophosphate pesticide whose presence in water poses serious ecological and health risks. Here, we report a sustainable adsorbent obtained by high-temperature carbonization of immature walnuts (Juglans regia). The adsorbent’s structure, surface chemistry, and charge properties were comprehensively characterized using FTIR, SEM-EDX, zeta potential measurement, BET analysis, and XPS. The synthesis yielded a mesoporous carbon material with a BET surface area of 303 m2 g−1. Its performance in CHP removal was assessed under batch and dynamic conditions. Adsorption followed pseudo-second-order kinetics (k2 = 0.122 mg min−1 g−1; contact time 0–120 min). Isotherm experiments performed at 20, 25, and 30 °C, with equilibrium data best described by the Langmuir and Sips models, reaching a maximum capacity of 43.2 mg g−1. Thermodynamic analysis indicated a spontaneous and endothermic process. The adsorbent demonstrated selectivity for CHP over chlorpyrifos-oxon (CPO) in binary mixtures, retained its efficiency over at least ten regeneration cycles with ethanol, and removed up to 90% of CHP toxicity, as measured by acetylcholinesterase inhibition. Dynamic filtration confirmed its applicability under flow conditions. These findings demonstrate that the investigated adsorbent is an effective, reusable, and selective adsorbent, offering a low-cost and eco-friendly approach to pesticide removal from contaminated waters.
Keywords:
chlorpyrifos; adsorption; biochar; walnut-derived carbon; water treatment; selectivity; regeneration 1. Introduction
The contamination of water bodies with organophosphorus pesticides (OPs) has become a global environmental and public health concern, primarily due to their extensive application in modern agriculture [1,2]. Among these compounds, chlorpyrifos (CHP) is widely used for pest control in crops, soil, and residential areas [3,4]. However, its chemical stability and moderate water solubility allow it to persist in the environment, where it can enter aquatic systems through surface runoff, leaching, or improper disposal practices [5,6].
A particularly troubling aspect of CHP pollution is its biotransformation into chlorpyrifos-oxon (CPO), a metabolite formed under oxidative conditions in water and living organisms [7]. This oxon derivative is significantly more toxic and reactive [8], with a much higher potential for acetylcholinesterase (AChE) inhibition, disrupting nerve function in both humans and wildlife [9]. Even at trace concentrations, these compounds can exert chronic toxic effects on aquatic ecosystems, including behavioral changes, reproductive failure, growth inhibition, and increased mortality in fish, amphibians, and invertebrates [10].
In terms of human exposure, CHP and its oxon metabolite CPO have been associated with neurological disorders, developmental delays in children, endocrine disruption, and possible carcinogenic effects [11,12,13]. The increasing detection of these substances in drinking water sources, rivers, and agricultural runoff has, therefore, necessitated the development of effective and sustainable removal strategies.
Traditional treatment methods, such as advanced oxidation processes, photodegradation, biodegradation, and chemical hydrolysis, can reduce the concentrations of such pollutants; however, they often suffer from significant limitations. These include high energy requirements, slow kinetics, incomplete mineralization, and the formation of hazardous by-products [2,14,15,16]. Moreover, some of these methods may not be suitable for decentralized or rural water treatment systems due to their complexity and cost.
Among various remediation techniques, adsorption has emerged as one of the most widely applied and effective methods for removing organic pollutants from water due to its operational simplicity, cost-effectiveness, and adaptability [17,18,19]. It relies on the interaction between pollutants and solid adsorbents, which can be natural materials or derived from organic and industrial waste [20,21]. The adsorption efficiency is influenced by the physical properties of the adsorbent, such as pore size and surface area, as well as chemical interactions determined by the functional groups present on both the adsorbent and the adsorbate [22,23]. For example, steric effects and molecular size differences significantly affect adsorption, as demonstrated by the markedly higher adsorption efficiency of methyl parathion compared to ethyl parathion on certain adsorbents [24].
The development of sustainable adsorbents has sparked interest in biochar, a carbon-rich material produced via pyrolysis of biomass [25,26]. Utilizing agricultural residues and plant-based waste for carbon material production aligns with the principles of the circular economy and promotes environmental sustainability. One promising yet underutilized biomass source is young (immature) walnuts (Juglans regia), typically discarded despite their richness in lignocellulosic compounds favorable for carbonization [27,28,29,30]. Preliminary studies suggest that carbonized agricultural biomass can exhibit adsorption capacities comparable to those of conventional activated carbon, due to the presence of various functional groups that facilitate interactions with organic molecules (e.g., –OH, –COOH) [31,32]. As young walnuts are harvested before full lignification, they offer a softer, more homogeneous matrix, which upon pyrolysis yields a highly porous carbon structure with well-developed micro- and mesoporosity, which is a key for the effective adsorption of small organic pollutants, such as organophosphate pesticides [32].
Although previous studies have demonstrated the potential of walnut-derived biochars for the removal of heavy metals and dyes [31], their application in organophosphate pesticide remediation remains insufficiently explored. This study addresses this gap by proposing carbon materials derived from young walnuts as selective and sustainable adsorbents for the removal of CHP from aqueous media. The use of carbon materials derived from agricultural waste and plant residues supports the Sustainable Development Goals, particularly SDG 6 (Clean Water and Sanitation) and SDG 12 (Responsible Consumption and Production) [33]. This approach mitigates environmental pollution, promotes waste valorization, reduces reliance on costly conventional adsorbents, and supports circular economy models.
In our previous study [34], carbons obtained from walnut liqueur pomace (an ethanol-extracted by-product) were investigated for CHP removal. In contrast, this work introduces a different precursor material (immature whole walnuts) that had not been tested previously for adsorption applications. Moreover, the present study addresses the removal of CHP from binary mixtures containing also its more toxic oxon metabolite CPO, with an emphasis on the adsorbent’s affinity and discrimination between structurally similar compounds. The novelty of this work further lies in the comprehensive regeneration study, where the adsorbent was tested over ten consecutive cycles using different eluents, providing a much deeper insight into its reusability compared to previous reports. In addition, the study includes full physicochemical characterization and systematic adsorption performance evaluation under both batch and dynamic conditions. Finally, the study includes an evaluation of the toxicological profiles of the aqueous phase before and after adsorption, providing insight into the real-world safety and effectiveness of the developed material.
2. Materials and Methods
2.1. Materials Synthesis and Characterization
For the synthesis of the adsorbent, young walnuts (Juglans regia) obtained in Belgrade, Serbia, were used as the precursor. A batch of 5 immature green walnuts, each with an average radius of approximately 2 cm, was selected for this purpose. To remove any remaining moisture, the walnuts were subsequently oven-dried at 90 °C for 24 h. The carbonization process was carried out using an electric tube furnace (Protherm Furnaces, Ankara, Turkey) under a continuous nitrogen flow. The temperature was increased at a rate of 5 °C min−1 until it reached 900 °C, after which it was maintained isothermally for 1 h. Following carbonization, the resulting adsorbent was ground manually using an agate mortar and sieved through a 100 µm mesh sieve (Cisa Cedaceria Industrial S.L., Barcelona, Spain) to obtain a uniform powder. The carbon powder was then thoroughly washed with 0.1 mol dm−3 NaOH, 0.1 mol dm−3 HCl, and deionized water (Milli-Q purification system, Merck KGaA, Darmstadt, Germany; resistivity 18.2 MΩ) to remove any remaining inorganic residues. The final carbon powder was then dispersed in a 50% ethanol solution to produce stock suspensions with a concentration of 2 mg mL−1, intended for use in subsequent experimental procedures. All chemicals, including NaOH (≥98%, Centrohem, Stara Pazova, Serbia), HCl (37%, Centrohem, Stara Pazova, Serbia), ethanol (≥99.8%, Merck, Darmstadt, Germany), and acetonitrile (≥99.8%, J.T. Baker, Phillipsburg, NJ, USA), were of analytical grade and used without further purification.
A comprehensive set of physicochemical techniques was employed to characterize the structural, morphological, and chemical features of the adsorbent under investigation. Surface morphology and elemental composition were analyzed using a Phenom ProX scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectroscopy (EDX) system, both manufactured by Thermo Fisher Scientific (Waltham, MA, USA).
To identify surface functional groups and molecular bonding characteristics, Fourier-transform infrared (FTIR) spectroscopy was carried out using a Nicolet iS20 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Spectral data were collected in the 4000–400 cm−1 range, with each spectrum acquired by averaging 64 scans at a resolution of 4 cm−1.
Zeta potential measurements were performed with a Nano ZS Zetasizer (Malvern Instruments, Malvern, UK), utilizing a 633 nm He-Ne laser to evaluate the surface charge properties of the adsorbents. Each measurement represents the average of three independent runs.
Textural parameters, including specific surface area (SBET), total pore volume (Vtot), average pore diameter (dav), and pore size distribution (PSD), were determined from nitrogen adsorption–desorption isotherms at −196.15 °C using an Autosorb-iQ gas sorption analyzer (Anton Paar QuantaTec Inc., Graz, Austria). The BET method was applied to estimate surface areas [35], while the NLDFT model was used for assessing PSD. Prior to analysis, all samples were degassed under vacuum at 300 °C for 5 h to eliminate residual adsorbates and ensure reliable measurements.
The samples were analyzed using a SPECS system (SPECS GmbH, Berlin, Germany) equipped with an XP50M X-ray source, a Focus 500 monochromator, and a PHOIBOS 100/150 analyzer. Analysis was carried out with an AlKα X-ray source (1486.74 eV) at 12.5 kV and 32 mA. Survey spectra (0–1000 eV binding energy) were collected with a constant pass energy of 40 eV, a step size of 0.5 eV, and a dwell time of 0.2 s in FAT mode. Detailed spectra of C 1s and O 1s peaks were recorded using a constant pass energy of 20 eV, a step size of 0.1 eV, and a dwell time of 2 s in FAT mode. The pressure during the experiment was held at 3 × 10−9 mbar. All peak positions were calibrated against the C 1s peak at 284.8 eV.
2.2. Adsorption Experiments
Adsorption studies were carried out under both static (batch) and dynamic flow conditions. The batch experiments aimed to assess the interaction between CHP (Pestanal, Sigma Aldrich, Søborg, Denmark) and the synthesized adsorbent. For each adsorption test, equal volumes (0.5 mL) of stock dispersions of the adsorbent (2 mg mL−1, pH 6 ± 0.01) and CHP solutions in 50% ethanol–water (prepared by diluting the stock solution of 0.1 mol dm−3 CHP in absolute ethanol with deionized water) at various concentrations were combined and agitated on a laboratory shaker (Orbital Shaker Incubator Grant-bio ES-20, Grant-Bio, Cambridgeshire, UK) for specific time intervals. After adsorption, samples were centrifuged at 14,500 rpm for 15 min, and the supernatants were filtered through nylon membrane filters (220 nm pore size; KX Syringe Filter, Kinesis, Cole Parmer, St. Neots, UK) to eliminate suspended particles.
Due to the very low solubility of CHP in pure water, a 50% ethanol–water mixture was employed to ensure complete solubilization of the pesticide. This solvent composition was consistently used in all experiments to avoid variability, and blank controls confirmed that ethanol itself did not contribute to adsorption.
The residual OP concentrations were quantified using Ultra-Performance Liquid Chromatography (UPLC), performed on a Waters ACQUITY UPLC system equipped with a photodiode array (PDA) detector and managed through Empower software (version 3) (Waters GmbH, Eschborn, Germany). Separation was achieved on a BEH C18 column (1.7 μm, 100 mm × 2.1 mm) under isocratic elution conditions, using a mobile phase of 20% deionized water with 10% acetonitrile and 80% pure acetonitrile (J.T. Baker, Phillipsburg, NJ, USA). The flow rate was 0.2 mL min−1, with an injection volume of 5 μL, and detection was performed at 205 nm. The detection wavelength was established experimentally from UV absorbance spectra. Blank experiments without an adsorbent were used to ensure data accuracy.
Experiments were conducted at environmentally relevant conditions (i.e., temperatures of 20, 25, and 30 °C; pH 6; and CHP concentrations ranging from 5 × 10−6 to 5 × 10−4 mol dm−3) to mimic potential field applications. All trials were performed in triplicate, and results were presented as mean values with error bars representing the highest observed deviations.
To investigate adsorption kinetics, 1 mg mL−1 dispersions of adsorbent were mixed with 5 × 10−5 mol dm−3 CHP solutions at 20 °C. Contact times ranged from 1 to 120 min. CHP concentrations in the supernatant were measured by UPLC, and the amount adsorbed was calculated from the concentration difference before and after exposure. The experimental data were fitted to various kinetic models, including pseudo-first-order (Equation (1)), pseudo-second-order (Equation (2)), Elovich (Equation (3)), and intraparticle diffusion (Equation (4)). The model equations are detailed below:
In these kinetic models, qt denotes the amount of adsorbate adsorbed at time t (in mg g−1), while qe represents the equilibrium adsorption capacity (mg g−1). The rate constants k1 (min−1) and k2 (g mg−1 min−1) correspond to the pseudo-first-order and pseudo-second-order models, respectively. For the Elovich model, the parameters α and β denote the initial adsorption rate (mg g−1 min−1) and the desorption constant (g mg−1), respectively. The intraparticle diffusion model incorporates the rate constant kid (mg g−1 min−0.5), and the boundary layer thickness parameter C (mg g−1).
For isotherm analysis, adsorption was carried out for 60 min using an adsorbent concentration of 1 mg mL−1 and CHP solutions spanning from 5 × 10−6 to 5 × 10−4 mol dm−3 at three temperatures (20, 25, and 30 °C). The data were interpreted using the Freundlich (Equation (5)), Langmuir (Equation (6)), Temkin (Equation (7)), Dubinin–Radushkevich (Equation (8)), and Sips (Equation (9)) models. The equations are provided below:
In these isotherm models, qe represents the equilibrium adsorption capacity (mg g−1), while Ce denotes the equilibrium adsorbate concentration (mg dm−3). The Freundlich isotherm employs constants KF ((dm3 mg−1)1/n and n, which characterize adsorption capacity and intensity, respectively. The Langmuir model uses the Langmuir constant KL (dm3 mg−1) and qmax (mg g−1), representing the maximum adsorption capacity. For the Temkin isotherm, bT (J g mol−1 mg−1) and KT (dm3 mg−1) relate to the heat of adsorption and equilibrium binding constant. The Dubinin–Radushkevich isotherm defines qDR (mg g−1) as the theoretical saturation capacity, and KDR (mol2 J−2) as the constant linked to the mean free energy per mole of adsorbent, with ε = RT × ln(1 + 1/Ce). The Sips isotherm incorporates constants KS (dm3 mg−1) and bS, representing the affinity of binding sites and heterogeneity of adsorption sites, respectively.
Thermodynamic parameters—including enthalpy change (ΔH0), entropy change (ΔS0), and Gibbs free energy change (ΔG0)—were evaluated to provide insight into the interactions between contaminant molecules and the adsorbent surface. The values of ΔH0 and ΔS0 were obtained from the slope and intercept, respectively, of the Van’t Hoff plot constructed according to Equation (11), where the standard Gibbs free energy change is expressed as:
The dimensionless standard distribution coefficient was calculated using Equation (12). To ensure its dimensionlessness, the equilibrium adsorption constant was multiplied by the ratio of the standard states C0 and q0, which correspond to 1 mol dm−3 for the contaminant concentration in solution and 1 mol kg−1 for the adsorbed phase, respectively:
Finally, the standard Gibbs free energy change was also independently determined using the Gibbs–Helmholtz equation (Equation (13)):
Dynamic adsorption behavior was evaluated using modified commercial syringe filters. A total of 1 mg of adsorbent was dispersed in 1 mL of 50% ethanol and loaded into nylon syringe filters (220 nm pore size; KX Syringe Filter, Kinesis, Cole Parmer, St. Neots, UK). Compressed air was applied to remove residual liquid before introducing the pesticide solution. Then, 1 mL of CHP solution (5 × 10−5 mol dm−3) was passed through each modified filter at a constant flow rate of 1 mL min−1. The filtered solution was collected and analyzed via UPLC to determine the amount of pesticide retained.
2.3. Regeneration of Adsorbent
The regeneration of the adsorbent was tested by rinsing the modified filters with 5 mL of absolute ethanol and acetonitrile for 1 min. This procedure was repeated over multiple cycles to evaluate the stability and reusability of the adsorbent.
2.4. Adsorption of CHP and CPO from Binary Mixtures
To evaluate the efficiency of the adsorbent in removing CHP and CPO (Pestanal, Sigma Aldrich, Søborg, Denmark) from a binary mixture, adsorption experiments were conducted using an aqueous solution containing 5 × 10−5 mol dm−3 of each compound. The adsorbent dosage was set to 1 mg mL−1, and the mixture was incubated for 1 h at 20 °C. After adsorption, the residual concentrations of CHP and CPO were determined by UPLC at 205 nm.
2.5. Toxicity Evaluation
As organophosphate pesticides are recognized inhibitors of AChE, the toxicity of CHP was assessed before and after adsorption by measuring its inhibitory effect on AChE activity. A modified version of the Ellman assay [36,37] was applied. Commercially sourced AChE (2.5 IU; electric eel, Sigma Aldrich, Taufkirchen, Germany) was pre-incubated with CHP solutions for 20 min in 5 × 10−2 mol dm−3 phosphate buffer (pH 8.0) at 37 °C. The enzymatic reaction was initiated by the addition of acetylthiocholine iodide (AChI, 0.075 mol dm−3) and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, 1 × 10−4 mol dm−3), both supplied by Sigma Aldrich, St. Louis, MO, USA. After 8 min, the reaction was stopped using 10% sodium dodecyl sulfate (SDS). Thiocholine, the enzymatic product, reacts with DTNB to produce a yellow-colored 5-thio-2-nitrobenzoate, whose absorbance was measured at 412 nm. Enzyme concentration was held constant to ensure consistent spectrophotometric readings.
The percentage of AChE inhibition, used as an indicator of CHP toxicity, was calculated using the equation:
where Acontrol represents the enzyme activity in the absence of CHP, and Asample denotes the activity following pesticide exposure.
3. Results
3.1. Physicochemical Properties of the Synthesized Adsorbent
SEM analysis conducted at three magnification levels (Figure 1(a1–a3)) provided insight into the surface morphology of the adsorbent. The obtained micrographs show a rough and irregular surface, with well-defined pores clearly visible. Moreover, the sharp contours of the structures further emphasize the distinct textural features of the adsorbent.
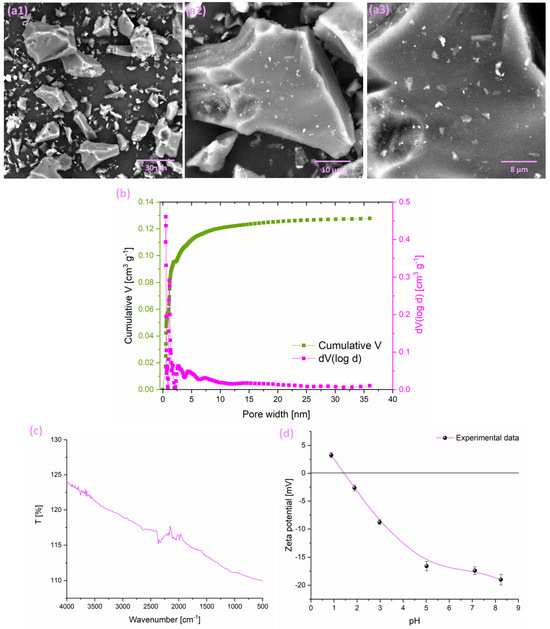
Figure 1.
(a) SEM images of the investigated adsorbent at (a1) 2000×, (a2) 5000×, and (a3) 10,000× magnification; (b) pore size distribution (adapted from [34]); (c) FTIR spectra; and (d) effect of pH on the zeta potential of adsorbent suspension.
The elemental composition of the investigated adsorbent, as determined by EDX analysis, shows that it is predominantly composed of carbon (80.82 at.%), indicating a primarily carbon-based structure. Oxygen (10.09 at.%) and nitrogen (7.19 at.%) are also present, originating from the precursor biomass and the carbonization process. Trace amounts of potassium (1.18 at.%), phosphorus (0.29 at.%), calcium (0.19 at.%), magnesium (0.16 at.%), sulfur (0.03 at.%), chlorine (0.02 at.%), and silicon (0.02 at.%) were also detected. These residual elements reflect the inherent mineral content of the walnut precursor and may influence the surface chemistry and performance of the carbonized adsorbent.
The gas adsorption analysis revealed (Figure 1b) that the adsorbent possesses a BET specific surface area of 303 m2 g−1 and a total pore volume of 0.139 cm3 g−1. The average pore diameter was calculated to be 2.08 nm, indicating porosity predominantly in the range of large micropores and small mesopores. These textural properties indicate a moderately developed porous structure, which is beneficial for adsorption applications as it provides abundant surface sites for interaction with target molecules.
The FTIR spectra of the adsorbent (Figure 1c) did not reveal distinct absorption bands corresponding to specific functional groups. This outcome is consistent with the high carbonization temperature of 900 °C applied during adsorbent preparation [34,38]. Such intense thermal treatment likely caused the decomposition of most organic functional groups, resulting in a mainly carbon-rich structure with only trace amounts of residual functionalities. Consequently, the typical vibrational signals associated with functional groups are either missing or considerably weakened.
Zeta potential measurements were performed on the investigated adsorbent at a concentration of 0.5 mg mL−1 to investigate its surface charge characteristics and to determine the isoelectric point (IEP). Titrations were carried out over a broad pH range from 1 to 14, using HCl and NaOH as titrating agents. The initial pH of the adsorbent’s stock suspension was measured at 6. As presented in Figure 1d, the isoelectric point of the adsorbent was identified at pH 1.42. Considering that the starting pH exceeds the IEP, it can be concluded that the adsorbent’s surface possesses a negative charge under the experimental conditions we applied for the adsorption measurements.
XPS measurements were used to determine surface composition and chemical bonds in the sample and are presented in Figure 2. Survey XPS spectra evidenced the presence of C (71.2 at.%), O (10.9 at.%), and K (17.9 at.%). No other elements are detected in the survey spectra. High-resolution C 1s spectra (Figure 2a) is deconvoluted into three components. The peak at 284.9 eV (45.4% of total C 1s emission) is attributed to C-C bonds, the peak at 285.5 eV (40.2% of total C 1s emission) is attributed to either C-O or C-OH bonds since both are located at approximately the same position and cannot be resolved in the C 1s spectra, and the final small peak at 287.5 eV is attributed to C=O bonds [39,40]. Two additional peaks at higher energies can be observed in the spectra, which correspond to K 2p 1/2 and K 2p 3/2 peaks. High-resolution spectra of O 1s (Figure 2b) is deconvoluted into three components. Peak at 531.5 eV (41.7% of total O 1s emission) is attributed to C=O bond. In the O 1s spectra, C-O and C-OH bonds can be resolved, and therefore, the peak at 533.2 eV (45.3% of total O 1s emission) is assigned to the C-O bond, while the peak at 536.6 eV (13.0% of total O 1s emission) is assigned to C-OH bonds [39,40].
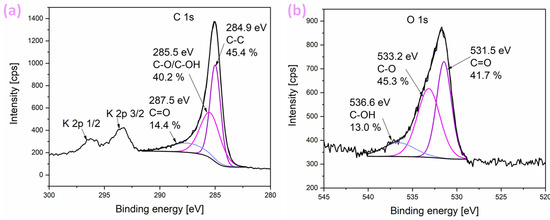
Figure 2.
XPS spectra of (a) C 1s spectra and (b) O 1s spectra. Relative fractions of each identified moiety are provided along with the corresponding assignation.
3.2. Adsorption Kinetics
The adsorption kinetics of the pesticide were investigated employing four kinetic models: pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion. The corresponding plots are shown in Figure 3, while the kinetic parameters obtained are summarized in Table 1.
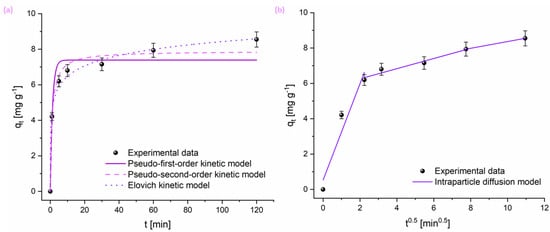
Figure 3.
Nonlinear model fitting of CHP adsorption kinetics on the adsorbent at 20 °C using: (a) pseudo-first-order, pseudo-second-order, and Elovich models and (b) the intraparticle diffusion model.

Table 1.
Kinetic parameters for the adsorption of CHP (5 × 10−5 mol dm−3) onto investigated adsorbent (1 mg mL−1) at 20 °C.
Based on the presented Figure 3, it can be observed that the equilibrium between the CHP and the investigated adsorbent was achieved after 60 min of contact time. Analysis of the data revealed that the pseudo-second-order model provided the best fit for the adsorption of CHP, as evidenced by the χ2 and R2 values. Additionally, the initial adsorption rate constant (α) derived from the Elovich model suggests a rapid uptake at the beginning, which gradually slows down as the adsorbent surface becomes saturated.
The intraparticle diffusion model further suggests that the adsorption process occurs through three distinct phases. Initially, pesticide molecules diffuse from the bulk solution to the external surface of the adsorbent. In the second phase, intraparticle diffusion occurs as molecules diffuse into the pores of the adsorbent. Finally, the process reaches adsorption equilibrium. During these stages, the boundary layer thickness increases, correlating with a higher amount of pesticide adsorbed on the surface. The intraparticle diffusion rate constant (kid) decreases over time, reflecting the gradual deceleration of the adsorption process. Moreover, the boundary layer thickness approaches a value similar to the equilibrium adsorption capacities (qe) predicted by both pseudo-first-order and pseudo-second-order models. The observed decline in kid values signals the completion of the adsorption process.
3.3. Adsorption Isotherms
The equilibrium data for CHP adsorption onto the adsorbent were analyzed using five isotherm models: the Freundlich, Langmuir, Temkin, Dubinin–Radushkevich, and Sips models. The corresponding parameters at 20, 25, and 30 °C are summarized in Table 2, while the corresponding plots are given in Figure 4.

Table 2.
Parameters for the adsorption of CHP onto the investigated adsorbent at 20, 25, and 30 °C.
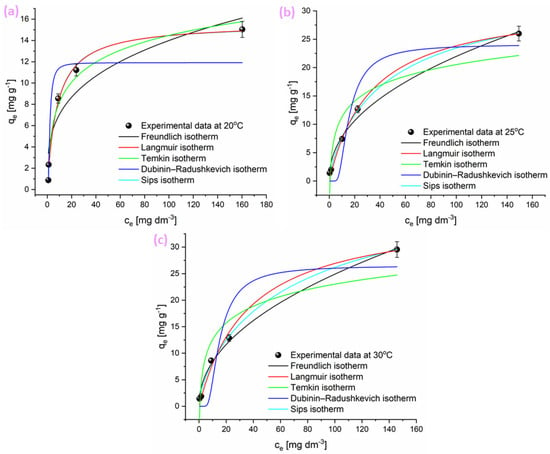
Figure 4.
Nonlinear model fitting of CHP adsorption isotherms on adsorbent at (a) 20, (b) 25, and (c) 30 °C using Langmuir, Freundlich, Temkin, Dubinin–Radushkevich, and Sips models.
Table 2 presents the key parameters characterizing the adsorption of CHP onto the investigated adsorbent, obtained through nonlinear fitting of various isotherm models. Among them, the Freundlich, Langmuir, and Sips isotherms exhibited the best agreement with the experimental data, as indicated by low χ2 values and high R2 values.
The n values obtained from the Freundlich model exceeded 1 across all temperatures, confirming that the adsorption of CHP onto the adsorbent is favorable at all temperatures. However, the gradual decrease in n with increasing temperature suggests a slight reduction in surface affinity, likely due to reduced adsorbate-adsorbent interactions or changes in surface energetics at higher temperatures. The KF constant also showed moderate variation, indicating a consistent but slightly temperature-dependent affinity of the adsorbent toward CHP.
By observing the Langmuir constant KL, which reflects the affinity between the adsorbate and the adsorbent, it can be seen that the KL values exhibited a decreasing trend with increasing temperature. This inverse relationship suggests a reduction in the binding affinity of the investigated adsorbent surface toward CHP molecules at higher temperatures. Such behavior may indicate that although the overall adsorption capacity increases due to enhanced molecular mobility and diffusion (as evidenced by rising qmax values), the individual interaction strength between active sites and adsorbate molecules weakens. This trend is characteristic of physisorption processes, where adsorption is primarily governed by weak van der Waals forces that are more easily disrupted by thermal agitation.
An increase in adsorption capacity with rising temperature was also observed. According to the Sips model, the maximum adsorption capacity ranged from 15.6 ± 0.2 to 43.2 ± 0.2 mg g−1, which aligns well with the values obtained from the Langmuir model, further reinforcing the reliability of the data. The heterogeneity parameter (bS) from the Sips model remained consistently below 1 at all temperatures, indicating that the investigated adsorbent surface is energetically heterogeneous with a broad distribution of adsorption sites.
The decline in the Temkin parameter (bT) with increasing temperature suggests a reduction in the strength of adsorbate–adsorbent interactions. This observation is further supported by the decreasing mean adsorption energy (E) values obtained from the Dubinin–Radushkevich model. These results indicate that the adsorption process is predominantly governed by physisorption, which is consistent with the previously stated.
3.4. Thermodynamic Analysis
The thermodynamic analysis of CHP adsorption onto the adsorbent provides important insights into the nature of the adsorption mechanism. The Van’t Hoff plot is presented in Figure 5, while corresponding parameters are presented in Table 3.
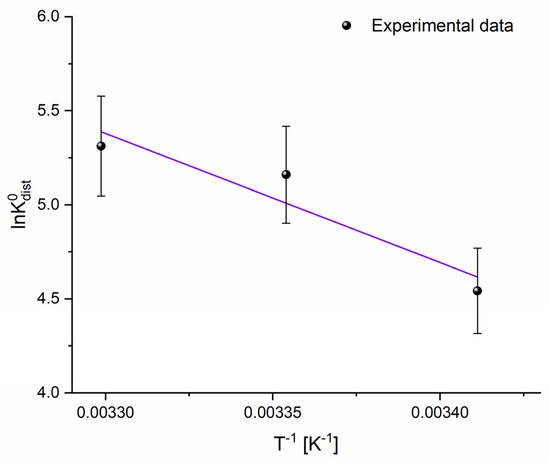
Figure 5.
Van’t Hoff plot.

Table 3.
Thermodynamic parameters of CHP adsorption onto the investigated adsorbent.
The positive ΔH0 value confirms the endothermic character of the process, implying that heat input facilitates adsorption. This observation is consistent with the trend of increasingly negative ΔG0 values with rising temperature, indicating that adsorption becomes more thermodynamically favorable at higher temperatures. The negative ΔG0 values across the studied temperature range confirm the spontaneous nature of the process. At the same time, the observed decrease from −(11 ± 2) to −(14 ± 1) kJ mol−1 further supports the temperature-dependent enhancement of adsorption efficiency. Such behavior is commonly attributed to the improved desolvation of the adsorbate at elevated temperatures, which exposes more active sites on the carbon surface and strengthens adsorbate–adsorbent interactions. Moreover, the positive ΔS0 value suggests an increase in randomness at the solid–liquid interface. These thermodynamic findings align well with previous kinetic and equilibrium studies, reinforcing the proposed adsorption mechanism. In this context, it is important to note that the use of a 50% ethanol–water solvent system, although necessary to dissolve CHP, may influence adsorption behavior by altering hydrophobic interactions and partially competing for adsorption sites. Nevertheless, since the solvent composition was kept constant in all experiments, the observed results reliably reflect the interaction between CHP and the adsorbent.
3.5. Selective Adsorption of CHP and CPO from Binary Mixtures
To further explore the adsorption behavior of the synthesized adsorbent in more complex systems, a mixture of CHP and CPO was investigated. As shown in the UPLC chromatogram (Figure 6), after 1 h of incubation with the adsorbent, a notable difference in the removal efficiency of the two compounds was observed. Specifically, the investigated adsorbent removed 44% of CHP, while only 11% of CPO was adsorbed under identical conditions. This result clearly demonstrates the selectivity of the adsorbent toward CHP. The higher adsorption of CHP relative to CPO is attributed to differences in hydrophobicity, surface affinity, and solvation. CHP is markedly more hydrophobic than CPO, which promotes stronger partitioning onto the largely hydrophobic carbon surface produced by high-temperature carbonization. In contrast, the substitution of the thiono group in CHP with a phosphoryl group in CPO increases molecular polarity, enhancing its affinity for the aqueous phase and lowering its interaction strength with the carbon surface. Hydration effects reinforce this difference. Using the Stokes–Einstein relationship, the hydrodynamic radius of solvated CHP in water can be estimated at ~0.3–0.6 nm (diameter ~0.6–1.2 nm). The greater polarity of CPO is expected to result in a more structured and extensive hydration shell, which will increase its effective solvated size, reduce the diffusion coefficient, and hinder penetration into smaller pores, as the studied adsorbent is dominantly microporous (Figure 1d). This larger solvation sphere also reduces the effectiveness of nonpolar surface interactions. Together, a higher hydrophobic affinity, weaker hydration, and smaller effective solvated size of CHP explain its preferential adsorption over CPO under the same experimental conditions.
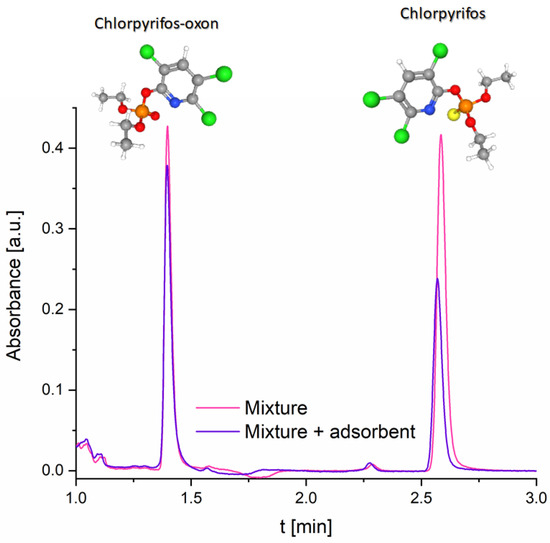
Figure 6.
UPLC chromatograms of a CHP and CPO mixture (5 × 10−5 mol dm−3) before and after adsorption using the investigated adsorbent; adsorbent dosage was 1 mg mL−1 and detection wavelength set at 205 nm. Structural formulas of CHP and CPO are also presented, with atoms color-coded as follows: grey—carbon, white—hydrogen, green—chlorine, blue—nitrogen, red—oxygen, orange—phosphorus, and yellow—sulfur.
Selective removal is advantageous not only for environmental remediation but also for applications in food safety, where targeted removal of toxic substances without affecting other constituents is essential.
In addition to its selectivity, the adsorption capacities obtained in this study for the adsorbent (15.6–36.6 mg g−1, based on the Langmuir model, and 15.6–43.2 mg g−1, based on the Sips model, depending on temperature) confirm its good performance in CHP removal. These values are significantly higher than those reported for several conventional biochars, such as those made from walnut shells or bagasse. They are comparable to materials like cashew nut shell biochar and sunflower seed shells. While still below the highest values achieved by modified or nanostructured adsorbents (e.g., cellulose-based carbon fibers or magnetic hydrochars), the results highlight young walnut as a competitive and sustainable precursor for the development of efficient adsorption materials. A detailed comparison with literature data is provided in Table 4.

Table 4.
Adsorption performance comparison with literature sources.
3.6. Dynamic Conditions, Regeneration, and Reusability of Adsorbent
The adsorption performance of the adsorbent was assessed under dynamic conditions using a filtration system with a 1 min contact time and an initial CHP concentration of 5 × 10−5 mol dm−3. Under static conditions, the adsorbent removed approximately 25% of CHP, whereas in the dynamic filtration setup, the removal reached around 10%. Although the removal efficiency in dynamic mode was lower, primarily due to the shorter contact time and continuous flow, the results confirm that the adsorbent can operate under filtration conditions and retain adsorption capacity in real-flow scenarios. It demonstrates its potential for integration into water treatment systems, where optimization of flow rate, adsorbent mass, and contact time could further enhance performance.
To assess the regeneration potential of the adsorbent, regeneration experiments were conducted using absolute ethanol and acetonitrile under dynamic filtration conditions. As illustrated by the data presented in Figure 7, the investigated adsorbent demonstrated remarkable stability and retention of adsorption capacity over at least ten adsorption–desorption cycles. After an initial slight decrease in adsorption efficiency between the initial adsorption and the first cycle, the removal performance remained essentially constant in subsequent cycles. This behavior highlights the robustness and good reusability of the synthesized adsorbent, which is a critical parameter for practical deployment in water treatment applications.
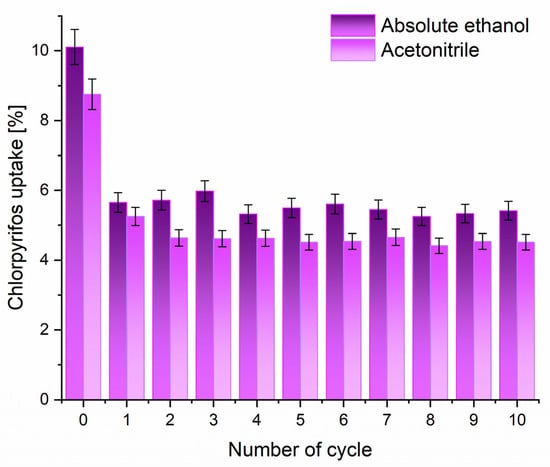
Figure 7.
Regeneration of investigated adsorbent (1 mg mL−1) after the adsorption of CHP (5 × 10−5 mol dm−3).
Although both absolute ethanol and acetonitrile demonstrated effective performance in regenerating this adsorbent, important distinctions arise when considering their environmental and safety profiles. In the case of acetonitrile, although it exhibits very good efficiency at regenerating the adsorbent, its use poses significant environmental and health concerns due to its toxicity, flammability, and challenges related to safe handling and disposal. In contrast, absolute ethanol, which is less toxic, biodegradable, and widely available, provided a comparable regeneration efficiency, making it a more sustainable and ecologically responsible choice.
Furthermore, the retention of adsorption capacity through multiple regeneration cycles without substantial degradation suggests that the pore structure and active sites of the investigated adsorbent remain largely intact, even after repeated solvent exposure. This stability indicates that the adsorbent’s physicochemical properties, such as surface area and pore volume, are resilient to solvent-induced structural changes, which is a key advantage over other carbonaceous adsorbents that often suffer from pore collapse or irreversible fouling.
3.7. Toxicity Assessment
To further assess the safety of the treated water, the residual neurotoxicity of CHP following adsorption was examined using the Ellman assay (Section 2.5). The initial CHP solution (1 × 10−5 mol dm−3) caused a 50% inhibition of AChE activity, reflecting its well-established neurotoxic properties [54]. In contrast, after exposure to the adsorbent, AChE inhibition dropped markedly to 10%, indicating a substantial reduction in the solution’s toxic potential.
This decrease in enzymatic inhibition demonstrates not only the efficient removal of the parent compound but also suggests that no more toxic metabolites, such as CPO, were generated during the adsorption process. These findings are particularly important from the perspective of water safety and human health risk assessment, as they confirm that the adsorbent not only eliminates the contaminant but also prevents the formation of harmful by-products.
4. Conclusions
This study demonstrates that the adsorbent obtained from immature walnuts via high-temperature carbonization is an efficient and sustainable adsorbent for the selective removal of CHP from water. The synthesized adsorbent exhibited a favorable mesoporous structure and suitable surface chemistry for pesticide adsorption. Adsorption kinetics and equilibrium modeling confirmed that the process is fast, spontaneous, and endothermic, with notable increases in capacity at higher temperatures. Importantly, the adsorbent displayed clear selectivity toward CHP over its more toxic oxon metabolite, highlighting its potential for targeted remediation in complex mixtures of contaminants. Dynamic filtration experiments confirmed its applicability under flow conditions, while regeneration studies showed that ethanol effectively restores adsorption capacity without compromising performance over multiple cycles. The significant reduction in acetylcholinesterase inhibition after treatment underscores its ability to improve water safety beyond mere contaminant removal. Owing to its low-cost, waste-derived origin, and simple preparation process, the walnut-derived carbon adsorbent offers a scalable solution for pesticide remediation. Its stability, reusability, and compatibility with dynamic filtration systems suggest strong potential for integration into existing municipal and decentralized water treatment units, enabling sustainable pollutant removal in both urban and rural settings.
Author Contributions
Conceptualization, T.T.; methodology, C.U., I.A.P., L.R. and T.L.-P.; validation, I.A.P. and T.L.-P.; formal analysis, R.K., T.T., V.M., V.J.A., L.R. and N.P.; investigation, R.K., T.T., V.M., V.J.A. and N.P.; resources, C.U., I.A.P. and T.L.-P.; data curation, R.K., T.T., V.M., V.J.A. and N.P.; writing—original draft preparation, L.R. and T.T.; writing—review and editing, C.U., I.A.P. and T.L.-P.; visualization, T.T.; supervision, T.L.-P.; project administration, T.L.-P.; funding acquisition, C.U., I.A.P. and T.L.-P. All authors have read and agreed to the published version of the manuscript.
Funding
T.L.-P., V.M., N.P., L.R. and T.T. acknowledge the support provided by the Serbian Ministry of Science, Technological Development and Innovations (contract number: 451-03-136/2025-03/200017). I.A.P. acknowledges the support provided by the Serbian Ministry of Science, Technological Development and Innovations (contract number: 451-03-137/2025-03/200146), and SASA (project no. F-49). V.J.A. acknowledges the support provided by the Serbian Ministry of Defence, Republic of Serbia (project name: Research on influence of characteristics of explosive ordnance on safety in Ministry of Defense and Army of Serbia, project code: VA-TT/1/22-24). C.U. gratefully acknowledges the financial support through the COMET Programme (Competence Centers for Excellent Technologies) funded by the Austrian ministries BMK and BMAW and the federal states of Upper Austria, Lower Austria, and Carinthia, operated by the Austrian Research Promotion Agency (FFG).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Christoph Unterwegerwas employed by the company Wood K Plus—Kompetenzzentrum Holz GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Mali, H.; Shah, C.; Raghunandan, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate pesticides an emerging environmental contaminant: Pollution, toxicity, bioremediation progress, and remaining challenges. J. Environ. Sci. 2023, 127, 234–250. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Wołejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A.; Pietruszyńska, M.; Wydro, U. Chlorpyrifos occurrence and toxicological risk assessment: A review. Int. J. Environ. Res. Public Health 2022, 19, 12209. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.Y.; Ma, N.L.; Lam, S.S.; Peng, W.; Low, F.; Lee, B.H.K.; Alstrup, A.K.O.; Sonne, C. A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: Prevalence, remediation and actions needed. J. Hazard. Mater. 2020, 400, 123006. [Google Scholar] [CrossRef]
- Solomon, K.R.; Williams, W.M.; Mackay, D.; Purdy, J.; Giddings, J.M.; Giesy, J.P. Properties and uses of chlorpyrifos in the United States. In Ecological Risk Assessment for Chlorpyrifos in Terrestrial and Aquatic Systems in the United States; Springer: Cham, Switzerland, 2014; pp. 13–34. [Google Scholar] [CrossRef]
- John, E.M.; Shaike, J.M. Chlorpyrifos: Pollution and remediation. Environ. Chem. Lett. 2015, 13, 269–291. [Google Scholar] [CrossRef]
- Bhende, R.S.; Jhariya, U.; Srivastava, S.; Bombaywala, S.; Das, S.; Dafale, N.A. Environmental distribution, metabolic fate, and degradation mechanism of chlorpyrifos: Recent and future perspectives. Appl. Biochem. Biotechnol. 2022, 194, 2301–2335. [Google Scholar] [CrossRef]
- Wu, J.; Laird, D.A. Abiotic transformation of chlorpyrifos to chlorpyrifos oxon in chlorinated water. Environ. Toxicol. Chem. 2003, 22, 261–264. [Google Scholar] [CrossRef]
- Jameson, R.R.; Seidler, F.J.; Slotkin, T.A. Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: Chlorpyrifos, chlorpyrifos oxon, and diazinon. Environ. Health Perspect. 2007, 115, 65–70. [Google Scholar] [CrossRef]
- NPIC. Chlorpyrifos Technical Fact Sheet. Available online: https://npic.orst.edu/factsheets/archive/chlorptech.html (accessed on 19 August 2025).
- Saunders, M.; Magnanti, B.L.; Correia Carreira, S.; Yang, A.; Alamo-Hernández, U.; Riojas-Rodriguez, H.; Calamandrei, G.; Koppe, J.G.; Krayer von Krauss, M.; Keune, H. Chlorpyrifos and neurodevelopmental effects: A literature review and expert elicitation on research and policy. Environ. Health 2012, 11, S5. [Google Scholar] [CrossRef] [PubMed]
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 2022, 185, 105138. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (US). Toxicological Profile for Chlorpyrifos; National Library of Medicine, Ed.; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 1997; Volume 2.
- Badawy, M.I.; Ghaly, M.Y.; Gad-Allah, T.A. Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. Desalination 2006, 194, 166–175. [Google Scholar] [CrossRef]
- Sud, D.; Kaur, P. Heterogeneous photocatalytic degradation of selected organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2365–2407. [Google Scholar] [CrossRef]
- Pehkonen, S.O.; Zhang, Q. The degradation of organophosphorus pesticides in natural waters: A critical review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 17–72. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Ali, S.; Zaman, W. Innovative adsorbents for pollutant removal: Exploring the latest research and applications. Molecules 2024, 29, 4317. [Google Scholar] [CrossRef]
- Badran, A.M.; Utra, U.; Yussof, N.S.; Bashir, M.J.K. Advancements in adsorption techniques for sustainable water purification: A focus on lead removal. Separations 2023, 10, 565. [Google Scholar] [CrossRef]
- Akhtar, M.; Sarfraz, M.; Ahmad, M.; Raza, N.; Zhang, L. Use of low-cost adsorbent for waste water treatment: Recent progress, new trend and future perspectives. Desalination Water Treat. 2025, 321, 100914. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. In Organic Pollutants—Monitoring, Risk and Treatment; IntechOpen: London, UK, 2013. [Google Scholar]
- Chaturvedi, P.; Giri, B.S.; Shukla, P.; Gupta, P. Recent advancement in remediation of synthetic organic antibiotics from environmental matrices: Challenges and perspective. Bioresour. Technol. 2021, 319, 124161. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Tabassum, N.; Rafique, U.; Balkhair, K.S.; Ashraf, M.A. Chemodynamics of methyl parathion and ethyl parathion: Adsorption models for sustainable agriculture. BioMed Res. Int. 2014, 2014, 831989. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Das, B.B.; Kanavaris, F. Biochar-concrete: A comprehensive review of properties, production and sustainability. Case Stud. Constr. Mater. 2024, 20, e02859. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Jaya Prasanna Kumar, D.; Narula, A.; Minnat Chistie, S.; Ullhas Naik, S. Production and beneficial impact of biochar for environmental application: A review on types of feedstocks, chemical compositions, operating parameters, techno-economic study, and life cycle assessment. Fuel 2023, 343, 127968. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zou, L.; Bai, Y.; Xiu, H. Research on the optimum carbonization process of walnut shell based on dynamic analysis. RSC Adv. 2023, 13, 13412–13422. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefinery 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Manterola-Barroso, C.; Godoy Sanchez, K.; Scheuermann, E.; Padilla-Contreras, D.; Morina, F.; Meriño-Gergichevich, C. Antioxidant and Physico-Structural Insights of Walnut (Juglans regia) and Hazelnut (Corylus avellana L.) Shells: Implications for Southern Chile By-Product Valorization. Resources 2025, 14, 82. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Coconut husk derived activated carbon via microwave induced activation: Effects of activation agents, preparation parameters and adsorption performance. Chem. Eng. J. 2012, 184, 57–65. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Available online: https://sdgresources.relx.com (accessed on 19 August 2025).
- Zlatković, M.; Kurtić, R.; Pašti, I.A.; Tasić, T.; Milanković, V.; Potkonjak, N.; Unterweger, C.; Lazarević-Pašti, T. Application of Carbon Materials Derived from Nocino Walnut Liqueur Pomace Residue for Chlorpyrifos Removal from Water. Materials 2025, 18, 3072. [Google Scholar] [CrossRef] [PubMed]
- Osterrieth, J.W.M.; Rampersad, J.; Madden, D.; Rampal, N.; Skoric, L.; Connolly, B.; Allendorf, M.D.; Stavila, V.; Snider, J.L.; Ameloot, R. How reproducible are surface areas calculated from the BET equation? Adv. Mater. 2022, 34, 2201502. [Google Scholar] [CrossRef] [PubMed]
- Lazarević-Pašti, T.D.; Bondžić, A.M.; Pašti, I.A.; Vasić, V.M. Indirect electrochemical oxidation of organophosphorous pesticides for efficient detection via acetylcholinesterase test. Pestic. Biochem. Physiol. 2012, 104, 236–242. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Milanković, V.; Tasić, T.; Brković, S.; Potkonjak, N.; Unterweger, C.; Pašti, I.; Lazarević-Pašti, T. Sustainable carbon materials from biowaste for the removal of organophosphorus pesticides, dyes, and antibiotics. J. Environ. Manag. 2025, 376, 124463. [Google Scholar] [CrossRef] [PubMed]
- Simões dos Reis, G.; Mayandi Subramaniyam, C.; Cárdenas, A.D.; Larsson, S.H.; Thyrel, M.; Lassi, U.; García-Alvarado, F. Facile Synthesis of Sustainable Activated Biochars with Different Pore Structures as Efficient Additive-Carbon-Free Anodes for Lithium- and Sodium-Ion Batteries. ACS Omega 2022, 7, 42570–42581. [Google Scholar] [CrossRef]
- Zhu, M.; Lan, J.; Zhang, X.; Sui, G.; Yang, X. Porous carbon derived from Ailanthus altissima with unique honeycomb-like microstructure for high-performance supercapacitors. New J. Chem. 2017, 41, 4281–4285. [Google Scholar] [CrossRef]
- Tulun, Ş.; Akgül, G.; Alver, A.; Celebi, H. Adaptive neuro-fuzzy interference system modelling for chlorpyrifos removal with walnut shell biochar. Arab. J. Chem. 2021, 14, 103443. [Google Scholar] [CrossRef]
- Pandey, P.; Kenchannavar, P.; Surenjan, A. Exploring the potential of cashew nut shell biochar for chlorpyrifos pesticide removal. Chem. Eng. Process.-Process Intensif. 2025, 213, 110307. [Google Scholar] [CrossRef]
- Asghar, A.; Mabarak, S.; Ashraf, B.; Rizwan, M.; Massey, S.; Asghar, B.H.; Shahid, B.; Rasheed, T. A sustainable approach for the removal of chlorpyrifos pesticide from aqueous phase using novel nano magnetized biochar. Inorg. Chem. Commun. 2024, 159, 111790. [Google Scholar] [CrossRef]
- Zgolli, A.; Fizer, M.; Mariychuk, R.; Dhaouadi, H. Insights into the adsorption mechanism of chlorpyrifos on activated carbon derived from prickly pear seeds waste: An experimental and DFT modeling study. Environ. Res. 2024, 263, 120221. [Google Scholar] [CrossRef]
- Jacob, M.M.; Ponnuchamy, M.; Kapoor, A.; Sivaraman, P. Bagasse based biochar for the adsorptive removal of chlorpyrifos from contaminated water. J. Environ. Chem. Eng. 2020, 8, 103904. [Google Scholar] [CrossRef]
- Joshi, V.; Jindal, M.K.; Sar, S.K. Approaching a discussion on the detachment of chlorpyrifos in contaminated water using different leaves and peels as bio adsorbents. Sci. Rep. 2023, 13, 11186. [Google Scholar] [CrossRef] [PubMed]
- Tasić, T.; Milanković, V.; Unterweger, C.; Fürst, C.; Breitenbach, S.; Pašti, I.A.; Lazarević-Pašti, T. Highly porous cellulose-based carbon fibers as effective adsorbents for chlorpyrifos removal: Insights and applications. C 2024, 10, 58. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Brković, S.; Potkonjak, N.; Unterweger, C.; Pašti, I.; Lazarević-Pašti, T. The adsorption of chlorpyrifos and malathion under environmentally relevant conditions using biowaste carbon materials. J. Hazard. Mater. 2024, 480, 135940. [Google Scholar] [CrossRef] [PubMed]
- Florence, C.N.; Adaobi, I.C.; Amaoge, O.-O.I.; Nkeiruka, N.C. Examining the Kinetics and Thermodynamics of Chlorpyrifos Adsorption unto Activated Mucuna pruriens Seed Shell. UNIZIK J. Eng. Appl. Sci. 2025, 4, 1631–1646. [Google Scholar]
- Kumari, S.; Sharma, A.; Dhiman, P.; Thakur, M.; Aloui, Z.; Selvaraj, M.; Kumar, A. Strategic synthesis of biowaste-derived magnetic hydrochar for adsorption and photocatalytic removal of Chlorpyrifos herbicides from simulated wastewater. Mater. Sci. Eng. B 2025, 314, 118009. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Q.; Liu, G.; Luo, X.; Li, F.; Zhang, Y.; Wang, Z. Characteristics and mechanisms of chlorpyrifos and chlorpyrifos-methyl adsorption onto biochars: Influence of deashing and low molecular weight organic acid (LMWOA) aging and co-existence. Sci. Total Environ. 2019, 657, 953–962. [Google Scholar] [CrossRef]
- Moradeeya, P.G.; Kumar, M.A.; Thorat, R.B.; Rathod, M.; Khambhaty, Y.; Basha, S. Nanocellulose for biosorption of chlorpyrifos from water: Chemometric optimization, kinetics and equilibrium. Cellulose 2017, 24, 1319–1332. [Google Scholar] [CrossRef]
- Rojas, R.; Morillo, J.; Usero, J.; Vanderlinden, E.; El Bakouri, H. Adsorption study of low-cost and locally available organic substances and a soil to remove pesticides from aqueous solutions. J. Hydrol. 2015, 520, 461–472. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Pašti, I.A.; Lazarević-Pašti, T. Resolving Coffee Waste and Water Pollution—A Study on KOH-Activated Coffee Grounds for Organophosphorus Xenobiotics Remediation. J. Xenobiotics 2024, 14, 1238–1255. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).