Influence of Prefermentative Cold Maceration on the Chemical and Sensory Properties of Red Wines Produced in Warm Climates
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Cultivars and Growing Conditions
2.2. Winemaking Procedure
2.3. Aging in Bottle
2.4. Analytical Determinations in Musts and Wines
2.5. Determination of Phenolic Compounds
2.6. Chromatic Characterization
2.7. Determining Volatile Compounds
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Musts and Wines Characterization
3.2. Influence on the Phenolic Contents and Color of Wines
3.3. Influence on the Aromatic Content
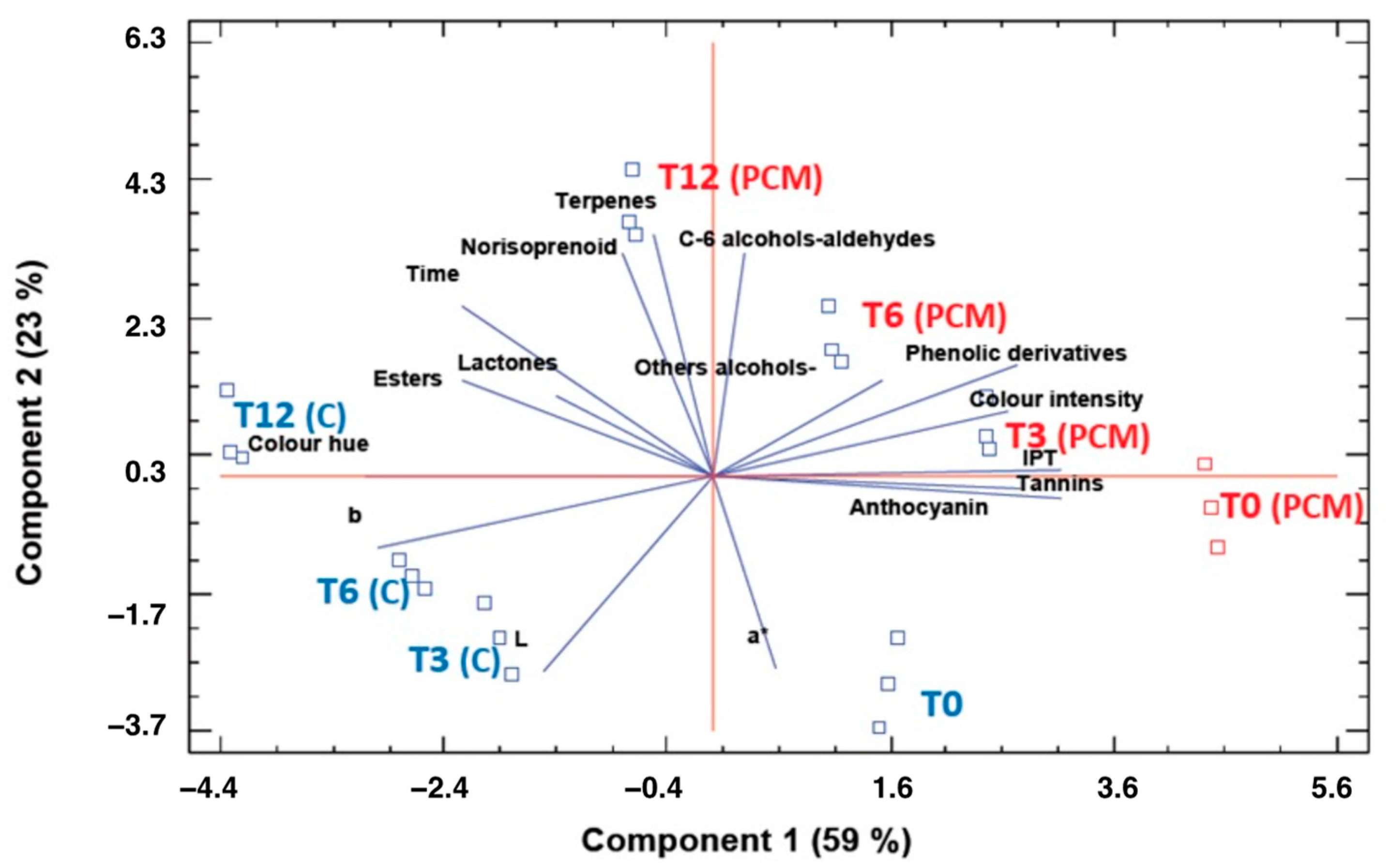
3.4. Aging in Bottle
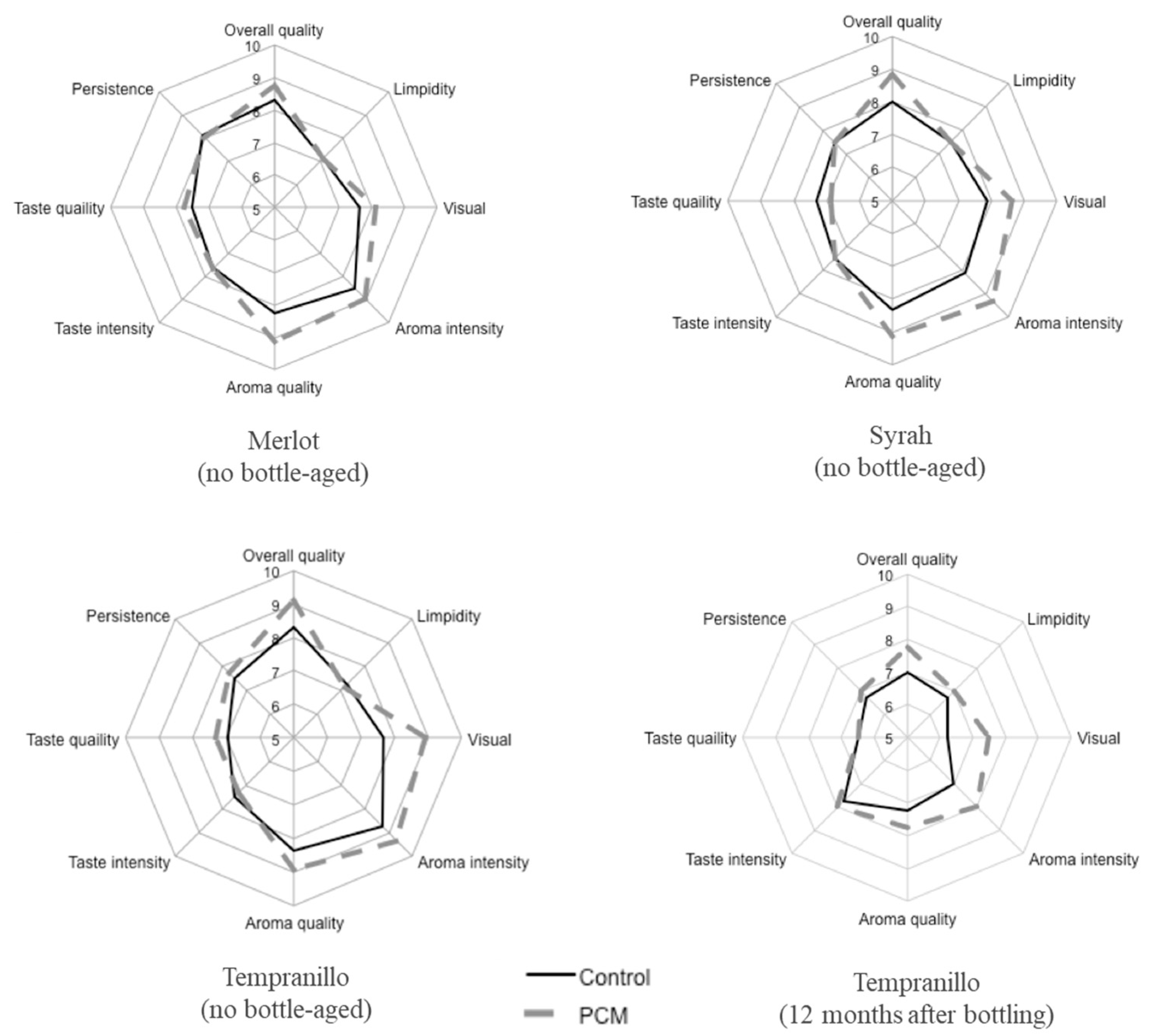
3.5. Sensory Analysis of Wines
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pons, A.; Allamy, L.; Schüttler, A.; Rauhut, D.; Thibon, C.; Darriet, P. What is the expected impact of climate change on wine aroma compounds and their precursors in grape? OENO One 2017, 51, 141–146. [Google Scholar] [CrossRef]
- Maza, M.A.; Martínez, J.M.; Delso, C.; Camargo, A.; Raso, J.; Álvarez, I. Pef-dependency on polyphenol extraction during maceration/fermentation of grenache grapes. Innov. Food Sci. Emerg. Technol. 2020, 60, 102303. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Pérez-Porras, P.; Bautista-Ortín, A.B.; Gómez-Plaza, E. Effects of combining high power ultrasound and enological enzymes on the composition of polysaccharides in red wine. LWT 2022, 170, 114060. [Google Scholar] [CrossRef]
- Ntuli, R.G.; Saltman, Y.; Ponangi, R.; Jeffery, D.W.; Bindon, K.; Wilkinson, K.L. Impact of fermentation temperature and grape solids content on the chemical composition and sensory profiles of cabernet sauvignon wines made from flash détente treated must fermented off-skins. Food Chem. 2022, 369, 130861. [Google Scholar] [CrossRef]
- Junqua, R.; Carullo, D.; Ferrari, G.; Pataro, G.; Ghidossi, R. Ohmic heating for polyphenol extraction from grape berries: An innovative prefermentary process. OENO One 2021, 55, 39–51. [Google Scholar] [CrossRef]
- Pérez-Porras, P.; Gómez-Plaza, E.; Muñoz García, R.; Díaz-Maroto, M.C.; Moreno-Olivares, J.D.; Bautista-Ortín, A.B. Prefermentative grape microwave treatment as a tool for increasing red wine phenolic content and reduce maceration time. Appl. Sci. 2022, 12, 8164. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Šimunek, M.; Petrović, M.; Bedić, H.; Herceg, Z.; Juretić, H. Aromatic profile and sensory characterisation of ultrasound treated cranberry juice and nectar. Ultrason. Sonochemistry 2017, 38, 783–793. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Shen, Y.; Fan, X.-H.; García Martín, J.F. Preliminary study of the effect of ultrasound on physicochemical properties of red wine. CyTA-J. Food 2016, 14, 55–64. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold maceration application in red wine production and its effects on phenolic compounds: A review. LWT 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of prefermentative maceration on the phenolic and volatile compounds in monastrell red wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Aleixandre, J.L.; Sánchez, N.; Aleixanre-Tudó, J.L.; Lizama, V.; García, M.J.; Alvarez, I. Phenolic composition of bobal red wines elaborated with pre-fermentative cold maceration. Ciência E Técnica Vitivinícola 2012, 27, 11. [Google Scholar]
- Santis, D.D.; Frangipane, M.T. Effect of prefermentative cold maceration on the aroma and phenolic profiles of a merlot red wine. Ital. J. Food Sci. 2010, 22, 47–53. [Google Scholar]
- Cai, J.; Zhu, B.Q.; Wang, Y.H.; Lu, L.; Lan, Y.B.; Reeves, M.J.; Duan, C.Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of cabernet sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E.; Barda, N. Effects of maceration length after prefermentative cold soak: Detailed chromatic, phenolic and sensory composition of cabernet sauvignon, malbec and merlot wines. J. Food Compos. Anal. 2021, 104, 104168. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Gómez-Plaza, E.; López-Roca, J.M.; Gil-Muñoz, R.; Bautista-Ortín, A.B. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J. Agric. Food Chem. 2011, 59, 5450–5455. [Google Scholar] [CrossRef] [PubMed]
- Busse-Valverde, N.; Gómez-Plaza, E.; López-Roca, J.M.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B. Effect of different enological practices on skin and seed proanthocyanidins in three varietal wines. J. Agric. Food Chem. 2010, 58, 11333–11339. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, M.J.; Gordillo, B.; Hernanz, D.; Escudero-Gilete, M.L.; González-Miret, M.L.; Heredia, F.J. Effect of the time of cold maceration on the evolution of phenolic compounds and color of syrah wines elaborated in warm climate. Int. J. Food Sci. Technol. 2014, 49, 1886–1892. [Google Scholar] [CrossRef]
- González-Neves, G.; Favre, G.; Piccardo, D.; Gil, G. Anthocyanin profile of young red wines of tannat, syrah and merlot made using maceration enzymes and cold soak. Int. J. Food Sci. Technol. 2016, 51, 260–267. [Google Scholar] [CrossRef]
- Ćurko, N.; Ganić, K.K.; Tomašević, M.; Gracin, L.; Jourdes, M.; Teissedre, P.-L. Effect of enological treatments on phenolic and sensory characteristics of red wine during aging: Micro-oxygenation, sulfur dioxide, iron with copper and gelatin fining. Food Chem. 2021, 339, 127848. [Google Scholar] [CrossRef]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E. Chemical, chromatic, and sensory attributes of 6 red wines produced with prefermentative cold soak. Food Chem. 2015, 174, 110–118. [Google Scholar] [CrossRef]
- Ribereau-Gayon, J. Le dosage des composés phénoliques totaux dans les vins rouges (The determination of total phenolic compounds in red wines). Chem. Anal. 1970, 52, 4. (In French) [Google Scholar]
- Ribereau-Gayon, P.; Stonestreet, E. Dógase des tannins du vin rouges et détermination du leur structure(Determination of the tannins in red wine and their structure)(French language). Chim. Anal. 1966, 48, 8. [Google Scholar]
- Gordillo, B.; López-Infante, M.I.; Ramírez-Pérez, P.; González-Miret, M.L.; Heredia, F.J. Influence of prefermentative cold maceration on the color and anthocyanic copigmentation of organic tempranillo wines elaborated in a warm climate. J. Agric. Food Chem. 2010, 58, 6797–6803. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Understanding cold maceration in red winemaking: A batch processing and multi-block data analysis approach. LWT 2019, 111, 147–157. [Google Scholar] [CrossRef]
- Caillé, S.; Samson, A.; Wirth, J.; Diéval, J.-B.; Vidal, S.; Cheynier, V. Sensory characteristics changes of red grenache wines submitted to different oxygen exposures pre and post bottling. Anal. Chim. Acta 2010, 660, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tomašević, M.; Gracin, L.; Ćurko, N.; Kovačević Ganić, K. Impact of pre-fermentative maceration and yeast strain along with glutathione and SO2 additions on the aroma of Vitis vinifera L. Pošip wine and its evaluation during bottle aging. LWT-Food Sci. Technol. 2017, 81, 67–76. [Google Scholar] [CrossRef]
- Recamales, A.F.; Gallo, V.; Hernanz, D.; González-Miret, M.L.; Heredia, F.J. Effect of time and storage conditions on major volatile compounds of zalema white wine. J. Food Qual. 2011, 34, 100–110. [Google Scholar] [CrossRef]
- Verzeletti, A.; Echeverrigaray, S.; Cardoso, A.; Vanderlinde, R.; Delamare, A.P.L. Evolution of aromatic compounds during the second fermentation and aging of brazilian sparkling wine. BIO Web Conf. 2016, 7, 02019. [Google Scholar] [CrossRef]




| Tempranillo | Merlot | Syrah | ||||
|---|---|---|---|---|---|---|
| Control | PCM | Control | PCM | Control | PCM | |
| Must characterization | ||||||
| Sugar content (°Bé) | 13.6 ± 0.00 b | 13.6 ± 0.00 b | 14.0 ± 0.01 c | 14.0 ± 0.01 c | 12.9 ± 0.00 a | 12.9 ± 0.01 a |
| pH | 3.69 ± 0.01 c | 3.75 ± 0.01 d | 3.51 ± 0.01 a | 3.57 ± 0.01 b | 3.56 ± 0.01 b | 3.71 ± 0.01 c |
| Total acidity (g tartaric acid/L) | 4.91 ± 0.07 b | 4.72 ± 0.03 a | 6.01 ± 0.03 e | 5.86 ± 0.05 d | 5.98 ± 0.07 d,e | 5.62 ± 0.04 c |
| Wine characterization | ||||||
| Alcoholic strength (% v/v) | 13.48 ± 0.05 c | 13.83 ± 0.03 d | 14.93 ± 0.07 e | 15.25 ± 0.04 f | 12.93± 0.04 a | 13.21 ± 0.02 b |
| pH in wine | 3.62 ± 0.01 a | 3.68 ± 0.01 b | 3.63 ± 0.01 a | 3.69 ± 0.01 b | 3.73 ± 0.02 c | 3.78 ± 0.01 d |
| Total acidity (g tartaric acid/L) | 7.05 ± 0.05 f | 6.82 ± 0.06 e | 6.6 ± 0.03 d | 6.48 ± 0.04 c | 6.31 ± 0.01 b | 6.20 ± 0.04 a |
| Volatile acidity (g acetic acid/L) | 0.26 ± 0.01 a | 0.35 ± 0.01 c | 0.42 ± 0.01 d | 0.32 ± 0.01 b | 0.40 ± 0.01 c | 0.37 ± 0.02 c |
| Tempranillo | Merlot | Syrah | ||||
|---|---|---|---|---|---|---|
| Control | PCM | Control | PCM | Control | PCM | |
| Phenolic content (TPI) | 60.15 ± 0.15 a | 62.73 ± 0.1 b | 94.8 ± 0.1 e | 97.55 ± 0.05 f | 63.1 ± 0.1 c | 66.2 ± 0.1 d |
| Tannins (g/L) | 2.69 ± 0.05 b | 2.78 ± 0.02 c | 4.65 ± 0.03 d | 4.76 ± 0.02 e | 2.35 ± 0.04 a | 2.63 ± 0.04 b |
| Anthocyanins (mg/L) | 454.7 ± 2.7 b | 537.0 ± 3.2 c | 372.7 ± 1.9 a | 453.2 ± 2.7 b | 546.6 ± 2.2 d | 676.5 ± 3.1 e |
| Color intensity | 13.75 ± 0.04 a | 15.74 ± 0.09 c | 17.17 ± 0.02 d | 19.26 ± 0.04 e | 14.32 ± 0.05 b | 17.19 ± 0.08 d |
| Hue | 0.68 ± 0.01 d | 0.62 ± 0.01 c | 0.74 ± 0.01 e | 0.68 ± 0.01 d | 0.57 ± 0.01 b | 0.52 ± 0.01 a |
| L* | 63.20 ± 0.12 e | 54.25 ± 0.09 d | 43.89 ± 0.13 b | 36.52 ± 0.22 a | 51.23 ± 0.16 c | 44.16 ± 0.2 b |
| a* | 34.69 ± 0.08 e | 30.51 ± 0.04 c | 28.60 ± 0.07 b | 24.73 ± 0.11 a | 36.65 ± 0.08 f | 33.25 ± 0.05 d |
| b* | 12.88 ± 0.13 e | 5.38 ± 0.06 c | 16.54 ± 0.08 f | 11.19 ± 0.03 d | 5.03 ± 0.14 b | −1.82 ± 0.07 a |
| Aroma Compound | Descriptor | Series | Odor Threshold | Merlot | Tempranillo | Syrah | |||
|---|---|---|---|---|---|---|---|---|---|
| Control | PCM | Control | PCM | Control | PCM | ||||
| C-6 alcohols and aldehydes | |||||||||
| Hexan-1-ol | Grass | H | 1100 | 35.41 ± 2.56 b | 34.12 ± 1.51b | 46.59 ± 2.98 c | 54.79 ± 2.51 d | 21.36 ± 1.92 a | 24.13 ± 1.86 a |
| [E] 2-hexen-1-ol | Green, fruity | H | 15,000 | 8.07 ± 0.36 b | 13.75 ± 0.29 d | 0.00 | 0.00 | 6.15 ± 0.41 a | 9.29 ± 0.45 c |
| [Z] 3-hexen-1-ol | Fresh grass | H | 400 | 6.06 ± 0.32 a | 10.21 ± 0.42 c | 16.56 ± 0.69 d | 16.31 ± 0.56 d | 7.89 ± 0.32 b | 11.36 ± 0.48 c |
| [E] 3-hexen-1-ol | Fresh, leaves | H | 400 | 6.83 ± 0.12 b | 0.68 ± 0.09 a | 19.41 ± 0.85 c | 22.61 ± 0.90 d | 20.08 ± 1.11 c | 20.59 ± 1.09 c,d |
| Hexanal | Grass, green | H | 4.5 | 16.29 ± 0.83 c | 18.17 ± 1.7 d | 3.26 ± 0.25 a | 4.18 ± 0.35 a | 6.52 ± 0.51 b | 7.16 ± 0.041 b |
| [E] 2-Hexenal | Herbaceous, green apple | H | 47 | 12.15 ± 0.61 b | 0.75 ± 0.03 a | 0.00 | 0.00 | 0.00 | 0.00 |
| Other alcohols and aldehydes | |||||||||
| Isobutanol | F | - | 2.41 ± 0.18 c | 1.96 ± 0.09 b | 3.18 ± 0.12 d | 2.51 ± 0.09 c,a | 1.24 ± 0.12 a | 1.93 ± 0,09 b | |
| Heptanal | Fruity | F | 6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.86 ± 0.05 b | 0.48 ± 0.02 a |
| [E] 2-Heptenal | Bitter almond, beer | N | 3 | 0.00 | 1.39 ± 0.11 a | 2.36 ± 0.14 c | 2.95 ± 0.15 c | 0.00 | 0.00 |
| 1-octanol | Jasmine, lemon | FL | 800 | 13.20 ± 0.83 d | 9.82 ± 0.56 c | 8.95 ± 0.66 c | 9.12 ± 0.5 c | 4.86 ± 0.23 b | 2.06 ± 0.11 a |
| Octanal | Nutmeg | S | 6 | 1.18 ± 0.21 a | 0.00 | 1.18 ± 0.21 a | 0.00 | 0.00 | 0.00 |
| Nonanal | Fatty, rancid | O | 1 | 0.10 ± 0.03 a | 0.00 | 0.10 ± 0.03 a | 0.00 | 0.29 ± 0.03 b | 0.36 ± 0.04 b |
| Methional | Boiled vegetables | O | 0.5 | 0.980.05 a | 1.21 ± 0.06 b | 0.0 | 0.00 | 0.00 | 0.00 |
| Phenolic derivatives | |||||||||
| β-Phenylethyl alcohol | Rose | FL | 10,000 | 11,777.87 ± 321.56 a | 18,564.70 ± 178.28 c | 13,562.32 ± 351.69 b | 19,684.21 ± 398.62 c | 21,814.12 ± 521.06 d | 26,250.31 ± 412.37 e |
| Benzyl alcohol | Floral, sweet | FL | 900 | 79.03 ± 7.03 a,b | 68.35 ± 4.52 a | 89.26 ± 6.35 b,c | 98.31 ± 6.1 b,c | 98.63 ± 6.08 c | 124.19 ± 10.9 d |
| Eugenol | Spiced, clove, licorice | S | 6 | 0.98 ± 0.06 a | 3.25 ± 0.28 b | 8.49 ± 0.32 c | 14.98 ± 0.61d | 0.00 | 0.00 |
| Guaiacol | Spiced, phenolic | S | 9.5 | 5.98 ± 0.36 a | 5.15 ± 0.41 a | 3.25 ± 0.21 a | 7.92 ± 0.33 a | 49.56 ± 3.7 b | 64.13 ± 3.59 c |
| Vanillin | Vanilla | SW | 60 | 0.00 | 0.00 | 1.89 ± 0.11 a | 2.95 ± 0.19 a | 12.98 ± 0.98 b | 20.36 ± 1.24 c |
| Terpenes and derivatives | |||||||||
| Citronellol | Floral rose, citrus like | F | 18 | 0.00 | 2.14 ± 0.07 a | 6.89 ± 0.45 c | 9.56 ± 0.7 d | 2.06 ± 0.14 a | 4.28 ± 0.26 b |
| [E] Geraniol | Floral | FL | 30 | 3.21 ± 0.12 a | 5.26 ± 0.42 b | 0.00 | 0.00 | 0.00 | 0.00 |
| A-terpineol | Sweet lilac | FL | 250 | 0.00 | 0.00 | 1.56 ± 0.09 a | 3.14 ± 0.1 b | 7.56 ± 0.25 c | 9.31 ± 0.4 d |
| Limonene | Lemon, citrus | F | 15 | 4.48 ± 0.15 a | 6.21 ± 0.41 b | 5.84 ± 0.21 b | 7.40 ± 0.39 c | 4.48 ± 0.15 a | 6.21 ± 0.41 b |
| pMenth1en4ol | Mint, menthol | B | No | 0.89 ± 0.06 a | 1.72 ± 0.04 c | 0.00 | 0.00 | 1.12 ± 0.07 b | 0.92 ± 0.08 a |
| Eucalyptol | Eucalyptus | B | 1.1 to 3.2 | 0.68 ± 0.02 b | 1.84 ± 0.07 c | 0.32 ± 0.02 a | 0.63 ± 0.05 b | 0.00 | 0.00 |
| [Z], [E] Linalool oxide | Floral | FL | 6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.96 ± 0.06 a | 2.25 ± 0.15 b |
| [E] Nerolidol | Floral, green | FL | 100 | 0.00 | 0.00 | 0.00 | 0.00 | 3.26 ± 0.21 a | 5.69 ± 0.32 b |
| [E,E] Farnesol | Sweet, floral | SW | 20 | 2.25 ± 0.11 a | 2.91 ± 0.09 b | 2.25 ± 0.11 a | 2.34 ± 0.09 a | 0.00 | 0.00 |
| Norisoprenoids | |||||||||
| Β-Damascenone | Apple, plum, fruity | F | 0.1 | 2.36 ± 0.18 a | 2.98 ± 0.14 b | 2.06 ± 0.18 a | 2.58 ± 0.14 a,b | 5.50 ± 0.21 c | 6.04 ± 0.31 c |
| Β-ionone | Violet | FL | 0.09 | 0.00 | 0.00 | 0.16 ± 0.02 a | 0.23 ± 0.02 a | 4.46 ± 0.24 b | 7.37 ± 0.41 c |
| 3-oxo-α-ionol | Honey | SW | No | 2.15 ± 0.13 a | 3.08 ± 0.2 b | 4.41 ± 0.21 c | 5.26 ± 0.18 d | 4.41 ± 0.21 c | 5.06 ± 0.18 d |
| Esters | |||||||||
| Isoamyl acetate | Banana | F | 30 | 14.56 ± 0.96 b | 19.31 ± 1.24 c | 8.91 ± 1.05 a | 8.43 ± 1.15 a | 8.23 ± 0.32 a | 10.14 ± 0.31 a |
| Ethyl butyrate | Acid fruit, apple | F | 20 | 3.65 ± 0.21 a | 3.96 ± 0.17 b | 0.00 | 0.00 | 0.00 | 0.00 |
| Ethyl isobutyrate | Red fruits, strawberry | RF | 15 | 215.63 ± 13.62 d | 275.48 ± 16.1 e | 110.26 ± 8.32 a | 168.11 ± 11.1 c | 118.95 ± 8.36 a,b | 142.87 ± 11.88 b,c |
| Ethyl 3-hydroxybutyrate | Rancid, phenolic | O | 67,000 | 12.87 ± 1.09 a | 11.96 ± 0.91 a | 0.00 | 0.00 | 0.00 | 0.00 |
| Ethyl 2-methylbutyrate | Sweet fruit, blueberry | RF | 18 | 40.15 ± 2.15 b | 65.23 ± 3.26 c | 21.65 ± 1.18 a | 40.06 ± 3.11 b | 0.00 | 0.00 |
| Hexyl acetate | Fruity, pear, plum | F | 670 | 23.62 ± 1.81 a | 38.74 ± 2.11 b | 0.00 | 0.00 | 0.00 | 0.00 |
| Ethyl pentanoate | Acid strawberry | RF | 1.5 | 0.91 ± 0.05 c | 0.87 ± 0.06 b,c | 0.76 ± 0.04 a,b | 0.89 ± 0.05 b,c | 0.72 ± 0.04 a | 0.78 ± 0.06 a,b,c |
| Ethyl 4-methylpentanoate | Strawberry | RF | 0.01 | 0.17 ± 0.02 a,b | 0.23 ± 0.03 b,c | 0.18 ± 0.02 b,c | 0.24 ± 0.02 c | 0.11 ± 0.02 a | 0.19 ± 0.02 b,c |
| Ethyl lactate | Dairy, butter | SW | 1546 | 99.3 ± 6.59 a | 114.37 ± 8.74 a,b | 253.32 ± 12.2 d | 278.13 ± 14.21 e | 126.32 ± 4.52 c | 111.96 ± 5.22 a,b |
| Ethyl hexanoate | Fruit, strawberry | F | 5 | 196.31 ± 12.37 a | 283.45 ± 18 c | 179.6 ± 10.63 a | 297.13 ± 14.32 c | 208.19 ± 10.25 a,b | 236.91 ± 11.1 b |
| Ethyl heptanoate | Fruity | F | 100,000 | 0.00 | 0.00 | 41.02 ± 2.63 a | 48.75 ± 2.98 b | 0.00 | 0.00 |
| Ethyl isovalerate | Sweet fruit, blackberry | RF | 3 | 73.31 ± 5.26 c | 84.73 ± 7.59 d | 42.35 ± 2.89 a | 48.36 ± 3.71 a,b | 56.65 ± 6.51 b | 71.54 ± 7.09 c |
| Ethyl octanoate | Fruit, pineapple | F | 5 | 165.37 ± 14.06 b | 206.96 ± 12.29 c | 106.32 ± 7.76 a | 141.15 ± 10.08 b | 253.85 ± 9.87 d | 264.86 ± 11.56 d |
| Ethyl decanoate | Fruit, nut | F | 200 | 47.94 ± 2.67 c | 58.13 ± 3.26 e | 32.28 ± 1.99 a | 37.69 ± 2.54 b | 52.63 ± 2.41 c,d | 56.78 ± 4.01 d,e |
| Diethyl succinate | Fruity | F | 1200 | 1395.79 ± 32.41 c | 902.78 ± 26.55 b | 936.24 ± 12.15 b | 728.96 ± 22.48 a | 2149.99 ± 51.29 d | 1325.26 ± 31.25 c |
| Phenyl etylacetate | Floral, rose | F | 250 | 538.92 ± 24.12 b,c | 624.79 ± 33.26 d | 422.63 ± 22.49 a | 493.57 ± 23.56 a,b | 484.71 ± 20.58 a,b | 608.6 ± 37.11 c,d |
| Ethyl vanillate | Vanilla | SW | 990 | 32.87 ± 2.46 a | 47.67 ± 3.65 | 56.32 ± 3.62 | 71.21 ± 4.51 | 28.21 ± 2.68 | 54.33 ± 4.27 |
| Lactones and enolones | |||||||||
| Γ-Butyrolactone | Butter, sweet | SW | 100,000 | 719.52 ± 8.69 d | 478.35 ± 18.56 c | 322.51 ± 20.14 b | 301.59 ± 15.63 b | 143.69 ± 7.89 a | 279.21 ± 8.15 b |
| Pantolactone | Sweet | SW | 2200 | 22.3 ± 1.59 a | 31.26 ± 2.01 b | 0.00 | 0.00 | 0.00 | 0.00 |
| Furaneol | Caramel, burnt sugar | SW | 37 | 48.89 ± 4.02 c | 54.36 ± 3.12 d | 23.19 ± 1.16 b | 26.31 ± 1.86 b | 13.59 ± 0.72 a | 11.26 ± 0.68 a |
| Homofuraneol | Cotton candy | SW | 40 | 52.85 ± 2.74 c | 67.5 ± 3.66 d | 19.68 ± 1.13 b | 22.36 ± 1.8 b | 9.11 ± 0.56 a | 9.95 ± 0.27 a |
| Aroma Compound | Tempranillo | |||||||
|---|---|---|---|---|---|---|---|---|
| Time Zero | 3 Months | 6 Months | 12 Months | |||||
| Control | PCM | Control | PCM | Control | PCM | Control | PCM | |
| C-6 alcohols and aldehydes | ||||||||
| Hexan-1-ol | 46.59 ± 2.98 a | 54.79 ± 1.51 b,c | 45.13 ± 1.46 a | 51.26 ± 3.27 a,b | 53.58 ± 0.75 b,c | 59.62 ± 2.03 c | 57.52 ± 3.25 b,c | 60.21 ± 3.21 c |
| [Z] 3-hexen-1-ol | 16.56 ± 0.69 a,b | 16.31 ± 0.56 a,b | 17.45 ± 0.35 a | 16.23 ± 0.27 a,b | 17.52 ± 0.87 a | 18.04 ± 1.1 b,c | 17.74 ± 1.23 a,b,c | 18.95 ± 0.23 c |
| [E] 3-hexen-1-ol | 19.41 ± 0.85 a | 22.61 ± 0.90 c | 19.75 ± 1.20 a,b | 19.01 ± 0.62 a | 20.59 ± 0.34 a,b,c | 21.78 ± 0.87 b,c | 20.36 ± 0.99 a,b | 22.69 ± 0.58 c |
| Hexanal | 3.26 ± 0.25 a | 4.18 ± 0.35 b | 2.50 ± 0.31 a | 5.21 ± 0.21 c,d | 5.29 ± 0.23 c,d | 5.40 ± 0.47 c,d | 4.72 ± 0.19 b,c | 5.56 ± 0.18 d |
| Other alcohols and aldehydes | ||||||||
| Isobutanol | 3.18 ± 0.12 b | 2.51 ± 0.0 a | 4.25 ± 0.10 c | 2.56 ± 0.16 a | 3.21 ± 0.18 b | 3.21 ± 0.12 b | 2.53 ± 0.04 a | 3.17 ± 0.1 b |
| [E] 2-Heptenal | 2.36 ± 0.14 a | 2.95 ± 0.15 b,c | 0.00 | 2.78 ± 0.1 b | 0.00 | 3.15 ± 0.20 c | 3.21 ± 0.12 c | 3.59 ± 0.14 d |
| 1-octanol | 8.95 ± 0.66 a | 9.12 ± 0.5 a | 10.87 ± 0.74 b | 9.51 ± 0.55 a,b | 9.45 ± 0.62 a,b | 9.32 ± 0.42 a,b | 8.42 ± 0.55 a | 8.54 ± 0.43 a |
| Octanal | 3.34 ± 0.21 a | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Phenolic derivatives | ||||||||
| β-Phenylethyl alcohol | 13,562.32 ± 351.69 d | 19,684.21 ± 398.62 g | 11,428.00 ± 92.09 b | 19,056 ± 221.65 g | 12,546 ± 281.62 c | 18,112 ± 195.38 f | 10,129.00 ± 295.53 a | 16,346 ± 254.85 e |
| Benzyl alcohol | 89.26 ± 6.35 a | 98.31 ± 6.1 a | 93.45 ± 7.03 a | 125.36 ± 5.54 b | 116.06 ± 2.34 b | 147.43 ± 6.28 c | 131.48 ± 5.26 b,c | 191.62 ± 7.43 d |
| Eugenol | 8.49 ± 0.32 ab | 14.98 ± 0.61 d | 7.26 ± 0.27 a | 15.85 ± 1.03 d | 9.87 ± 0.41 b,c | 15.18 ± 0.48 d | 10.51 ± 0.87 c | 16.35 ± 0.56 d |
| Guaiacol | 3.25 ± 0.21 a | 7.92 ± 0.33 c | 5.86 ± 0.19 b | 8.41 ± 0.32 c | 4.72 ± 0.30 b | 9.75 ± 0.26 d | 8.05 ± 0.23 c | 11.22 ± 0.9 e |
| Vanillin | 1.89 ± 0.1 a,b | 2.95 ± 0.19 b,c | 1.93 ± 0.14 a,b | 3.79 ± 0.2 c,d | 2.63 ± 0.1 a | 4.23 ± 0.12 c,d | 3.87 ± 0.38 c,d | 5.47 ± 0.64 d |
| Terpenes and derivatives | ||||||||
| Citronellol | 6.89 ± 0.45 c | 9.56 ± 0.7 e | 5.13 ± 0.26 b | 8.95 ± 0.31 e | 4.45 ± 0.21 b | 8.53 ± 0.67 d,e | 3.06 ± 0.1 a | 7.32 ± 0.26 c,d |
| α-terpineol | 1.56 ± 0.1 b | 3.14 ± 0.1 e | 1.12 ± 0.09 a | 2.66 ± 0.18 d | 0.00 | 2.39 ± 0.11 c,d | 7.56 ± 0.25 f | 2.12 ± 0.07 c |
| Limonene | 5.84 ± 0.21 c,d | 7.40 ± 0.39 e | 5.58 ± 0.2 c | 6.36 ± 0.22 d | 4.12 ± 0.26 b | 5.28 ± 0.18 c | 3.26 ± 0.16 a | 5.69 ± 0.14 c,d |
| Eucalyptol | 0.32 ± 0.1 a | 0.63 ± 0.1 b,c | 0.00 | 0.52 ± 0.1 b | 0.00 | 0.77 ± 0.06 c | 0.00 | 0.00 |
| [Z], [E] Linalool oxide | 0.00 | 0.00 | 3.23 ± 0.11 a,b | 2.63 ± 0.12 a | 5.48 ± 0.41 c | 3.15 ± 0.18 a,b | 5.79 ± 0.33 c | 3.56 ± 0.21 b |
| Hotrienol | 0.00 | 0.00 | 0.00 | 2.28 ± 0.08 a | 3.56 ± 0.21 b | 3.69 ± 0.16 b | 4.21 ± 0.24 c | 11.52 ± 0.62 d |
| [E,E] Farnesol | 2.25 ± 0.11 b,c,d | 2.34 ± 0.0 c,d | 2.59 ± 0.15 d | 2.11 ± 0.12 a,b,c | 3.69 ± 0.16 e | 1.85 ± 0.16 a | 3.85 ± 0.20 e | 1.93 ± 0.09 a,b |
| p-ment-1-en-7,8-diol | 0.00 | 0.00 | 0.00 | 1.12 ± 0.0 a | 1.25 ± 0.07 a | 1.96 ± 0.21 b | 3.84 ± 0.26 d | 2.58 ± 0.17 c |
| 8-hidroxy-linalool | 0.00 | 0.00 | 0.95 ± 0.07 d | 0.33 ± 0.1 a | 0.56 ± 0.1 b | 0.78 ± 0.03 c | 2.73 ± 0.11 f | 1.45 ± 0.23 e |
| Norisoprenoids | ||||||||
| β -Damascenone | 2.06 ± 0.18 d | 2.58 ± 0.14 e | 1.85 ± 0.14 c | 2.41 ± 0.13 e | 1.26 ± 0.09 b | 2.10 ± 0.19 d | 0.78 ± 0.06 a | 2.13 ± 0.15 d |
| β -ionone | 0.16 ± 0.2 a,b | 0.23 ± 0.2 b,c | 0.18 ± 0.02 a,b,c | 0.26 ± 0.01 c | 0.12 ± 0.2 a | 0.19 ± 0.07 a,b,c | 0.00 | 0.24 ± 0.04 b,c |
| 3-oxo-α-ionol | 4.41 ± 0.21 a | 5.26 ± 0.18 b,c | 4.59 ± 0.32 a,b | 4.48 ± 0.22 a,b | 4.96 ± 0.39 a,b,c | 6.62 ± 0.21 d | 5.68 ± 0.42 c | 8.47 ± 0.89 e |
| Vitispirane | 0.00 | 0.00 | 0.00 | 0.00 | 3.25 ± 0.27 a | 4.52 ± 0.26 b | 6.58 ± 0.33 c | 8.44 ± 0.22 d |
| Actinidol | 0.00 | 0.00 | 0.00 | 0.63 ± 0.06 a | 0.00 | 1.47 ± 0.08 c | 0.95 ± 0.07 b | 5.33 ± 0.34 d |
| TDN | 0.00 | 0.00 | 0.00 | 0.00 | 1.14 ± 0.13 a | 1.52 ± 0.09 b | 2.06 ± 0.14 c | 3.69 ± 0.26 d |
| Esters | ||||||||
| Isoamylacetate | 8.91 ± 0.65 c | 8.43 ± 1.15 c | 8.43 ± 0.79 c | 6.25 ± 0.87 b | 6.32 ± 0.58 b | 5.41 ± 0.22 a,b | 5.17 ± 0.35 a,b | 4.12 ± 0.15 a |
| Ethylisobutyrate | 110.26 ± 4.32 a | 168.11 ± 9.1 c | 115.36 ± 4.38 a | 155.36 ± 7.87 b,c | 121.85 ± 7.67 a | 178.23 ± 9.22 c | 132.46 ± 9.21 a,b | 160.38 ± 5.89 c |
| Ethyl 2-methylbutyrate | 21.65 ± 0.68 a | 40.06 ± 3.11 b,c | 25.42 ± 2.11 a | 45.22 ± 1.45 cd | 36.59 ± 2.15 b | 51.39 ± 2.14 d | 41.25 ± 3.01 b,c | 59.98 ± 3.96 e |
| Ethylpentanoate | 0.76 ± 0.05 c,d | 0.89 ± 0.05 d,e | 0.48 ± 0.13 a,b | 1.02 ± 0.066 | 0.66 ± 0.03 b,c | 0.91 ± 0.03 d,e | 0.41 ± 0.05 a | 0.67 ± 0.05 c |
| Ethyl 4-methylpentanoate | 0.18 ± 0.02 a | 0.24 ± 0.02 b | 0.22 ± 0.02 a,b | 0.59 ± 0.08 d | 0.25 ± 0.02 b | 1.04 ± 0.04 f | 0.36 ± 0.03 c | 0.93 ± 0.03 e |
| Ethyllactate | 253.32 ± 10.2 a,b | 278.13 ± 9.21 b,c,d | 221.22 ± 17.45 a | 299.4 ± 11.23 c,d,e | 278.95 ± 4.95 b,c,d | 265.31 ± 6.13 b,c | 315.44 ± 18.7 e | 303.4 ± 9.85 d,e |
| Ethylhexanoate | 179.6 ± 8.63 b | 297.13 ± 14.32 d | 162.15 ± 8.73 a,b | 284.11 ± 9.85 cd | 142.26 ± 11.28 a | 270.52 ± 3.15 c,d | 151.13 ± 12.36 a,b | 261.37 ± 7.74 c |
| Ethylheptanoate | 41.02 ± 2.63 e | 48.75 ± 1.98 f | 28.59 ± 1.98 c | 33.25 ± 1.28 d | 13.25 ± 1.14 a | 21.64 ± 1.64 b | 0.00 | 11.66 ± 0.49 a |
| Ethylisovalerate | 42.35 ± 2.89 a | 48.36 ± 3.71 a,b | 45.26 ± 2.15 a,b | 52.33 ± 3.05 b,c | 39.87 ± 0,86 a | 60.22 ± 4.13 c,d | 47.12 ± 3.86 a,b | 63.4 ± 2.94 d |
| Ethyloctanoate | 106.32 ± 7.76 b,c | 141.15 ± 10.08 d | 95.26 ± 6.59 a,b | 121.49 ± 6.03 c,d | 112.22 ± 8.48 b,c | 108.11 ± 3.12 b,c | 83.19 ± 4.55 a | 91.34 ± 7.02 a,b |
| Ethyldecanoate | 32.28 ± 1.99 c,d,e | 37.69 ± 2.54 e,f | 42.58 ± 2.63 f | 35.6 ± 1.76 d,e | 28,25 ± 0.98 b,c | 31.26 ± 2.09 c,d | 16.78 ± 1.02 a | 24.11 ± 2.12 b |
| Diethylsuccinate | 936.24 ± 12.15 c | 728.96 ± 22.48 a | 1165 ± 42.36 d | 848.51 ± 20.56 b | 1545 ± 35.21 e | 991.2 ± 12.13 c | 1913.22 ± 29.97 f | 1234.46 ± 34.77 d |
| Phenyletylacetate | 422.63 ± 22.49 c | 493.57 ± 23.56 d | 369.52 ± 21.65 b,c | 411.56 ± 6.89 c | 315.21 ± 16.16 b | 368.77 ± 21.65 b,c | 236.98 ± 8.70 a | 315.43 ± 17.4 b |
| Ethylvanillate | 56.32 ± 3.62 c,d | 71.21 ± 4.51 d | 48.25 ± 2.69 b,c | 67.14 ± 2.26 e,f | 46.95 ± 2.87 b | 59.98 ± 3.54 d,e | 28.21 ± 1.22 a | 46.58 ± 1.87 b |
| Lactones and enolones | ||||||||
| γ-Butyrolactone | 322.51 ± 20.14 a,b,c | 301.59 ± 15.63 a,b,c | 341.25 ± 15.80 c | 294.51 ± 14.54 a,b | 318.95 ± 25.1 a,b,c | 287.14 ± 8.32 a | 336.4 ± 9.07 b,c | 309.23 ± 11.41 a,b,c |
| Pantolactone | 0.00 | 0.00 | 0.00 | 0.00 | 2.36 ± 0.19 a | 3.69 ± 1.54 a | 3.14 ± 0.22 a | 14.23 ± 1.09 b |
| Furaneol | 23.19 ± 1.16 c,d | 26.31 ± 1.86 d,e | 19.86 ± 1.59 b,c | 27.98 ± 0.98 e,f | 17.45 ± 0.97 b | 31.25 ± 1.54 f,g | 11.29 ± 0.88 a | 35.18 ± 1.76 g |
| Homofuraneol | 19.68 ± 1.13 a | 22.36 ± 1.8 a | 20.59 ± 1.56 a | 23.56 ± 1.12 a | 23.69 ± 0.77 a | 32.55 ± 0.22 b | 28.75 ± 2.06 b | 43.96 ± 1.58 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasanta, C.; Cejudo, C.; Gómez, J.; Caro, I. Influence of Prefermentative Cold Maceration on the Chemical and Sensory Properties of Red Wines Produced in Warm Climates. Processes 2023, 11, 374. https://doi.org/10.3390/pr11020374
Lasanta C, Cejudo C, Gómez J, Caro I. Influence of Prefermentative Cold Maceration on the Chemical and Sensory Properties of Red Wines Produced in Warm Climates. Processes. 2023; 11(2):374. https://doi.org/10.3390/pr11020374
Chicago/Turabian StyleLasanta, Cristina, Cristina Cejudo, Juan Gómez, and Ildefonso Caro. 2023. "Influence of Prefermentative Cold Maceration on the Chemical and Sensory Properties of Red Wines Produced in Warm Climates" Processes 11, no. 2: 374. https://doi.org/10.3390/pr11020374
APA StyleLasanta, C., Cejudo, C., Gómez, J., & Caro, I. (2023). Influence of Prefermentative Cold Maceration on the Chemical and Sensory Properties of Red Wines Produced in Warm Climates. Processes, 11(2), 374. https://doi.org/10.3390/pr11020374







