Changes in Volatile Compounds during Grape Brandy Production from ‘Cabernet Sauvignon’ and ‘Syrah’ Grape Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Varieties
2.2. Base Wine Production
2.3. Distillation
2.4. Aging
2.5. Chemical Composition Analysis of Musts and Base Wine
2.6. GC-FID Analysis of Primary Volatile Compounds
2.7. Gas Chromatography–Mass Spectrometry (GC/MS) Analysis
3. Results and Discussion
3.1. Musts
3.2. Base Wines
3.3. Wine Spirit
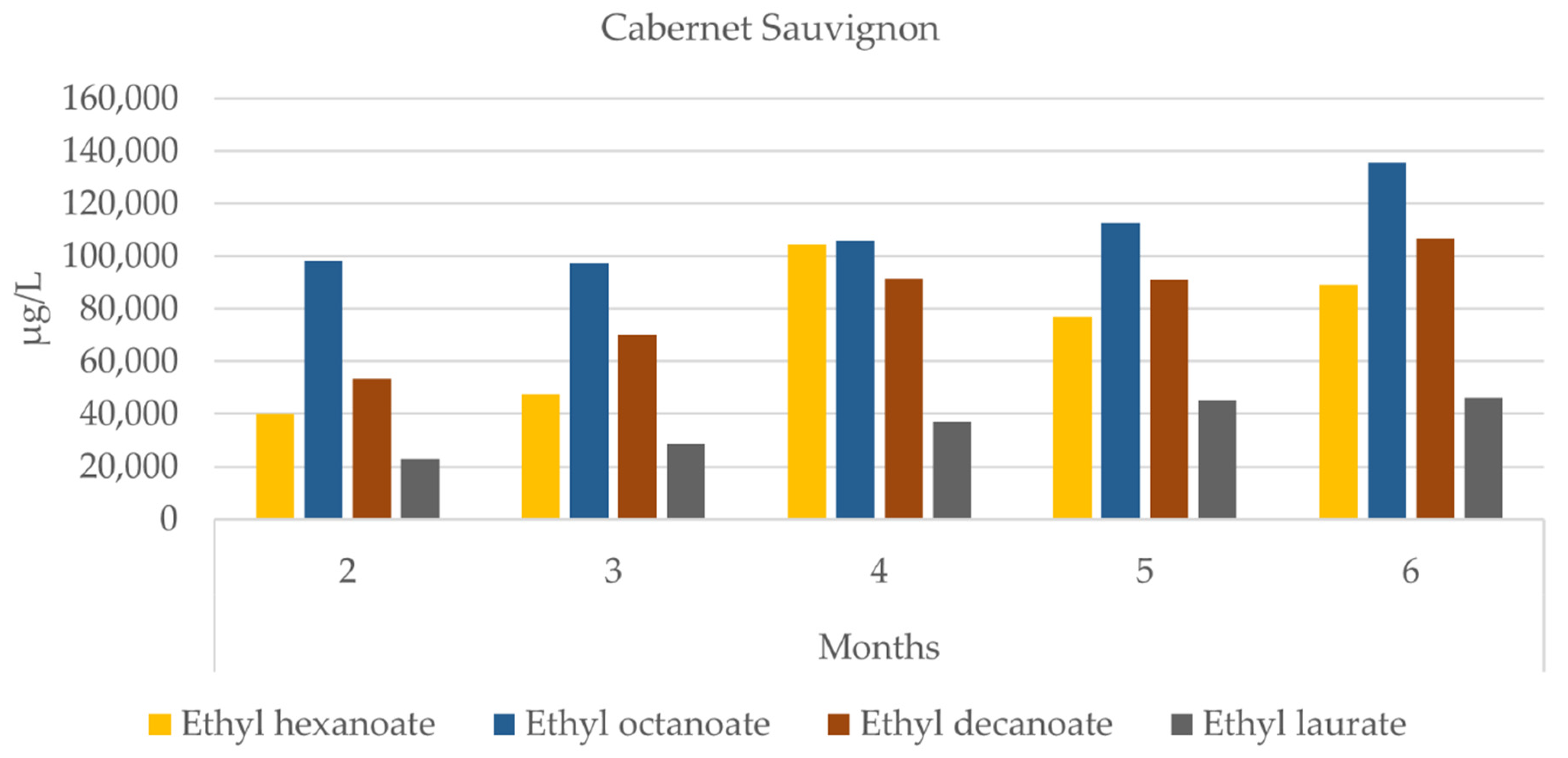
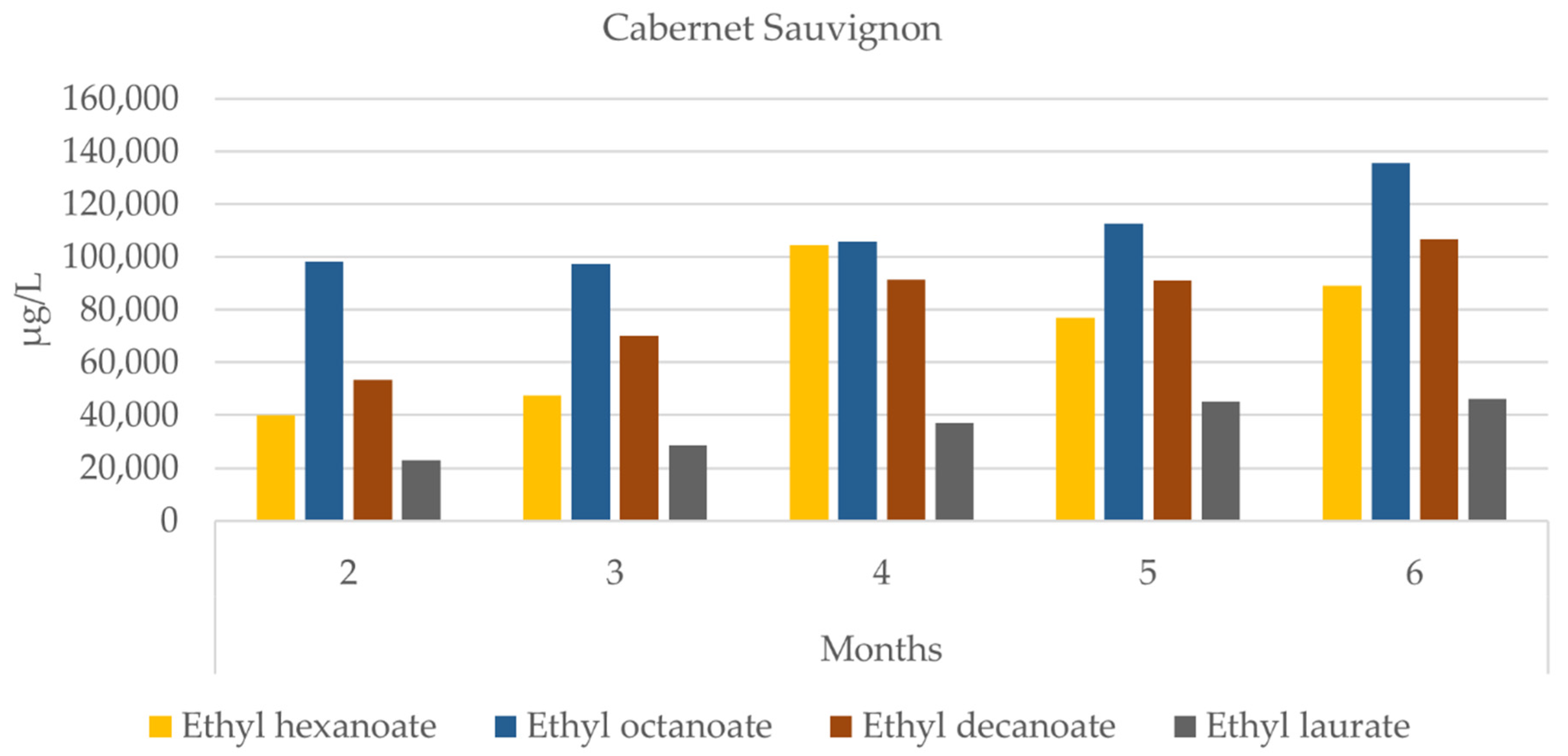
3.4. Grape Brandy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- EU. The Regulation (EC) No 787/2019 of the European Parliament and of the Council; EU: Brussels, Belgium, 2019. [Google Scholar]
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food Agric. 2014, 94, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Vergara, M.; Alvarez-Marin, A.; Carvajal-Cortes, S.; Salinas-Flores, S. Implementation of a Cleaner Production Agreement and impact analysis in the grape brandy (pisco) industry in Chile. J. Clean. Prod. 2015, 96, 110–117. [Google Scholar] [CrossRef]
- Sánchez-Guillén, M.M.; Schwarz-Rodríguez, M.; Rodríguez-Dodero, M.C.; García-Moreno, M.V.; Guillén-Sánchez, D.A.; García-Barroso, C. Discriminant ability of phenolic compounds and short chain organic acids profiles in the determination of quality parameters of Brandy de Jerez. Food Chem. 2019, 286, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Aylott, R. Analytical Strategies Supporting Protected Designations of Origin for Alcoholic Beverages, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 60, ISBN 9780444595621. [Google Scholar]
- Ramos, M.C.; Jones, G.V. Relationships between Cabernet Sauvignon phenology and climate in two Spanish viticultural regions: Observations and predicted future changes. J. Agric. Sci. 2018, 156, 1079–1089. [Google Scholar] [CrossRef]
- Nan, L.; Liu, L.; Li, Y.; Huang, J.; Wang, Y.; Wang, C.; Wang, Z.; Xu, C. Comparison of Aroma Compounds in Cabernet Sauvignon Red Wines from Five Growing Regions in Xinjiang in China. J. Food Qual. 2021, 2021, 5562518. [Google Scholar] [CrossRef]
- Geffroy, O.; Morère, M.; Lopez, R.; Pasquier, G.; Condoret, J.S. Investigating the Aroma of Syrah Wines from the Northern Rhone Valley Using Supercritical CO2-Dearomatized Wine as a Matrix for Reconstitution Studies. J. Agric. Food Chem. 2020, 68, 11512–11523. [Google Scholar] [CrossRef]
- Milicevic, B.; Banovic, M.; Kovacevic-Ganic, K.; Gracin, L. Impact of grape varieties on wine distillates flavour. Food Technol. Biotechnol. 2002, 40, 227–232. [Google Scholar]
- Flamini, R. Mass Spectrometry in Grape and Wine Chemistry Wiley-Interscience Series in Mass Spectrometry; Wiley-Blackwell: Hoboken, NJ, USA, 2009; ISBN 9780470392478. [Google Scholar]
- Mayr Marangon, C.; De Rosso, M.; Carraro, R.; Flamini, R. Changes in volatile compounds of grape pomace distillate (Italian grappa) during one-year ageing in oak and cherry barrels. Food Chem. 2021, 344, 128658. [Google Scholar] [CrossRef]
- Louw, L.; Lambrechts, M.G. Grape-Based Brandies: Production, Sensory Properties and Sensory Evaluation; Woodhead Publishing Limited: Sawston, UK, 2012. [Google Scholar]
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Brandy and Cognac: Manufacture and Chemical Composition, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780123849533. [Google Scholar]
- Costa, C.; Graça, A.; Fontes, N.; Teixeira, M.; Gerós, H.; Santos, J.A. The interplay between atmospheric conditions and grape berry quality parameters in Portugal. Appl. Sci. 2020, 10, 4943. [Google Scholar] [CrossRef]
- Ubalde, J.M.; Sort, X.; Zayas, A.; Poch, R.M. Effects of Soil and Climatic Conditions on Grape Ripening and Wine Quality of Cabernet Sauvignon. J. Wine Res. 2010, 21, 1–17. [Google Scholar] [CrossRef]
- Ruffner, H.P.; Hawker, J.S.; Hale, C.R. Temperature and enzymic control of malate metabolism in berries of Vitis vinifera. Phytochemistry 1976, 15, 1877–1880. [Google Scholar] [CrossRef]
- Keller, M. Managing grapevines to optimise fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Poudel, P.R.; Mochioka, R.; Beppu, K.; Kataoka, I. Influence of temperature on berry composition of interspecific hybrid wine grape “Kadainou R-1” (Vitis ficifolia var. ganebu × V. vinifera ‘Muscat of Alexandria’). J. Jpn. Soc. Hortic. Sci. 2009, 78, 169–174. [Google Scholar] [CrossRef][Green Version]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Antunes, C.; Ramos, A.; Serra, A.T.; Figueira, M.E.; Duarte, C.M.M.; de Carvalho, A.; Bronze, M.R. Characterization of traditional and exotic apple varieties from Portugal. Part 1—Nutritional, phytochemical and sensory evaluation. J. Funct. Foods 2010, 2, 35–45. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2020; ISBN 9782850380037. [Google Scholar]
- Wang, M.L.; Choong, Y.M.; Su, N.W.; Lee, M.H. A rapid method for determination of ethanol in alcoholic beverages using capillary gas chromatography. J. Food Drug Anal. 2003, 11, 133–140. [Google Scholar] [CrossRef]
- Ordinance on analytical methods for spirits and alcoholic beverages. MAFWM 2005, 11, 1–10.
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Jackson, R.S. Specific and Distinctive Wine Styles. In Wine Science; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780123814685. [Google Scholar]
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef]
- Rizzon, L.A.; Miele, A. Physicochemical Characteristics of the Brazilian Cabernet Sauvignon Wine as a Function of the Vintage. Available online: https://www.alice.cnptia.embrapa.br/bitstream/doc/542397/1/OIVBudapest2007ArticleRizzonandMiele.pdf (accessed on 27 April 2022).
- Souza, S.C.; Theodoro, K.H.; Souza, É.R.; Da Motta, S.; Glória, M.B.A. Bioactive amines in Brazilian wines: Types, levels and correlation with physico-chemical parameters. Braz. Arch. Biol. Technol. 2005, 48, 53–62. [Google Scholar] [CrossRef]
- Eliete Iochims dos Santos, C.; Raquel Manfredi da Silva, L.; Appel Boufleur, L.; Debastiani, R.; Alberici Stefenon, C.; Amaral, L.; Lúcia Yoneama, M.; Dias, J.F. Elemental characterisation of Cabernet Sauvignon wines using Particle-Induced X-ray Emission (PIXE). Food Chem. 2010, 121, 244–250. [Google Scholar] [CrossRef]
- De Carvalho, E.S.S.; Biasoto, A.C.T.; Nassur, R.d.C.M.R.; Barros, A.P.A.; Leão, P.C.S.; Lima, E.d.S.; De Camargo, A.C.; Mamede, M.E.d.O. Physicochemical characteristics, phenolic profile, and antioxidant capacity, of Syrah tropical wines: Effects of vineyard management practices. J. Food Bioact. 2020, 9, 70–78. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, Y.; Li, J.; Fan, W.; Jiang, W. Profile of volatile compounds in 11 brandies by headspace solid-phase microextraction followed by gas chromatography-mass spectrometry. J. Food Sci. 2009, 74, C90–C99. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V. Volatile Aroma Compounds and Wine Sensory Attributes. In Managing Wine Quality; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–28. [Google Scholar]
| ‘Cabernet Sauvignon’ | ‘Syrah’ | |
|---|---|---|
| Soluble solids content (°Oe) | 94.00 | 90.00 |
| pH | 3.32 | 3.52 |
| Total acidity (g/L) | 9.00 | 7.50 |
| ‘Cabernet Sauvignon’ | ‘Syrah’ | |
|---|---|---|
| φ (alcohol)/% | 13.18 | 12.63 |
| γ (total dry extract) (g/L) | 30.70 | 26.90 |
| γ(reducing sugars) (g/L) | 2.64 | 2.78 |
| Total acidity/(g/L) | 7.35 | 6.60 |
| Volatile acidity (g/L) | 0.72 | 0.62 |
| Non-volatile acidity (g/L) | 6.13 | 5.98 |
| pH | 3.41 | 3.54 |
| γ (free SO2) (mg/L) | - | - |
| γ(total SO2) (mg/L) | - | - |
| 1st Distillation | ||
|---|---|---|
| ‘Cabernet Sauvignon’ | ‘Syrah’ | |
| Volume (mL) | 28,720.00 | 27,000.00 |
| ‘heads’ (mL) | 143.50 | 133.00 |
| φ (alcohol)/% | 68.00 | 64.00 |
| ‘heart’ (mL) | 7525.00 | 7800.00 |
| φ (alcohol)/% | 39.02 | 39.00 |
| ‘tails’ (mL) | 1105.00 | 1065.00 |
| 2nd distillation | ||
| Volume (mL) | 7525.00 | 7800.0 |
| ‘heads’ (mL) | 73.00 | 78.00 |
| φ (alcohol)/% | 85.00 | 85.00 |
| ‘heart’ (mL) | 3300.00 | 3200.00 |
| φ (alcohol)/% | 74.00 | 72.00 |
| ‘tails’ (mL) | 965.00 | 1000.00 |
| φ (alcohol)/% | 26.00 | 24.00 |
| Recovery (%) of Ethanol | 64.55 | 67.57 |
| Compound | ‘Cabernet Sauvignon’ | ‘Syrah’ |
|---|---|---|
| Alcohols | ||
| 3-methylbutanol | n.d. * | 99.13 ± 2.5 |
| 1-hexanol | 431.39 ± 29.27 | 355.90 ± 19.16 |
| 1-octen-3-ol | n.d. | 40.87 ± 3.9 |
| 3-octanol | n.d. | 21.95 ± 0.42 |
| 1-octanol | n.d. | 16.17 ± 0.85 |
| Hotrienol | n.d. | 49.40 ± 6.59 |
| Dodecanol | 6.73 ± 0.13 | 5.58 ± 0.23 |
| Total | 438.12 | 589.00 |
| Aldehydes and ketones | ||
| 2-hexenal | 35.96 ± 5.36 | n.d. |
| Benzeneacetaldehyde | 8.39 ± 0.42 | 13.09 ± 0.21 |
| 3-octanone | n.d. | 24.41 ± 1.25 |
| Decanal | n.d. | 9.04 ± 0.3 |
| Dodecanal | 4.61 ± 0.23 | 4.47 ± 0.28 |
| Alpha-hexylcinnamic aldehyde | 2.47 ± 0.18 | n.d. |
| Total | 51.43 | 51.01 |
| Esters | ||
| Ethyl hexanoate | n.d. | 23.60 ± 2.72 |
| 1-hexyl acetate | 19.28 ± 0.83 | 13.67 ± 2.04 |
| Ethyl 2-hexenoate | n.d. | 27.15 ± 2.6 |
| Ethyl palmitate | n.d. | 49.93 ± 1.15 |
| Ethyl linoleate | n.d. | 23.05 ± 1.34 |
| Ethyl oleate | n.d. | 23.92 ± 0.59 |
| Ethyl myristate | n.d. | 4.22 ± 0.44 |
| Isopropyl myristate | 24.69 ± 0.35 | 14.22 ± 1.46 |
| Ethyl laurate | n.d. | 19.80 ± 0 |
| Phenethyl acetate | 22.56 ± 1.95 | n.d. |
| Ethyl decanoate | n.d. | 4.20 ± 0.01 |
| Total | 66.53 | 203.76 |
| Terpenes and norisoprenoides | ||
| Linalool | 9.40 ± 0.49 | 10.71 ± 0.38 |
| Gamma-terpinene | n.d. | 11.42 ± 0.34 |
| β-damascenone | 97.93 ± 1.73 | 23.04 ± 1.27 |
| Geranyl acetone | 9.94 ± 0.71 | 8.30 ± 0.23 |
| Trans-Caryophyllene | n.d. | 8.60 ± 0.16 |
| α-ionone | 1.74 ± 0.21 | n.d. |
| β-ionone | 2.66 ± 0.35 | 3.71 ± 0.32 |
| Perilla alcohol | 6.65 ± 0.08 | 5.13 ± 0.39 |
| Total | 128.29 | 70.91 |
| Acids | ||
| Hexanoic acid | 8.64 ± 0.72 | n.d. |
| Nonanoic acid | 11.61 ± 0.62 | 5.48 ± 0.24 |
| Decanoic acid | 7.96 ± 0.74 | 6.43 ± 0.13 |
| Dodecanoic acid | 1.60 ± 0.11 | n.d. |
| Tetradecanoic acid | 1.97 ± 0.02 | n.d. |
| Total | 31.77 | 11.91 |
| Furans | ||
| Phellandral | 5.84 ± 0.35 | n.d. |
| Total | 5.84 | 0 |
| Volatile phenols | ||
| 4-ethyl phenol | n.d. | 183.58 ± 12.54 |
| 4-vinylphenol | n.d. | 14.40 ± 0.09 |
| Total | 0.00 | 197.98 |
| Aromatic compounds | ||
| Benzaldehyde | n.d. | 9.34 ± 0.98 |
| 2-phenylethanol | n.d. | 180.50 ± 6 |
| Nonanone | 6.55 ± 0.09 | 4.11 ± 0.47 |
| p-cymene | 7.56 ± 0.47 | 22.76 ± 2.43 |
| Dihydro-methyl-jasmonate | 6.82 ± 0.11 | 4.80 ± 0.22 |
| Total | 20.93 | 221.51 |
| Compound | ‘Cabernet Sauvignon’ | ‘Syrah’ |
|---|---|---|
| Alcohols | ||
| 3-methylbutanol | 10,498.70 ± 93.32 | 8442.74 ± 360.6 |
| 1-hexanol | 226.47 ± 61.74 | 153.43 ± 0.69 |
| 2,3-butanediol | 692.05 ± 105.59 | 319.85 ± 87.05 |
| Total | 11,417.22 | 8916.02 |
| Esters | ||
| Ethyl acetate | 646.82 ± 12.76 | 344.03 ± 0.99 |
| Ethyl hexanoate | 2041.08 ± 107.86 | 1977.14 ± 20.37 |
| Ethyl octanoate | 1648.75 ± 1.45 | 1408.73 ± 0.30 |
| Ethyl palmitate | 489.90 ± 32.02 | 205.57 ± 6.70 |
| Isoamyl acetate | 374.94 ± 42.51 | n.d. * |
| Ethyl myristate | 121.34 ± 13.86 | 82.14 ± 0.74 |
| Isopropyl myristate | 188.23 ± 7.77 | 216.46 ± 10.43 |
| Ethyl laurate | 880.41 ± 45.65 | 247.63 ± 0.16 |
| Phenethyl acetate | 201.90 ± 10.07 | 81.13 ± 2.45 |
| Total | 6593.37 | 4562.83 |
| Terpenes | ||
| Linalool | 276.59 ± 8.67 | n.d. |
| β-damascenone | 17.93 ± 1.41 | 18.56 ± 0.43 |
| Total | 294.52 | 18.56 |
| Acids | ||
| Acetic acid | 514.15 ± 41.44 | 175.38 ± 1.85 |
| Hexanoic acid | 357.36 ± 9.92 | n.d. |
| Octanoic acid | 3963.74 ± 89.26 | 1865.10 ± 3.84 |
| Capric acid | 45.50 ± 59.55 | 933.24 ± 4.64 |
| Caproic acid | n.d. | 196.84 ± 1.89 |
| Myristic acid | 70.64 ± 1.88 | 99.82 ± 0.29 |
| Decanoic acid | 244.96 ± 6.29 | 340.47 ± 5.76 |
| Dodecanoic acid | 291.53 ± 5.90 | 180.18 ± 3.54 |
| Total | 5487.88 | 3791.03 |
| Aromatic compounds | ||
| 2-phenylethanol | 1101.44 ± 51.94 | 1154.43 ± 64.29 |
| Total | 1101.44 | 1154.43 |
| Compounds | 1st Distillation | |
|---|---|---|
| ‘Cabernet Sauvignon’ | ‘Syrah’ | |
| Esters | ||
| Isoamylacetate | 1699.26 ± 56.88 | 3127.20 ± 783.19 |
| Ethyl hexanoate | 4493.33 ± 309.36 | n.d. * |
| Ethyl octanoate | 6630.85 ± 9.96 | 5997.03 ± 69.93 |
| Phenethyl acetate | 7145.27 ± 295.87 | n.d. |
| Ethyl decanoate | 5792.73 ± 17.29 | 2499.78 ± 94.52 |
| Ethyl laurate | 2320.88 ± 16.66 | 877.65 ± 42.86 |
| Ethyl myristate | 61.59 ± 2.16 | n.d. |
| Total | 28,143.91 | 12,501.66 |
| Alcohols | ||
| 3-methylbutanol | 31,173.29 ± 2.73 | 85,481.35 ± 2194.48 |
| 1-hexanol | 1196.89 ± 42.45 | 5286.73 ± 12.69 |
| 2-phenylethanol | 271.72 ± 12.32 | n.d. |
| Total | 32,641.90 | 90,768.08 |
| Acids | ||
| Acetic acid | n.d. | 1473.06 ± 64.84 |
| Decanoic acid | 279.77 ± 2.67 | n.d. |
| Myristic acid | 101.13 ± 0.12 | n.d. |
| Total | 380.90 | 1473.06 |
| 2nd distillation | ||
| ‘Cabernet Sauvignon’ | ‘Syrah’ | |
| Esters | ||
| Ethyl hexanoate | 1524.36 ± 69.18 | n.d. |
| Ethyl octanoate | 7148.06 ± 99.8 | 3566.46 ± 88.04 |
| Phenethyl acetate | n.d. | 313.23 ± 2.27 |
| Ethyl decanoate | 1815.29 ± 75.47 | 607.09 ± 0.23 |
| Ethyl laurate | 762.20 ± 10.44 | n.d. |
| Ethyl palmitate | 622.22 ± 5.62 | n.d. |
| Total | 11,872.13 | 4486.78 |
| Alcohols | ||
| 3-methylbutanol | 24,464.58 ± 723.11 | 80,593.73 ± 341.83 |
| 1-hexanol | 1716.89 ± 2.41 | 741.65 ± 9.01 |
| 2-phenylethanol | n.d. | 871.01 ± 23.17 |
| Total | 26,181.47 | 82,206.39 |
| Acids | ||
| Acetic acid | n.d. | 1988.67 ± 77.52 |
| Hexanoic acid | 1270.72 ± 7.59 | n.d. |
| Total | 1270.72 | 1988.67 |
| Final distillate | ||
| ‘Cabernet Sauvignon’ | ‘Syrah’ | |
| Esters | ||
| Isoamyl acetate | 799.76 ± 47.5 | 1131.90 ± 17.06 |
| Ethyl hexanoate | 1038.98 ± 22.58 | n.d. |
| N-Hexyl acetate | 111.50 ± 5.91 | n.d. |
| Ethyl octanoate | 739.28 ± 42.26 | 2879.02 ± 4.63 |
| Ethyl decanoate | 169.31 ± 0.83 | 1159.59 ± 28.27 |
| Ethyl laurate | 106.17 ± 2.6 | 172.26 ± 1.97 |
| Ethyl myristate | 79.76 ± 3.25 | n.d. |
| Ethyl palmitate | 99.90 ± 3.01 | n.d. |
| Total | 3144.66 | 6342.77 |
| Alcohols | ||
| 3-methylbutanol | 2317.22 ± 4.71 | 8084.11 ± 250.25 |
| 1-hexanol | 205.39 ± 7.06 | 584.88 ± 27.49 |
| 2-phenylethanol | n.d. | 542.57 ± 11.82 |
| Total | 205.39 | 14,697.92 |
| Acids | ||
| Acetic acid | n.d. | 1771.4 ± 73.24 |
| Total | 0 | 1771.4 |
| 2nd Distillation | Final Distillate | |||
|---|---|---|---|---|
| ‘Heads’ | ‘Heart’ | ‘Tails’ | ||
| ‘Cabernet Sauvignon’ | ||||
| Ethanol (% vol.) | 87.58 ± 0.17 | 73.18 ± 0.26 | 27.51 ± 0.43 | 55.56 ± 0.47 |
| Methanol (mg/L a.a.) | 1267.60 ± 120.00 | 440.0 ± 9.60 | 0.0 ± 0.00 | 400.00 ± 1.00 |
| Acetaldehyde (mg/L a.a.) | 499.30 ± 51.90 | 103.30 ± 0.60 | 0.0 ± 0.00 | 83.40 ± 14.40 |
| Ethyl acetate (mg/L a.a.) | 82,242.10 ± 107.00 | 512.90 ± 11.00 | 0.0 ± 0.00 | 447.30 ± 12.30 |
| 2-methylpropanol (mg/L a.a.) | 284.90 ± 402.90 | 42.60 ± 0.10 | 107.40 ± 1.60 | 35.80 ± 4.80 |
| 2-butanol (mg/L a.a.) | 7146.60 ± 439.90 | 539.80 ± 38.00 | 25.80 ± 1.20 | 451.60 ± 94.70 |
| Isoamyl alcohol (mg/L a.a.) | 13,417.40 ± 274.60 | 1928.20 ± 11.80 | 256.10 ± 9.00 | 1676.80 ± 45.90 |
| ‘Syrah’ | ||||
| Ethanol (% vol.) | 81.74 ± 0.22 | 70.61 ± 1.77 | 30.24 ± 0.32 | 55.32 ± 0.22 |
| Methanol (mg/L a.a.) | 145.30 ± 9.200 | 403.70 ± 9.40 | 0.00 ± 0.00 | 403.70 ± 1.60 |
| Acetaldehyde (mg/L a.a.) | 1110.30 ± 10.6 | 49.40 ± 0.20 | 94.90 ± 72.40 | 52.00 ± 0.20 |
| Ethyl acetate (mg/L a.a.) | 8399.00 ± 11.70 | 426.00 ± 10.30 | 341.10 ± 5.80 | 367.00 ± 1.00 |
| 2-methylpropanol (mg/L a.a.) | 0.00 ± 0.00 | 52.50 ± 74.30 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 2-butanol (mg/L a.a.) | 1042.10 ± 24.20 | 846.80 ± 40.30 | 29.20 ± 20.20 | 662.90 ± 52.50 |
| Isoamyl alcohol (mg/L a.a.) | 1324.60 ± 10.40 | 1798.00 ± 33.20 | 483.20 ± 5.90 | 1590.00 ± 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončarić, A.; Patljak, M.; Blažević, A.; Jozinović, A.; Babić, J.; Šubarić, D.; Pichler, A.; Flanjak, I.; Kujundžić, T.; Miličević, B. Changes in Volatile Compounds during Grape Brandy Production from ‘Cabernet Sauvignon’ and ‘Syrah’ Grape Varieties. Processes 2022, 10, 988. https://doi.org/10.3390/pr10050988
Lončarić A, Patljak M, Blažević A, Jozinović A, Babić J, Šubarić D, Pichler A, Flanjak I, Kujundžić T, Miličević B. Changes in Volatile Compounds during Grape Brandy Production from ‘Cabernet Sauvignon’ and ‘Syrah’ Grape Varieties. Processes. 2022; 10(5):988. https://doi.org/10.3390/pr10050988
Chicago/Turabian StyleLončarić, Ante, Mićo Patljak, Ante Blažević, Antun Jozinović, Jurislav Babić, Drago Šubarić, Anita Pichler, Ivana Flanjak, Toni Kujundžić, and Borislav Miličević. 2022. "Changes in Volatile Compounds during Grape Brandy Production from ‘Cabernet Sauvignon’ and ‘Syrah’ Grape Varieties" Processes 10, no. 5: 988. https://doi.org/10.3390/pr10050988
APA StyleLončarić, A., Patljak, M., Blažević, A., Jozinović, A., Babić, J., Šubarić, D., Pichler, A., Flanjak, I., Kujundžić, T., & Miličević, B. (2022). Changes in Volatile Compounds during Grape Brandy Production from ‘Cabernet Sauvignon’ and ‘Syrah’ Grape Varieties. Processes, 10(5), 988. https://doi.org/10.3390/pr10050988








