Innovation and Winemaking By-Product Valorization: An Ohmic Heating Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Fractionation of Grape By-Products
2.4. Characterization of the Raw Material
2.4.1. Proximate Composition
2.4.2. Total Pectin
2.4.3. Cellulose, Hemicellulose, and Lignin
2.4.4. Soluble Sugars
2.5. Extraction Methodologies to Phenolic Compound Recovery
2.5.1. CONV Method
2.5.2. OH Technology
2.6. Bioactive Characterization
2.6.1. Total Antioxidant Capacity and Total Phenolic Content
2.6.2. Bound Phenols
2.6.3. Phenolic Compound Quantification Using HPLC-DAD
2.7. Statistical Analysis
3. Results
3.1. Fractionation Approach
3.2. Proximate Composition
3.3. Bioactivity Characterization
Antioxidant Capacity and Total Polyphenolic Content after CONV Extraction
3.4. Impact of OH vs. CONV Method on Phytochemical Composition of Grape Bagasse
3.4.1. Protein Content
3.4.2. Dietary Fiber
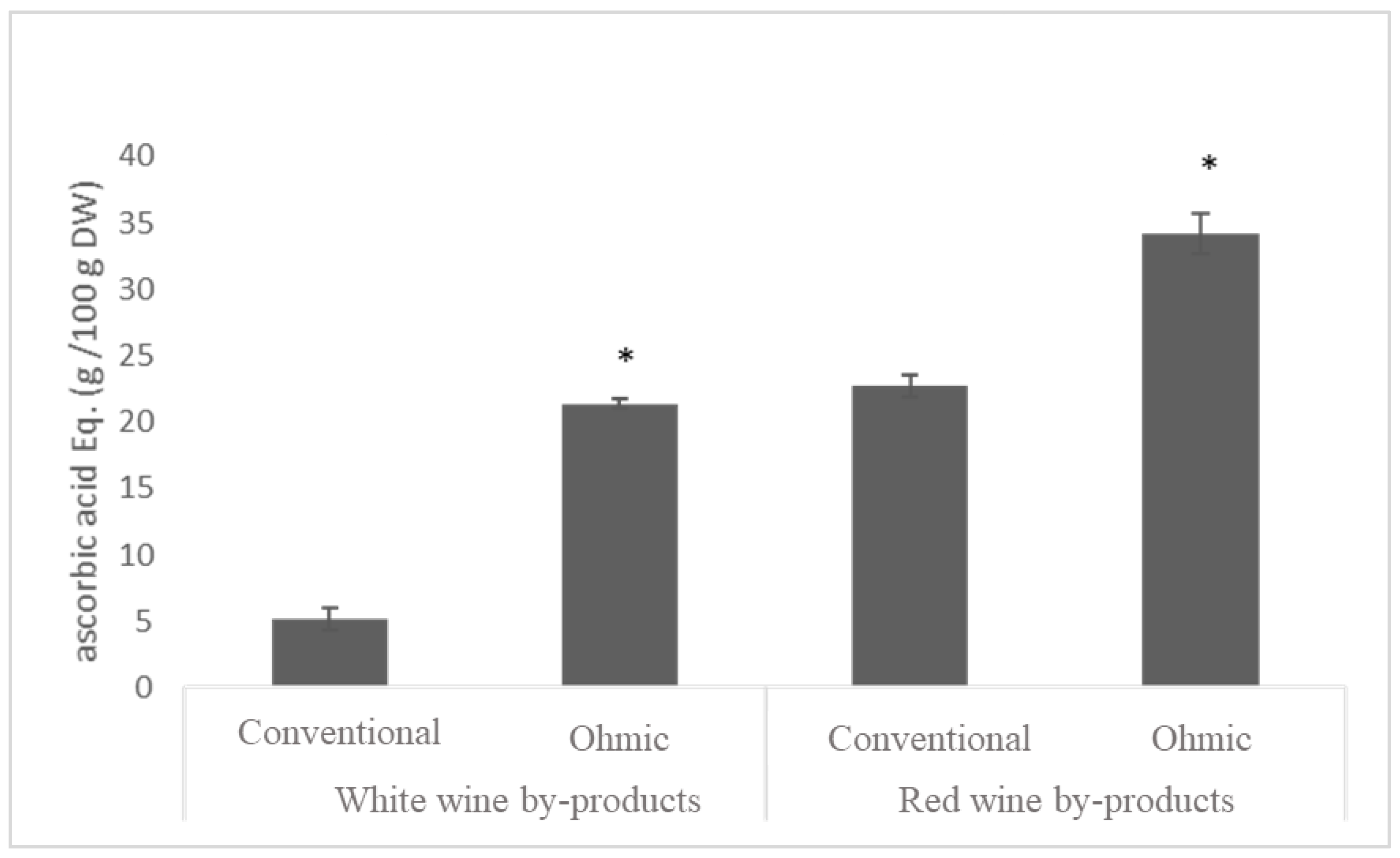
3.5. Bioactivity Characterization
3.5.1. Total Phenolic Content and Antioxidant Activity of Free and Bound Phenolics
3.5.2. Identification of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Organisation OIV. 2019 Statistical Report on World Vitiviniculture; Organisation Internationale de la Vigne et du Vin Publication: France, Paris, 2019; pp. 3–20. [Google Scholar]
- Fereres, E.; Goldhamer, D.A.; Sadras, V. Yield Response to Water of Fruit Trees and Vines: Guidelines. FAO Irrigation and Drainage Paper 66. 2012. Available online: https://www.oiv.int/public/medias/5116/booklet-fao-oiv-grapes-focus.pdf (accessed on 1 October 2022).
- FAO FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 9 December 2022).
- Coelho, M.C.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. The Use of Emergent Technologies to Extract Added Value Compounds from Grape By-Products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.-M.; Cantos-Villar, E. Grapevine Cane’s Waste Is a Source of Bioactive Stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Delgado Adámez, J.; Gamero Samino, E.; Valdés Sánchez, E.; González-Gómez, D. In Vitro Estimation of the Antibacterial Activity and Antioxidant Capacity of Aqueous Extracts from Grape-Seeds (Vitis vinifera L.). Food Control 2012, 24, 136–141. [Google Scholar] [CrossRef]
- Lee, H.S.; Coates, G.A. Characterization of Color Fade during Frozen Storage of Red Grapefruit Juice Concentrates. J. Agric. Food Chem. 2002, 50, 3988–3991. [Google Scholar] [CrossRef] [PubMed]
- Dharmadhikari, M. Composition of Grapes. Vineyard VInt. Age View Mo. State Univ. 1994, 9, 3–8. [Google Scholar]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical Composition of Grape Stalks of Vitis vinifera L. from Red Grape Pomaces. Ind. Crops Prod 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Valiente, C.; Arrigoni, E.; Esteban, R.M.; Amado, R. Grape Pomace as a Potential Food Fiber. J. Food Sci. 1995, 60, 818–820. [Google Scholar] [CrossRef]
- Zacharof, M.-P. Grape Winery Waste as Feedstock for Bioconversions: Applying the Biorefinery Concept. Waste Biomass Valorization 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Abdrabba, S.; Hussein, S. Chemical Composition of Pulp, Seed and Peel of Red Grape From Libya. Glob. J. Sci. Res. 2015, 3, 6–11. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Raposo, R.; Cayuela, J.M.; Zafrilla, P.; Piñeiro, Z.; Moreno-Rojas, J.M.; Mulero, J.; Puertas, B.; Giron, F.; Guerrero, R.F.; et al. Valorization of Grape Stems. Ind. Crops Prod. 2015, 63, 152–157. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-Chemical Properties of Cell Wall Materials Obtained from Ten Grape Varieties and Their Byproducts: Grape Pomaces and Stems. LWT—Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds—Biological Activity; Soto-Hernández, M., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Guida, V.; Ferrari, G.; Pataro, G.; Chambery, A.; di Maro, A.; Parente, A. The Effects of Ohmic and Conventional Blanching on the Nutritional, Bioactive Compounds and Quality Parameters of Artichoke Heads. LWT—Food Sci. Technol. 2013, 53, 569–579. [Google Scholar] [CrossRef]
- Ianni, A.; Martino, G. Dietary Grape Pomace Supplementation in Dairy Cows: Effect on Nutritional Quality of Milk and Its Derived Dairy Products. Foods 2020, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel Application and Industrial Exploitation of Winery By-Products. Bioresour Bioprocess 2018, 5, 46. [Google Scholar] [CrossRef]
- Truong, L.; Morash, D.; Liu, Y.; King, A. Food Waste in Animal Feed with a Focus on Use for Broilers. Int. J. Recycl. Org. Waste Agric. 2019, 8, 417–429. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture Waste Valorisation as a Source of Antioxidant Phenolic Compounds within a Circular and Sustainable Bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Hanušovský, O.; Gálik, B.; Bíro, D.; Šimko, M.; Juráček, M.; Rolinec, M.; Zábranský, L.; Philipp, C.; Puntigam, R.; Slama, J.A.; et al. The Nutritional Potential of Grape By-Products from the Area of Slovakia and Austria. Emir. J. Food Agric. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Kurosawa, A. Multigas Mitigation: An Economic Analysis Using GRAPE Model. Energy J. 2006, 27, 275–288. [Google Scholar] [CrossRef]
- Vidović, S.; Vladić, J.; Nastić, N.; Jokić, S. Subcritical and Supercritical Extraction in Food By-Product and Food Waste Valorization. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 705–721. ISBN 9780081005965. [Google Scholar]
- Galanakis, C.M. Food Waste Recovery; Elsevier: Chania, Greece, 2014. [Google Scholar] [CrossRef]
- el Darra, N.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R. Extraction of Polyphenols from Red Grape Pomace Assisted by Pulsed Ohmic Heating. Food Bioproc. Tech. 2013, 6, 1281–1289. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Genisheva, Z.; Oliveira, H.; de Freitas, V.; Teixeira, J.A.; Vicente, A.A. Effects of Ohmic Heating on Extraction of Food-Grade Phytochemicals from Colored Potato. LWT—Food Sci. Technol. 2016, 74, 493–503. [Google Scholar] [CrossRef]
- Maroun, R.G.; Rajha, H.N.; Vorobiev, E.; Louka, N. Emerging Technologies for the Recovery of Valuable Compounds from Grape Processing By-Products. In Handbook of Grape Processing By-Products: Sustainable Solutions; Galanakis, C.M.B.T., Ed.; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128098714. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019; ISBN 0-935584-89-7. [Google Scholar]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical Composition of Dietary Fiber and Polyphenols of Five Different Varieties of Wine Grape Pomace Skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, D.C. Determination of Structural Carbohydrates and Lignin in Biomass. In NREL Laboratory Analytical Procedures; Golden, CO, USA, 2012; pp. 1–3. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 1 October 2022).
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Gomes, M.H.; Almeida, D.P.F.; Pintado, M. Effect of Modified Atmosphere on Polyphenols during Storage of Pasteurised Strawberry Purées. LWT—Food Sci. Technol. 2015, 60, 377–384. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Lopes, C.; Almeida, D.P.F.; Pintado, M. Incorporation of Strawberries Preparation in Yoghurt: Impact on Phytochemicals and Milk Proteins. Food Chem. 2015, 171, 370–378. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.M.E. Production of a Food Grade Blueberry Extract Rich in Anthocyanins: Selection of Solvents, Extraction Conditions and Purification Method. J. Food Meas. Charact. 2017, 11, 1248–1253. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese Medicinal Plants: Dependence of Final Antioxidant Capacity and Phenol Content on Extraction Features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of Tomato By-Products’ Bioactive Compounds Using Ohmic Technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Xavier, A.M.R.B.; Evtuguin, D.V.; Lopes, L.P.C. Integrated Utilization of Grape Skins from White Grape Pomaces. Ind. Crops Prod. 2013, 49, 286–291. [Google Scholar] [CrossRef]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of Grape Pomace: Drying Behavior and Ultrasound Extraction of Phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Rocha, C.B.; Noreña, C.P.Z. Microwave-Assisted Extraction and Ultrasound-Assisted Extraction of Bioactive Compounds from Grape Pomace. Int. J. Food Eng. 2020, 16, 20190191. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of Solvent and Ultrasound-Assisted Extraction for Different Anthocyanin Rich Fruit and Their Effects on Anthocyanin Compositions. LWT—Food Sci. Technol. 2016, 72, 229–238. [Google Scholar] [CrossRef]
- da Porto, C.; Porretto, E.; Decorti, D. Comparison of Ultrasound-Assisted Extraction with Conventional Extraction Methods of Oil and Polyphenols from Grape (Vitis vinifera L.) Seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Bonfigli, M.; Godoy, E.; Reinheimer, M.A.; Scenna, N.J. Comparison between Conventional and Ultrasound-Assisted Techniques for Extraction of Anthocyanins from Grape Pomace. Experimental Results and Mathematical Modeling. J. Food Eng. 2017, 207, 56–72. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; De Lima, M.A.C.; Alves, R.E.; Gonçalves, A.L.D.S.; Souza, A.P.C. Chemical Characterization of Winemaking Byproducts from Grape Varieties Cultivated in Vale Do São Francisco, Brazil. Food Sci. Technol. 2018, 38, 577–583. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, A.; Hejtmánková, K.; Horníčková, Š.; Pivec, V.; Skala, O.; Dědina, M.; Přibyl, J. Towards Complex Utilisation of Winemaking Residues: Characterisation of Grape Seeds by Total Phenols, Tocols and Essential Elements Content as a by-Product of Winemaking. Ind. Crops Prod. 2013, 49, 445–453. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical Composition of Grape Canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Bravo, L.; Saura-Calixto, F. Characterization of Dietary Fiber and the in Vitro Indigestible Fraction of Grape Pomace. Am. J. Enol. Vitic. 1998, 49, 135–141. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Dietary Fibre Content and Antioxidant Activity of Manto Negro Red Grape (Vitis vinifera): Pomace and Stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Sousa, E.C.; DuarteAlexandrino, C.; Carioca, J.O.B.; Rodrigues, S.P.; Uchôa-Thomaz, A.M.A.; Rodrigues, L.L.; Lima, A.; Ferreira, P.A.T.; Morais, S.M.; Rodrigues, A.L.M.; et al. Chemical Composition and Bioactive Compounds of Grape Pomace (Vitis vinifera L.), Benitaka Variety, Grown in the Semiarid Region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Williams, P.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Oligosaccharides of Cabernet Sauvignon, Syrah and Monastrell Red Wines. Food Chem. 2015, 179, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Karnopp, A.R.; Margraf, T.; Maciel, L.G.; Santos, J.S.; Granato, D. Chemical Composition, Nutritional and in Vitro Functional Properties of by-Products from the Brazilian Organic Grape Juice Industry. Int. Food Res. J. 2017, 24, 207–214. [Google Scholar]
- Tahergorabi, R.; Hosseini, S.V. Chapter 2—Proteins, Peptides, and Amino Acids A2—Galanakis, Charis M. BT—Nutraceutical and Functional Food Components. In Nutraceutical and Functional Food Components; Academic Press: Cambridge, MA, USA, 2017; pp. 15–38. ISBN 978-0-12-805257-0. [Google Scholar]
- Llobera, A.; Cañellas, J. Antioxidant Activity and Dietary Fibre of Prensal Blanc White Grape (Vitis vinifera) by-Products. Int. J. Food Sci. Technol. 2008, 43, 1953–1959. [Google Scholar] [CrossRef]
- Costa, J.R.; Amorim, M.; Vilas-Boas, A.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M. Impact of: In Vitro Gastrointestinal Digestion on the Chemical Composition, Bioactive Properties, and Cytotoxicity of Vitis vinifera L. Cv. Syrah Grape Pomace Extract. Food Funct. 2019, 10, 1856–1869. [Google Scholar] [CrossRef]
- Karovičová, J.; Kohajdová, Z.; Minarovičová, L.; Kuchtová, V. The Chemical Composition of Grape Fibre. Potravinarstvo 2015, 9, 53–57. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Towards Comprehensive Utilization of Winemaking Residues: Characterization of Grape Skins from Red Grape Pomaces of Variety Touriga Nacional. Ind. Crops Prod. 2013, 43, 25–32. [Google Scholar] [CrossRef]
- Sauvignon, C.; Words, K.E.Y.; Morgues, A.; Sauvi, C.; Valley, N.; Napa, T.; Division, C. Changes in Pectin Content of Cabernet Sauvignon Grape Berries During Maturation. Water 1990, 41, 111–115. [Google Scholar]
- Güzel, M.; Akpınar, Ö. Valorisation of Fruit By-Products: Production Characterization of Pectins from Fruit Peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- van Buggenhout, S.; Sila, D.N.; Duvetter, T.; van Loey, A.; Hendrickx, M. Pectins in Processed Fruits and Vegetables: Part III—Texture Engineering. Compr. Rev. Food Sci. Food Saf. 2009, 8, 105–117. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Alonso, Á.M.; Guillén, D.A.; Barroso, C.G.; Puertas, B.; García, A. Determination of Antioxidant Activity of Wine Byproducts and Its Correlation with Polyphenolic Content. J. Agric. Food Chem. 2002, 50, 5832–5836. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; De Faveri, D.M. Antioxidants from Grape Stalks and Marc: Influence of Extraction Procedure on Yield, Purity and Antioxidant Power of the Extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic Compounds, Antioxidant Activity and Insulinotropic Effect of Extracts Prepared from Grape (Vitis Vinifera L.) Byproducts. J. Food Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape Stem Extracts: Polyphenolic Content and Assessment of Their in Vitro Antioxidant Properties. LWT—Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Thimothe, J.; Bonsi, I.A.; Padilla-Zakour, O.I.; Koo, H. Chemical Characterization of Red Wine Grape (Vitis vinifera and Vitis Interspecific Hybrids) and Pomace Phenolic Extracts and Their Biological Activity against Streptococcus Mutans. J. Agric. Food Chem. 2007, 55, 10200–10207. [Google Scholar] [CrossRef]
- Comuzzo, P.; Voce, S.; Grazioli, C.; Tubaro, F.; Marconi, M.; Zanella, G.; Querzè, M. Pulsed Electric Field Processing of Red Grapes (Cv. Rondinella): Modifications of Phenolic Fraction and Effects on Wine Evolution. Foods 2020, 9, 414. [Google Scholar] [CrossRef]
- Iylia Arina, M.Z.; Harisun, Y. Effect of Extraction Temperatures on Tannin Content and Antioxidant Activity of Quercus Infectoria (Manjakani). Biocatal. Agric. Biotechnol. 2019, 19, 101104. [Google Scholar] [CrossRef]
- Abreu, J.; Quintino, I.; Pascoal, G.; Postingher, B.; Cadena, R.; Teodoro, A. Antioxidant Capacity, Phenolic Compound Content and Sensory Properties of Cookies Produced from Organic Grape Peel (Vitis labrusca) Flour. Int. J. Food Sci. Technol. 2019, 54, 1215–1224. [Google Scholar] [CrossRef]
- Ueno, T.; Masuda, H.; Ho, C.-T. Formation Mechanism of P-Methylacetophenone from Citral via a Tert-Alkoxy Radical Intermediate. J. Agric. Food Chem. 2004, 52, 5677–5684. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The Wine Proteins. Trends Food Sci. Technol. 2001, 12, 230–239. [Google Scholar] [CrossRef]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P.; Bordignon-Luiz, M.T. Bioactive Compounds and Antioxidant Activity of Vitis Vinifera and Vitis Labrusca Grapes: Evaluation of Different Extraction Methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Kayser, J.J.; Arnold, P.; Steffen-Heins, A.; Schwarz, K.; Keppler, J.K. Functional Ethanol-Induced Fibrils: Influence of Solvents and Temperature on Amyloid-like Aggregation of Beta-Lactoglobulin. J. Food Eng. 2020, 270, 109764. [Google Scholar] [CrossRef]
- Shao, Q. Methanol Concentration Dependent Protein Denaturing Ability of Guanidinium/Methanol Mixed Solution. J. Phys. Chem. B 2014, 118, 6175–6185. [Google Scholar] [CrossRef]
- Pereira, R.N.; Teixeira, J.A.; Vicente, A.A. Exploring the Denaturation of Whey Proteins upon Application of Moderate Electric Fields: A Kinetic and Thermodynamic Study. J. Agric. Food Chem. 2011, 59, 11589–11597. [Google Scholar] [CrossRef]
- Pereira, R.N.; Vicente, A.A. Environmental Impact of Novel Thermal and Non-Thermal Technologies in Food Processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Martins, A.J.; Ramos, O.L.; Malcata, F.X.; Teixeira, J.A.; Vicente, A.A.; Pereira, R.N. Influence of Moderate Electric Fields on Gelation of Whey Protein Isolate. Food Hydrocoll. 2015, 43, 329–339. [Google Scholar] [CrossRef]
- Pereira, R.N.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Effects of Electric Fields on Protein Unfolding and Aggregation: Influence on Edible Films Formation. Biomacromolecules 2010, 11, 2912–2918. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. The Effect of Chemical Treatments of Fibers on the Mechanical and Thermo-Mechanical Properties of the Pineapple Leaf Fiber (PALF) Reinforced Poly(Lactic Acid) (PLA) Laminated Composites. In Proceedings of the American Society for Composites—21st Technical Conference of the American Society for Composites 2006, Dearborn, MI, USA, 17–20 September 2006; Volume 2, pp. 888–900. [Google Scholar]
- Chang, M.-C.; Morris, W.C. The Effect of Heat Treatments on Dietary Fiber as Assessed by Scanning Electron Microscopy. J. Food Process. Preserv. 1990, 14, 335–343. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Ain, H.B.U.; Saeed, F.; Ahmed, A.; Khan, M.A.; Niaz, B.; Tufail, T. Improving the Physicochemical Properties of Partially Enhanced Soluble Dietary Fiber through Innovative Techniques: A Coherent Review. J. Food Process. Preserv. 2019, 43, e13917. [Google Scholar] [CrossRef]
- Jesus, M.S.; Ballesteros, L.F.; Pereira, R.N.; Genisheva, Z.; Carvalho, A.C.; Pereira-Wilson, C.; Teixeira, J.A.; Domingues, L. Ohmic Heating Polyphenolic Extracts from Vine Pruning Residue with Enhanced Biological Activity. Food Chem. 2020, 316, 126298. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Rangel-Hernández, J.; Morales-Sánchez, E.; Loarca-Piña, G.; Gaytán-Martínez, M. Changes on the Phytochemicals Profile of Instant Corn Flours Obtained by Traditional Nixtamalization and Ohmic Heating Process. Food Chem. 2019, 276, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Roda-Serrat, M.C.; Andrade, T.A.; Rindom, J.; Lund, P.B.; Norddahl, B.; Errico, M. Optimization of the Recovery of Anthocyanins from Chokeberry Juice Pomace by Homogenization in Acidified Water. Waste Biomass Valorization 2021, 12, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Sabino, L.B.; Alves Filho, E.G.; Fernandes, F.A.N.; de Brito, E.S.; da Silva Júnior, I.J. Optimization of Pressurized Liquid Extraction and Ultrasound Methods for Recovery of Anthocyanins Present in Jambolan Fruit (Syzygium cumini L.). Food Bioprod. Process. 2021, 127, 77–89. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of Novel Technologies on Polyphenols during Food Processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P.; Moontree, T. Ohmic Heating-Assisted Extraction of Anthocyanins from Black Rice Bran to Prepare a Natural Food Colourant. Innov. Food Sci. Emerg. Technol. 2015, 27, 102–110. [Google Scholar] [CrossRef]
- Salari, S.; Jafari, S.M. The Influence of Ohmic Heating on Degradation of Food Bioactive Ingredients. Food Eng. Rev. 2020, 12, 191–208. [Google Scholar] [CrossRef]

| Chemical Components | R-GB | LF-GB | SF-GB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WGS | RGS | WGB | RGB | W_OH | W_CONV | R_OH | R_CONV | W_OH | W_CONV | R_OH | R_CONV | ||
| Proximate composition (g/100 g) | Moisture | 0.98 ± 0.12 | 0.97 ± 0.19 | 0.97 ± 0.10 | 0.92 ± 0.09 | 2.97 ± 0.32 | 3.03 ± 0.21 | 2.70 ± 0.17 | 2.96 ± 0.15 | 0.58 ± 0.04 | 0.72 ± 0.08 | 0.63 ± 0.05 | 0.76 ± 0.08 |
| Ash | 6.40 ± 0.02 | 6.30 ± 0.01 | 1.23 ± 0.01 | 2.50 ± 0.01 * | 13.50 ± 0.09 | 12.71 ± 0.12 | 6.21 ± 0.08 | 10 ± 0.07 | 1.29 ± 0.09 | 1.20 ± 0.01 | 1.93 ± 0.01 | 2.44 ± 0.01 | |
| Protein | 7.31 ± 0.01 | 7.30 ± 0.02 | 8.34 ± 0.02 | 8.51 ± 0.02 | 5.02 ± 0.32 | 2.18 ± 0.12 | 5.05 ± 0.15 | 4.26 ± 0.05 | 8.51 ± 0.02 | 11.3 ± 0.5 | 8.34 ± 0.02 | 9.95 ± 0.25 | |
| Fat | 2.15 ± 0.12 | 2.96 ± 0.15 | 14.14 ± 0.17 | 12.58 ± 0.23 | 1.25 ± 0.14 | 2.49 ± 0.08 | 3.08 ± 0.12 | 4.87 ± 0.03 | 15.02 ± 0.45 | 12.18 ± 0.2 | 11.86 ± 0.31 | 9.46 ± 0.21 | |
| Crude Fiber | 76.91 ± 0.80 ** | 72.28 ± 0.73 | 57.82 ± 0.76 * | 55.98 ± 0.96 | 21.02 ± 0.54 | 16.98 ± 0.25 | 20.98 ± 0.23 | 15 ± 0.76 | 68.98 ± 0.69 | 70.56 ± 0.45 | 70 ± 0.44 | 71.02 ± 0.53 | |
| Carbohydrates | 6.33 ± 0.04 | 7.80 ± 0.13 ** | 11.03 ± 0.29 | 14.28 ± 0.05 * | 56.26 ± 0.35 | 62.58 ± 0.34 | 62.03 ± 0.12 | 62.55 ± 0.23 | 5.76 ± 0.09 | 4.06 ± 0.12 | 6.98 ± 0.13 | 8 ± 0.15 | |
| Structural Carbohydrates | Cellulose (as glucose) | 16.33 ± 0.04 | 17.33 ± 0.69 ** | 5.42 ± 0.68 | 6.77 ± 0.04 * | 0.50244 | ND | ND | ND | 6.03 ± 0.96 | 5.41 ± 0.68 | 8.12 ± 0.68 | 7.69 ± 0.40 |
| Hemicellulose | 6.70 ± 0.44 | 6.85 ± 0.28 | 6.74 ± 0.16 | 8.38 ± 0.09 * | ND | ND | ND | ND | 7.83 ± 0.65 * | 6.73 ± 0.16 | 11.23 ± 0.51 * | 9.52 ± 0.11 | |
| Lignin | 30.54 ± 0.10 | 30.24 ± 0.09 | 40.46 ± 0.09 | 40.84 ± 0.40 | ND | ND | ND | ND | 21.53 ± 1.81 * | 18.44 ± 0.72 | 21.97 ± 1.8 | 21.37 ± 4.00 | |
| Insoluble | 21.24 ± 0.39 | 21.07 ± 0.04 | 22.31 ± 0.08 | 22.41 ± 0.22 | ND | ND | ND | ND | 20.45 ± 0.10 | 16.91 ± 0.09 | 17.53 ± 0.07 | 17.59 ± 0.07 | |
| Soluble | 9.33 ± 0.15 | 9.17 ± 0.10 | 18.15 ± 0.12 | 18.42 ± 0.16 | ND | ND | ND | ND | 1.57 ± 0.08 | 1.52 ± 0.04 | 4.44 ± 0.17 | 3.78 ± 0.15 | |
| Pectins | TSP | 13.72 ± 0.50 | 14.21 ± 0.60 | 7.75 ± 0.15 | 8.77 ± 0.19 | 4.09 ± 0.21 | 2.12 ± 0.23 | 5.49 ± 0.12 | 6.08 ± 0.41 | 3.75 ± 0.22 | 5.75 ± 0.49 | 3.77 ± 0.36 | 2.77 ± 0.24 |
| WSP | 7.84 ± 0.75 | 6.53 ± 0.73 | 3.04 ± 0.18 | 3.12 ± 0.06 | 1.98 ± 0.41 | 1.24 ± 0.17 | 2.12 ± 0.31 | 1.41 ± 0.12 | 4.80 ± 0.32 * | 3.00 ± 0.18 | 5.03 ± 0.14 * | 3.51 ± 0.18 | |
| CSP | 4.42 ± 0.19 | 6.47 ± 0.61 | 1.51 ± 0.06 | 2.88 ± 0.08 | ND | ND | ND | ND | 3.20 ± 0.69 * | 1.50 ± 0.06 | 4.76 ± 0.12 * | 3.25 ± 0.24 | |
| HSP | 1.46 ± 0.06 | 1.02 ± 0.06 | 3.20 ± 0.15 | 2.80 ± 0.28 | ND | ND | ND | ND | 4.20 ± 0.23 * | 3.18 ± 0.15 | 4.53 ± 0.56 | 3.14 ± 0.81 | |
| Soluble sugars | total soluble sugars | 6.74 ± 0.3 | 1.04 ± 0.98 | 10.57 ± 1.76 | 17.24 ± 1.25 | 12.36 ± 0.85 | 11.01 ± 1.3 | 21.91 ± 0.56 | 23.5 ± 0.31 | 1.79 ± 0.03 | 0.44 ± 0.01 | 4.67 ± 0.08 | 6.26 ± 0.06 |
| Samples | Bagasse | Stems | ||
|---|---|---|---|---|
| White Grape | Red Grape | White Grape | Red Grape | |
| Total phenolic compound | 4.0 ± 0.0 | 6.6 ± 0.1 * | 3.0 ± 0.1 | 9.8 ± 0.0 * |
| Total antioxidant activity | 5.2 ± 0.66 | 22.6 ± 0.8 * | 8.3 ± 0.7 | 34.6 ± 0.9 * |
| Compound (μg/g)c | White Grape Bagasse | Red Grape Bagasse | ||
|---|---|---|---|---|
| Free Phenolic Compound (LF) | CONV | OH | CONV | OH |
| (-)-Epicatechin | 145.30 ± 18.10 * | 38.95 ± 2.01 | n.d. | n.d. |
| Syringic acid | 1.14 ± 0.33 | 0.98 ± 0.03 | n.d. | n.d. |
| Ferulic acid | 0.39 ± 0.07 * | n.d. | ||
| p-coumaric acid | 0.80 ± 0.02 * | n.d. | n.d. | n.d. |
| Caffeic acid | 0.79 ± 0.01 * | n.d | n.d | n.d |
| Gallic acid | 7.46 ± 2.37 | 8.63 ± 1.81 | 10.83 ± 1.85 | 28.64 ± 0.96 * |
| Esculin | n.d. | n.d | n.d. | 1.77 ± 0.06 * |
| Catechin hydrate | 0.98 ± 0.07 | 1.63 ± 0.35 * | ||
| Vanillic acid | 0.23 ± 0.32 | 1.36 ± 0.05 | ||
| Delphinidin-3-o-glucoside | n.d. | n.d. | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Petunidin-o-glucoside | n.d. | n.d. | 132.64 ± 1.45 | 133.80 ± 0.16 |
| Peonidin-3-o-glucoside | n.d. | n.d. | 0.02 ± 0.001 | 0.02 ± 0.01 |
| bound phenolic compound (SF) | ||||
| Gallic Acid | 15.30 ± 0.98 | 21.57 ± 1.84 | 26.71 ± 1.15 | 54.20 ± 3.24 * |
| Protocatechuic acid | n.d. | n.d. | 5.35 ± 0.21 * | n.d. |
| Catechin | n.d. | n.d. | 13.40 ± 0.52 | 32.70 ± 2.50 * |
| Vanillic acid | n.d. | n.d. | 4.67 ± 0.30 | 6.94 ± 0.61 * |
| Caffeic acid | n.d. | n.d. | n.d. | 0.03 ± 0.01 |
| Syringic acid | n.d. | n.d. | 0.88 ± 0.01 | 1.76 ± 0.01 |
| p-coumaric acid | n.d. | n.d. | 2.62 ± 0.03 | 3.06 ± 0.01 |
| Rutin | n.d. | n.d. | 0.04 ± 0.01 | 0.01 ± 0.01 |
| Phloretin | n.d. | n.d. | 1.24 ± 0.01 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.C.; Ghalamara, S.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Innovation and Winemaking By-Product Valorization: An Ohmic Heating Approach. Processes 2023, 11, 495. https://doi.org/10.3390/pr11020495
Coelho MC, Ghalamara S, Pereira R, Rodrigues AS, Teixeira JA, Pintado ME. Innovation and Winemaking By-Product Valorization: An Ohmic Heating Approach. Processes. 2023; 11(2):495. https://doi.org/10.3390/pr11020495
Chicago/Turabian StyleCoelho, Marta C., Soudabeh Ghalamara, Ricardo Pereira, António S. Rodrigues, José A. Teixeira, and Manuela E. Pintado. 2023. "Innovation and Winemaking By-Product Valorization: An Ohmic Heating Approach" Processes 11, no. 2: 495. https://doi.org/10.3390/pr11020495
APA StyleCoelho, M. C., Ghalamara, S., Pereira, R., Rodrigues, A. S., Teixeira, J. A., & Pintado, M. E. (2023). Innovation and Winemaking By-Product Valorization: An Ohmic Heating Approach. Processes, 11(2), 495. https://doi.org/10.3390/pr11020495










