Modelling of Drinking Water Recarbonization in Fluidized Bed Reactor
Abstract
:1. Introduction
2. Materials and Methods
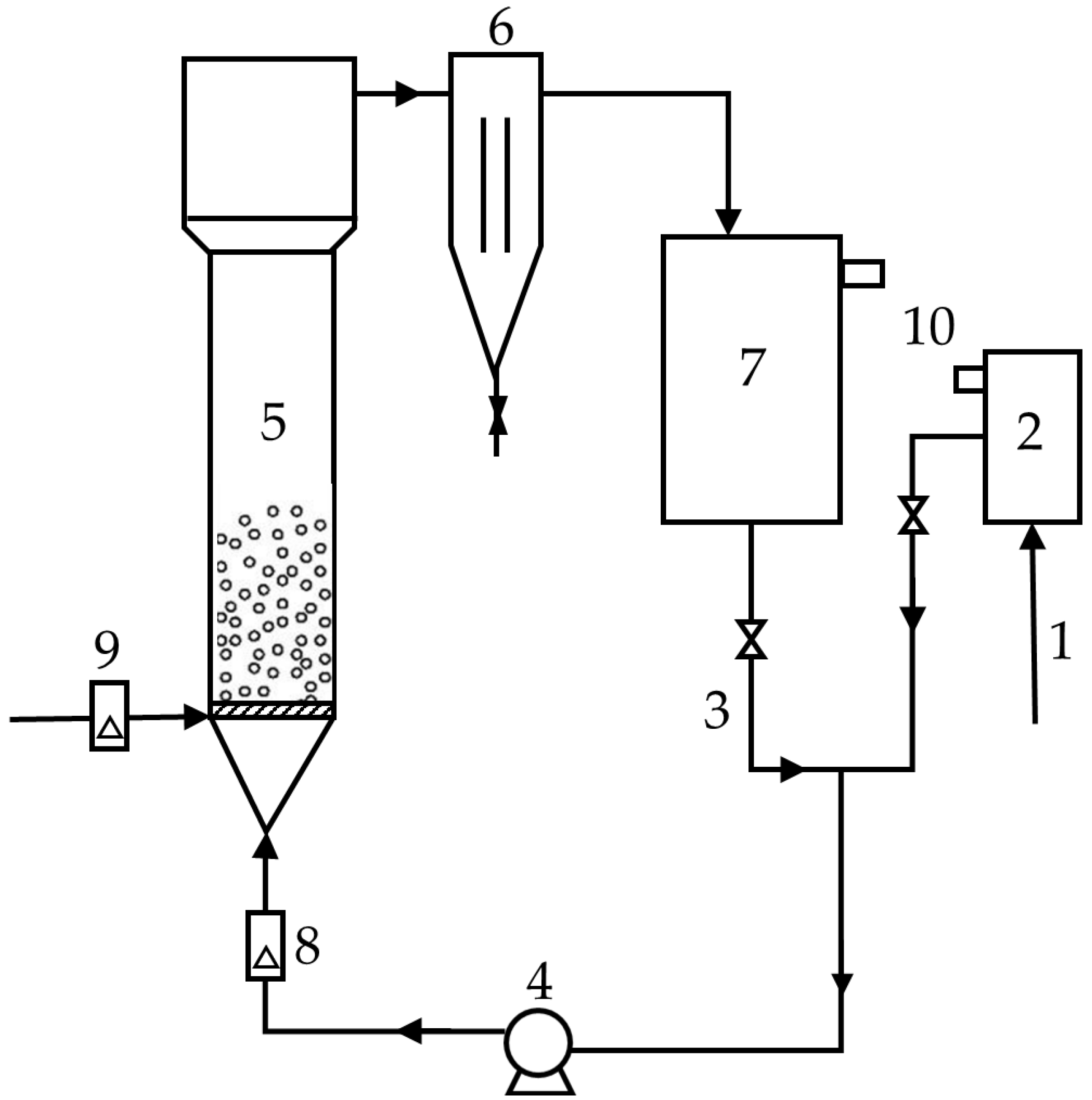
2.1. Fluidized Bed Reactor
Hydraulics of Fluidized Bed Reactor
2.2. Materials Used
2.3. Water Saturation with Calcium and Magnesium
2.4. Applied Analytical Methods
2.5. Multiple Regression Analysis
2.6. Processing of Experimental Data
3. Results
3.1. Lab-Scale FBRR
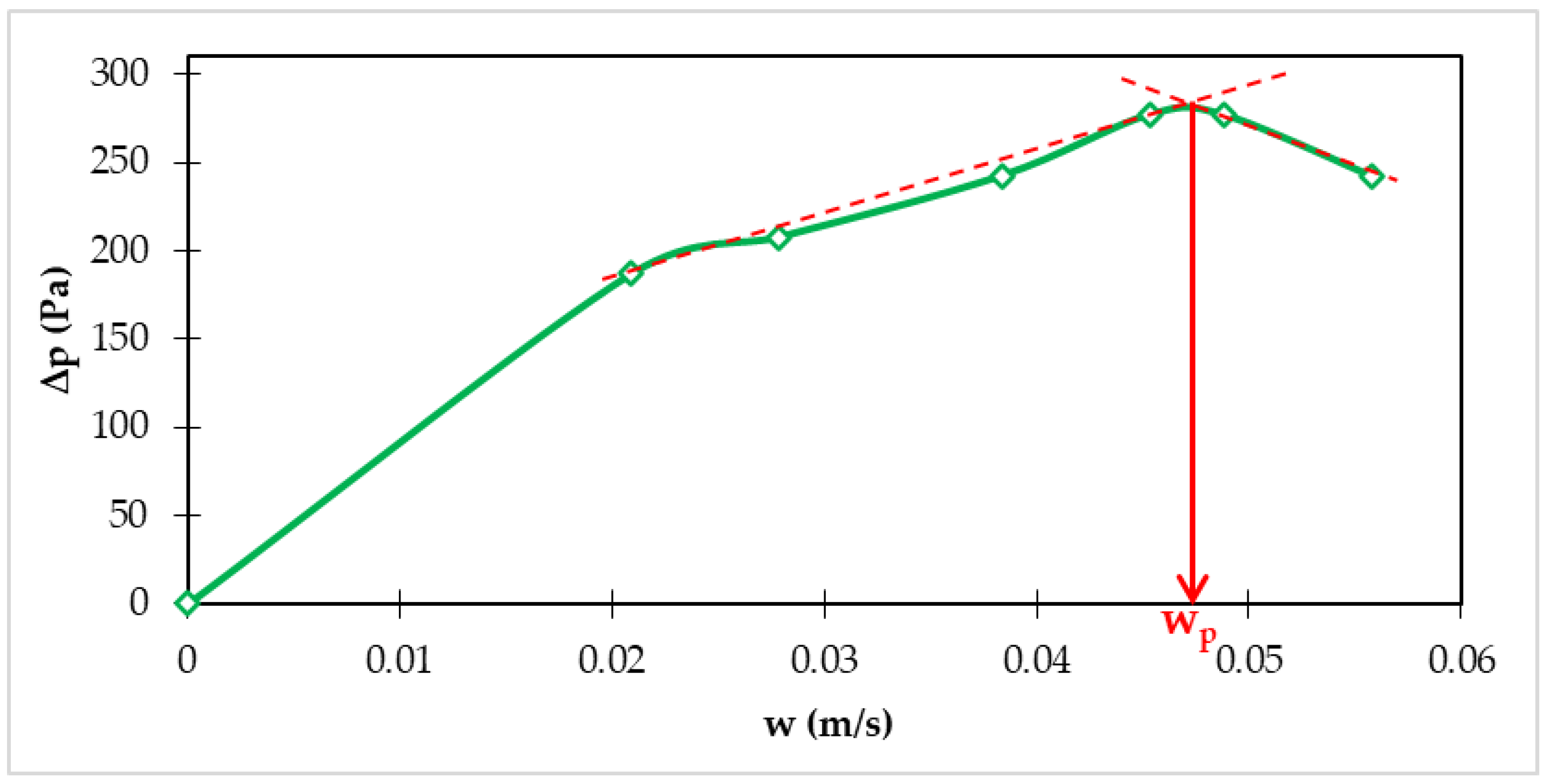
3.1.1. Hydraulic Characteristics
- Particle mass in the bed: mc = 0.36 kg;
- Voidage of fixed bed: ε = 0.4217;
- Volume of fixed bed of solids: Vs = 0.266 × 10−3 m3;
- Cross-sectional area of the column: Ap = 1.8 × 10−3 m2.
3.1.2. Saturation of Treated Water in the Reactor
3.1.3. Regression Analysis of Experimental Results
3.1.4. Optimizing the Conditions of the Recarbonization Process in Laboratory FBRR
3.2. Pilot Reactor
3.2.1. Statistical Processing of Experimental Results
- c(Ca2+ + Mg2+),
- c(Ca2+)/c(Mg2+),
- c(Mg2+),
- c(Ca2+).
- Reduces the sum of the molar concentration of calcium and magnesium ions in drinking water,
- Reduces the molar concentration of calcium ions in drinking water,
- Reduces the ratio of the molar content of calcium and magnesium in drinking water.
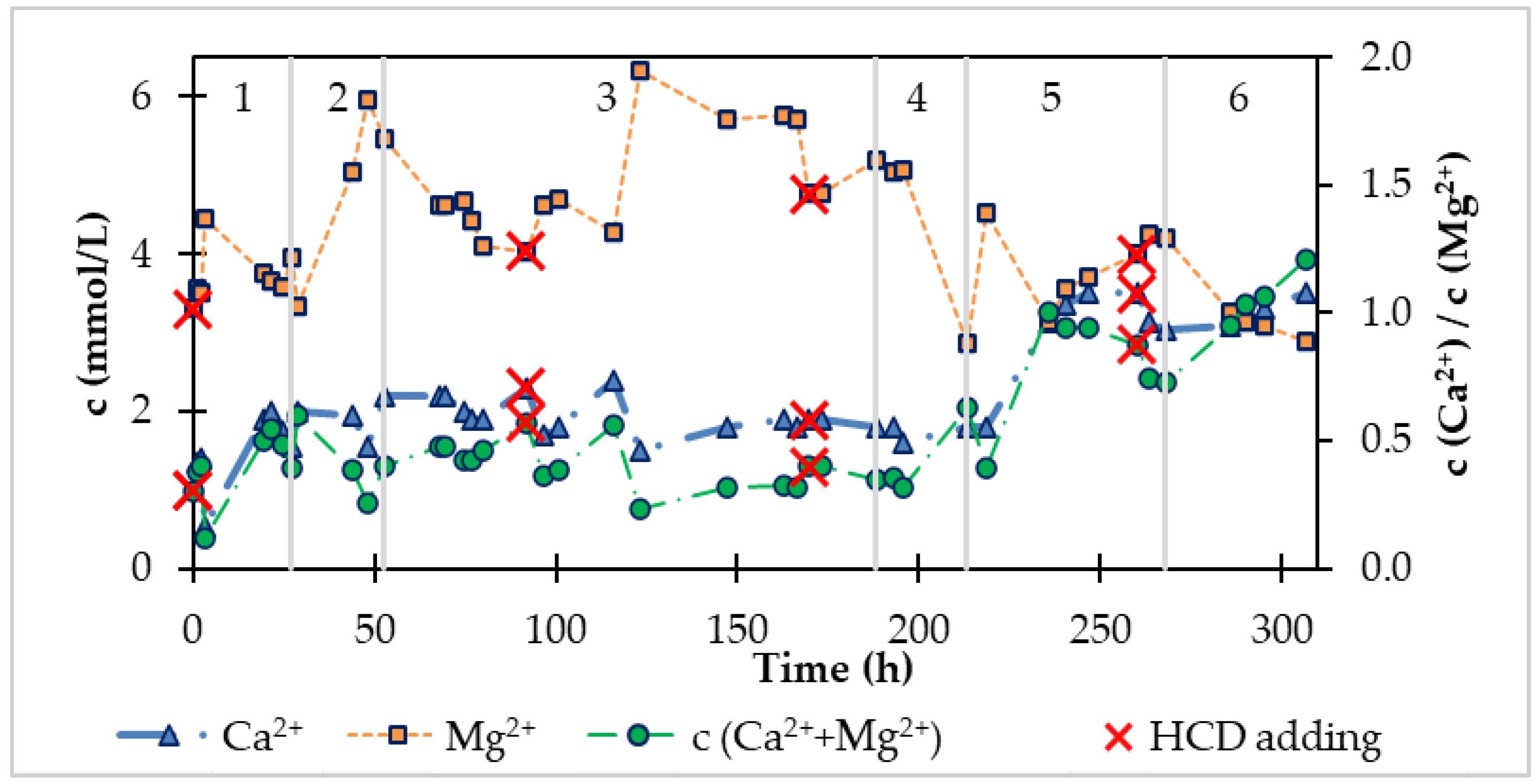
- As the CO2 flow increases, the ratio of the molar content of Ca2+ and Mg2+ decreases,
- With increasing CO2 flow, the molar concentration of Mg2+ in drinking water also increases,
- At the same time as the CO2 flow increases, the molar concentration of Ca2+ in drinking water decreases.
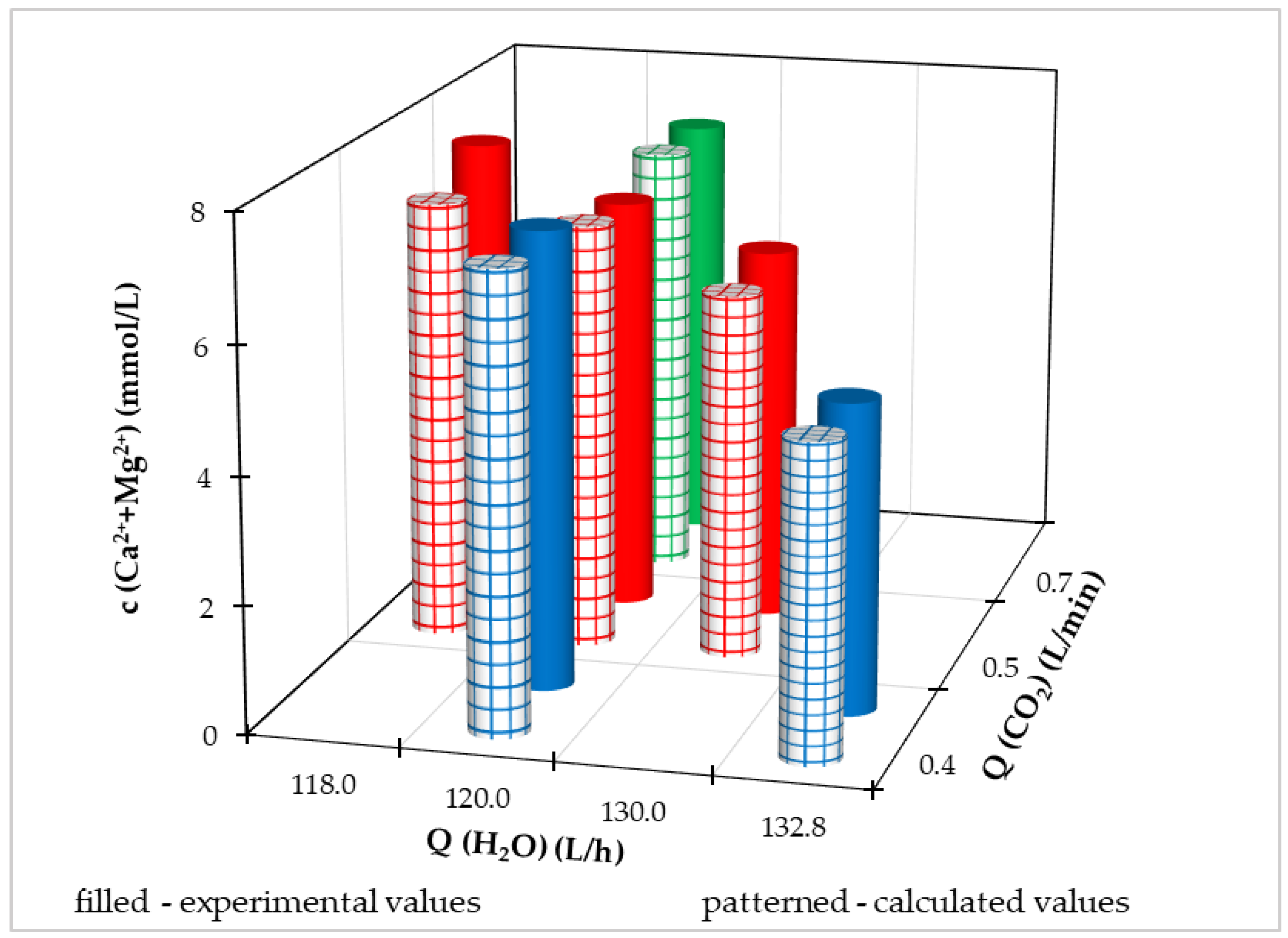
3.2.2. Optimization of the Process in the Pilot FBRR
- Q(CO2) = 0.4–0.7 L/min,
- Q(H2O) = 118.0–132.8 L/h.
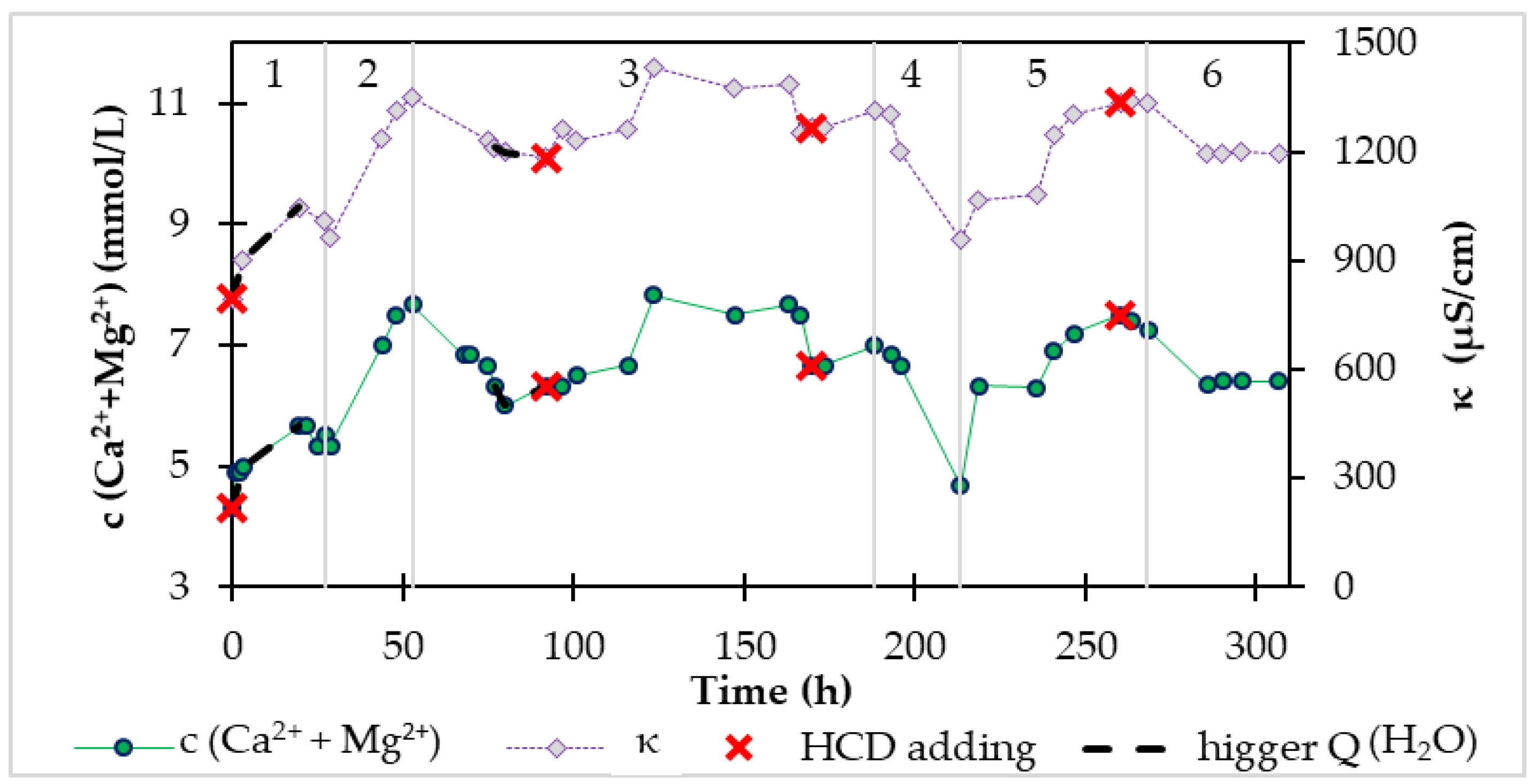
3.2.3. Comparison of the Used Two Recarbonization Materials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rylander, R.; Bonevik, H.; Rubenowitz, E. Magnesium and Calcium in Drinking Water and Cardiovascular Mortality. Scand. J. Work. Environ. Health 1991, 17, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y. Calcium and Magnesium in Drinking Water and Risk of Death from Cerebrovascular Disease. Stroke 1998, 29, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A. The High Heart Health Value of Drinking-Water Magnesium. Med. Hypotheses 2013, 81, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Orsini, N.; Levitan, E.B.; Brzozowska, A.; Roszkowski, W.; Wolk, A. Dietary Calcium and Magnesium Intake and Mortality: A Prospective Study of Men. Am. J. Epidemiol. 2010, 171, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Cotruvo, J. Drinking Water Quality and Contaminants Guidebook; Chapman and Hall/CRC: Milton, MA, USA, 2018; ISBN 9781351110464. [Google Scholar]
- Rubenowitz, E.; Axelsson, G.; Rylander, R. Magnesium in Drinking Water and Death from Acute Myocardial Infarction. Am. J. Epidemiol. 1996, 143, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Rosborg, I. Drinking Water Minerals and Mineral Balance; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 531. [Google Scholar]
- Jiang, L.; He, P.; Chen, J.; Liu, Y.; Liu, D.; Qin, G.; Tan, N. Magnesium Levels in Drinking Water and Coronary Heart Disease Mortality Risk: A Meta-Analysis. Nutrients 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Barloková, D.; Ilavský, J.; Kapusta, O.; Šimko, V. Importance of Calcium and Magnesium in Water—Water Hardening. IOP Conf. Ser. Earth Environ. Sci. 2017, 92, 012002. [Google Scholar] [CrossRef]
- Rapant, S.; Letkovičová, A.; Jurkovičová, D.; Kosmovský, V.; Kožíšek, F.; Jurkovič, Ľ. Differences in Health Status of Slovak Municipalities Supplied with Drinking Water of Different Hardness Values. Environ. Geochem. Health 2021, 43, 2665–2677. [Google Scholar] [CrossRef]
- Whelton, A.J.; Dietrich, A.M.; Burlingame, G.A.; Schechs, M.; Duncan, S.E. Minerals in Drinking Water: Impacts on Taste and Importance to Consumer Health. Water Sci. Technol. 2007, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; De Luca, A. Remineralization and Stabilization of Desalinated Water. In Pathways and Challenges for Efficient Desalination; IntechOpen: London, UK, 2022. [Google Scholar]
- Birnhack, L.; Shlesinger, N.; Lahav, O. A Cost-Effective Method for Improving the Quality of Inland Desalinated Brackish Water Destined for Agricultural Irrigation. Desalination 2010, 262, 152–160. [Google Scholar] [CrossRef]
- Bang, D.P. Upflow Limestone Contactor for Soft and Desalinated Water. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2012. [Google Scholar]
- Lehmann, O.; Birnhack, L.; Lahav, O. Design Aspects of Calcite-Dissolution Reactors Applied for Post Treatment of Desalinated Water. Desalination 2013, 314, 1–9. [Google Scholar] [CrossRef]
- Van Schagen, K.M. Model-Based Control of Drinking-Water Treatment Plants. Ph.D. Thesis, Technical University of Delft, Delft, The Netherlands, 2009. [Google Scholar]
- Kramer, O.; Jobse, M.A.; Baars, E.T.; van der Helm, A.W.C.; Colin, M.G.; Kors, L.J.; van Vugt, W.H. Model-Based Prediction of Fluid Bed State in Full-Scale Drinking Water Pellet Softening Reactors. In Proceedings of the 2nd IWA New Developments in IT & Water Conference, Amsterdam, The Netherlands, 8–10 February 2015; pp. 8–10. [Google Scholar]
- Derco, J.; Dudáš, J.; Luptáková, A.; Vrabeľ, M. Method of Increasing the Content of Mineral Substances in Water and Equipment for Performing This Method. (Industrial Property Office of the Slovak Republic—WebRegisters PP 50035-2018). Available online: https://wbr.indprop.gov.sk/WebRegistre/Patent/Detail/50035-2018 (accessed on 6 November 2023).
- Ca and Mg Extraction from Rocks: Laboratory Tests. Available online: https://fns.uniba.sk/fileadmin/prif/envi/kge/LifeWater/PDF/Sum_of_Laboratory_tests_of_water_recarbonation_Ca_Mg.pdf (accessed on 3 November 2023).
- Richardson, J.F.; Zaki, W.N. Sedimentation and Fluidisation: Part I. Trans. Instn. Chem. Eng. 1954, 32, 35–53. [Google Scholar] [CrossRef]
- Dudáš, J.; Derco, J.; Kurák, T.; Šoltýsová, N.; Jelemenský, Ľ.; Vrabeľ, M. Design, Scale-Up, and Construction of Drinking Water Recarbonization Fluidized Bed Reactor System. Processes 2022, 10, 2068. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S.; American Public Health Association; American Water Works Association. Water Environment Federation Standard Methods for Examination of Water and Wastewater 2012; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Mulcahy, L.T.; Shieh, W.K.; Lamotta, E.J. Simplified Mathematical Models for a Fludized Bed Biofilm Reactors. Am. Inst. Chem. Eng. 1981, 209, 273–285. [Google Scholar]
- Olejko, Š. Study of Drinking Water Treatability and Environmental Aspects of Water Flows. In Sub-Task 02: Treatment of Drinking Water Mineralization; Final Report of the Task 1999; VÚVH: Bratislava, Slovakia, 1999. [Google Scholar]
- Dang, J.S.; Harvey, D.M.; Jobbagy, A.; Grady, C.P.L. Evaluation of Biodegradation Kinetics with Respirometric Data. Res. J. Water Pollut. Control Fed. 1989, 61, 1711–1721. [Google Scholar]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Ministry of Health of the Slovak Republic. Decree No. 247/2017 Coll. Slovak Standard Values for Drinking Water. 2017. Available online: https://www.slov-lex.sk/pravne-predpisy/SK/ZZ/2017/247/20171015 (accessed on 6 November 2023).
- Pitter, P. Hydrochemistry, 5th ed.; University of Chemistry and Technology: Prague, Czech Republic, 2015; p. 792. [Google Scholar]









| a | b | 3c | D | cd | n | RXY |
|---|---|---|---|---|---|---|
| 34.2 | 0.71 | 4.97 | 6.39 | 0.23 | 3.18 | 0.70 |
| HCD Refill Time [h] | HCD Dose [g] | Hight of Fluidized Bed [cm] |
|---|---|---|
| 0 | 360.0 | - |
| 9 | 29.1 | 15.9 |
| 53 | 36.8 | 14.9 |
| Time (h) | pH (-) | Κ (µS/cm) | c(Ca2+ + Mg2+) (mmol/L) | cm (Ca2+) (mg/L) | cm (Mg2+) (mg/L) |
|---|---|---|---|---|---|
| 0 | 7.00 | 672 | 4.10 | 40.08 | 75.33 |
| 64 | 7.51 | 4900 | 36.75 | 84.17 | 842.00 |
| cmax (mmol/L) | dC (1/min) | RXY | ||
|---|---|---|---|---|
| 37.10 | 3.95∙10−2 | 118.60 | 4.77 | 0.9611 |
| Parameter | Value |
|---|---|
| P0 | 33.994 |
| P1 | 2.790 |
| P2 | −0.847 |
| P11 | 10.000 |
| P22 | 0.007 |
| P12 | −0.021 |
| Parameter | Value |
|---|---|
| P0 | −0.1249 |
| P1 | −0.8076 |
| P2 | 0.0194 |
| P11 | −0.4775 |
| P22 | −0.0002 |
| P12 | 0.0160 |
| Value | c(Ca2+ + Mg2+) (mmol/L) | Q(CO2) (L/min) | Q(H2O) (mL/min) | c(Ca2+)/c(Mg2+) |
|---|---|---|---|---|
| Maximum | 16.20 | 0.54 | 76.00 | 0.01 |
| Minimum | 10.90 | 0.24 | 57.20 | 0.18 |
| Value | c(Ca2+)/c(Mg2+) | Q(CO2) (L/min) | Q(H2O) mL/min) | c(Ca2+ + Mg2+) |
|---|---|---|---|---|
| Maximum | 0.20 | 0.24 | 47.10 | 11.60 |
| Minimum | 0.05 | 0.54 | 70.00 | 14.80 |
| Area | Q(CO2) (L/min) |
|---|---|
| 1 | 0.4 |
| 2 | 0.7 |
| 3 | 0.5 |
| 4 | 0.3 |
| 5 | 0.5 |
| 6 | 0.4 |
| Dependent Variables Y | ||||
|---|---|---|---|---|
| Parameter | c(Ca2+ + Mg2+) | c(Ca2+)/c(Mg2+) | c(Mg2+) | c(Ca2+) |
| P0 | 90.785 | 238.760 | 29.965 | 288.433 |
| P1 | −118.575 | −67.015 | −75.558 | −86.230 |
| P2 | −0.788 | −3.546 | −0.123 | −4.160 |
| P11 | 16.279 | 2.524 | 16.645 | −3.577 |
| P22 | 1.09 × 10−3 | 1.31 × 10−2 | −5.17 × 10−4 | 1.49 × 10−2 |
| P12 | 0.834 | 0.526 | 0.500 | 0.724 |
| RXY | 0.9635 | 0.9929 | 0.9796 | 0.9357 |
| RXY2 | 0.9597 | 0.8755 | 0.9858 | 0.9283 |
| Dependent Variables | Value | Q(CO2) (L/min) | Q(H2O) (L/h) |
|---|---|---|---|
| c(Ca2+ + Mg2+) | 7.59 mmol/L | 0.40 | 118.00 |
| c(Mg2+) | 4.30 mmol/L | 0.40 | 118.00 |
| c(Ca2+) | 3.53 mmol/L | 0.40 | 118.00 |
| c(Ca2+)/c(Mg2+) | 1.14 | 0.40 | 118.00 |
| Dependent Variables | Value | Q(CO2) (L/min) | Q(H2O) (L/h) |
|---|---|---|---|
| c(Ca2+ + Mg2+) | 4.93 mmol/L | 0.40 | 132.80 |
| c(Mg2+) | 3.52 mmol/L | 0.40 | 132.80 |
| c(Ca2+) | 1.30 mmol/L | 0.40 | 130.24 |
| c(Ca2+)/c(Mg2+) | 6.93 × 10−4 | 0.40 | 127.35 |
| Magno Dol (Akdolit) | Semidol | |
|---|---|---|
| Average inflow of freshwater (L/h) | 121.8 | 119.7 |
| Average c(Mg2+) (mg/L) | 105.3 | 146.2 |
| Average c(Ca2+) (mg/L) | 86.5 | 70.5 |
| Average outflow Mg2+ (g/h) | 12.8 | 16.6 |
| Average outflow Ca2+ (g/h) | 10.5 | 8.5 |
| Magno Dol (Akdolit) | Semidol | |
|---|---|---|
| Reaction time (h) | 306.6 | 167.0 |
| Total added HCD (kg) | 31.2 | 23.1 |
| Reacted amount of HCD (kg) | 15.9 | 18.4 |
| Reacted HCD to added HCD (%) | 51.0 | 79.9 |
| Total consumption of CO2 (kg) | 35.3 | 18.2 |
| Total production of Mg2+ (kg) | 3.9 | 2.8 |
| Total production of Ca2+ (kg) | 3.2 | 1.4 |
| Average production of Mg2+ per added HCD (g/kg) | 125.8 | 119.8 |
| Average production of Ca2+ per added HCD (g/kg) | 103.2 | 61.2 |
| Average production of Mg2+ per reacted HCD (g/kg) | 246.8 | 149.9 |
| Average production of Ca2+ per reacted HCD (g/kg) | 202.5 | 76.5 |
| CO2 consumption per added HCD (kg/kg) | 1.1 | 0.8 |
| CO2 consumption per reacted HCD (kg/kg) | 2.2 | 1.0 |
| Magno Dol (Akdolit) | Semidol | |
|---|---|---|
| HCD consumption (kg/year) | 874.7 | 917.9 |
| CO2 consumption (kg/year) | 989.6 | 721.2 |
| Magno Dol (Akdolit) | Semidol | |
|---|---|---|
| Released Ca2+ (kg/year)) | 90.3 | 56.2 |
| Increases c(Ca2+) (mg/L) | 8.2 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derco, J.; Šoltýsová, N.; Kurák, T.; Vajíčeková, A.; Dudáš, J. Modelling of Drinking Water Recarbonization in Fluidized Bed Reactor. Processes 2023, 11, 3209. https://doi.org/10.3390/pr11113209
Derco J, Šoltýsová N, Kurák T, Vajíčeková A, Dudáš J. Modelling of Drinking Water Recarbonization in Fluidized Bed Reactor. Processes. 2023; 11(11):3209. https://doi.org/10.3390/pr11113209
Chicago/Turabian StyleDerco, Ján, Nikola Šoltýsová, Tomáš Kurák, Anna Vajíčeková, and Jozef Dudáš. 2023. "Modelling of Drinking Water Recarbonization in Fluidized Bed Reactor" Processes 11, no. 11: 3209. https://doi.org/10.3390/pr11113209
APA StyleDerco, J., Šoltýsová, N., Kurák, T., Vajíčeková, A., & Dudáš, J. (2023). Modelling of Drinking Water Recarbonization in Fluidized Bed Reactor. Processes, 11(11), 3209. https://doi.org/10.3390/pr11113209







