Kinetic Investigation of the Oxidative Thermal Decomposition of Levonorgestrel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Preparation
2.2. FTIR Analysis
2.3. Thermal Analysis
2.4. Kinetic Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. Thermal Analysis
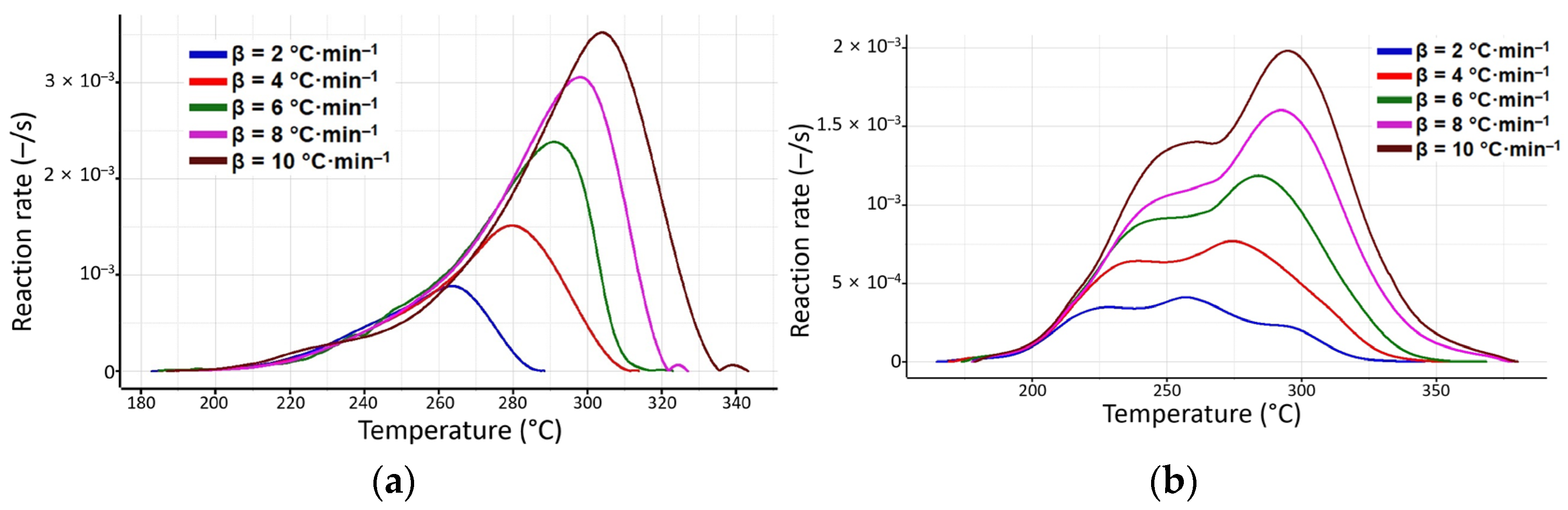
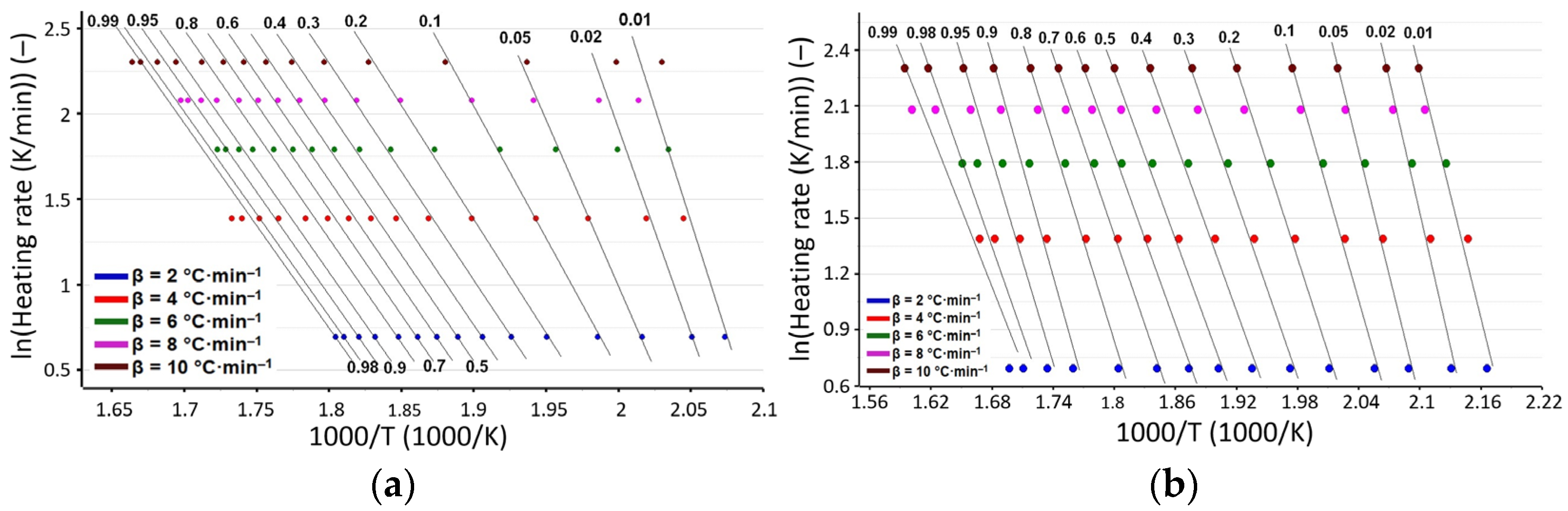
3.3. Kinetic Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodante, F.; Catalani, G.; Vecchio, S. Kinetic analysis of single or multi-step decomposition processes. Limits introduced by statistical analysis. J. Therm. Anal. Calorim. 2002, 68, 689–713. [Google Scholar]
- Vecchio, S.; Rodante, F.; Tomassetti, M. Thermal stability of disodium and calcium phosphomycin and the effects of the excipients evaluated by thermal analysis. J. Pharm. Biomed. Anal. 2001, 24, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Szilagyi, I.M.; Pielichowska, K.; Raftopoulos, K.N.; Šimon, P.; Melnikov, A.P.; Ivanov, D.A. Good laboratory practice in thermal analysis and calorimetry. J. Therm. Anal. Calorim. 2023, 148, 2211–2231. [Google Scholar] [CrossRef]
- Wan, Y.; He, H.; Li, F.; Zhang, P.; Gao, X.; Wang, Y.; Gan, Z.; Li, Y. Thermal stability, thermodynamics and kinetic study of (R)-(–)-phenylephrine hydrochloride in nitrogen and air environments. J. Therm. Anal. Calorim. 2023, 148, 2483–2499. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Drug Stability and Chemical Kinetics; Springer: Singapore, 2020; pp. 1–284. [Google Scholar]
- Ciou, H.H.; Lee, T.H.; Wang, H.C.; Ding, Y.R.; Tseng, C.J.; Wang, P.H.; Tsai, M.H.; Tzeng, S.L. Repurposing gestrinone for tumor suppressor through P21 reduction regulated by JNK in gynecological cancer. Transl. Res. 2022, 243, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-S.; Frey, N. Ulipristal Versus Levonorgestrel for Emergency Contraception: A Review of Comparative Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018.
- Dinehart, E.; Lathi, R.B.; Aghajanova, L. Levonorgestrel IUD: Is there a long-lasting effect on return to fertility? J. Assist. Reprod. Genet. 2020, 37, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Basaraba, C.N.; Westhoff, C.L.; Pike, M.C.; Nandakumar, R.; Cremers, S. Estimating systemic exposure to levonorgestrel from an oral contraceptive. Contraception 2017, 95, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Fotherby, K. Pharmacokinetics of gestagens: Some problems. Am. J. Obstet. Gynecol. 1990, 163, 323–328. [Google Scholar] [CrossRef]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental methods in chemical engineering: Thermogravimetric analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Topuz, F.; Kilic, M.E.; Durgun, E.; Szekely, G. Fast-dissolving antibacterial nanofibers of cyclodextrin/antibiotic inclusion complexes for oral drug delivery. J. Colloid Interface Sci. 2021, 585, 184–194. [Google Scholar] [CrossRef]
- Pires, F.Q.; Pinho, L.A.; Freire, D.O.; Silva, I.C.R.; Sa-Barreto, L.L.; Cardozo-Filho, L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Thermal analysis used to guide the production of thymol and Lippia origanoides essential oil inclusion complexes with cyclodextrin. J. Therm. Anal. Calorim. 2019, 137, 543–553. [Google Scholar] [CrossRef]
- Szente, L.; Puskás, I.; Sohajda, T.; Varga, E.; Vass, P.; Nagy, Z.K.; Farkas, A.; Várnai, B.; Béni, S.; Hazai, E. Sulfobutylether-beta-cyclodextrin-enabled antiviral remdesivir: Characterization of electrospun- and lyophilized formulations. Carbohydr. Polym. 2021, 264, 118011. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.R.; Kenguva, G.; Giri, L.; Kar, A.; Dandela, R. Binary to ternary drug-drug molecular adducts of the antihypertensive drug ketanserin (KTS) with advanced physicochemical properties. Chem. Commun. 2023, 59, 4640–4643. [Google Scholar] [CrossRef] [PubMed]
- Gunnam, A.; Nangia, A.K. Solubility improvement of curcumin with amino acids. CrystEngComm 2021, 23, 3398–3410. [Google Scholar] [CrossRef]
- Nugrahani, I.; Jessica, M.A. Amino acids as the potential co-former for co-crystal development: A review. Molecules 2021, 26, 3279. [Google Scholar] [CrossRef] [PubMed]
- Caillard, D.; Martin, J.L. (Eds.) Chapter 6 Experimental studies of Peierls-Nabarro-type friction forces in metals and alloys. In Pergamon Materials Series; Elsevier: Pergamon, Turkey, 2003; Volume 8, pp. 159–224. [Google Scholar]
- Krisyuk, B.E.; Sypko, T.M.; Zyuzin, I.N. Mechanism of thermal decomposition of 1-tert-butyl- and 1-ethyl-2-methoxydiazene-1-oxides. FirePhysChem 2023, 3, 142–148. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Pérez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee recommendations for analysis of thermal decomposition kinetics. Thermochim. Acta 2023, 719, 179384. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Achilias, D.; Fernandez-Francos, X.; Galukhin, A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of thermal polymerization kinetics. Thermochim. Acta 2022, 714, 179243. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Vyazovkin, S. The Status of Pyrolysis Kinetics Studies by Thermal Analysis: Quality Is Not as Good as It Should and Can Readily Be. Thermo 2022, 2, 435–452. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Friedman, H.L. New methods for evaluating kinetic parameters from thermal analysis data. J. Polym. Sci. Part B Polym. Lett. 1969, 7, 41–46. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0470011130. [Google Scholar]
- Norgestrel on Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Norgestrel (accessed on 22 August 2023).
- Baul, B.; Ledeţi, A.; Cîrcioban, D.; Ridichie, A.; Vlase, T.; Vlase, G.; Peter, F.; Ledeţi, I. Thermal Stability and Kinetics of Degradation of Moxonidine as Pure Ingredient vs. Pharmaceutical Formulation. Processes 2023, 11, 1738. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Kinetics in Solids. Annu. Rev. Phys. Chem. 1997, 48, 125–149. [Google Scholar] [CrossRef]
- Vyazovkin, S.V.; Lesnikovich, A.I. An approach to the solution of the inverse kinetic problem in the case of complex processes. Part 1. Methods employing a series of thermoanalytical curves. Thermochim. Acta 1990, 165, 273–280. [Google Scholar] [CrossRef]




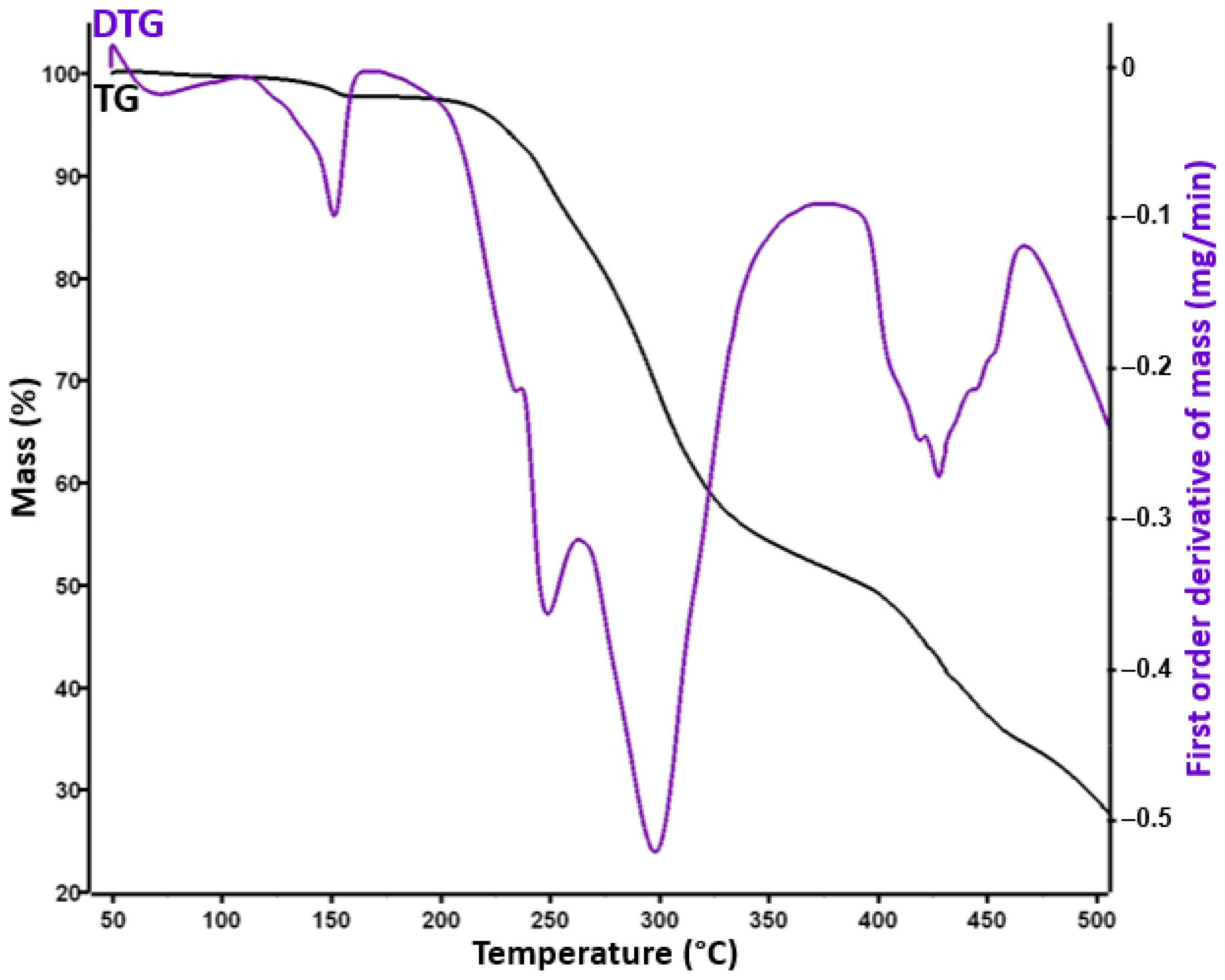




| Sample | Decomposition Step | Ti (°C) | Tf (°C) | Tmax·DTG (°C) | Tpeak·DSC (°C) | Δm (%) |
|---|---|---|---|---|---|---|
| LNG | I | 192 | 355 | 306 | 243 | 28.3 |
| II | 355 | 483 | 433; 443 | - | 41.04 | |
| LNGTAB | I | 50 | 115 | 68 | 67.1 | 1.8 |
| II | 115 | 165 | 151 | 149.8 | 3.72 | |
| III | 165 | 267 | 242 | 215; 229 | 19.9 | |
| IV | 267 | 384 | 306 | - | 45.4 | |
| LNGMIX | I | 51 | 110 | 71 | - | 0.48 |
| II | 110 | 178 | 151 | 149 | 1.88 | |
| III | 178 | 263 | 233; 248 | 214; 238 | 13.34 | |
| IV | 263 | 380 | 297 | - | 32.61 | |
| V | 380 | 466 | 418; 427 | - | 17.17 | |
| Lactose monohydrate | I | 100 | 171 | 146 | 147 | 4.99 |
| II | 218 | 264 | 236 | 220; 239 | 8.7 | |
| III | 264 | 391 | 306 | - | 60.6 | |
| Silicon dioxide | - | - | - | - | - | - |
| Magnesium stearate | I | 68 | 117 | 101 | 91; 116 | 3.24 |
| II | 204 | 437 | 404; 413 | - | 76.44 | |
| III | 437 | 520 | 460 | - | 10.18 | |
| Starch | I | 30 | 141 | 72 | 105 | 10.11 |
| II | 228 | 346 | 303 | - | 59.12 | |
| Talc | - | - | - | - | - | - |
| β (°C·min−1) | DTG Temperature Interval for “Process 1” (°C) for Kinetic Analysis of Samples | |
|---|---|---|
| LNG | LNGMIX | |
| 2 | 182–288 | 164–345 |
| 4 | 184–313 | 169–356 |
| 6 | 184–322 | 173–368 |
| 8 | 187–327 | 177–378 |
| 10 | 192–355 | 178–380 |
| α | Variation in Ea (kJ·mol−1) vs. α for Process 1 for | |||
|---|---|---|---|---|
| LNG | LNGMIX | |||
| FR | FWO | FR | FWO | |
| 0.05 | 99.1 | 153.8 | 145.1 | 175.6 |
| 0.1 | 82.7 | 122.1 | 124.9 | 152.1 |
| 0.15 | 80.8 | 110.9 | 119.7 | 141.1 |
| 0.2 | 82.3 | 105.3 | 115.1 | 133.9 |
| 0.25 | 84.8 | 102.2 | 112.3 | 128.4 |
| 0.3 | 87.3 | 100.4 | 111.9 | 124.5 |
| 0.35 | 89.5 | 99.4 | 111.5 | 121.7 |
| 0.4 | 91.1 | 98.8 | 110.8 | 119.7 |
| 0.45 | 91.9 | 98.5 | 112.7 | 118.6 |
| 0.5 | 91.9 | 98.2 | 115.6 | 118.4 |
| 0.55 | 91.5 | 98.0 | 119.3 | 118.9 |
| 0.6 | 91.0 | 97.8 | 125.6 | 120.1 |
| 0.65 | 90.5 | 97.5 | 133.9 | 122.4 |
| 0.7 | 90.1 | 97.3 | 144.9 | 126.2 |
| 0.75 | 89.9 | 97.0 | 158.7 | 132.1 |
| 0.8 | 89.9 | 96.7 | 171.5 | 140.5 |
| 0.85 | 89.8 | 96.3 | 172.6 | 149.7 |
| 0.9 | 89.6 | 95.9 | 151.4 | 154.0 |
| 0.95 | 89.1 | 95.2 | 110.3 | 144.5 |
| Ēa/kJ·mol−1 | 89 ± 1 | 103 ± 3 | 130 ± 5 | 134 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridichie, A.; Ledeţi, A.; Peter, F.; Ledeţi, I.; Muntean, C.; Rădulescu, M. Kinetic Investigation of the Oxidative Thermal Decomposition of Levonorgestrel. Processes 2023, 11, 3210. https://doi.org/10.3390/pr11113210
Ridichie A, Ledeţi A, Peter F, Ledeţi I, Muntean C, Rădulescu M. Kinetic Investigation of the Oxidative Thermal Decomposition of Levonorgestrel. Processes. 2023; 11(11):3210. https://doi.org/10.3390/pr11113210
Chicago/Turabian StyleRidichie, Amalia, Adriana Ledeţi, Francisc Peter, Ionuţ Ledeţi, Cornelia Muntean, and Matilda Rădulescu. 2023. "Kinetic Investigation of the Oxidative Thermal Decomposition of Levonorgestrel" Processes 11, no. 11: 3210. https://doi.org/10.3390/pr11113210









