Comparison in Content of Total Polyphenol, Flavonoid, and Antioxidant Capacity from Different Organs and Extruded Condition of Moringa oleifera Lam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Formulations
2.2. Extract Preparation
2.3. Estimation of Total Phenolic Content
2.4. Estimation of Flavonoid Content
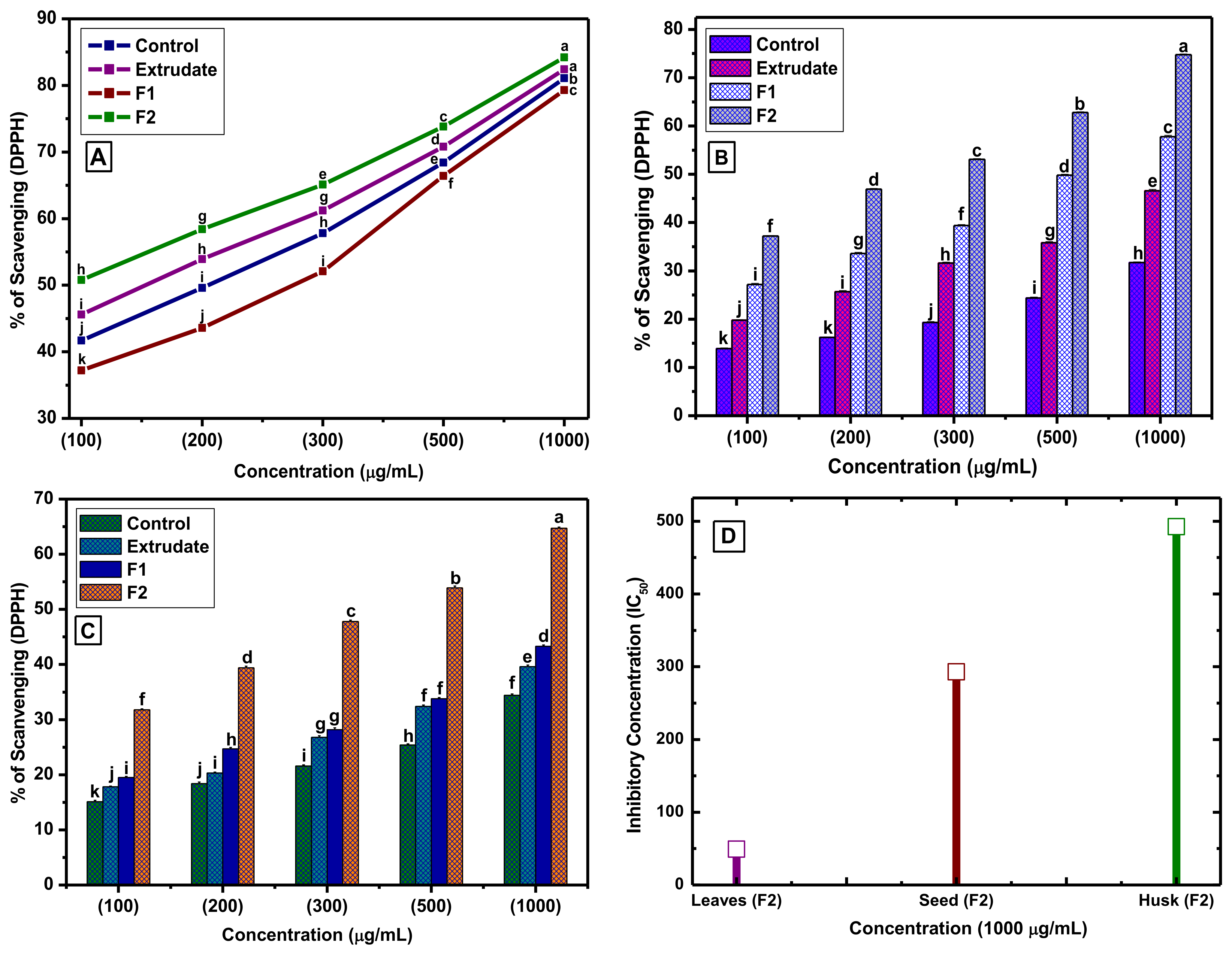
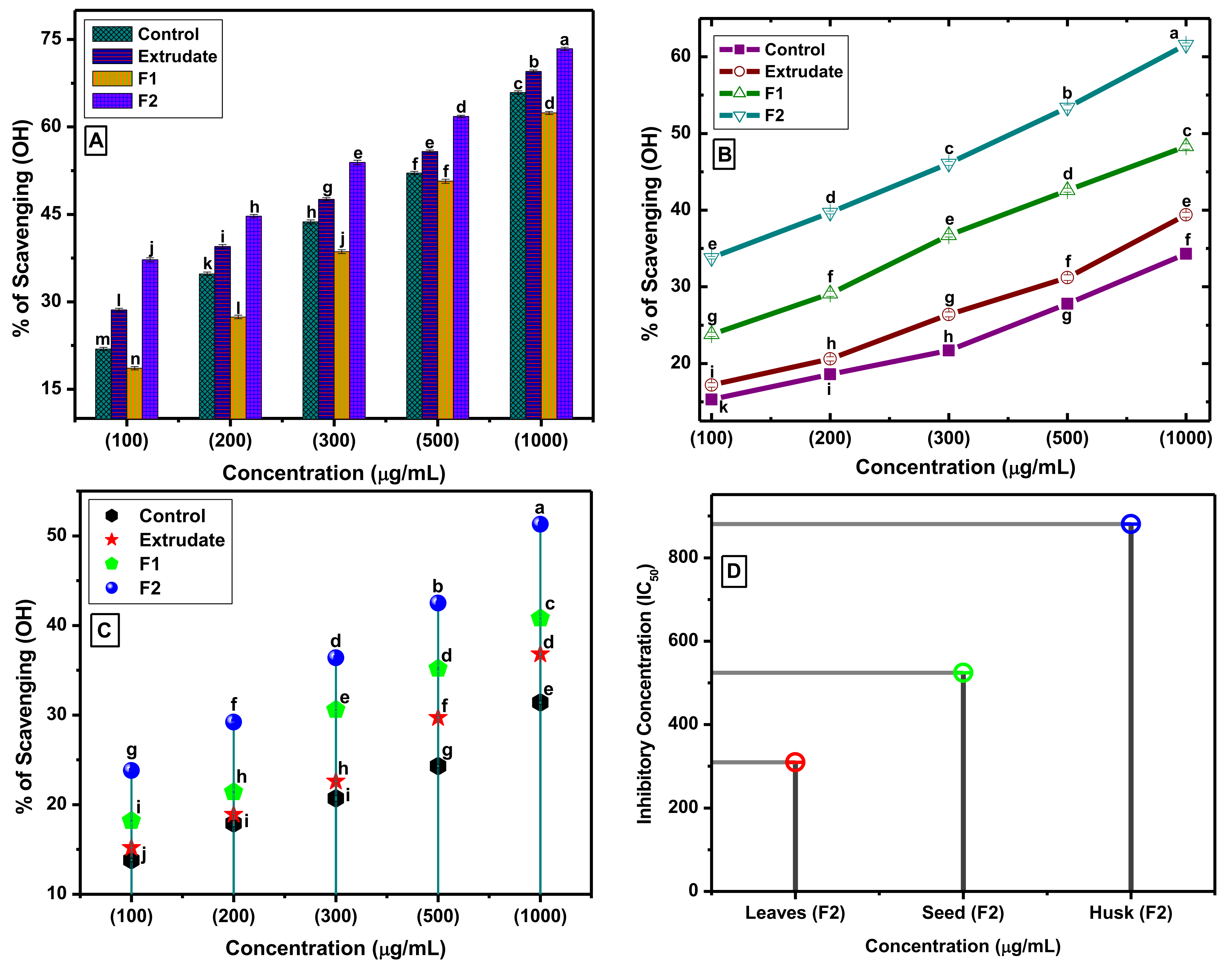
2.5. DPPH and H2O2 Free Radical Scavenging Capacity
2.6. Statistical Analysis
3. Result and Discussion
3.1. Total Phenolic and Flavonoid Contents
3.2. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, M.; Mohammad, K.I.; Hossain Manik, M.E. Anticancer Agents in Combination with Statins. J. Bioequiv. Availab. 2017, 9, 463–466. [Google Scholar] [CrossRef]
- Harman, D. Aging: Phenomena and theories. Ann. N. Y. Acad. Sci. 1998, 854, 1–7. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Bergman, M.; Varshavsky, L.; Gottlieb, H.; Grossman, S. The antioxidant activity of aqueous spinach extract: Chemical identification of active fractions. Phytochemistry 2001, 58, 143–152. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant profile of various solvent extracts of Carissa opaca leaves: An edible plant. Chem. Cent. J. 2017, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, T.J.; Alvarez, V.A. Bionanocomposite films developed from corn starch and natural and modified nano-clays with or without added blueberry extract. Food Hydrocoll. 2018, 77, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [Green Version]
- De Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey protein layer applied on biodegradable packaging film to improve barrier properties while maintaining biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Patel, A.; Sahu, D.; Dashora, A.; Garg, R.; Agraval, P.; Patel, P.; Patel, P.; Patel, G. A review of hot melt extrusion technique. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2194–2198. [Google Scholar]
- Azad, M.O.K.; Adnan, M.; Kang, W.S.; Lim, J.D.; Lim, Y.S. A technical strategy to prolong anthocyanins thermal stability in formulated purple potato (Solanum tuberosum L. cv Bora valley) processed by hot-melt extrusion. Int. J. Food Sci. Technol. 2021; in press. [Google Scholar] [CrossRef]
- Repka, M.A.; Bandari, S.; Kallakunta, V.R.; Vo, A.Q.; McFall, H.; Pimparade, M.B.; Bhagurkar, A.M. Melt extrusion with poorly soluble drugs—An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef]
- Diplock, A.T. Safety of antioxidant vitamins and beta-carotene. Am. J. Clin. Nutr. 1995, 62, 1510S–1516S. [Google Scholar] [CrossRef]
- Sejidov, F.T.; Mansoori, Y.; Goodarzi, N. Esterification reaction using solid heterogeneous acid catalysts under solvent-less condition. J. Mol. Catal. A Chem. 2005, 240, 186–190. [Google Scholar] [CrossRef]
- Pielichowski, K.; Świerz-Motysia, B. Influence of polyesterurethane plasticizer on the kinetics of poly(vinyl chloride) decomposition process. J. Therm. Anal. Calorim. 2006, 83, 207–212. [Google Scholar] [CrossRef]
- Verreck, G. The Influence of Plasticizers in Hot-Melt Extrusion. In Hot-Melt Extrusion: Pharmaceutical Applications; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 93–112. ISBN 9780470711187. [Google Scholar]
- Mahfuz, S.; Piao, X.S. Application of moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals 2019, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, M.R.; Karim, R. Moringa oleifera is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef]
- Mutar, Y.S.; Al-Rawi, K.F.; Mohammed, M.T. Moringa oleifera: Nutritive importance and its medicinal application, as a Review. Egypt. J. Chem. 2021, 64, 6827–6834. [Google Scholar] [CrossRef]
- Su, B.; Chen, X. Current Status and Potential of Moringa oleifera Leaf as an Alternative Protein Source for Animal Feeds. Front. Vet. Sci. 2020, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Kalam Azad, M.O.; Jeong, D.I.; Adnan, M.; Salitxay, T.; Heo, J.W.; Naznin, M.T.; Lim, J.D.; Cho, D.H.; Park, B.J.; Park, C.H. Effect of different processing methods on the accumulation of the phenolic compounds and antioxidant profile of broomcorn millet (Panicum miliaceum L.) flour. Foods 2019, 8, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, M.; Oh, K.K.; Azad, M.O.K.; Shin, M.H.; Wang, M.-H.; Cho, D.H. Kenaf (Hibiscus cannabinus L.) Leaves and Seed as a Potential Source of the Bioactive Compounds: Effects of Various Extraction Solvents on Biological Properties. Life 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Kalam Azad, M.O.; Adnan, M.; Sung, I.J.; Lim, J.D.; Baek, J.; Lim, Y.S.; Park, C.H. Development of value-added functional food by fusion of colored potato and buckwheat flour through hot melt extrusion. J. Food Process. Preserv. 2021, e15312. [Google Scholar] [CrossRef]
- Ashour, E.A.; Majumdar, S.; Alsheteli, A.; Alshehri, S.; Alsulays, B.; Feng, X.; Gryczke, A.; Kolter, K.; Langley, N.; Repka, M.A. Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine. J. Pharm. Pharmacol. 2016, 68, 989–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, Y.; Ryu, G.-H. Effects of extrusion cooking on physicochemical properties of white and red ginseng (powder). J. Ginseng Res. 2014, 38, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of extrusion cooking on functional properties and in vitro starch digestibility of barley-based extrudates from fruit and vegetable by-products. J. Food Sci. 2009, 74, E77–E86. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Adnan, M.; Azad, M.O.K.; Ju, H.S.; Son, J.M.; Park, C.H.; Shin, M.H.; Alle, M.; Cho, D.H. Development of biopolymer-mediated nanocomposites using hot-melt extrusion to enhance the bio-accessibility and antioxidant capacity of kenaf seed flour. Appl. Nanosci. 2020, 10, 1305–1317. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, X.; Zhang, M.; Hu, X.; Yu, C.; Zhu, Z.; Shao, Y. Effects of different extrusion temperatures on extrusion behavior, phenolic acids, antioxidant activity, anthocyanins and phytosterols of black rice. RSC Adv. 2018, 8, 7123–7132. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014, 66, 148–166. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, M.; Li, Y.; Pang, H.; Lin, L.; Liu, X.; Wu, C. The utilization of drug–polymer interactions for improving the chemical stability of hot-melt extruded solid dispersions. J. Pharm. Pharmacol. 2014, 66, 285–296. [Google Scholar] [CrossRef]
- Liu, X.; Lu, M.; Guo, Z.; Huang, L.; Feng, X.; Wu, C. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm. Res. 2012, 29, 806–817. [Google Scholar] [CrossRef]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Impact of liposomal encapsulation on degradation of anthocyanins of black carrot extract by adding ascorbic acid. Food Funct. 2017, 8, 1085–1093. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion formation and stabilization by biomolecules: The leading role of cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Herrero, J.A.; Frutos, M.J. Influence of rutin and ascorbic acid in colour, plum anthocyanins and antioxidant capacity stability in model juices. Food Chem. 2015, 173, 495–500. [Google Scholar] [CrossRef]
- Heinonen, J.; Farahmandazad, H.; Vuorinen, A.; Kallio, H.; Yang, B.; Sainio, T. Extraction and purification of anthocyanins from purple-fleshed potato. Food Bioprod. Process. 2016, 99, 136–146. [Google Scholar] [CrossRef]
- Riedl, K.M.; Hagerman, A.E. Tannin—Protein complexes as radical scavengers and radical sinks. J. Agric. Food Chem. 2001, 49, 4917–4923. [Google Scholar] [CrossRef]
- Brennan, C.; Brennan, M.; Derbyshire, E.; Tiwari, B.K. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci. Technol. 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Chen, L. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
| Materials | Mixing Ratio (w/w) and Preparation of Formulation | |||
|---|---|---|---|---|
| Control | Extrudate | F1 | F2 | |
| M. oleifera (leaves, seed, and husk) | 100 | 100 | 83 | 83 |
| Whey protein isolated (WPI) | NA | NA | 10 | NA |
| Lecithin | NA | NA | 5 | 5 |
| Ascorbyl Palmitate (AP) | NA | NA | NA | 10 |
| Vitamin E | NA | NA | 2 | 2 |
| Processing status | Non extrusion | Extrusion | Extrusion | Extrusion |
| HME barrel temperature (°C) | NA | 70-80-80-70 | 70-80-80-70 | 70-80-80-70 |
| Total Phenolic Content (mg/g) | |||
|---|---|---|---|
| Concentration (1 mg/mL) | Leaves | Seed | Husk |
| Control | 8.73 + 0.04 bd | 3.17 + 0.28 cd | 1.74 + 0.02 d |
| Extrudate | 8.96 + 0.03 b | 5.29 + 0.16 c | 3.86 + 0.04 bc |
| F1 | 8.98 + 0.05 c | 6.04 + 0.18 b | 4.05 + 0.08 b |
| F2 | 11.27 + 0.09 a | 10.13 + 0.14 a | 6.06 + 0.13 a |
| Total Flavonoid Content (mg/g) | |||
|---|---|---|---|
| Concentration (1 mg/mL) | Leaves | Seed | Husk |
| Control | 9.60 + 0.03 bd | 2.62 + 0.08 c | 1.65 + 0.15 cd |
| Extrudate | 9.82 + 0.04 b | 3.73 + 0.05 bc | 1.87 + 0.08 c |
| F1 | 9.94 + 0.06 bc | 3.95 + 0.02 b | 2.11 + 0.05 b |
| F2 | 10.61 + 0.05 a | 9.82 + 0.05 a | 3.72 + 0.07 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-O.; Park, C.-I.; Jin, S.-J.; Park, M.-R.; Choi, I.-Y.; Park, C.-H.; Adnan, M. Comparison in Content of Total Polyphenol, Flavonoid, and Antioxidant Capacity from Different Organs and Extruded Condition of Moringa oleifera Lam. Processes 2022, 10, 819. https://doi.org/10.3390/pr10050819
Park M-O, Park C-I, Jin S-J, Park M-R, Choi I-Y, Park C-H, Adnan M. Comparison in Content of Total Polyphenol, Flavonoid, and Antioxidant Capacity from Different Organs and Extruded Condition of Moringa oleifera Lam. Processes. 2022; 10(5):819. https://doi.org/10.3390/pr10050819
Chicago/Turabian StylePark, Min-Ook, Choon-Il Park, Se-Jong Jin, Mi-Ri Park, Ik-Young Choi, Cheol-Ho Park, and Md. Adnan. 2022. "Comparison in Content of Total Polyphenol, Flavonoid, and Antioxidant Capacity from Different Organs and Extruded Condition of Moringa oleifera Lam" Processes 10, no. 5: 819. https://doi.org/10.3390/pr10050819
APA StylePark, M.-O., Park, C.-I., Jin, S.-J., Park, M.-R., Choi, I.-Y., Park, C.-H., & Adnan, M. (2022). Comparison in Content of Total Polyphenol, Flavonoid, and Antioxidant Capacity from Different Organs and Extruded Condition of Moringa oleifera Lam. Processes, 10(5), 819. https://doi.org/10.3390/pr10050819








