Abstract
Pulmonary vein stenosis is a rare and frequently lethal childhood disease. There are few known genetic associations, and the pathophysiology is not well known. Current treatments include surgery, interventional cardiac catheterization, and more recently, medications targeting cell proliferation, which are not uniformly effective. We present a patient with PVS and a PIK3CA mutation, who demonstrated a good response to the targeted inhibitor, alpelisib.
1. Introduction
Pulmonary vein stenosis (PVS) is a critical narrowing of one or more pulmonary vein(s) that affects an estimated 1.7 per 100,000 children under the age of two years [1]. Risk factors for the development of PVS include congenital heart disease, especially total anomalous pulmonary venous connection, prematurity with bronchopulmonary dysplasia (BPD), and select genetic syndromes. In some children, the stenosis can be improved with surgery, such as sutureless marsupialization repair, or catheter-based interventions, such as balloon dilation and stenting. However, the disease often becomes recurrent and progressive, sometimes leading to complete vein atresia, and results in pulmonary hypertension, heart failure, and death [2,3,4,5]. Mortality rates are up to 83% in patients with three or more stenosed veins [6].
The etiology of PVS is poorly understood, but histopathological examination of the vessel lesions demonstrates neo-intimal hyperplasia and proliferation of myofibroblasts, and immunohistochemistry demonstrates strong receptor tyrosine kinase expression [7,8]. To delay disease progression, drug therapies directed at pathways related to cellular proliferation have been trialed over the past decade [9]. Current targeted medical treatments in clinical use include losartan (transforming growth factor beta pathway) [10], imatinib (tyrosine kinase inhibition), bevacizumab (vascular endothelia growth factor A inhibition) [11], and sirolimus (mammalian target of rapamycin (mTOR) pathway) [12,13,14]. We present a case of a term infant with congenital heart disease and recurrent PVS who was found to have a mutation in the phosphatidylinositol 3-kinase protein kinase B mTOR (PI3K–AKT–mTOR) pathway, and who was successfully treated with alpelisib, an inhibitor of this pathway.
2. Case Presentation
A three-week-old female presented with respiratory distress, hypotonia, rapid head growth and cardiomegaly on chest X-ray. She was born at term via c-section with a birthweight of 3.7 kg (80 percentile) due to failure to progress after an unremarkable pregnancy. She spent five days in the neonatal intensive care unit for hypoglycemia and respiratory distress requiring a high flow nasal cannula. The parents brought her to the pediatrician two weeks after discharge for poor feeding and lethargy.
On admission, the echocardiogram showed a large perimembranous ventricular septal defect (VSD) and normal pulmonary veins without stenosis. The head ultrasound revealed multiple small bilateral ventricular cysts and further brain MRI showed megalencephaly with diffuse hypo/dysmyelination consistent with megalencephalic leukodystrophy. A renal ultrasound showed scattered echogenic foci in the upper and interpolar medullary pyramids. Bloodwork was normal except for indirect hyperbilirubinemia. A genetics evaluation was performed, and diagnostic exome sequencing was sent. The patient improved with furosemide therapy and was discharged home after seven days with feeding therapy.
At the three-week follow up, the patient was well with good weight gain and no respiratory distress. However, the echocardiogram showed new evidence of left lower pulmonary vein (LLPV) stenosis with a mean gradient of 6 mmHg. A CT angiogram confirmed narrowing of the common trunk of the left-sided pulmonary veins (Table 1). The following week, oral feeds had decreased, work of breathing had increased, and oxygen saturation was 82%. A repeat echocardiogram revealed the LLPV gradient had increased to 12 mmHg and new pulmonary hypertension with bidirectional shunting. She underwent surgical VSD closure and left-sided pulmonary vein sutureless repair (Figure 1). She also underwent a complete evaluation for pulmonary hypertension, including a feeding evaluation that diagnosed aspiration, and a sleep study that diagnosed obstructive sleep apnea. Oral feedings were stopped, and she was discharged with tube feeding and oxygen. A postoperative echo showed no residual gradient through the left pulmonary veins and normal pulmonary pressure.

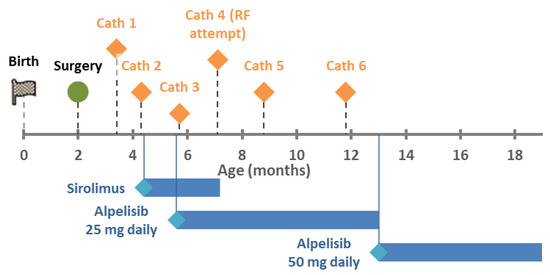

Table 1.
Patient parameters at time of PVS diagnosis.
Table 1.
Patient parameters at time of PVS diagnosis.
| General | |
|---|---|
| Body weight | 4.8 kg |
| Age | 2 months |
| Sex | Female |
| Ethnicity | Asian (Korean) |
| Echocardiogram | |
| Pulmonary vein gradient | 5.6 mmHg |
| RV | Dilated with normal systolic function |
| LV | Normal size and function |
| TR gradient | 3.9 m/s TR jet in setting of large VSD |
| Interventricular septal position | Flattened |
| Atria | Normal size |
| Electrocardiogram | |
| Rhythm | Normal sinus rhythm |
| Heart rate | 146 per minute |
| QRS duration | 70 ms |
| PR interval | 100 ms |
| QTc interval | 451 ms |
| T wave | Normal |

Figure 1.
Timeline of treatments.
Three weeks post-discharge, an echocardiogram showed LLPV re-stenosis with a mean gradient of 12 mmHg. The RV pressures remained normal with a TR jet velocity of 2.7 m/s. Over the next six weeks, she underwent two catheter-based left pulmonary balloon venoplasties for progression of venous stenosis with gradients over 9 mmHg. She was started on sirolimus with a target level of 6–10 mcg/L. Despite this, the LLPV progressed to atresia by her third interventional catheterization, and a fourth catheterization was planned for the purpose of re-canalization. In the interim, she was admitted for urosepsis. After that, the fourth catheterization proceeded, but was unsuccessful and resulted in perforation of her descending aorta. This was further complicated by culture negative sepsis, and sirolimus was stopped.
Trio exome sequencing revealed a pathogenic germline PIK3CA mutation (c.1345C > T, p.Pro449Ser). She was initiated on alpelisib at five months of age. The fifth and sixth catheterizations occurred over the following five months at progressively increasing intervals for echocardiographic gradients over 9 mmHg. There have been no further catheterizations at nineteen months of age. Echocardiograms demonstrated a stable LUPV gradient of 7–8 mmHg and TR jet velocities between 2.7–3.0 m/s.
Developmentally, she has made remarkable progress despite her initial neurological evaluations. At one year, she was able to sit unassisted, and at 18 months, she could stand and cruise as well as smile, clap, babble, and sign. A swallow study at ten months showed significant improvement and thin liquids were re-introduced. A repeat brain MRI at 14 months of age showed interval myelination compared to her study at 1 month and no cortical malformations. She was weaned off nighttime oxygen after tonsillectomy and adenoidectomy at 15 months of age.
3. Discussion
3.1. Case Discussion
Like many patients with severe PVS, this patient presented as a neonate with congenital heart disease but initially normal pulmonary veins and underwent surgical repair, which was not curative. She also had a large left-to-right shunt, which in certain patients has been demonstrated to increase the risk for recurrent PVS, and may prove to be an independent risk factor [15]. In addition, she was not diagnosed or treated for aspiration, another known risk factor for PVS [16], until after surgery. Pulmonary hypertension was first noted due to bidirectional shunting across the VSD at the same time as PVS was first seen. Fortunately, after VSD closure and treatment for aspiration and sleep apnea, pulmonary hypertension has been mild. Pulmonary hypertension has remained mild in the setting of residual PVS, and no pulmonary hypertension medical therapy has been indicated. Other markers of initial severe disease included symptoms of respiratory and cardiac failure, and rapid progression of one pulmonary vein to atresia. Additionally, the brain findings carried a poor neurodevelopmental prognosis.
Given the child’s complex clinical presentation with multi-system involvement, trio exome sequencing (ES) was performed. ES is a good first- or second-tier test for individuals with congenital anomalies, as recommended by the College of Medical Genetics and Genomics [17]. Knowledge of genetic conditions can contribute to treatment; for example, patients with Trisomy 21 and Smith–Lemli–Optiz syndrome are known to have rapid progression of PVS, and in Trisomy 21, a higher risk for pulmonary hypertension and mortality, so early treatment should be aggressive [5]. In this case, the genetics revealed both a potential pathophysiology and a novel treatment option, showing the benefit of increased genetic testing in patients with PVS. As is also common, she underwent surgical intervention initially, followed by catheterization-based therapy. The usual standard of care is to limit catheter interventions to balloon angioplasty due to the complications of stent placement in infants [18]. The unfortunate rapid progression of the LLPV to atresia led to the decision for stent placement in the LUPV when stenosis started to progress.
Over the past several years, medical therapy of PVS has become more common since reports of imatinib and sirolimus demonstrated improved mortality. After our patient had rapid progression leading to the second catheterization, we elected to add medical therapy to delay progression. Sirolimus was initially chosen over imatinib due to overlap in known PIK3CA mutation and the mTOR pathway (see Section 3.2 below). However, there was no obvious improvement in PVS since two further catheterization procedures were performed over the following two months and the LLPV progressed to atresia. In addition, attempts at blood draw to check sirolimus levels became difficult and were sometimes unsuccessful. Sirolimus was continued after initiation of alpelisib due to the unknown efficacy of alpelisib but stopped after three months due to frequent serious infections, which did not recur after discontinuation.
Alpelisib was started after a delay in obtaining the medication (see Section 3.3 below). Discounting cath #3, which was performed two days after initiation, and cath #4, which was not performed due to interval worsening, there have only been two cardiac catheterization procedures—at 3 and 6 months after initiation—with 13 months of stability after that. In addition, she appears to have made neurodevelopmental improvements as well. There have been no side effects to the drug, to date. Thus, alpelisib appears to be associated with a significant improvement in her PVS phenotype.
3.2. PIK3CA
3.2.1. PI3K–AKT–mTOR Signaling Pathway
The PI3K–AKT–mTOR pathway regulates cell proliferation and overgrowth (Figure 2).
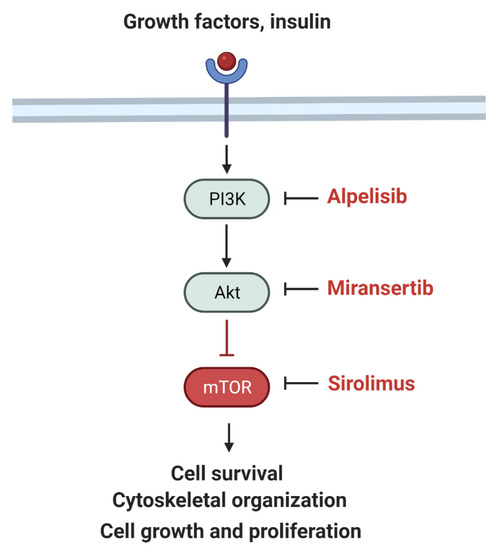
Figure 2.
Simplified schematic of the PI3K signaling pathway and inhibitors including alpelisib (PI3K inhibitor), miransertib (AKT inhibitor), and sirolimus (MTOR inhibitor). Adapted from “mTOR signaling pathway” by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender—accessed on 15 March 2022.
3.2.2. PIK3CA Mutations and Clinical Phenotypes
PIK3CA mutations cause constitutive activation of the PI3K–AKT–mTOR pathway leading to cell proliferation and overgrowth [19]. Mutations most commonly arise post-zygotically, are exclusively activating (never loss of function), and concentrated at one of several mutational “hotspots”. Mutations are well known to cause cancer in adults [20]. In children, PIK3CA mutations in similar locations cause PIK3CA related overgrowth spectrum (PROS). PROS describes a growing number of phenotypes featuring congenital overgrowth with hyperplasia in a wide variety of tissues including brain, body, and vessels [20,21].
Congenital heart defects have been reported in children with PIK3CA related megalencephaly with capillary malformations (MCAP), including ventricular septal defect, atrial septal defect, patent ductus arteriosus, persistent left SVC, aortic arch anomalies, plus tachyarrhythmias [22]. Germline PIK3CA mutations are rare, and previously were considered lethal in the embryonic stage. In our patient, the constellation of the child’s features, including megalencephaly, white matter abnormalities, and hypotonia, broadly fit within PROS. Further, the PIK3CA variant identified in this child has been previously reported in individuals with features of PROS as well, supporting a causal association. Please see Figure 3 for gene structure and mutation location.
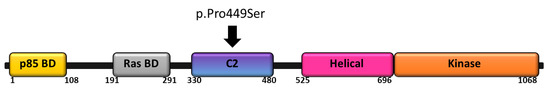
Figure 3.
Schematic representation of PIK3CA including known functional domains. The c.1345C > T (p.Pro449Ser) variant that lies within the C2 domain is shown.
3.3. Alpelisib
Alpelisib (BYL719) is a select PI3K inhibitor that has been recently approved for PIK3CA-mutated hormone receptor positive breast cancer and has been used in children with somatic features of PROS [23]. Known side effects include glucose dysregulation and rash. It is commercially available due to the approval for adults with breast cancer. In children, it is being compassionately studied in those older than 2 years with PROS (ClinicalTrials.gov Identifier: NCT04980833 and NCT04085653). Because our patient was under age 2 years, she was not eligible for research studies, and despite discussion with the manufacturer, she was not eligible for compassionate use. She finally was able to obtain the medication with private insurance authorization and appeal. The enteral dosage for ages 2–18 is 50 mg daily. We started with 25 mg daily and increased to 50 mg daily at 13 months of life.
3.4. Future Directions
We hypothesize that PIK3CA mutations could play a role in pulmonary vein stenosis. The pathophysiology of PVS could be considered a form of recurrent local “overgrowth” or localized hyperplasia, like other known phenotypes of PIK3CA mutations. Identification of mosaic mutations as a cause of PVS would be paradigm-shifting in the care of affected children. If PIK3CA mutations are identified, alpelisib may be a consideration as a targeted therapy to prevent the progression of PVS. This highlights the importance of multi-disciplinary care including genetics, and multi-center studies to advance PVS knowledge.
4. Conclusions
This is the first report of a patient with PVS and a PIK3CA mutation who appears to have responded to a targeted inhibitor, alpelisib. Future research should investigate whether PIK3CA mutation contribute to other patients with PVS and whether alpelisib may be a useful medical therapy.
- Main concern: PVS is a rare and frequently lethal childhood disease with few known genetic associations or treatments.
- Main discovery: We present a patient with PVS and a PIK3CA mutation, who demonstrated a good response to the targeted inhibitor, alpelisib.
- Main take-home message: Future patients with PVS may benefit from further research with whole exome sequencing and alpelisib therapy.
Author Contributions
Writing—original draft preparation, D.Y. and K.F.; writing—review and editing, D.Y., K.F. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by Jordan’s Guardian Angels, the Sunderland Foundation, and the Brotman Baty Institute (to G.M.M.).
Informed Consent Statement
Written informed consent has been obtained from the patient’s guardian to publish this paper.
Acknowledgments
We wish to thank Emma Jackson ARNP, Anne Davis, RN, Kelly Merrill, RN, and Wendy Mowbray MA for contribution to the patient’s care.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drossner, D.M.; Kim, D.W.; Maher, K.O.; Mahle, W.T. Pulmonary vein stenosis: Prematurity and associated conditions. Pediatrics 2008, 122, e656–e661. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Nealon, E.; Armstrong, A.K.; Cua, C.L.; Mitchell, C.; Krishnan, U.; Vanderlaan, R.D.; Song, M.K.; Viola, N.; Smith, C.V.; et al. Pulmonary Vein Stenosis in Infants: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Pediatr. 2018, 198, 36–45.e33. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.B.; Levy, P.T.; Stiver, C.A.; Boe, B.A.; Baird, C.W.; Callahan, R.M.; Smith, C.V.; Vanderlaan, R.D.; Backes, C.H. Primary pulmonary vein stenosis during infancy: State of the art review. J. Perinatol. 2021, 41, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Latson, L.A.; Prieto, L.R. Congenital and acquired pulmonary vein stenosis. Circulation 2007, 115, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, A.H.; Yamada, J.M.; Miller, D.T.; McEnaney, K.; Ireland, C.; Roberts, A.E.; Gauvreau, K.; Jenkins, K.J.; Chen, M.H. Clinical Syndromic Phenotypes and the Potential Role of Genetics in Pulmonary Vein Stenosis. Children 2021, 8, 128. [Google Scholar] [CrossRef]
- Breinholt, J.P.; Hawkins, J.A.; Minich, L.A.; Tani, L.Y.; Orsmond, G.S.; Ritter, S.; Shaddy, R.E. Pulmonary vein stenosis with normal connection: Associated cardiac abnormalities and variable outcome. Ann. Thorac. Surg. 1999, 68, 164–168. [Google Scholar] [CrossRef]
- Pogoriler, J.E.; Kulik, T.J.; Casey, A.M.; Baird, C.W.; Mullen, M.P.; Jenkins, K.J.; Vargas, S.O. Lung Pathology in Pediatric Pulmonary Vein Stenosis. Pediatr. Dev. Pathol. 2016, 19, 219–229. [Google Scholar] [CrossRef]
- Riedlinger, W.F.; Juraszek, A.L.; Jenkins, K.J.; Nugent, A.W.; Balasubramanian, S.; Calicchio, M.L.; Kieran, M.W.; Collins, T. Pulmonary vein stenosis: Expression of receptor tyrosine kinases by lesional cells. Cardiovasc. Pathol. 2006, 15, 91–99. [Google Scholar] [CrossRef]
- Kanaan, U.B.; Mahle, W.T. New Paradigms for Pulmonary Vein Stenosis Treatment: When Surgery and Transcatheter Therapy Aren’t Good Enough. J. Pediatr. 2018, 198, 12–13. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Ide, H.; Fu, Y.Y.; Teichert, A.M.; Kato, H.; Weisel, R.D.; Maynes, J.T.; Coles, J.G.; Caldarone, C.A. Losartan ameliorates “upstream” pulmonary vein vasculopathy in a piglet model of pulmonary vein stenosis. J. Thorac. Cardiovasc. Surg. 2014, 148, 2550–2557. [Google Scholar] [CrossRef] [Green Version]
- Callahan, R.; Kieran, M.W.; Baird, C.W.; Colan, S.D.; Gauvreau, K.; Ireland, C.M.; Marshall, A.C.; Sena, L.M.; Vargas, S.O.; Jenkins, K.J. Adjunct Targeted Biologic Inhibition Agents to Treat Aggressive Multivessel Intraluminal Pediatric Pulmonary Vein Stenosis. J. Pediatr. 2018, 198, 29–35.e25. [Google Scholar] [CrossRef]
- Bromberg-Marin, G.; Tsimikas, S.; Mahmud, E. Treatment of recurrent pulmonary vein stenoses with endovascular stenting and adjuvant oral sirolimus. Catheter. Cardiovasc. Interv. 2007, 69, 362–368. [Google Scholar] [CrossRef]
- Callahan, R.; Esch, J.J.; Wang, G.; Ireland, C.M.; Gauvreau, K.; Jenkins, K.J. Systemic Sirolimus to Prevent In-Stent Stenosis in Pediatric Pulmonary Vein Stenosis. Pediatr. Cardiol. 2020, 41, 282–289. [Google Scholar] [CrossRef]
- Patel, J.D.; Briones, M.; Mandhani, M.; Jones, S.; Suthar, D.; Gray, R.; Pettus, J.; McCracken, C.; Thomas, A.; Petit, C.J. Systemic Sirolimus Therapy for Infants and Children with Pulmonary Vein Stenosis. J. Am. Coll. Cardiol. 2021, 77, 2807–2818. [Google Scholar] [CrossRef]
- Choi, C.; Gauvreau, K.; Levy, P.; Callahan, R.; Jenkins, K.J.; Chen, M. Longer Exposure to Left-to-Right Shunts Is a Risk Factor for Pulmonary Vein Stenosis in Patients with Trisomy 21. Children 2021, 8, 19. [Google Scholar] [CrossRef]
- Niccum, M.; Callahan, R.; Gauvreau, K.; Jenkins, K.J. Aspiration Is Associated with Poor Treatment Response in Pediatric Pulmonary Vein Stenosis. Children 2021, 8, 783. [Google Scholar] [CrossRef]
- Manickam, K.; McClain, M.R.; Demmer, L.A.; Biswas, S.; Kearney, H.M.; Malinowski, J.; Massingham, L.J.; Miller, D.; Yu, T.W.; Hisama, F.M.; et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 2029–2037. [Google Scholar] [CrossRef]
- Kuo, J.A.; Petit, C.J. Pulmonary Vein Stenosis in Children: A Programmatic Approach Employing Primary and Anatomic Therapy. Children 2021, 8, 663. [Google Scholar] [CrossRef]
- Gymnopoulos, M.; Elsliger, M.A.; Vogt, P.K. Rare cancer-specific mutations in PIK3CA show gain of function. Proc. Natl. Acad. Sci. USA 2007, 104, 5569–5574. [Google Scholar] [CrossRef] [Green Version]
- Douzgou, S.; Rawson, M.; Baselga, E.; Danielpour, M.; Faivre, L.; Kashanian, A.; Keppler-Noreuil, K.M.; Kuentz, P.; Mancini, G.M.S.; Maniere, M.C.; et al. A standard of care for individuals with PIK3CA-related disorders: An international expert consensus statement. Clin. Genet. 2022, 101, 32–47. [Google Scholar] [CrossRef]
- Keppler-Noreuil, K.M.; Rios, J.J.; Parker, V.E.; Semple, R.K.; Lindhurst, M.J.; Sapp, J.C.; Alomari, A.; Ezaki, M.; Dobyns, W.; Biesecker, L.G. PIK3CA-related overgrowth spectrum (PROS): Diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am. J. Med. Genet. A 2015, 167a, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Mirzaa, G.; Timms, A.E.; Conti, V.; Boyle, E.A.; Girisha, K.M.; Martin, B.; Kircher, M.; Olds, C.; Juusola, J.; Collins, S.; et al. PIK3CA-associated developmental disorders exhibit distinct classes of mutations with variable expression and tissue distribution. JCI Insight 2016, 1, e87623. [Google Scholar] [CrossRef] [Green Version]
- Venot, Q.; Blanc, T.; Rabia, S.H.; Berteloot, L.; Ladraa, S.; Duong, J.P.; Blanc, E.; Johnson, S.C.; Hoguin, C.; Boccara, O.; et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 2018, 558, 540–546. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).