Amplitude-Integrated EEG Monitoring in Pediatric Intensive Care: Prognostic Value in Meningitis before One Year of Age
Abstract
1. Introduction
2. Materials and Methods
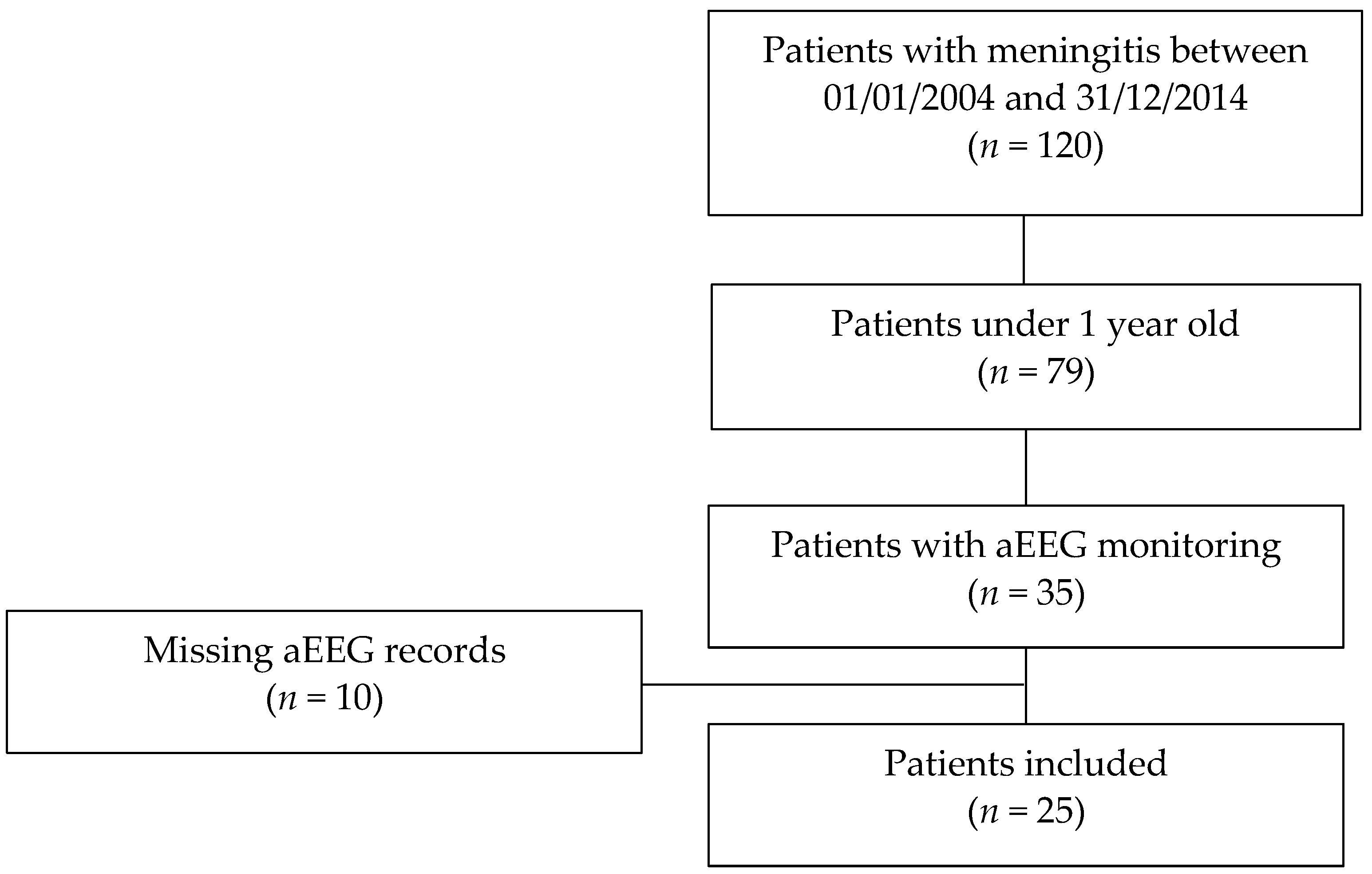
2.1. Study and Population
2.2. Demographic, Clinical, and Biological Data
2.3. aEEG Data
2.4. Statistics
2.5. Ethics Conflict of Interest
3. Results
3.1. Population and Epidemiological Characteristics
3.2. Characteristics of Meningitis and Hospitalization
3.3. Patient Outcome
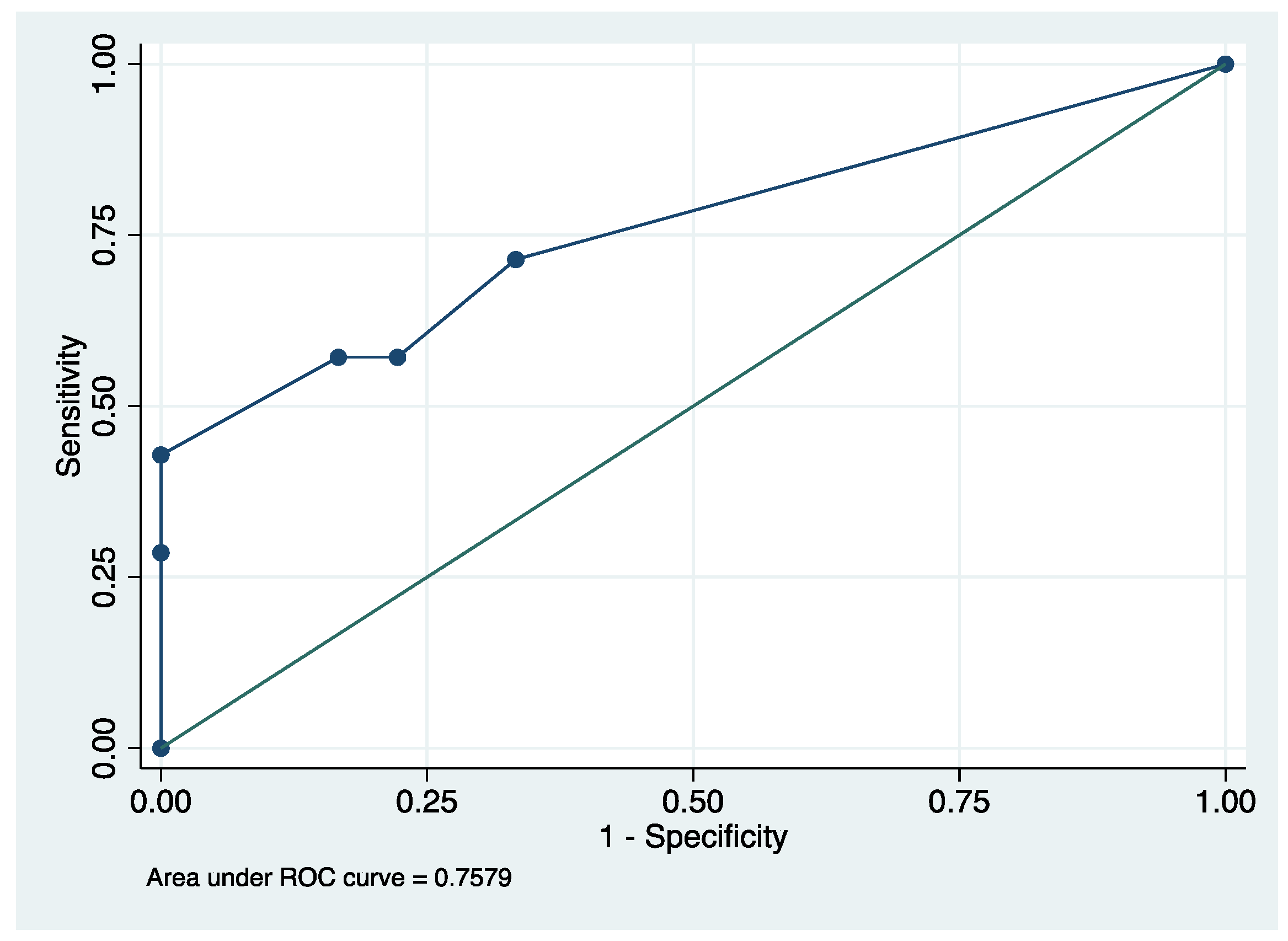
3.4. Description and Prognostic Value of aEEG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 789–858. [Google Scholar] [CrossRef]
- GBD Results Tool n.d. Available online: https://gbd2017.healthdata.org/gbd-search/?params=gbd-api-2017-permalink/d5ef08e91af2473443be2e62c24610fc (accessed on 15 March 2022).
- Lautaret, S.; Gennai, S.; Sellier, E.; Wintenberger, C.; Francois, P.; Carpentier, F.; Pavese, P. Suspicion de méningite: évaluation de la prise en charge aux urgences. La Presse Médicale 2013, 42, e69–e77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ben Hamouda, H.; Khalifa, A.B.H.; Hamza, M.; Ayadi, A.; Soua, H.; Khedher, M.; Sfar, M. Aspects cliniques et évolutifs des méningites bactériennes néonatales. Arch. Pédiatrie 2013, 20, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Bidet, P.; Mariani-Kurkdjian, P.; Bonacorsi, S. Méningites néonatales. Rev. Francoph. Des Lab. 2015, 470, 55–63. [Google Scholar] [CrossRef]
- Xu, M.; Hu, L.; Huang, H.; Wang, L.; Tan, J.; Zhang, Y.; Chen, C.; Zhang, X.; Huang, L. Etiology and Clinical Features of Full-Term Neonatal Bacterial Meningitis: A Multicenter Retrospective Cohort Study. Front. Pediatr. 2019, 7, 31. [Google Scholar] [CrossRef]
- Hoen, B.; Varon, E.; de Debroucker, T.; Fantin, B.; Grimprel, E.; Wolff, M.; Duval, X. Management of acute community-acquired bacterial meningitis (excluding newborns). Long version with arguments. Médecine Mal. Infect. 2019, 49, 405–441. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- Gaschignard, J.; Levy, C.; Romain, O.; Cohen, R.; Bingen, E.; Aujard, Y.; Boileau, P. Neonatal Bacterial Meningitis. Pediatr. Infect. Dis. J. 2011, 30, 212–217. [Google Scholar] [CrossRef]
- Ouchenir, L.; Renaud, C.; Khan, S.; Bitnun, A.; Boisvert, A.-A.; McDonald, J.; Bowes, J.; Brophy, J.; Barton, M.; Ting, J.; et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics 2017, 140, e20170476. [Google Scholar] [CrossRef]
- Briand, C.; Levy, C.; Baumie, F.; Joao, L.; Béchet, S.; Carbonnelle, E.; Grimprel, E.; Cohen, R.; Gaudelus, J.; de Pontual, L. Outcomes of bacterial meningitis in children. Médecine Et Mal. Infect. 2016, 46, 177–187. [Google Scholar] [CrossRef]
- Svendsen, M.B.; Kofoed, I.R.; Nielsen, H.; Schønheyder, H.C.; Bodilsen, J. Neurological sequelae remain frequent after bacterial meningitis in children. Acta Paediatr. 2020, 109, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.-C. Signes évocateurs de méningite chez le nourrisson. Médecine Mal. Infect. 2009, 39, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Bosis, S.; Mayer, A.; Esposito, S. Meningococcal disease in childhood: Epidemiology, clinical features and prevention. J. Prev. Med. Hyg. 2015, 56, E121–E124. [Google Scholar] [PubMed]
- Dubos, F. Stratégie de prise en charge (diagnostic, surveillance, suivi) d’une méningite présumée bactérienne de l’enfant. Médecine Mal. Infect. 2009, 39, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Zainel, A.; Mitchell, H.; Sadarangani, M. Bacterial Meningitis in Children: Neurological Complications, Associated Risk Factors, and Prevention. Microorganisms 2021, 9, 535. [Google Scholar] [CrossRef]
- Theodoridou, K.; Vasilopoulou, V.A.; Katsiaflaka, A.; Theodoridou, M.N.; Roka, V.; Rachiotis, G.; Hadjichristodoulou, C.S. Association of treatment for bacterial meningitis with the development of sequelae. Int. J. Infect. Dis. 2013, 17, e707–e713. [Google Scholar] [CrossRef]
- Larsen, F.T.B.D.; Brandt, C.T.; Larsen, L.; Klastrup, V.; Wiese, L.; Helweg-Larsen, J.; Riber, M.; Hansen, B.R.; Andersen, C.Ø.; Nielsen, H.; et al. Risk factors and prognosis of seizures in adults with community-acquired bacterial meningitis in Denmark: Observational cohort studies. BMJ Open 2019, 9, e030263. [Google Scholar] [CrossRef]
- Frenkel, N.; Friger, M.; Meledin, I.; Berger, I.; Marks, K.; Bassan, H.; Shany, E. Neonatal seizure recognition–Comparative study of continuous-amplitude integrated EEG versus short conventional EEG recordings. Clin. Neurophysiol. 2011, 122, 1091–1097. [Google Scholar] [CrossRef]
- Sanchez, S.M.; Carpenter, J.; Chapman, K.E.; Dlugos, D.J.; Gallentine, W.B.; Giza, C.C.; Goldstein, J.L.; Hahn, C.D.; Kessler, S.K.; Loddenkemper, T.; et al. Pediatric ICU EEG Monitoring. J. Clin. Neurophysiol. 2013, 30, 156–160. [Google Scholar] [CrossRef]
- Theda, C. Use of amplitude integrated electroencephalography (aEEG) in patients with inborn errors of metabolism—A new tool for the metabolic geneticist. Mol. Genet. Metab. 2010, 100, S42–S48. [Google Scholar] [CrossRef]
- Maynard, D.; Prior, P.F.; Scott, D.F. Device for continuous monitoring of cerebral activity in resuscitated patients. BMJ 1969, 4, 545–546. [Google Scholar] [CrossRef]
- Mehta, B.; Griffiths, N.; Spence, K.; Laing, S. Inter-observer reliability in reading amplitude-integrated electroencephalogram in the newborn intensive care unit. J. Paediatr. Child Health 2017, 53, 1007–1012. [Google Scholar] [CrossRef]
- Bourgoin, P.; Barrault, V.; Loron, G.; Roger, A.; Bataille, E.; Leclair-Visonneau, L.; Joram, N.; Chenouard, A. Interrater Agreement Between Critical Care Providers for Background Classification and Seizure Detection After Implementation of Amplitude-Integrated Electroencephalography in Neonates, Infants, and Children. J. Clin. Neurophysiol. 2020, 37, 259–262. [Google Scholar] [CrossRef]
- Kobayashi, K.; Mimaki, N.; Endoh, F.; Inoue, T.; Yoshinaga, H.; Ohtsuka, Y. Amplitude-integrated EEG colored according to spectral edge frequency. Epilepsy Res. 2011, 96, 276–282. [Google Scholar] [CrossRef]
- Mastrangelo, M.; Fiocchi, I.; Fontana, P.; Gorgone, G.; Lista, G.; Belcastro, V. Acute neonatal encephalopathy and seizures recurrence: A combined aEEG/EEG study. Seizure 2013, 22, 703–707. [Google Scholar] [CrossRef]
- Toso, P.A.; González, A.J.; Pérez, M.E.; Kattan, J.; Fabres, J.G.; Tapia, J.L.; González, H.S. Clinical utility of early amplitude integrated EEG in monitoring term newborns at risk of neurological injury. J. Pediatr. 2014, 90, 143–148. [Google Scholar] [CrossRef]
- van der Heide, M.J.; Roze, E.; van der Veere, C.N.; ter Horst, H.J.; Brouwer, O.F.; Bos, A.F. Long-term neurological outcome of term-born children treated with two or more anti-epileptic drugs during the neonatal period. Early Hum. Dev. 2012, 88, 33–38. [Google Scholar] [CrossRef]
- Shellhaas, R.A.; Barks, A. Impact of Amplitude-Integrated Electroencephalograms on Clinical Care for Neonates with Seizures. Pediatr. Neurol. 2012, 46, 32–35. [Google Scholar] [CrossRef]
- Soubasi, V.; Mitsakis, K.; Sarafidis, K.; Griva, M.; Nakas, C.T.; Drossou, V. Early abnormal amplitude-integrated electroencephalography (aEEG) is associated with adverse short-term outcome in premature infants. Eur. J. Paediatr. Neurol. 2012, 16, 625–630. [Google Scholar] [CrossRef]
- Bruns, N.; Sanchez-Albisua, I.; Weiß, C.; Tschiedel, E.; Dohna-Schwake, C.; Felderhoff-Müser, U.; Müller, H. Amplitude-Integrated EEG for Neurological Assessment and Seizure Detection in a German Pediatric Intensive Care Unit. Front. Pediatr. 2019, 7. [Google Scholar] [CrossRef]
- Viniker, D.; Maynard, D.; Scott, D. Cerebral Function Monitor Studies in Neonates. Clin. Electroencephalogr. 1984, 15, 185–192. [Google Scholar] [CrossRef]
- Bruns, N.; Felderhoff-Müser, U.; Dohna-Schwake, C. aEEG as a useful tool for neuromonitoring in critically ill children—Current evidence and knowledge gaps. Acta Paediatr. 2021, 110, 1132–1140. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Q.; Wei, H.; Dong, W.; Fan, Y.; Hua, Z. Prognostic Value of Clinical Tests in Neonates With Hypoxic-Ischemic Encephalopathy Treated With Therapeutic Hypothermia: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Fogtmann, E.P.; Plomgaard, A.M.; Greisen, G.; Gluud, C. Prognostic Accuracy of Electroencephalograms in Preterm Infants: A Systematic Review. Pediatrics 2017, 139, e20161951. [Google Scholar] [CrossRef]
- Fiser, D.H.; Long, N.; Roberson, P.K.; Hefley, G.; Zolten, K.; Brodie-Fowler, M. Relationship of Pediatric Overall Performance Category and Pediatric Cerebral Performance Category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit. Care Med. 2000, 28, 2616–2620. [Google Scholar] [CrossRef]
- Fiser, D.H. Assessing the outcome of pediatric intensive care. J. Pediatr. 1992, 121, 68–74. [Google Scholar] [CrossRef]
- Zaritsky, A.; Nadkarni, V.; Hazinski, M.F.; Foltin, G.; Quan, L.; Wright, J.; Fiser, D.; Zideman, D.; O’Malley, P.; Chameides, L.; et al. Recommended guidelines for uniform reporting of pediatric advanced life support: The Pediatric Utstein Style: A statement for Healthcare Professionals from a Task Force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Resuscitation 1995, 30, 95–115. [Google Scholar] [CrossRef]
- Holmberg, M.J.; Moskowitz, A.; Raymond, T.T.; Berg, R.A.; Nadkarni, V.M.; Topjian, A.A.; Grossestreuer, A.V.; Donnino, M.W.; Andersen, L.W. Derivation and Internal Validation of a Mortality Prediction Tool for Initial Survivors of Pediatric In-Hospital Cardiac Arrest*. Pediatr. Crit. Care Med. 2018, 19, 186–195. [Google Scholar] [CrossRef]
- Hellström-Westas, L.; Rosén, I.; de Vries, L.; Greisen, G. Amplitude-integrated EEG Classification and Interpretation in Preterm and Term Infants. NeoReviews 2006, 7, e76–e87. [Google Scholar] [CrossRef]
- Bourgoin, P.; Barrault, V.; Joram, N.; Visonneau, L.L.; Toulgoat, F.; Anthoine, E.; Loron, G.; Chenouard, A. The Prognostic Value of Early Amplitude-Integrated Electroencephalography Monitoring After Pediatric Cardiac Arrest*. Pediatr. Crit. Care Med. 2020, 21, 248–255. [Google Scholar] [CrossRef]
- Williams, R.P.; Banwell, B.; Berg, R.A.; Dlugos, D.J.; Donnelly, M.; Ichord, R.; Kessler, S.K.; Lavelle, J.; Massey, S.L.; Hewlett, J.; et al. Impact of an ICU EEG monitoring pathway on timeliness of therapeutic intervention and electrographic seizure termination. Epilepsia 2016, 57, 786–795. [Google Scholar] [CrossRef]
- Fernández, I.S.; Sansevere, A.J.; Guerriero, R.; Buraniqi, E.; Pearl, P.L.; Tasker, R.; Loddenkemper, T. Time to electroencephalography is independently associated with outcome in critically ill neonates and children. Epilepsia 2017, 58, 420–428. [Google Scholar] [CrossRef]
- Abend, N.S.; Arndt, D.H.; Carpenter, J.L.; Chapman, K.E.; Cornett, K.M.; Gallentine, W.B.; Giza, C.C.; Goldstein, J.L.; Hahn, C.D.; Lerner, J.T.; et al. Electrographic seizures in pediatric ICU patients: Cohort study of risk factors and mortality. Neurology 2013, 81, 383–391. [Google Scholar] [CrossRef]
- Hellström-Westas, L. Amplitude-integrated electroencephalography for seizure detection in newborn infants. Semin. Fetal Neonatal Med. 2018, 23, 175–182. [Google Scholar] [CrossRef]
- al Naqeeb, N.; Edwards, A.D.; Cowan, F.M.; Azzopardi, D. Assessment of Neonatal Encephalopathy by Amplitude-integrated Electroencephalography. Pediatrics 1999, 103, 1263–1271. [Google Scholar] [CrossRef]
- Thomas, E. Place de l’EEG Continu en Réanimation Pédiatrique: Indications, Intérêts et Limites. Etude Prospective sur 20 cas. Master’s Thesis, Université de Lorraine, Nancy, France, 2011. [Google Scholar]
- Rundgren, M.; Rosén, I.; Friberg, H. Amplitude-integrated EEG (aEEG) predicts outcome after cardiac arrest and induced hypothermia. Intensiv. Care Med. 2006, 32, 836–842. [Google Scholar] [CrossRef]
- Rundgren, M.; Westhall, E.; Cronberg, T.; Rosén, I.; Friberg, H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit. Care Med. 2010, 38, 1838–1844. [Google Scholar] [CrossRef]
- Kessler, S.K.; Topjian, A.A.; Gutierrez-Colina, A.M.; Ichord, R.N.; Donnelly, M.; Nadkarni, V.M.; Berg, R.A.; Dlugos, D.J.; Clancy, R.R.; Abend, N.S. Short-Term Outcome Prediction by Electroencephalographic Features in Children Treated with Therapeutic Hypothermia After Cardiac Arrest. Neurocrit. Care 2010, 14, 37–43. [Google Scholar] [CrossRef]
- Topjian, A.A.; Sánchez, S.M.; Shults, J.; Berg, R.A.; Dlugos, D.J.; Abend, N.S. Early Electroencephalographic Background Features Predict Outcomes in Children Resuscitated from Cardiac Arrest*. Pediatr. Crit. Care Med. 2016, 17, 547–557. [Google Scholar] [CrossRef]
- Gui, J.; He, S.; Zhuang, J.; Sun, Y.; Liu, Y.; Liang, S.; Chen, C.; Ren, Y.; Wang, B.; Chen, J. Peri- and Post-operative Amplitude-integrated Electroencephalography in Infants with Congenital Heart Disease. Indian Pediatr. 2020, 57, 133–137. [Google Scholar] [CrossRef]
- Gui, J.; Liang, S.; Sun, Y.; Liu, Y.; Chen, C.; Wang, B.; Zhong, J.; Yu, Y.; He, S. Effect of perioperative amplitude-integrated electroencephalography on neurodevelopmental outcomes following infant heart surgery. Exp. Ther. Med. 2020, 20, 2879–2887. [Google Scholar] [CrossRef]
- Bruns, N.; Felderhoff-Müser, U.; Dohna-Schwake, C.; Woelfle, J.; Müller, H. aEEG Use in Pediatric Critical Care—An Online Survey. Front. Pediatr. 2020, 8, 3. [Google Scholar] [CrossRef]
- Gupta, A. Febrile Seizures. Contin. Lifelong Learn. Neurol. 2016, 22, 51–59. [Google Scholar] [CrossRef]
- Shany, E. The influence of phenobarbital overdose on aEEG recording. Eur. J. Paediatr. Neurol. 2004, 8, 323–325. [Google Scholar] [CrossRef]
- Guan, Q.; Li, S.; Li, X.; Yang, H.P.; Wang, Y.; Liu, X.Y. Feasibility of using amplitude-integrated electroencephalogram to identify epileptic seizures by pediatric intensive care unit medical staff independently. Chin. J. Pediatr. 2016, 54, 823–828. [Google Scholar] [CrossRef]
- Du Pont-Thibodeau, G.; Sanchez, S.M.; Jawad, A.F.; Nadkarni, V.M.; Berg, R.A.; Abend, N.S.; Topjian, A.A. Seizure Detection by Critical Care Providers Using Amplitude-Integrated Electroencephalography and Color Density Spectral Array in Pediatric Cardiac Arrest Patients. Pediatr. Crit. Care Med. 2017, 18, 363–369. [Google Scholar] [CrossRef]
- Sisman, J.; Campbell, D.E.; Brion, L.P. Amplitude-Integrated EEG in Preterm Infants: Maturation of Background Pattern and Amplitude Voltage with Postmenstrual Age and Gestational Age. J. Perinatol. 2005, 25, 391–396. [Google Scholar] [CrossRef]
- Burdjalov, V.F.; Baumgart, S.; Spitzer, A.R. Cerebral Function Monitoring: A New Scoring System for the Evaluation of Brain Maturation in Neonates. Pediatrics 2003, 112, 855–861. [Google Scholar] [CrossRef]
- Olischar, M.; Klebermass, K.; Kuhle, S.; Hulek, M.; Kohlhauser, C.; Rücklinger, E.; Pollak, A.; Weninger, M. Reference Values for Amplitude-Integrated Electroencephalographic Activity in Preterm Infants Younger Than 30 Weeks’ Gestational Age. Pediatrics 2004, 113, e61–e66. [Google Scholar] [CrossRef]

| Patient Characteristics | Favorable Outcome Group n = 18 | Unfavorable Outcome Group n = 7 | p |
|---|---|---|---|
| Sex 1, men | 10 (55.6) | 2 (28.6) | 0.378 |
| Age 3, days | 22 ± 60 | 61 ± 86 | 0.471 |
| Weight 2, g | 3732 ± 1494 | 4187 ± 2837 | 0.602 |
| Size 2, cm | 51.6 ± 7.0 | 52.9 ± 9.4 | 0.725 |
| Head circumference 2, cm | 35.6 ± 4.5 | 36.2 ± 5.5 | 0.795 |
Center referring the patient to intensive care
| 6 (33.3) 12 (66.7) | 1 (14.3) 6 (85.7) | 0.626 |
| Neurological history 1 | 2 (11.1) | 0 (0) | 1 |
| Characteristics 1 | Favorable Outcome Group n = 18 | Unfavorable Outcome Group n = 7 | p |
|---|---|---|---|
| Symptoms 1 | |||
| Neurologicals exclusive * | 1 (5.6) | 1 (14.3) | |
| Generals exclusive ** | 6 (33.3) | 1 (14,3) | 0.518 |
| Both | 11 (61.1) | 5 (71.4) | |
| Time from symptoms onset to diagnosis 2 | 37.1 ± 35.8 | 45 ± 24.7 | 0.683 |
| <12 h 1 | 5 (27.8) | 3 (42.9) | |
| 12–24 h 1 | 5 (27.8) | 2 (28.6) | 0.853 |
| >24 h 1 | 8 (44.4) | 2 (28.6) | |
| Initial CRP (mg/mL) 2 | 78.1 ± 101.5 | 150.9 ± 118.9 | 0.138 |
| Initial PCT (ng/mL) 2 (0 3/4 3) | 25.8 ± 22.8 | 117.3 ± 48.1 | <0.001 |
| Initial leukocytes (/mm3) 2 | 9.0 ± 7.6 | 6.4 ± 5.1 | 0.414 |
| Positive blood culture 1 | 12 (66.7) | 5 (71.4) | 0.819 |
| First lumbar puncture 1 | 17 *** | 7 | - |
| Leukocytes (/mm3) 2 (3 3/0 3) | 2055.9 ± 2341.1 | 4135.9 ± 7357.4 | 0.323 |
| Hematite (/mm3) 2 (4 3/1 3) | 2829.1 ± 4978.5 | 4963.7 ± 3049.4 | 0.459 |
| Proteins (g/l) 2 (3 3/0 3) | 2.41 ± 1.57 | 5.08 ± 2.60 | 0.007 |
| Glucose (g/l) 2 (3 3/0 3) | 1.39 ± 1.13 | 0.62 ± 0.96 | 0.138 |
| Lactates (mmol/l) 2 (8 3/4 3) | 6.54 ± 4.28 | 10.53 ± 2.22 | 0.156 |
| Chlore (mmol/l) 2 (4 3/3 3) | 115.5 ± 5.8 | 109.3 ± 8.4 | 0.104 |
| Cerebrospinal fluid sterile 1 Positive bacteriology 1 | 2 15 | 0 7 | |
| 4 (22.2) 5 (27.8) 1 (5.5) 1 (5.5) 2 (11.1) 1 (5.5) 1 (5.5) | 2 (28.6) 2 (28.6) 0 0 3 (42.9) 0 0 | |
| Time from initial symptoms to treatment 1 | |||
| <6 h | 5 (27.8) | 2 (28.6) | |
| 6–12 h | 4 (22.2) | 2 (28.6) | 1 |
| >12 h | 8 (44.4) | 3 (42.9) | |
| Total duration of antibiotic 2, 4 | 16.7 ± 8.1 | 20.3 ± 6.6 | 0.305 |
| Mean time of parenteral nutrition weaning 2, 4 (1 3/3 3) | 4.3 ± 7.1 | 13.6 ± 12.5 | 0.043 |
| Length of stay in PICU 2, 4 | 7.9 ± 4.7 | 20.4 ± 5.9 | <0.001 |
| Total length of stay at hospital 2, 4 | 22.4 ± 22.1 | 33.0 ± 12.7 | 0.247 |
| aEEG Characteristics | Favorable Outcome Group (n = 18) | Unfavorable Outcome Group (n = 7) | p | OR (IC 95%) |
|---|---|---|---|---|
| Background score over first 24 h 2 | 10 ± 3 | 16 ± 7 | 0.008 | 6.66 (0.95–46.56) |
| <161 | 15 (83.3) | 3 (42.9) | 0.066 | |
| ≥161 | 3 (16.7) | 4 (57.1) | ||
| Electrical epileptic seizures 1 | 15.60 (1.48–164.38) | |||
| Yes | 5 (27.8) | 6 (85.7) | 0.021 | |
| No | 13 (72.2) | 1 (14.3) | ||
| Electrical status epilepticus 1 | 20 (2.21–180.90) | |||
| Yes | 2 (11.1) | 5 (71.4) | 0.007 | |
| No | 16 (88.9) | 2 (28.6) | ||
| Recovery of a modulation 1 | ||||
| Yes | 15 (83.3) | 5 (71.4) | 0.597 | 0.5 (0.06–3.91) |
| No | 3 (16.7) | 2 (28.6) | ||
| Recovery of a normal background 1 | ||||
| Yes | 11 (61.1) | 1 (14.3) | 0.073 | 0.11 (0.01–1.08) |
| No | 7 (38.9) | 6 (85.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, J.; Grosjean, C.; Bednarek, N.; Loron, G. Amplitude-Integrated EEG Monitoring in Pediatric Intensive Care: Prognostic Value in Meningitis before One Year of Age. Children 2022, 9, 668. https://doi.org/10.3390/children9050668
Beck J, Grosjean C, Bednarek N, Loron G. Amplitude-Integrated EEG Monitoring in Pediatric Intensive Care: Prognostic Value in Meningitis before One Year of Age. Children. 2022; 9(5):668. https://doi.org/10.3390/children9050668
Chicago/Turabian StyleBeck, Jonathan, Cecile Grosjean, Nathalie Bednarek, and Gauthier Loron. 2022. "Amplitude-Integrated EEG Monitoring in Pediatric Intensive Care: Prognostic Value in Meningitis before One Year of Age" Children 9, no. 5: 668. https://doi.org/10.3390/children9050668
APA StyleBeck, J., Grosjean, C., Bednarek, N., & Loron, G. (2022). Amplitude-Integrated EEG Monitoring in Pediatric Intensive Care: Prognostic Value in Meningitis before One Year of Age. Children, 9(5), 668. https://doi.org/10.3390/children9050668







