Lung and Pleural Findings of Children with Pulmonary Vein Stenosis with and without Aspiration: MDCT Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Institutional Review Board Approval
2.2. Patient Population
2.3. Pulmonary Vein Stenosis and Aspiration Diagnostic Criteria
2.4. Thoracic MDCT Technical Factors
2.4.1. Types of MDCT Scanners
2.4.2. Thoracic MDCT Technical Parameters
2.5. Thoracic MDCT Image Review
2.6. Thoracic MDCT Study Image Assessment
2.6.1. Lung Evaluation on Thoracic MDCT Study
2.6.2. Pleural Evaluation on Thoracic MDCT Study
2.7. Statistical Analysis
3. Results
3.1. Patient Information
3.2. Thoracic MDCT Findings
3.2.1. Lung Findings on Thoracic MDCT Study
3.2.2. Pleural Findings on Thoracic MDCT Study
3.3. Interobserver Agreement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McLennan, D.I.; Solano, E.C.R.; Handler, S.S.; Lincoln, J.; Mitchell, M.E.; Kirkpatrick, E.C. Pulmonary vein stenosis: Moving from past pessimism to future optimism. Front. Pediatr. 2021, 9, 747812. [Google Scholar] [CrossRef]
- Jadcherla, A.V.; Backes, C.H.; Cua, C.L.; Smith, C.V.; Levy, P.T.; Ball, M.K. Primary pulmonary vein stenosis: A new look at rare but challenging disease. Neoreviews 2022, 22, e296–e308. [Google Scholar] [CrossRef]
- Frank, D.B.; Levy, P.T.; Stiver, C.A.; Boe, B.A.; Baird, C.W.; Callahan, R.M.; Smith, C.V.; Vanderlaan, R.D.; Backes, C.H. Primary pulmonary vein stenosis during infancy: State of the art review. J. Perinatol. 2021, 41, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Suntharos, P.; Prieto, L.R. Treatment of congenital and acquired pulmonary vein stenosis. Curr. Cardiol. Rep. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Vanderlaan, R.D.; Rome, J.; Hirsch, R.; Ivy, D.; Caldarone, C.A. Pulmonary vein stenosis: Treatment and challenges. J. Thorac. Cardiovasc. Surg. 2021, 161, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Hump, T.; Fineman, J.; Qureshi, A.M. The many faces and outcomes of pulmonary vein stenosis in early childhood. Pediatr. Pulmonol. 2021, 56, 649–655. [Google Scholar] [CrossRef]
- Riedlinger, W.F.J.; Juraszek, A.L.; Jenkins, K.J.; Nugent, A.W.; Balasubramanian, S.; Calicchio, M.L.; Kieran, M.W.; Collins, T. Pulmonary vein stenosis: Expression of receptor tyrosine kinases by lesion cells. Cardiovasc. Pathol. 2006, 15, 91–99. [Google Scholar] [CrossRef]
- Sadr, I.M.; Tan, P.E.; Kieran, M.W.; Jenkins, K.J. Mechanism of pulmonary vein stenosis in infants with normally connected veins. Am. J. Cardiol. 2000, 86, 577–579. [Google Scholar] [CrossRef]
- Balasubaramanian, S.; Rehman, M.; Gauvreau, K.; Jenkins, K.J. Bilateral disease and early age at presentation are associated with shorter survival in patients with congenital heart disease and intraluminal pulmonary vein stenosis. Congenit. Heart Dis. 2012, 7, 378–386. [Google Scholar] [CrossRef]
- Rosenblum, J.M.; Altin, H.F.; Gillespie, S.E.; Bauser-Heaton, H.; Kanter, K.A.; Sinha, R.; Cory, M.; Alsoufi, B. Management outcomes of primary pulmonary vein stenosis. J. Thorac. Cardiovasc. Surg. 2020, 159, 1029–1036.e1. [Google Scholar] [CrossRef]
- Quinonez, L.G.; Gauvreau, K.; Borisuk, M.; Ireland, C.; Marshall, A.M.; Mayer, J.E.; Jenkins, K.J.; Fynn-Thompson, F.E.; Baird, C.W. Outcomes of surgery for young children with multivessel pulmonary vein stenosis. J. Thorac. Cardiovasc. Surg. 2015, 150, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, R.; Kieran, M.W.; Baird, C.W.; Colan, S.D.; Gauvreau, K.; Ireland, C.M.; Marshall, A.C.; Sena, L.M.; Vargas, S.O.; Jenkins, K.J. Adjunct targeted biologic inhibition agents to treat aggressive multivessel intraluminal pediatric pulmonary vein stenosis. J. Pediatr. 2018, 198, 29–35. [Google Scholar] [CrossRef]
- Mahgoub, L.; Kaddoura, T.; Kameny, A.R.; Lopez Ortego, P.; Vanderlaan, R.D.; Kakadekar, A.; Dicke, F.; Rebeyka, I.; Calderone, C.A.; Redington, A.; et al. Pulmonary vein stenosis of ex-premature infants with pulmonary hypertension and bronchopulmonary dysplasia, epidemiology, and survival from a multicenter cohort. Pediatr. Pulmonol. 2017, 52, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- DiLorenzo, M.P.; Santo, A.; Rome, J.J.; Zhang, H.; Faerber, J.A.; Mercer-Rosa, L.; Hopper, R.K. Pulmonary vein stenosis: Outcomes in children with congenital heart disease and prematurity. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lo Rito, M.; Gazzaz, T.; Wilder, T.J.; Vanderlaan, R.D.; Van Arsdell, G.S.; Honjo, O.; Yoo, S.J.; Caldarone, C.A. Pulmonary vein stenosis: Severity and location predict survival after surgical repair. J. Thorac. Cardiovasc. Surg. 2016, 151, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Kalfa, D.; Belli, E.; Bacha, E.; Lambert, V.; di Carlo, D.; Kostolny, M.; Salminen, J.; Nosal, M.; Poncelet, A.; Horer, J.; et al. Primary pulmonary vein stenosis: Outcomes, risk factors, and severity score in a multicentric study. Ann. Thorac. Surg. 2017, 104, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Niccum, M.; Callahan, R.; Gauvreau, K.; Jenkins, K.J. Aspiration is associated with poor treatment response in pediatric pulmonary vein stenosis. Children 2021, 8, 783. [Google Scholar] [CrossRef]
- Lee, E.Y.; Jenkins, K.J.; Muneeb, M.; Marshall, A.C.; Tracy, D.A.; Zurakowski, D.; Boiselle, P.M. Proximal pulmonary vein stenosis detection in pediatric patients: Value of multiplanar and 3D VR imaging evaluation. Pediatr. Radiol. 2013, 43, 929–936. [Google Scholar] [CrossRef]
- Lee, E.Y.; Dorkin, H.; Vargas, S.O. Congenital pulmonary malformations in pediatric patients: Review and update on etiology, classification, and imaging findings. Radiol. Clin. N. Am. 2011, 49, 921–948. [Google Scholar] [CrossRef]
- Lee, E.Y.; Boiselle, P.M.; Cleveland, R.H. Multidetector CT evaluation of congenital lung anomalies. Radiology 2008, 247, 632–648. [Google Scholar] [CrossRef]
- Lee, E.Y.; Jenkins, K.J.; Vargas, S.O.; Callahan, R.; Park, H.J.; Gauthier, Z.; Winant, A.J. Thoracic multidetector computed tomography angiography of primary pulmonary vein stenosis in children: Evaluation of characteristic extravascular findings. J. Thorac. Imaging 2021, 36, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Callahan, R.; Vargas, S.O.; Jenkins, K.J.; Park, H.J.; Gauthier, Z.; Winant, A.J. Extravascular MDCT findings of pulmonary vein stenosis in children with cardiac septal defect. Children 2021, 8, 667. [Google Scholar] [CrossRef]
- Lee, E.Y.; Vargas, S.O.; Jenkins, K.J.; Callahan, R.; Park, H.J.; Gauthier, Z.; Winant, A.J. Secondary pulmonary vein stenosis due to total anomalous pulmonary venous connection repair in children: Extravascular MDCT findings. Children 2021, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Muller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 3, 697–722. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.; Khatwa, U.; McAdam, A.J.; Bastos, M.D.; Mahmood, S.A.; Ervoes, J.P.; Boiselle, P.M. Streptococcus milleri group pleuropulmonary infection in children: Computed tomography findings and clinical features. J. Comput. Assist. Tomogr. 2010, 43, 927–932. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 14.1 User Guide; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Winant, A.J.; Vargas, S.O.; Jenkins, K.J.; Callahan, R.; Rameh, V.; Krone, K.A.; Johnston, P.R.; Keochakian, M.L.; Lee, E.Y. Winant, A.J.; et al. Pleuropulmonary MDCT Findings: Comparison between Children with Pulmonary Vein Stenosis and Prematurity-Related Lung Disease. Children 2022, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Tutor, J.D. Dysphagia and chronic pulmonary aspiration in children. Pediatr. Rev. 2020, 41, 236–244. [Google Scholar] [CrossRef]
- Torres-Silva, C.A. Chronic pulmonary aspiration in children: Diagnosis and management. Curr. Probl. Pediatr. Adolesc. Health Care 2018, 48, 74–81. [Google Scholar] [CrossRef]
- Boesch, R.P.; Daines, C.; Wilging, J.P.; Kaul, A.; Cohen, A.P.; Wood, R.E.; Amin, R.S. Advances in the diagnosis and management of chronic pulmonary aspiration in children. Eur. Respir. J. 2006, 28, 847–861. [Google Scholar] [CrossRef] [Green Version]
- Liszewski, M.C.; Laya, B.F.; Zucker, E.J.; Restrepo, R.; Lee, E.Y. Pediatric Thoracic Imaging; Lee, E.Y., Ed.; Wolters Kluwer Press: Philadelphia, PA, USA, 2019; pp. 1–98. [Google Scholar]
- Laya, B.F.; Amini, B.; Zucker, E.J. Pediatric Radiology: Practical Imaging Evaluation of Infants and Children; Lee, E.Y., Ed.; Wolters Kluwer Press: Philadelphia, PA, USA, 2018; pp. 358–458. [Google Scholar]
- Pogoriler, J.E.; Kulik, T.J.; Casey, A.M.; Baird, C.W.; Mullen, M.P.; Jenkins, K.J.; Vargas, S.O. Lung pathology in pediatric pulmonary vein stenosis. Pediatr. Dev. Pathol. 2016, 30, 219–229. [Google Scholar] [CrossRef]
- Callahan, R.; Gauthier, Z.; Toba, S.; Sanders, S.P.; Porras, D.; Vargas, S.O. Correlation of intravascular ultrasound with histology in pediatric pulmonary vein stenosis. Children 2021, 8, 193. [Google Scholar] [CrossRef] [PubMed]

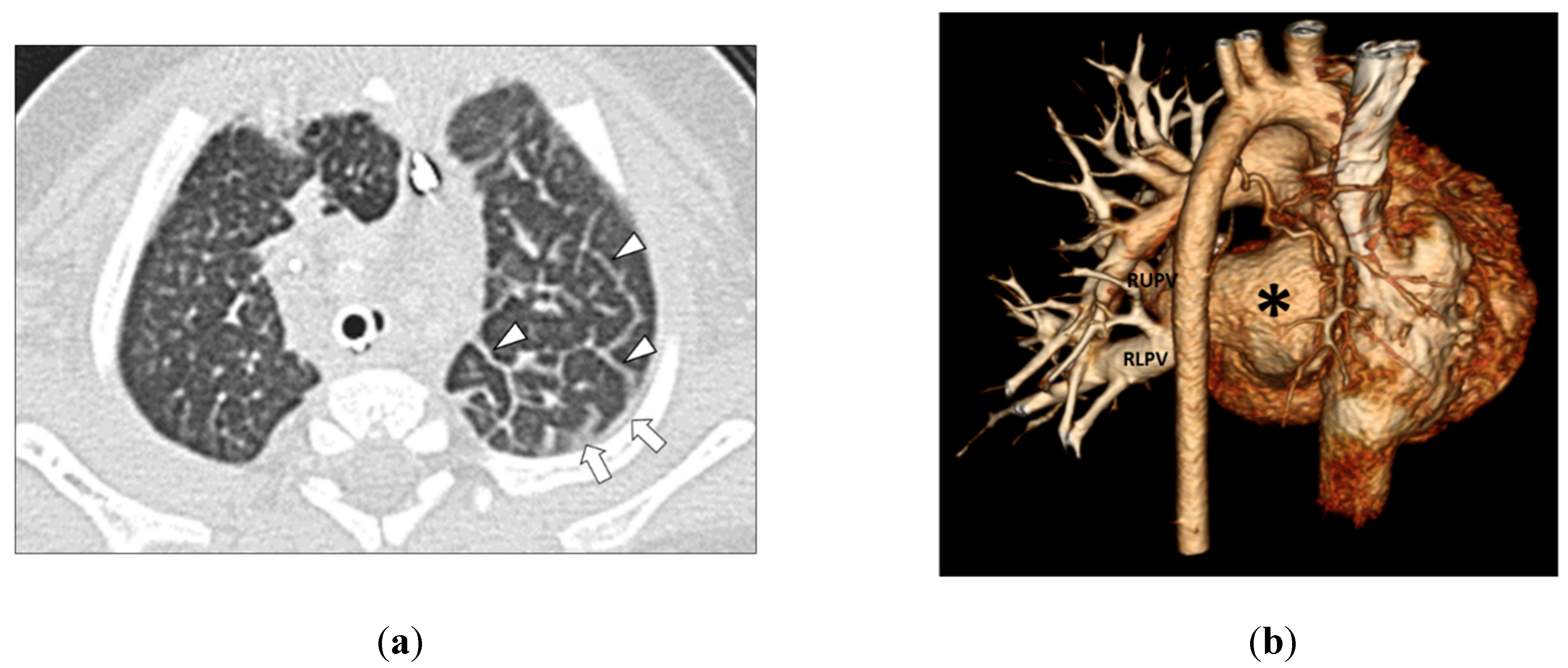
| Types of Lung and Pleural Abnormalities on Thoracic MDCT Studies | Group 1 (PVS with Aspiration) Number (Percentage) of Abnormalities (n = 19) | Group 2 (PVS without Aspiration) Number (Percentage) of Abnormalities (n = 45) | p Value |
|---|---|---|---|
| Lung Abnormalities | |||
| GGO | 17/19 (89.5%) | 37/45 (82.2%) | 0.710 |
| Consolidation | 16/19 (84.5%) | 3/45 (6.7%) | <0.001 |
| Nodule | 0/19 (0%) | 0/45 (0%) | 0.525 |
| Mass | 0/19 (0%) | 0/45 (0%) | 0.525 |
| Cyst(s) | 0/19 (0%) | 1/45 (2.2%) | 1.000 |
| Septal Thickening | 16/19 (84.5%) | 36/45 (80%) | 1.000 |
| Fibrosis | 0/19 (0%) | 0/45 (0%) | 0.525 |
| Pleural Abnormalities | |||
| Pleural Thickening | 17/19 (89.5%) | 37/45 (82.2%) | 0.710 |
| Pleural Effusion | 0/19 (0%) | 1/45 (2.2%) | 1.000 |
| Pneumothorax | 0/19 (0%) | 1/45 (2.2%) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winant, A.J.; Callahan, R.; Vargas, S.O.; Jenkins, K.J.; Rameh, V.; Johnston, P.R.; Niccum, M.; Keochakian, M.L.; Lee, E.Y. Lung and Pleural Findings of Children with Pulmonary Vein Stenosis with and without Aspiration: MDCT Evaluation. Children 2022, 9, 543. https://doi.org/10.3390/children9040543
Winant AJ, Callahan R, Vargas SO, Jenkins KJ, Rameh V, Johnston PR, Niccum M, Keochakian ML, Lee EY. Lung and Pleural Findings of Children with Pulmonary Vein Stenosis with and without Aspiration: MDCT Evaluation. Children. 2022; 9(4):543. https://doi.org/10.3390/children9040543
Chicago/Turabian StyleWinant, Abbey J., Ryan Callahan, Sara O. Vargas, Kathy J. Jenkins, Vanessa Rameh, Patrick R. Johnston, Maria Niccum, Mirjam L. Keochakian, and Edward Y. Lee. 2022. "Lung and Pleural Findings of Children with Pulmonary Vein Stenosis with and without Aspiration: MDCT Evaluation" Children 9, no. 4: 543. https://doi.org/10.3390/children9040543
APA StyleWinant, A. J., Callahan, R., Vargas, S. O., Jenkins, K. J., Rameh, V., Johnston, P. R., Niccum, M., Keochakian, M. L., & Lee, E. Y. (2022). Lung and Pleural Findings of Children with Pulmonary Vein Stenosis with and without Aspiration: MDCT Evaluation. Children, 9(4), 543. https://doi.org/10.3390/children9040543








