The Utility of Umbilical Cord Pulse Oximetry—A Translational Study with Four Minutes of Deferred Cord Clamping Using an Asphyxiated Preterm Ovine Model
Abstract
Highlights
- Umbilical pulse oximetry is a novel method to measure umbilical cord saturations in newborns.
- Umbilical saturations and umbilical heart rate through pulse oximetry, although postductal, had a significant correlation with arterial saturations and invasive carotid heart rate measurements.
- Umbilical cord pulse oximetry could serve as an alternative to measure oxygen saturation while undergoing a longer duration of deferred cord clamping.
- Umbilical cord pulse oximetry may help decide the duration of deferred cord clamping in the future.
Abstract
1. Introduction
2. Materials and Methods
2.1. Preterm Ovine Model
2.2. Pulse Oximetry
2.3. Protocol
2.4. Data Analysis
3. Results
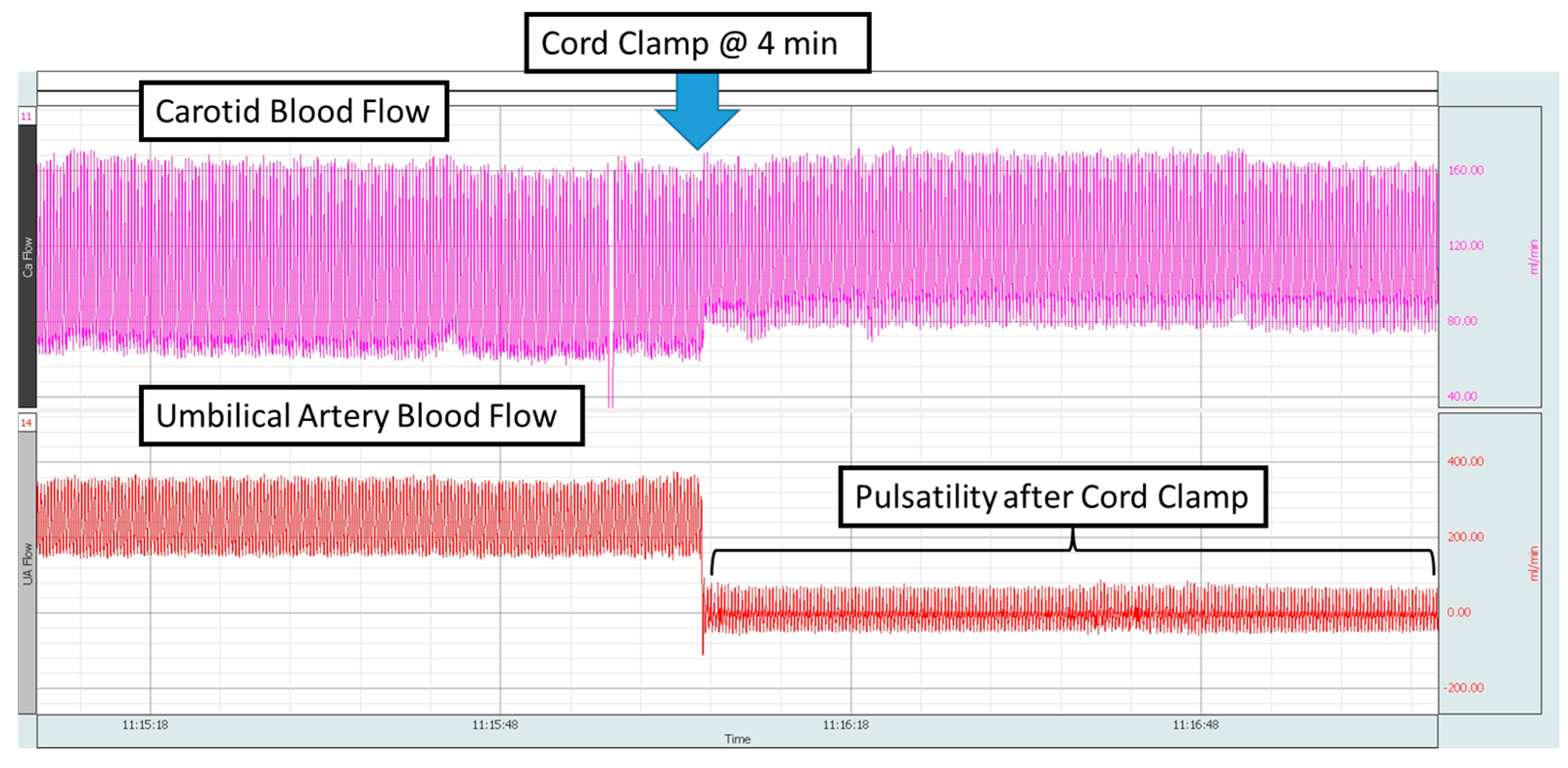
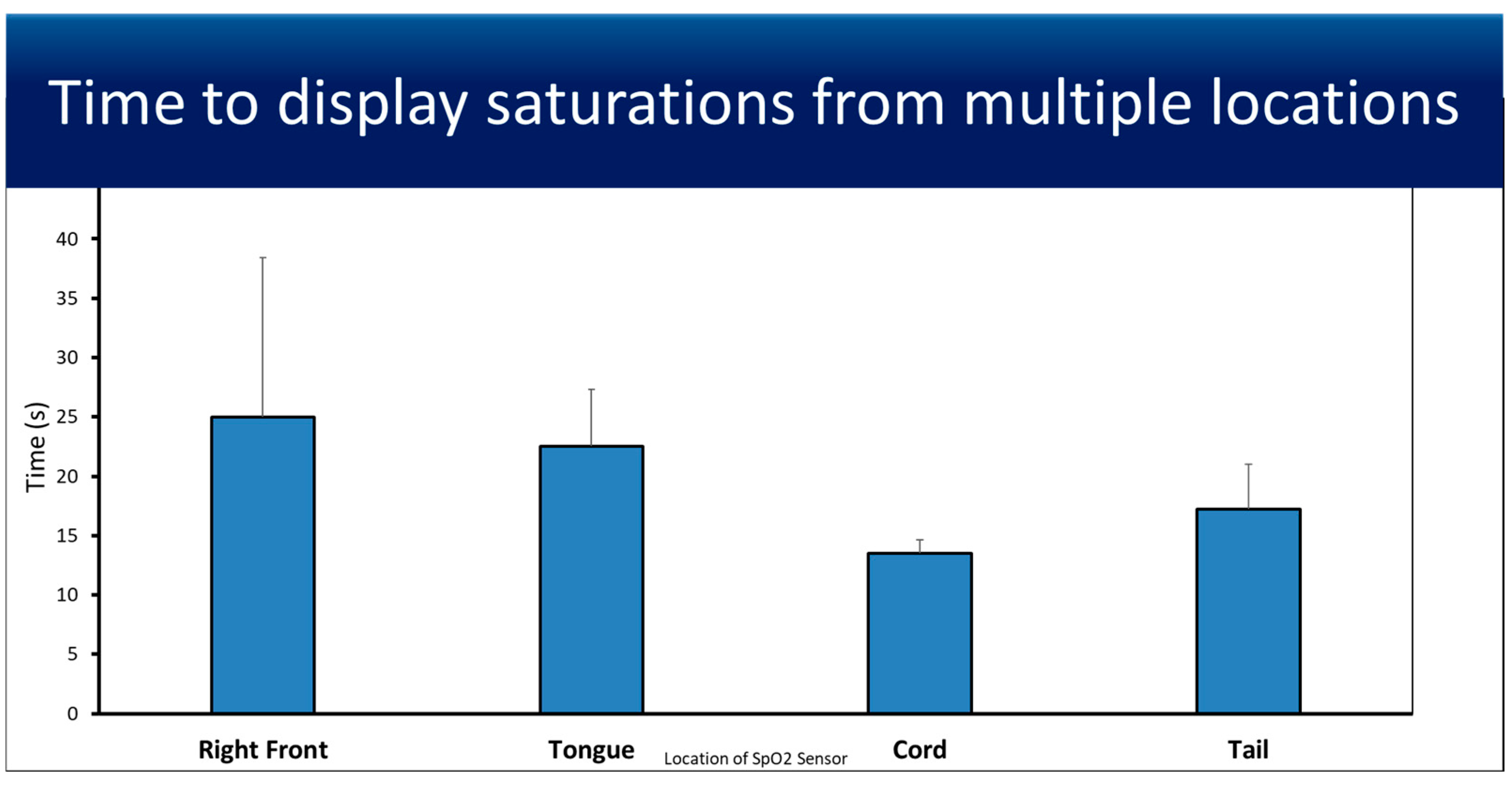
3.1. Feasibility
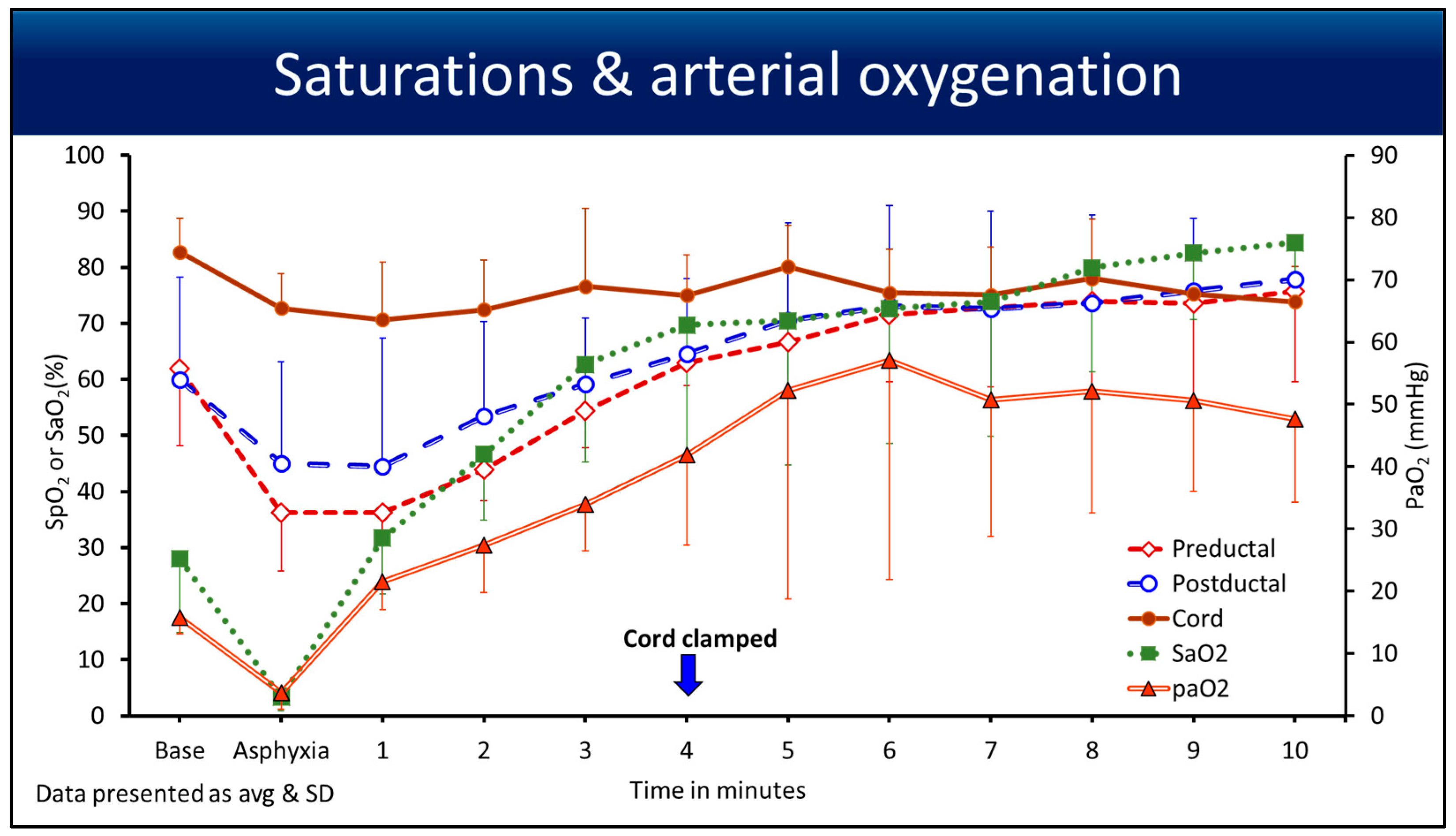
3.2. Saturations
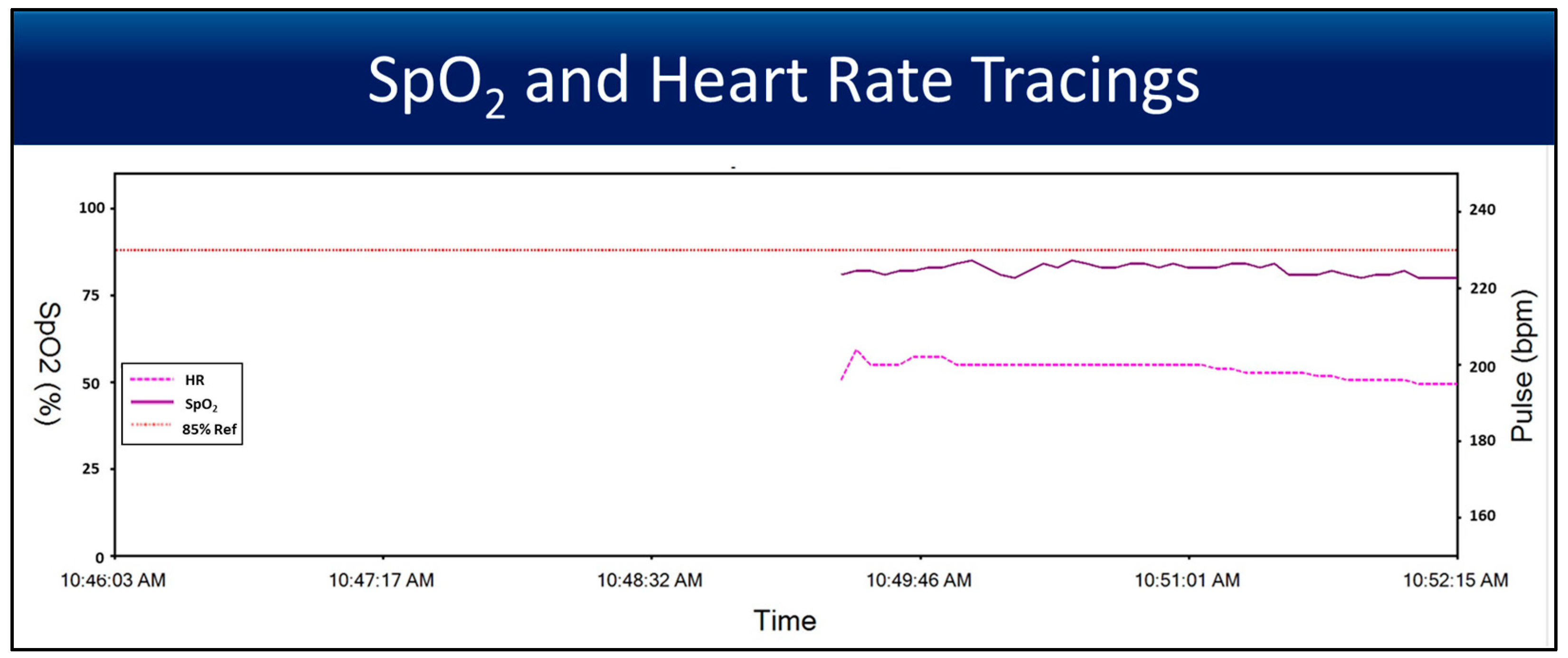
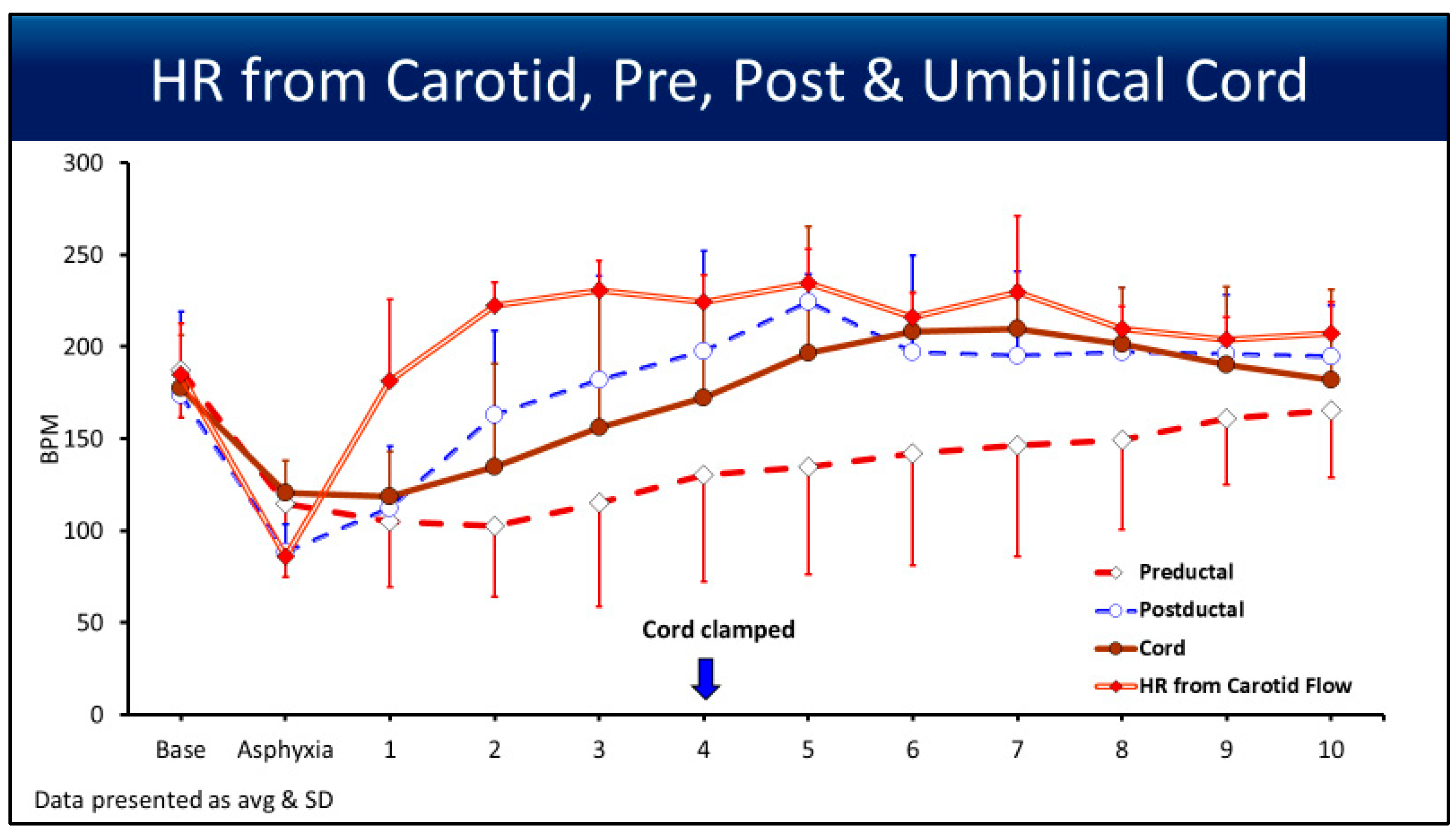
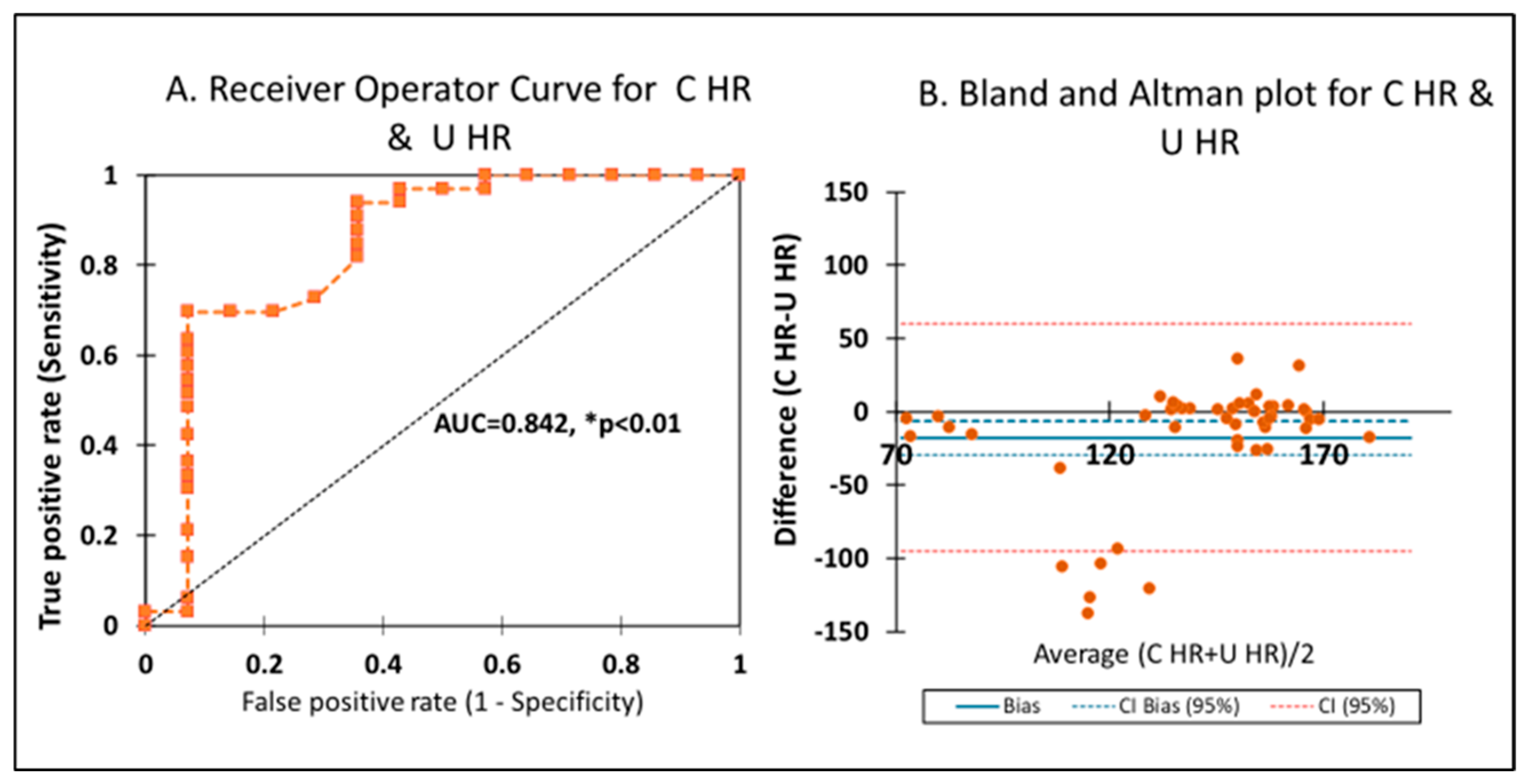
3.3. Heart Rate
4. Discussion
4.1. Point of Interest: How Can You Measure the SpO2 and HR for 10 min if the Cord Is Clamped at 4 min?
4.2. Point of Interest: Can Umbilical Cord Pulse Oximetry Be Helpful to Determine the Duration of Deferred Cord Clamping?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UCP | umbilical cord pulse oximetry |
| SpO2 | preductal saturations |
| SaO2 | arterial saturations |
| U SpO2 | umbilical saturations |
| C HR | carotid heart rate |
| U HR | umbilical heart rate |
| CA | carotid artery |
| UV | umbilical vein |
| UA | umbilical artery |
References
- Weiner, G.M.; Zaichkin, J. Textbook of Neonatal Resuscitation, 8th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- Wyckoff, M.H.; Sawyer, T.; Lakshminrusimha, S.; Collins, A.; Ohls, R.K.; Leone, T.A. Resuscitation 2020: Proceedings from the NeoHeart 2020 International Conference. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 77–88. [Google Scholar] [CrossRef]
- Yamada, N.K.; Szyld, E.; Strand, M.L.; Finan, E.; Illuzzi, J.L.; Kamath-Rayne, B.D.; Kapadia, V.S.; Niermeyer, S.; Schmolzer, G.M.; Williams, A.; et al. 2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2024, 149, e157–e166. [Google Scholar] [CrossRef]
- Ortega, R.; Hansen, C.J.; Elterman, K.; Woo, A. Videos in clinical medicine. Pulse oximetry. N. Engl. J. Med. 2011, 364, e33. [Google Scholar] [CrossRef]
- Wackernagel, D.; Blennow, M.; Hellstrom, A. Accuracy of pulse oximetry in preterm and term infants is insufficient to determine arterial oxygen saturation and tension. Acta Paediatr. 2020, 109, 2251–2257. [Google Scholar] [CrossRef]
- FDA In Brief: FDA Warns About Limitations and Accuracy of Pulse Oximeters. 2021. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-about-limitations-and-accuracy-pulse-oximeters (accessed on 25 October 2021).
- Vesoulis, Z.; Tims, A.; Lodhi, H.; Lalos, N.; Whitehead, H. Racial discrepancy in pulse oximeter accuracy in preterm infants. J. Perinatol. 2022, 42, 79–85. [Google Scholar] [CrossRef]
- Sjoding, M.W.; Dickson, R.P.; Iwashyna, T.J.; Gay, S.E.; Valley, T.S. Racial Bias in Pulse Oximetry Measurement. N. Engl. J. Med. 2020, 383, 2477–2478. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Blanchet, M.A.; Mercier, G.; Delobel, A.; Nayet, E.; Bouchard, P.A.; Simard, S.; L’Her, E.; Branson, R.D.; Lellouche, F. Accuracy of Multiple Pulse Oximeters in Stable Critically Ill Patients. Respir. Care 2023, 68, 565–574. [Google Scholar] [CrossRef]
- Allgoewer, A.; Mayer, B. Sample size estimation for pilot animal experiments by using a Markov Chain Monte Carlo approach. Altern. Lab. Anim. 2017, 45, 83–90. [Google Scholar] [CrossRef]
- Laws, T.R.; Maishman, T.C. Considerations in the design of animal infection pilot studies. Front. Cell Infect. Microbiol. 2022, 12, 948464. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Gugino, S.; Helman, J.; Koenigsknecht, C.; Nielsen, L.; Bradley, N.; Nair, J.; Agrawal, V.; Bawa, M.; Mari, A.; et al. Resuscitation with an Intact Cord Enhances Pulmonary Vasodilation and Ventilation with Reduction in Systemic Oxygen Exposure and Oxygen Load in an Asphyxiated Preterm Ovine Model. Children 2021, 8, 307. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Manja, V.; Mathew, B.; Suresh, G.K. Oxygen targeting in preterm infants: A physiological interpretation. J. Perinatol. 2015, 35, 8–15. [Google Scholar] [CrossRef]
- Barr, P.; Courtman, S.P. Cardiopulmonary resuscitation in the newborn intensive care unit. J. Paediatr. Child. Health 1998, 34, 503–507. [Google Scholar] [CrossRef]
- Kapadia, V.; Oei, J.L.; Finer, N.; Rich, W.; Rabi, Y.; Wright, I.M.; Rook, D.; Vermeulen, M.J.; Tarnow-Mordi, W.O.; Smyth, J.P.; et al. Outcomes of delivery room resuscitation of bradycardic preterm infants: A retrospective cohort study of randomised trials of high vs low initial oxygen concentration and an individual patient data analysis. Resuscitation 2021, 167, 209–217. [Google Scholar] [CrossRef]
- Saugstad, O.D.; Kapadia, V.; Oei, J.L. Oxygen in the First Minutes of Life in Very Preterm Infants. Neonatology 2021, 118, 218–224. [Google Scholar] [CrossRef]
- Apgar, V. A Proposal for a New Method of Evaluation of the Newborn Infant. Anesth. Analg. 2015, 120, 1056–1059. [Google Scholar] [CrossRef]
- Rottgering, J.G.; de Man, A.M.E.; Schuurs, T.C.; Wils, E.J.; Daniels, J.M.; van den Aardweg, J.G.; Girbes, A.R.J.; Smulders, Y.M. Determining a target SpO2 to maintain PaO2 within a physiological range. PLoS ONE 2021, 16, e0250740. [Google Scholar] [CrossRef]
- Shah, B.A.; Wlodaver, A.G.; Escobedo, M.B.; Ahmed, S.T.; Blunt, M.H.; Anderson, M.P.; Szyld, E.G. Impact of electronic cardiac (ECG) monitoring on delivery room resuscitation and neonatal outcomes. Resuscitation 2019, 143, 10–16. [Google Scholar] [CrossRef]
- Seidler, A.L.; Libesman, S.; Hunter, K.E.; Barba, A.; Aberoumand, M.; Williams, J.G.; Shrestha, N.; Aagerup, J.; Sotiropoulos, J.X.; Montgomery, A.A.; et al. Short, medium, and long deferral of umbilical cord clamping compared with umbilical cord milking and immediate clamping at preterm birth: A systematic review and network meta-analysis with individual participant data. Lancet 2023, 402, 2223–2234. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Gugino, S.; Koenigsknecht, C.; Helman, J.; Nielsen, L.; Bradley, N.; Nair, J.; Sankaran, D.; Bawa, M.; Rawat, M.; et al. Placental transfusion during neonatal resuscitation in an asphyxiated preterm model. Pediatr. Res. 2022, 92, 678–684. [Google Scholar] [CrossRef]
- Andersson, O.; Rana, N.; Ewald, U.; Malqvist, M.; Stripple, G.; Basnet, O.; Subedi, K.; Kc, A. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 min of birth (Nepcord III)—A randomized clinical trial. Matern. Health Neonatol. Perinatol. 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Kc, A.; Singhal, N.; Gautam, J.; Rana, N.; Andersson, O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 min of birth—Randomized clinical trial. Matern. Health Neonatol. Perinatol. 2019, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Sanchez, C.; Baixauli-Alacreu, S.; Canada-Martinez, A.J.; Solaz-Garcia, A.; Alemany-Anchel, M.J.; Vento, M. Delayed vs Immediate Cord Clamping Changes Oxygen Saturation and Heart Rate Patterns in the First Minutes after Birth. J. Pediatr. 2020, 227, 149–156.e1. [Google Scholar] [CrossRef] [PubMed]

| Multiplicity | Weight (kg) | Gestational Age (days) | Gender |
|---|---|---|---|
| Twin | 2.75 | 126 | M |
| Single | 3.1 | 125 | M |
| Single | 3.31 | 126 | F |
| Single | 3.8 | 126 | F |
| Single | 2.47 | 126 | M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helman, J.; Bawa, M.; Gugino, S.; Bradley, N.; Nielsen, L.; Prasath, A.; Blanco, C.; Kasu, M.D.; Abbasi, H.; Rawat, M.; et al. The Utility of Umbilical Cord Pulse Oximetry—A Translational Study with Four Minutes of Deferred Cord Clamping Using an Asphyxiated Preterm Ovine Model. Children 2025, 12, 1205. https://doi.org/10.3390/children12091205
Helman J, Bawa M, Gugino S, Bradley N, Nielsen L, Prasath A, Blanco C, Kasu MD, Abbasi H, Rawat M, et al. The Utility of Umbilical Cord Pulse Oximetry—A Translational Study with Four Minutes of Deferred Cord Clamping Using an Asphyxiated Preterm Ovine Model. Children. 2025; 12(9):1205. https://doi.org/10.3390/children12091205
Chicago/Turabian StyleHelman, Justin, Mausma Bawa, Sylvia Gugino, Nicole Bradley, Lori Nielsen, Arun Prasath, Clariss Blanco, Mary Divya Kasu, Hamza Abbasi, Munmun Rawat, and et al. 2025. "The Utility of Umbilical Cord Pulse Oximetry—A Translational Study with Four Minutes of Deferred Cord Clamping Using an Asphyxiated Preterm Ovine Model" Children 12, no. 9: 1205. https://doi.org/10.3390/children12091205
APA StyleHelman, J., Bawa, M., Gugino, S., Bradley, N., Nielsen, L., Prasath, A., Blanco, C., Kasu, M. D., Abbasi, H., Rawat, M., & Chandrasekharan, P. (2025). The Utility of Umbilical Cord Pulse Oximetry—A Translational Study with Four Minutes of Deferred Cord Clamping Using an Asphyxiated Preterm Ovine Model. Children, 12(9), 1205. https://doi.org/10.3390/children12091205







