Injectable Hyaluronic Acid and Amino Acids Complex for Pediatric Hard-to-Heal Wounds: A Prospective Case Series and Therapeutic Protocol
Highlights
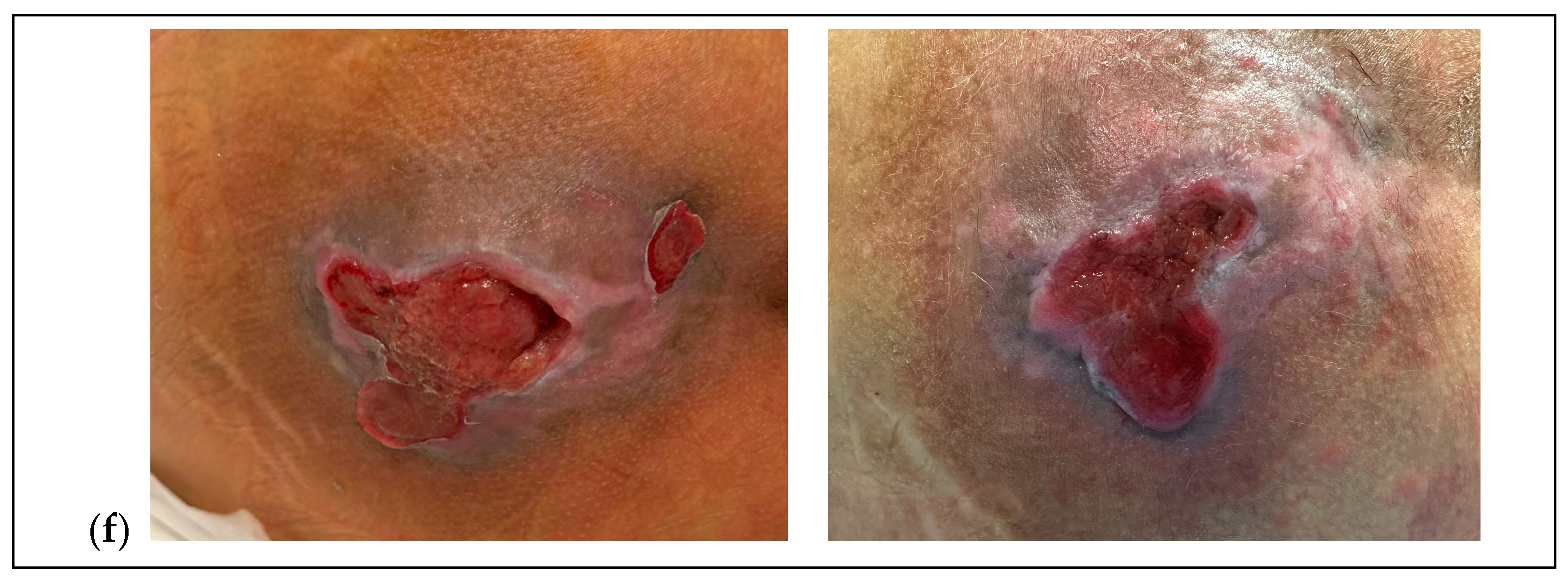
- Complex soft-tissue injuries in pediatric patients, which may delay healing beyond six months due to a recalcitrant, chronic, or stalled wound, can be effectively treated with an injectable therapy combining hyaluronic acid and six amino acids in a single formulation. Regardless of the etiopathogenesis, the results allow for tissue repair and regeneration up to complete re-epithelialization.
- The results obtained, with no pain caused by the therapy itself, show a reduction and disappearance of pain from the chronic condition after just the first two injections, and complete repair of the complex lesion within a maximum of six weeks from the start of treatment. A six-month follow-up confirms stable outcomes with no relapse.
Abstract
1. Introduction
1.1. Defining Hard-to-Heal Wounds in Pediatric Patients
1.2. The Fragile, Immature Skin in Pediatric Patients
1.3. The Role and Properties of HA in Pediatric Skin
1.4. Rationale for the Injectable Route
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Population and Inclusion Criteria
2.3. Intervention: Injectable HA+6AA Protocol
2.4. Co-Interventions. Indications and Standardization
2.5. Outcomes and Endpoints
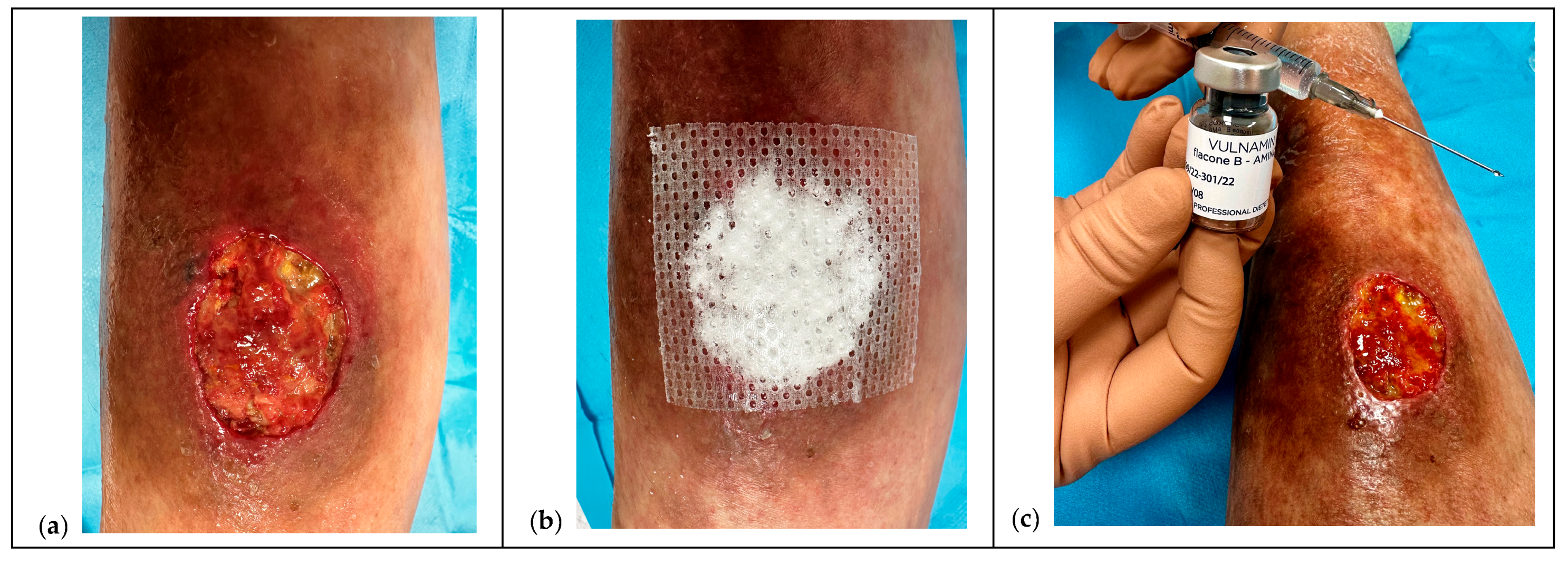
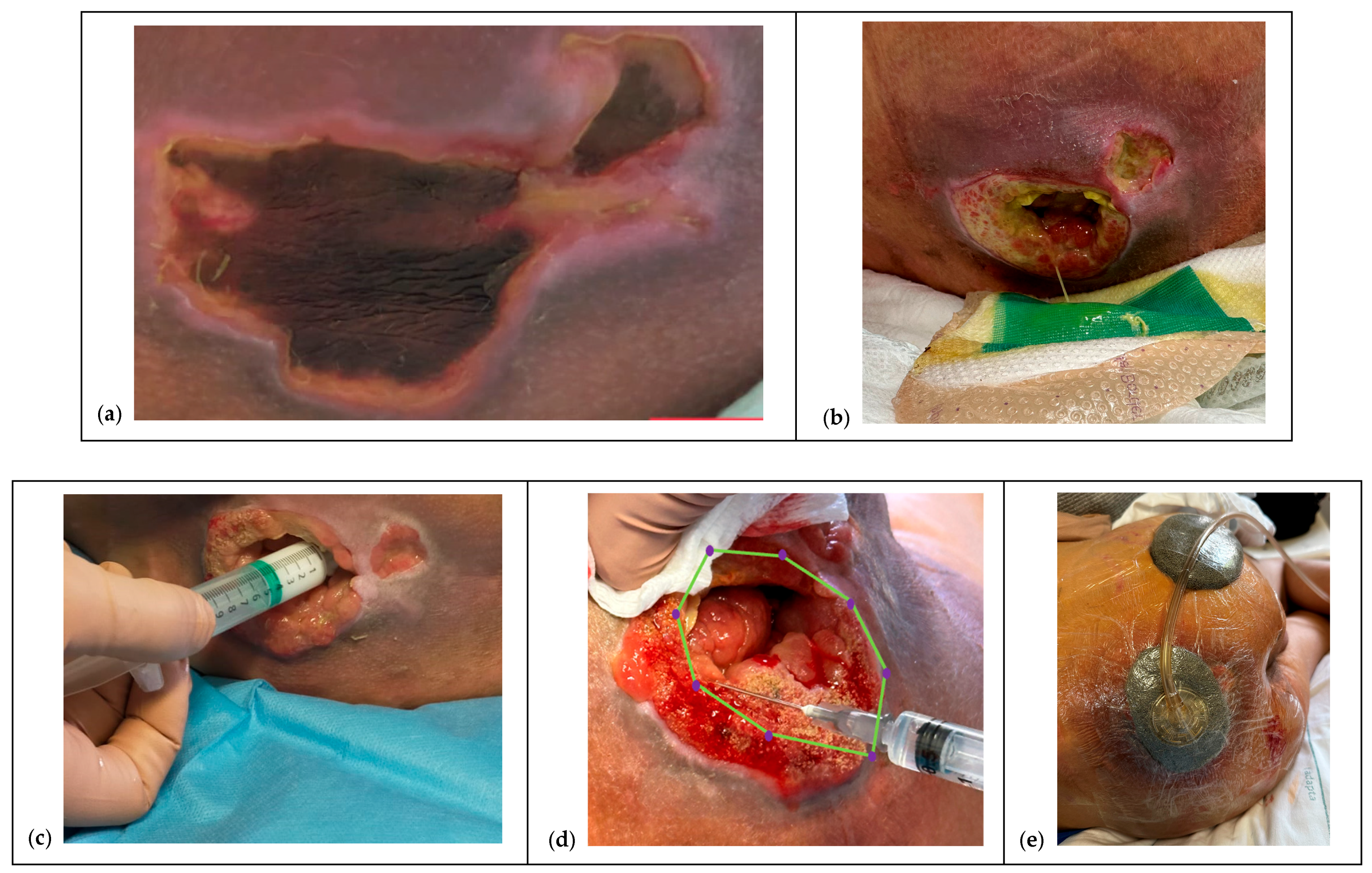
2.6. The Protocol
2.6.1. Pretreatment and NPWT Bridge
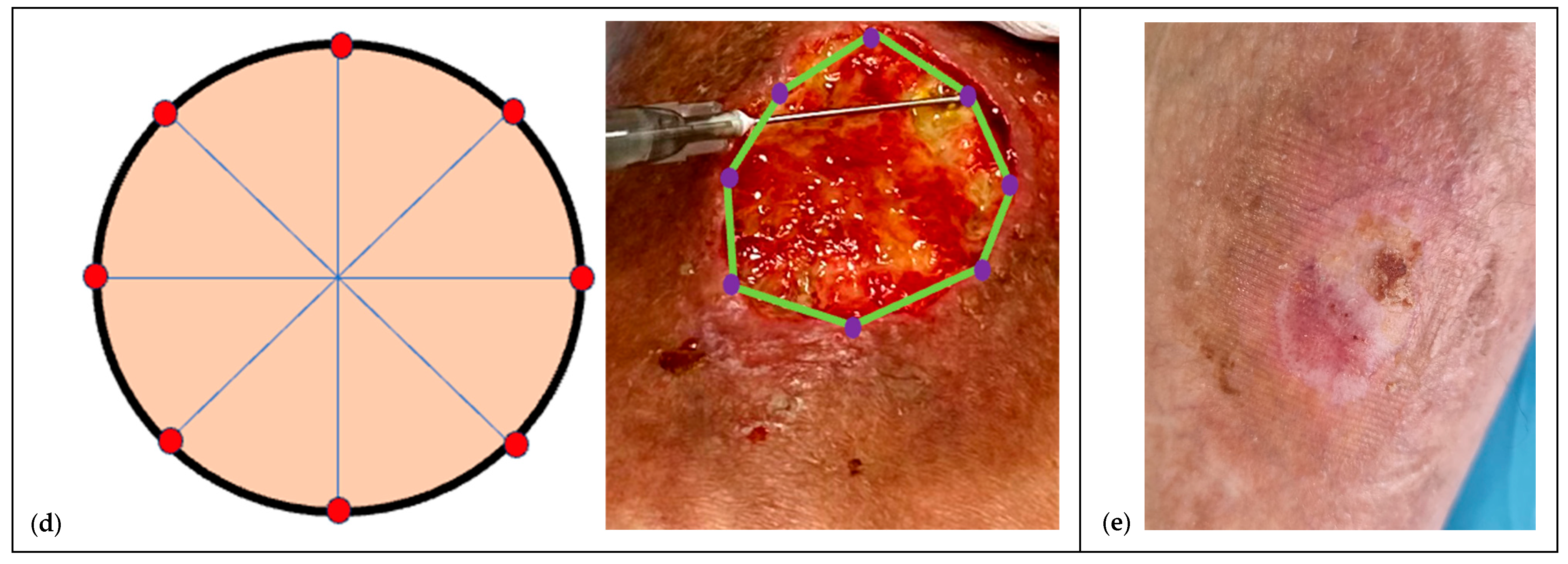
2.6.2. Pre-Infiltration Mapping
2.6.3. Mechanistic Rationale
2.6.4. Ten-Points Operating Checklist
- Stressless. Minimize procedure-related stress and pain through caregiver/patient counseling and stepwise measures adopted by the clinical team: (a) active distraction in all needle procedures; (b) topical anesthesia (cream or flush dispersion) when needed; (c) deep sedation only in selected cases (severe agitation, autism spectrum disorder, complex comorbidities). Intranasal agents were used occasionally at the team’s discretion [25].
- Clean cleanse. Gently cleanse the wound bed using non-ionic, no-rinse solutions (ozonated oils or surfactants), avoiding anionic or cationic products [22].
- Disinfection. Disinfect periwound skin with 2% chlorhexidine and the wound and periwound edges with PHMB; maintain 10 min contact time. Apply with gentle, non-traumatic sponges [22].
- Syringe and needles. Prepare two needles (19 G for aspiration; 21–23 G for inoculation). Keep needles out of the child’s sight; use distraction to reduce procedural discomfort.
- Accurate preparation. Expose the lesion in full: assess it as a volume (ragged walls, thick base, possible fistulous tracts) rather than a simple area and perimeter.
- Body position. Choose the most comfortable position close to a parent or caregiver (often in-arms). Use cartoons or music therapy; in newborns, soothing light or color stimulation can be considered.
- Moving the wound. Gently tension the periwound to visualize undermining and areas of greater tissue loss or necrosis.
- Mapping. Mark undermined and poorly granulating areas with a dermographic pencil to define depots (to spacing depth see SOP).
- Avoid bleeding. Under magnification (microsurgical loupes), avoid vascular injury and fragile early granulation. Areas of tissue with less granulation are selected first, and if bleeding occurs, moderate compression is applied (sometimes gauze compresses can be soaked in hydrogen peroxide and left on for 15–20 s). Significant bleeding has never been observed.
- Waiting time. Allow a few seconds between sequential depots; withdraw the needle fully and re-enter gently for each inoculation.Analgesia and topical anesthesia. The entire procedure can be performed after taking oral paracetamol 30 min beforehand and using a topical lidocaine hydrochloride spray or cream, covering it with a polyurethane film and waiting at least 15–20 min.Documentation and consent. Injections of HA+6AA formulations are administered clockwise or counterclockwise, and each injection must be well documented for subsequent applications, using photographs taken after obtaining written consent from the parents or caregivers (see Ethical considerations).
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, C.; Atkin, L.; Swanson, T.; Tachi, M.; Tan, Y.K.; de Ceniga, M.V.; Weir, D.; Wolcott, R.; Ĉernohorská, J.; Ciprandi, G.; et al. Defying hard-to-heal wounds with an early antibiofilm intervention strategy: Wound hygiene. J. Wound Care 2020, 29 (Suppl. S3), S1–S26. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Ferreira, P.L.; Lourenço, C.; Alves, P.J.P.; Marques, J.M.N.D.; de Sá, L.O. Chronic wound assessment: Cultural and lin-guistic adaptation for European Portuguese of RESVECH-2 scale. J. Tissue Viability 2022, 31, 783–789. [Google Scholar] [CrossRef]
- Atkin, L.; Bućko, Z.; Montero, E.C.; Cutting, K.; Moffatt, C.; Probst, A.; Romanelli, M.; Schultz, G.S.; Tettelbach, W. Implementing TIMERS: The race against hard-to-heal wounds. J. Wound Care 2019, 28, S1–S50. [Google Scholar] [CrossRef]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D.; et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef] [PubMed]
- A McNamara, S.; A Hirt, P.; A Weigelt, M.; Nanda, S.; de Bedout, V.; Kirsner, R.S.; A Schachner, L. Traditional and advanced therapeutic modalities for wounds in the paediatric population: An evidence-based review. J. Wound Care 2020, 29, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Webb, R. Hard-to-heal wounds: TIMERS for action. J. Wound Care 2019, 28, 131. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Gray, L.; Marsh, Z. Wound Care in Children and Adolescents. Nurs. Clin. N. Am. 2025, 60, 49–56. [Google Scholar] [CrossRef]
- Teare, J. A home-care team in paediatric wound care. J. Wound Care 1997, 6, 295–296. [Google Scholar] [CrossRef]
- Mancl, K.A.; Kirsner, R.S.; Ajdic, D. Wound biofilms: Lessons learned from oral biofilms. Wound Repair Regen. 2013, 21, 352–362. [Google Scholar] [CrossRef]
- Nie, A.M.P.; Johnson, D.M.; Reed, R.C. Neonatal Skin Structure: Pressure Injury Staging Challenges. Adv. Ski. Wound Care 2022, 35, 149–154. [Google Scholar] [CrossRef]
- Arnal-Forné, M.; Molina-García, T.; Ortega, M.; Marcos-Garcés, V.; Molina, P.; Ferrández-Izquierdo, A.; Sepulveda, P.; Bodí, V.; Ríos-Navarro, C.; Ruiz-Saurí, A. Changes in human skin composition due to intrinsic aging: A histologic and morphometric study. Histochem. Cell Biol. 2024, 162, 259–271. [Google Scholar] [CrossRef]
- Steen, E.H.; Wang, X.; Boochoon, K.S.; Ewing, D.C.B.; Strang, H.E.; Kaul, A.B.; Masri, L.; Balaji, S.; Hollier, L.H.J.; Keswani, S. Wound Healing and Wound Care in Neonates: Current Therapies and Novel Options. Adv. Ski. Wound Care 2020, 33, 294–300. [Google Scholar] [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications-A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef]
- Ciprandi, G.; Nicolosi, B. Bridging strategies for pediatric wound care: Hyaluronic acid and amino acids. In Neonatal and Pediatric Wound Care: A Contemporary Perspective on Innovations and Best Practices; Ciprandi, G., Beeckman, D., Eds.; Edizioni Minerva Medica: Torino, Italy, 2025; pp. 141–175. [Google Scholar]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- International Guideline. 2025. Guideline. Available online: https://internationalguideline.com/2025 (accessed on 14 September 2025).
- I Merkel, S.; Voepel-Lewis, T.; Shayevitz, J.R.; Malviya, S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr. Nurs. 1997, 23, 293–297. [Google Scholar] [PubMed]
- Huskisson, E.C. Measurement of pain. Lancet 1974, 2, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Solodiuk, J.; Curley, M.A.Q. Pain assessment in nonverbal children with severe cognitive impairments: The Individualized Numeric Rating Scale (INRS). J. Pediatr. Nurs. 2003, 18, 295–299. [Google Scholar] [CrossRef]
- Vercammen, Y.; Dauwe, D.; De Vlieger, G.; Houthoofd, S.; Desmet, L.; Casaer, M.P. Povidone Iodine Disinfection Associated with Hypothyroidism and Potentially Contributing to Prolonged Kidney Failure. Case Rep. Crit. Care 2021, 2021, 5528210. [Google Scholar] [CrossRef]
- Castiello, G.; Caravella, G.; Ghizzardi, G.; Conte, G.; Magon, A.; Fiorini, T.; Ferraris, L.; Devecchi, S.; Calorenne, V.; Andronache, A.A.; et al. Efficacy of Polyhexamethylene Biguanide in Reducing Post-Operative Infections: A Systematic Review and Meta-Analysis. Surg. Infect. 2023, 24, 692–702. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Woo, K.; Ayello, E.A. Increased bacterial burden and infection: The story of NERDS and STONES. Adv. Ski. Wound Care 2006, 19, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, B.; Parente, E.; Petronici, A.; Fioravanti, L.; Cavalieri, S.; Nobile, V.; Reggiardo, G.; Coletta, R.; Moroni, M.; Ciprandi, G. Moisture-associated skin damage and its management in neonatal and infant populations: A retrospective study in Italy. J. Wound Care 2025, 34, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Taddio, A.; McMurtry, C.M.; Shah, V.; Riddell, R.P.; Chambers, C.T.; Noel, M.; MacDonald, N.E.; Rogers, J.; Bucci, L.M.; Mousmanis, P.; et al. Reducing pain during vaccine injections: Clinical practice guideline. CMAJ 2015, 187, 975–982. [Google Scholar] [CrossRef]
- Żur, S.; Sokal, A.; Staśkiewicz-Bartecka, W.; Kiciak, A.; Grajek, M.; Krupa-Kotara, K.; Kowalski, O.; Białek-Dratwa, A. Nutrition for Children with Down Syndrome—Current Knowledge, Challenges, and Clinical Recommendations—A Narrative Review. Healthcare 2025, 13, 2222. [Google Scholar] [CrossRef]
- de Jesus, L.E.; Martins, A.B.; Oliveira, P.B.; Gomes, F.; Leve, T.; Dekermacher, S. Negative pressure wound therapy in pediatric surgery: How and when to use. J. Pediatr. Surg. 2018, 53, 585–591. [Google Scholar] [CrossRef]
- Ciprandi, G.; Crucianelli, S.; Grussu, F.; Spuntarelli, G.; Marino, S.F.M.; Urbani, U.; Bernaschi, P.; Sisto, A.; Rizzo, M.I.; Zama, M. Meeting the Challenges in Pediatric Wound Care: Our 15-Year Experience with Dialkylcarbamoyl Chloride-Coated Dressing Technology in Acute and Chronic Wounds. Chronic Wound Care Manag. Res. 2022, 9, 23–33. [Google Scholar] [CrossRef]
- Nicolosi, B.; Parente, E. Use of antimicrobial Dialkyl Carbamoyl Chloride (DACC) surface dressings for the treatment of infected post-surgical complications in neonates with low risk of adverse reactions: Case series in the AOU Meyer NICU. Inferm. J. 2023, 2, 39–45. [Google Scholar] [CrossRef]
- Yıldırım, S.; Özener, H.Ö.; Doğan, B.; Kuru, B. Effect of topically applied hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J. Periodontol. 2018, 89, 36–45. [Google Scholar] [CrossRef]
- Özker, E.; Krakowiecki, A.; Cassino, R.; Pezzuto, C.; Chadwick, P.; Romanelli, M. Unique combination of hyaluronic acid and amino acids in the management of patients with a range of moderate-to-severe chronic wounds: Evidence from international clinical trials. Int. Wound J. 2024, 21 (Suppl. S1), 4–8. [Google Scholar] [CrossRef]
- David-Raoudi, M.; Tranchepain, F.; Deschrevel, B.; Vincent, J.C.; Bogdanowicz, P.; Boumediene, K.; Pujol, J.P. Differential effects of hyalu-ronan and its fragments on fibroblasts: Relation to wound healing. Wound Repair Regen. 2008, 16, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.Z.; Houtrow, A.J.; Arango, P.; Kuhlthau, K.A.; Simmons, J.M.; Neff, J.M. Family-centered care: Current applications and future directions in pediatric health care. Matern. Child Health J. 2012, 16, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.H.; Ding, S.; Wu, J.H.; Wang, F. Family caregivers’ experiences of caring for neonates undergoing enterostomy in China: A qualitative study. Nurs. Open 2023, 10, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Beccari, T.; Marks, R.S.; Brown, R.; Lorusso, L.; Pheby, D.; Miertus, S.; Herbst, K.L.; Stuppia, L.; Henehan, G. Ethics committees for clinical experimentation at international level with a focus on Italy. Acta Biomed. 2020, 91 (Suppl. S13), e2020016. [Google Scholar]
- Clinical trials—Regulation EU No 536/2014—European Commission. 2025. Available online: https://health.ec.europa.eu/medicinal-products/clinical-trials/clinical-trials-regulation-eu-no-5362014_en (accessed on 14 September 2025).
- Gazzetta Ufficiale. Available online: https://www.gazzettaufficiale.it/eli/id/2018/09/04/18G00129/sg (accessed on 2 December 2019).

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age between 4 and 16 years | Presence of uncontrolled systemic or local acute infection requiring urgent surgical intervention |
| Presence of at least one hard-to-heal wound (defined as <30% reduction in area at 4 weeks and non-healing >6 up to 12 months from onset) | Severe immunosuppression or ongoing chemotherapy/radiotherapy incompatible with the protocol |
| Wound staged according to EPUAP/NPIAP/PPPIA guidelines (2025) [17] | Uncorrected coagulation disorders or conditions contraindicating infiltration therapy |
| Availability of written informed consent from parents/legal guardians and assent from children and adolescents when applicable | Inability to obtain informed consent/assent. Concerns about the family’s ability to complete the outpatient protocol due to logistical constraints. |
| Presence of congenital or acquired chronic conditions (neurological or metabolic disorders) | Known hypersensitivity to HA+AA |
| Outcome | Measurement Method | Timing of Assessment |
|---|---|---|
| Primary outcome | Time to complete re-epithelialization: defined as 100% epithelial coverage of the wound without exudate, confirmed at two consecutive assessments at least 7–14 days apart. | Weekly during treatment; at 1-, 3-, and 6-month follow-up |
| Percentage area reduction at 4 weeks | Relative reduction in wound surface area compared to baseline (measured by standardized digital photography with calibration and planimetry). | Baseline and week 4 |
| Time to 50% wound area reduction | Number of weeks required to achieve a 50% reduction in wound area compared to baseline. | Weekly until endpoint |
| Pain intensity | Measured with age-appropriate validated scales: FLACC (<7 years), INRS (cognitively impaired), VAS 0–10 (≥7 years). | Baseline; before each injection session; at follow-up |
| Exudate amount and odor | Assessed using a 0–3 ordinal scale (0 = absent, 3 = abundant/severe). | At each dressing/injection change |
| Local wound infection | Evaluated according to IWII NERDS/STONEES clinical criteria. | At each assessment |
| Use of systemic antibiotics | Recorded if systemic antimicrobial therapy was required during treatment. | Throughout treatment and follow-up |
| Tolerability and adverse events | Documented presence of procedural pain, local bleeding requiring intervention, infection exacerbation, nodule formation, or systemic/local hypersensitivity reactions. | During and after each injection session |
| Variable | Result |
|---|---|
| Age (years) | 11 [6.5–16] |
| Sex | M 8 (53.3%), F 7 (46.7%) |
| Wound duration (months) | 8 [6–12] |
| Etiology | PUs 6 (40.0%)
LLU 4 (26.7%) Surgical dehiscence 3 (20.0%) Traumatic wounds 2 (13.3%) |
| Comorbidities | Neurological 5 (33.3%) Genetic/syndromic 3 (20.0%) Autoimmune 2 (13.3%) Others 5 (33.3%) |
| Wound location | Sacral/Gluteal region 6 (40.0%) LLU 7 (46.7%) Trunk/Others 2 (13.3%) |
| Stage | III 8 (53.3%) IV 6 (40.0%) Mixed 1 (6.7%) |
| Co-Intervention | Patients Receiving n/N (%) | Typical Timing of Use |
|---|---|---|
| NPWT | 11/15 (73.3) | Before and during injections (2–4 dressing changes) in cases of extensive tissue loss |
| DACC dressing | 3/15 (20.0) | Before injections in wounds with local signs of critical colonization (IWII stage 1–2 according to NERDS/STONEES) * |
| HA+AA gel/cream | 0/15 (0.0) | Pretreatment phase to induce osmotic debridement prior to infiltration |
| Week | Number at Risk |
|---|---|
| 0 | 15 |
| 1 | 15 |
| 2 | 15 |
| 3 | 15 |
| 4 | 15 |
| 5 | 15 |
| 6 | 13 |
| 7 | 7 |
| 8 | 5 |
| 9 | 2 |
| 10 | 1 |
| 11 | 1 |
| 12 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciprandi, G.; Nicolosi, B.; Storti, G.; Marino, S.F.; Scarpa, C.; Bassetto, F. Injectable Hyaluronic Acid and Amino Acids Complex for Pediatric Hard-to-Heal Wounds: A Prospective Case Series and Therapeutic Protocol. Children 2025, 12, 1554. https://doi.org/10.3390/children12111554
Ciprandi G, Nicolosi B, Storti G, Marino SF, Scarpa C, Bassetto F. Injectable Hyaluronic Acid and Amino Acids Complex for Pediatric Hard-to-Heal Wounds: A Prospective Case Series and Therapeutic Protocol. Children. 2025; 12(11):1554. https://doi.org/10.3390/children12111554
Chicago/Turabian StyleCiprandi, Guido, Biagio Nicolosi, Gabriele Storti, Simone F. Marino, Carlotta Scarpa, and Franco Bassetto. 2025. "Injectable Hyaluronic Acid and Amino Acids Complex for Pediatric Hard-to-Heal Wounds: A Prospective Case Series and Therapeutic Protocol" Children 12, no. 11: 1554. https://doi.org/10.3390/children12111554
APA StyleCiprandi, G., Nicolosi, B., Storti, G., Marino, S. F., Scarpa, C., & Bassetto, F. (2025). Injectable Hyaluronic Acid and Amino Acids Complex for Pediatric Hard-to-Heal Wounds: A Prospective Case Series and Therapeutic Protocol. Children, 12(11), 1554. https://doi.org/10.3390/children12111554












