Ultrasound in Pediatric Inflammatory Bowel Disease—A Review of the State of the Art and Future Perspectives
Abstract
1. Introduction
2. Diagnostic Imaging and Surveillance Approaches in Pediatric IBD
3. US in Pediatric Inflammatory Bowel Disease
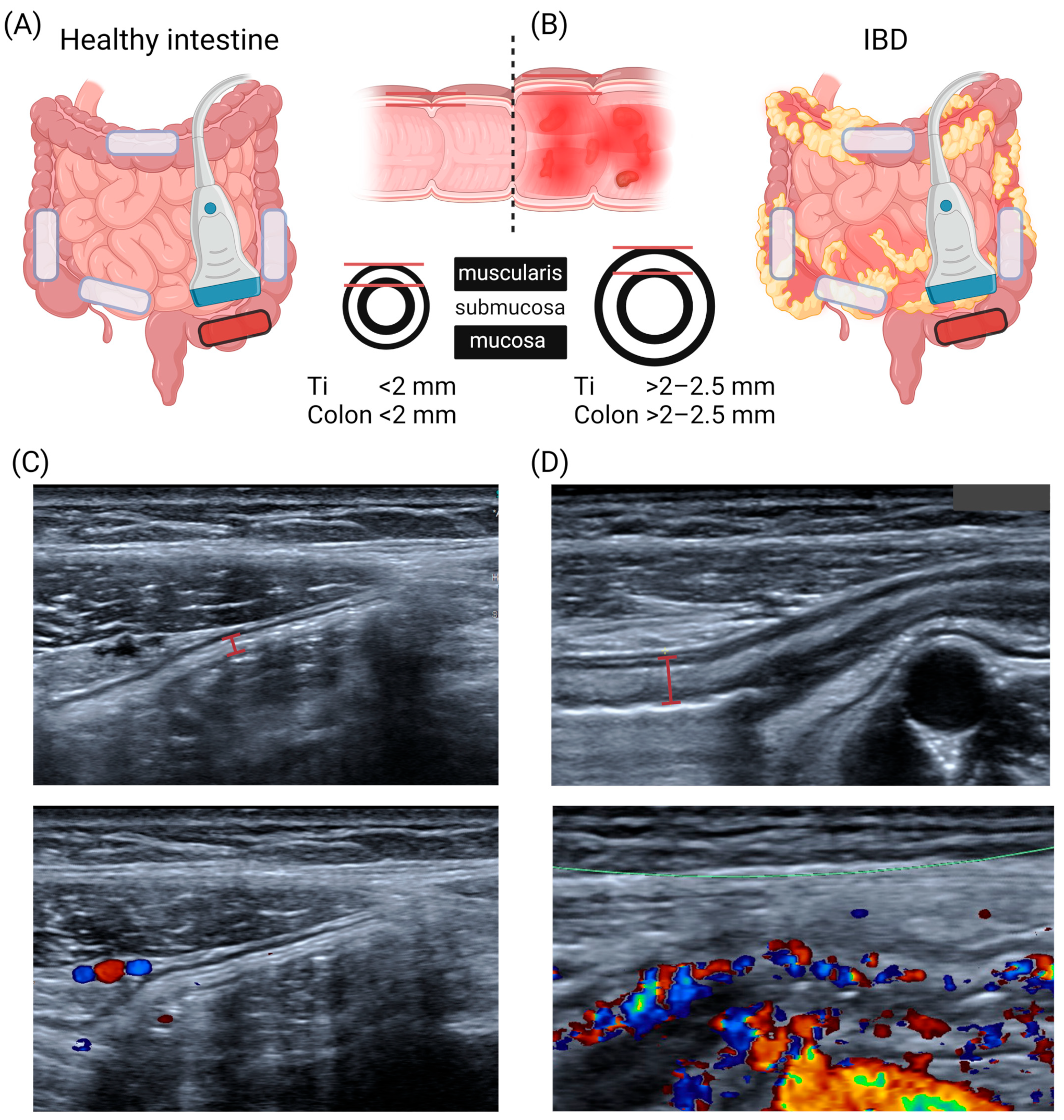
3.1. US Anatomy of the Intestinal Wall
3.2. Current US Information Used in IBD Diagnostics
3.3. Bowel Wall Thickness (BWT)
3.4. US Doppler Signals
3.5. Mesenterial or “Creeping Fat”
3.6. Fibrostenosis and Intestinal Strictures
3.7. US Scoring Systems
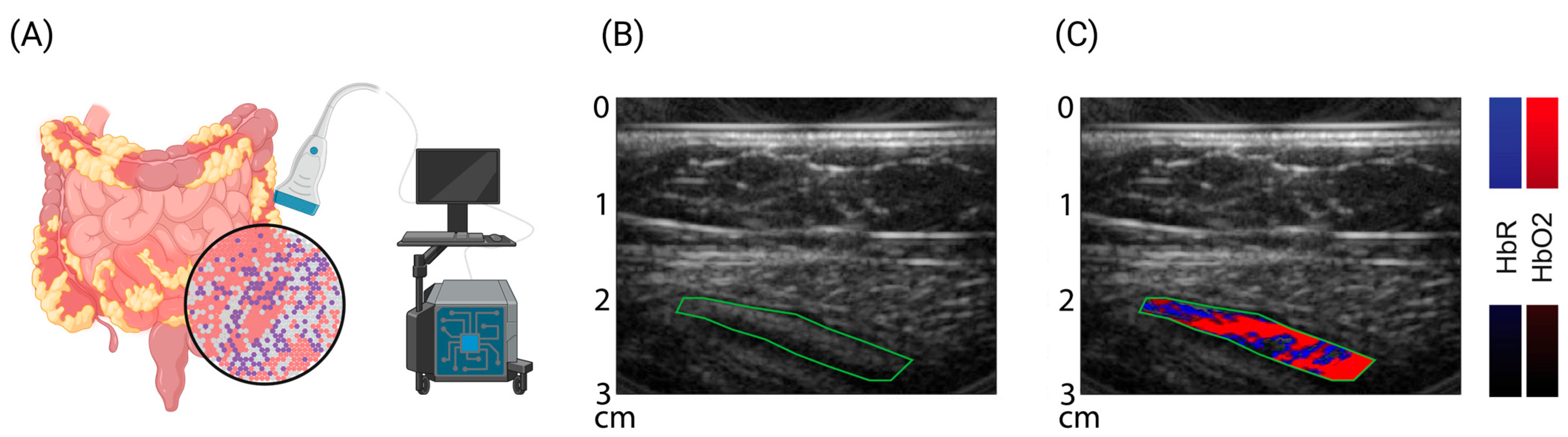
4. Novel US-Based Imaging Technologies: Optoacoustic Imaging (OAI)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, H.; Kaschitzki, A.; Alberts, C.; Bodammer, P.; Bannert, K.; Koller, T.; Warnke, P.; Kreikemeyer, B.; Lamprecht, G. Alterations in the mucosa-associated bacterial composition in Crohn’s disease: A pilot study. Int. J. Color. Dis. 2016, 31, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Host-microbiota interactions in inflammatory bowel disease. Nat. Reviews. Gastroenterol. Hepatol. 2020, 17, 76–77. [Google Scholar] [CrossRef]
- Ruel, J.; Ruane, D.; Mehandru, S.; Gower-Rousseau, C.; Colombel, J.F. IBD across the age spectrum: Is it the same disease? Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Van Limbergen, J.; Russell, R.K.; Drummond, H.E.; Aldhous, M.C.; Round, N.K.; Nimmo, E.R.; Smith, L.; Gillett, P.M.; McGrogan, P.; Weaver, L.T.; et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008, 135, 1114–1122. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Ko, C.W.; Singh, S.; Feuerstein, J.D.; Falck-Ytter, C.; Falck-Ytter, Y.; Cross, R.K.; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 2019, 156, 748–764. [Google Scholar] [CrossRef]
- Buderus, S.; Scholz, D.; Behrens, R.; Classen, M.; De Laffolie, J.; Keller, K.M.; Zimmer, K.P.; Koletzko, S.; Group, C.-G.S. Inflammatory bowel disease in pediatric patients: Characteristics of newly diagnosed patients from the CEDATA-GPGE Registry. Dtsch. Arztebl. Int. 2015, 112, 121–127. [Google Scholar] [CrossRef][Green Version]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting cytokines in inflammatory bowel disease. Sci. Transl. Med. 2022, 14, eabq4473. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G. Resolution of inflammation in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2017, 2, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Present, D.H.; Rutgeerts, P.; Targan, S.; Hanauer, S.B.; Mayer, L.; van Hogezand, R.A.; Podolsky, D.K.; Sands, B.E.; Braakman, T.; DeWoody, K.L.; et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N. Engl. J. Med. 1999, 340, 1398–1405. [Google Scholar] [CrossRef]
- Feagan, B.G.; Greenberg, G.R.; Wild, G.; Fedorak, R.N.; Pare, P.; McDonald, J.W.; Dube, R.; Cohen, A.; Steinhart, A.H.; Landau, S.; et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N. Engl. J. Med. 2005, 352, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Panes, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Fortinsky, K.J.; Gozdyra, P.; Van den Heuvel, M.; Van Limbergen, J.; Griffiths, A.M. Epidemiology of pediatric inflammatory bowel disease: A systematic review of international trends. Inflamm. Bowel Dis. 2011, 17, 423–439. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Carroll, M.W.; Singh, H.; Otley, A.R.; Vutcovici, M.; El-Matary, W.; Nguyen, G.C.; Griffiths, A.M.; et al. Trends in Epidemiology of Pediatric Inflammatory Bowel Disease in Canada: Distributed Network Analysis of Multiple Population-Based Provincial Health Administrative Databases. Am. J. Gastroenterol. 2017, 112, 1120–1134. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e312. [Google Scholar] [CrossRef]
- Ruemmele, F.M. Pediatric inflammatory bowel diseases: Coming of age. Curr. Opin. Gastroenterol. 2010, 26, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Dabritz, J.; Gerner, P.; Enninger, A.; Classen, M.; Radke, M. Inflammatory Bowel Disease in Childhood and Adolescence. Dtsch. Arztebl. Int. 2017, 114, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohns Colitis 2020, 15, 171–194. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 257–291. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis-An Evidence-based Consensus Guideline From the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef] [PubMed]
- De Bie, C.I.; Buderus, S.; Sandhu, B.K.; de Ridder, L.; Paerregaard, A.; Veres, G.; Dias, J.A.; Escher, J.C.; ESPGHAN, E.P.I.W.G.o. Diagnostic workup of paediatric patients with inflammatory bowel disease in Europe: Results of a 5-year audit of the EUROKIDS registry. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Dolinger, M.T.; Kayal, M. Intestinal ultrasound as a non-invasive tool to monitor inflammatory bowel disease activity and guide clinical decision making. World J. Gastroenterol. WJG 2023, 29, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Dolinger, M.T.; Aronskyy, I.; Kellar, A.; Spencer, E.; Pittman, N.; Dubinsky, M.C. Early Intestinal Ultrasound Response to Biologic Therapy Predicts Endoscopic Remission in Children with Ileal Crohn’s Disease: Results from the Prospective Super Sonic Study. J. Crohns Colitis 2023, 2023, jjad216. [Google Scholar] [CrossRef]
- Dolinger, M.T.; Aronskyy, I.; Kellar, A.; Gao, M.; Spencer, E.A.; Pittman, N.; Dubinsky, M.C. Determining the accuracy of intestinal ultrasound scores as a pre-screening tool in Crohn’s disease clinical trials. Am. J. Gastroenterol. 2023, 2023, 2632. [Google Scholar] [CrossRef]
- Van Wassenaer, E.A.; Benninga, M.A.; van Limbergen, J.L.; D’Haens, G.R.; Griffiths, A.M.; Koot, B.G.P. Intestinal Ultrasound in Pediatric Inflammatory Bowel Disease: Promising, but Work in Progress. Inflamm. Bowel Dis. 2022, 28, 783–787. [Google Scholar] [CrossRef]
- Ilvemark, J.; Hansen, T.; Goodsall, T.M.; Seidelin, J.B.; Al-Farhan, H.; Allocca, M.; Begun, J.; Bryant, R.V.; Carter, D.; Christensen, B.; et al. Defining Transabdominal Intestinal Ultrasound Treatment Response and Remission in Inflammatory Bowel Disease: Systematic Review and Expert Consensus Statement. J. Crohns Colitis 2022, 16, 554–580. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Yoon, H.M.; Jung, A.Y.; Lee, J.S.; Cho, Y.A. Diagnostic Performance of Diffusion-weighted Imaging for Evaluation of Bowel Inflammation in Paediatric Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohns Colitis 2022, 16, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.M.; Suh, C.H.; Kim, J.R.; Lee, J.S.; Jung, A.Y.; Kim, K.M.; Cho, Y.A. Diagnostic Performance of Magnetic Resonance Enterography for Detection of Active Inflammation in Children and Adolescents With Inflammatory Bowel Disease: A Systematic Review and Diagnostic Meta-analysis. JAMA Pediatr. 2017, 171, 1208–1216. [Google Scholar] [CrossRef]
- Schreiber-Dietrich, D.; Chiorean, L.; Cui, X.W.; Braden, B.; Kucharzik, T.; Jungert, J.; Kosiak, W.; Stenzel, M.; Dietrich, C.F. Particularities of Crohn’s disease in pediatric patients: Current status and perspectives regarding imaging modalities. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.A.; Petit, P.; Augdal, T.A.; Avni, E.F.; Bruno, C.; Damasio, M.B.; Darge, K.; Kjucevsek, D.; Franchi-Abella, S.; Ibe, D.; et al. European Society of Paediatric Radiology abdominal imaging task force: Statement on imaging in very early onset inflammatory bowel disease. Pediatr. Radiol. 2019, 49, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.; Bouhnik, Y.; Reinisch, W.; Stoker, J.; Taylor, S.A.; Baumgart, D.C.; Danese, S.; Halligan, S.; Marincek, B.; Matos, C.; et al. Imaging techniques for assessment of inflammatory bowel disease: Joint ECCO and ESGAR evidence-based consensus guidelines. J. Crohns Colitis 2013, 7, 556–585. [Google Scholar] [CrossRef]
- Van Wassenaer, E.A.; van Rijn, R.R.; de Voogd, F.A.E.; Noels, F.L.; Deurloo, E.E.; van Schuppen, J.; Verbeke, J.; Gecse, K.B.; D’Haens, G.R.; Benninga, M.A.; et al. A Healthcare Physician Can Be Trained to Perform Intestinal Ultrasound in Children With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 74, e143–e147. [Google Scholar] [CrossRef]
- Hudson, A.S.; Huynh, H.Q.; Novak, K.L.; Ma, H.; Kuc, A.; Kim, J.; Almeida, P.; Carroll, M.W.; Wine, E.; Isaac, D.M. Pediatric Patient and Caregiver Satisfaction With the Use of Transabdominal Bowel Ultrasound in the Assessment of Inflammatory Bowel Diseases. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 33–37. [Google Scholar] [CrossRef]
- Van Wassenaer, E.A.; van der Klift, R.R.; Staphorst, M.S.; van der Lee, J.H.; Benninga, M.A.; Koot, B.G.P. The child’s perception on monitoring inflammatory bowel disease activity. Eur. J. Pediatr. 2022, 181, 1143–1149. [Google Scholar] [CrossRef]
- Elliott, C.L.; Maclachlan, J.; Beal, I. Paediatric bowel ultrasound in inflammatory bowel disease. Eur. J. Radiol. 2018, 108, 21–27. [Google Scholar] [CrossRef]
- Strobel, D.; Goertz, R.S.; Bernatik, T. Diagnostics in inflammatory bowel disease: Ultrasound. World J. Gastroenterol. WJG 2011, 17, 3192–3197. [Google Scholar]
- Hirche, T.O.; Russler, J.; Schroder, O.; Schuessler, G.; Kappeser, P.; Caspary, W.F.; Dietrich, C.F. The value of routinely performed ultrasonography in patients with Crohn disease. Scand. J. Gastroenterol. 2002, 37, 1178–1183. [Google Scholar] [CrossRef]
- Bremner, A.R.; Griffiths, M.; Argent, J.D.; Fairhurst, J.J.; Beattie, R.M. Sonographic evaluation of inflammatory bowel disease: A prospective, blinded, comparative study. Pediatr. Radiol. 2006, 36, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; de Horatio, L.T.; Terrin, G.; Romano, M.T.; Miele, E.; Staiano, A.; Rapacciuolo, L.; Polito, G.; Bisesti, V.; Manguso, F.; et al. Combined use of noninvasive tests is useful in the initial diagnostic approach to a child with suspected inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Van Wassenaer, E.A.; de Voogd, F.A.E.; van Rijn, R.R.; van Der Lee, J.H.; Tabbers, M.M.; van Etten-Jamaludin, F.S.; Gecse, K.B.; Kindermann, A.; De Meij, T.G.J.; D’Haens, G.R.; et al. Diagnostic Accuracy of Transabdominal Ultrasound in Detecting Intestinal Inflammation in Paediatric IBD Patients-a Systematic Review. J. Crohns Colitis 2019, 13, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.L.; Nylund, K.; Maaser, C.; Petersen, F.; Kucharzik, T.; Lu, C.; Allocca, M.; Maconi, G.; de Voogd, F.; Christensen, B.; et al. Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A Reliability and Inter-rater Variability Study on Intestinal Ultrasonography in Crohn’s Disease. J. Crohns Colitis 2021, 15, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Van Wassenaer, E.A.; de Voogd, F.A.E.; van Rijn, R.R.; van der Lee, J.H.; Tabbers, M.M.; van Etten-Jamaludin, F.S.; Kindermann, A.; de Meij, T.G.J.; Gecse, K.B.; D’Haens, G.R.; et al. Bowel ultrasound measurements in healthy children—Systematic review and meta-analysis. Pediatr. Radiol. 2020, 50, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Haber, H.P.; Stern, M. Intestinal ultrasonography in children and young adults: Bowel wall thickness is age dependent. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2000, 19, 315–321. [Google Scholar] [CrossRef]
- Ramsden, W.H.; Moya, E.F.; Littlewood, J.M. Colonic wall thickness, pancreatic enzyme dose and type of preparation in cystic fibrosis. Arch. Dis. Child. 1998, 79, 339–343. [Google Scholar] [CrossRef]
- Pohl, M.; Krackhardt, B.; Posselt, H.G.; Lembcke, B. Ultrasound studies of the intestinal wall in patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 317–320. [Google Scholar] [CrossRef]
- Chiorean, L.; Schreiber-Dietrich, D.; Braden, B.; Cui, X.; Dietrich, C.F. Transabdominal ultrasound for standardized measurement of bowel wall thickness in normal children and those with Crohn’s disease. Med. Ultrason. 2014, 16, 319–324. [Google Scholar] [CrossRef][Green Version]
- De Voogd, F.; van Wassenaer, E.A.; Mookhoek, A.; Bots, S.; van Gennep, S.; Lowenberg, M.; D’Haens, G.R.; Gecse, K.B. Intestinal Ultrasound Is Accurate to Determine Endoscopic Response and Remission in Patients With Moderate to Severe Ulcerative Colitis: A Longitudinal Prospective Cohort Study. Gastroenterology 2022, 163, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Limberg, B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z. Fur Gastroenterol. 1999, 37, 495–508. [Google Scholar]
- Spalinger, J.; Patriquin, H.; Miron, M.C.; Marx, G.; Herzog, D.; Dubois, J.; Dubinsky, M.; Seidman, E.G. Doppler US in patients with crohn disease: Vessel density in the diseased bowel reflects disease activity. Radiology 2000, 217, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Scholbach, T.; Herrero, I.; Scholbach, J. Dynamic color Doppler sonography of intestinal wall in patients with Crohn disease compared with healthy subjects. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 524–528. [Google Scholar] [CrossRef]
- Ravnic, D.J.; Konerding, M.A.; Tsuda, A.; Huss, H.T.; Wolloscheck, T.; Pratt, J.P.; Mentzer, S.J. Structural adaptations in the murine colon microcirculation associated with hapten-induced inflammation. Gut 2007, 56, 518–523. [Google Scholar] [CrossRef]
- Ha, C.W.Y.; Martin, A.; Sepich-Poore, G.D.; Shi, B.; Wang, Y.; Gouin, K.; Humphrey, G.; Sanders, K.; Ratnayake, Y.; Chan, K.S.L.; et al. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 2020, 183, 666–683.e617. [Google Scholar] [CrossRef] [PubMed]
- Eder, P.; Adler, M.; Dobrowolska, A.; Kamhieh-Milz, J.; Witowski, J. The Role of Adipose Tissue in the Pathogenesis and Therapeutic Outcomes of Inflammatory Bowel Disease. Cells 2019, 8, 628. [Google Scholar] [CrossRef]
- Rivera, E.D.; Coffey, J.C.; Walsh, D.; Ehrenpreis, E.D. The Mesentery, Systemic Inflammation, and Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 226–234. [Google Scholar] [CrossRef]
- Goncalves, P.; Magro, F.; Martel, F. Metabolic inflammation in inflammatory bowel disease: Crosstalk between adipose tissue and bowel. Inflamm. Bowel Dis. 2015, 21, 453–467. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, X.T.; Zhou, Y.; Yan, Y.Q.; Ran, Z.H.; Zhu, J. Creeping fat in patients with ileo-colonic Crohn’s disease correlates with disease activity and severity of inflammation: A preliminary study using energy spectral computed tomography. J. Dig. Dis. 2018, 19, 475–484. [Google Scholar] [CrossRef]
- Li, X.H.; Feng, S.T.; Cao, Q.H.; Coffey, J.C.; Baker, M.E.; Huang, L.; Fang, Z.N.; Qiu, Y.; Lu, B.L.; Chen, Z.H.; et al. Degree of Creeping Fat Assessed by Computed Tomography Enterography is Associated with Intestinal Fibrotic Stricture in Patients with Crohn’s Disease: A Potentially Novel Mesenteric Creeping Fat Index. J. Crohns Colitis 2021, 15, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; O’Leary, D.P. The mesentery: Structure, function, and role in disease. Lancet Gastroenterol. Hepatol. 2016, 1, 238–247. [Google Scholar] [CrossRef]
- Coffey, J.C.; Byrnes, K.G.; Walsh, D.J.; Cunningham, R.M. Update on the mesentery: Structure, function, and role in disease. Lancet Gastroenterol. Hepatol. 2022, 7, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Coffey, C.J.; Kiernan, M.G.; Sahebally, S.M.; Jarrar, A.; Burke, J.P.; Kiely, P.A.; Shen, B.; Waldron, D.; Peirce, C.; Moloney, M.; et al. Inclusion of the Mesentery in Ileocolic Resection for Crohn’s Disease is Associated With Reduced Surgical Recurrence. J. Crohns Colitis 2018, 12, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, G.; Rodriguez-Justo, M.; Higginson, A.; Bassett, P.; Windsor, A.; Cohen, R.; Halligan, S.; Taylor, S.A. Inflammation and fibrosis in Crohn’s disease: Location-matched histological correlation of small bowel ultrasound features. Abdom. Radiol. 2021, 46, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Stenke, E.; Bourke, B.; Knaus, U. Crohn’s Strictures—Moving Away from the Knife. Front. Pediatr. 2017, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e346. [Google Scholar] [CrossRef]
- Freeman, H.J. Natural history and long-term clinical course of Crohn’s disease. World J. Gastroenterol. 2014, 20, 31–36. [Google Scholar] [CrossRef]
- Mak, J.W.Y.; Ng, S.C. Epidemiology of fibrostenosing inflammatory bowel disease. J. Dig. Dis. 2020, 21, 332–335. [Google Scholar] [CrossRef]
- Kugathasan, S.; Denson, L.A.; Walters, T.D.; Kim, M.O.; Marigorta, U.M.; Schirmer, M.; Mondal, K.; Liu, C.; Griffiths, A.; Noe, J.D.; et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: A multicentre inception cohort study. Lancet 2017, 389, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Bettenworth, D.; Rieder, F. Medical therapy of stricturing Crohn’s disease: What the gut can learn from other organs—A systematic review. Fibrogenesis Tissue Repair 2014, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.; Ribeiro, H.; Maconi, G. Bowel Thickening in Crohn’s Disease: Fibrosis or Inflammation? Diagnostic Ultrasound Imaging Tools. Inflamm. Bowel Dis. 2017, 23, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jiang, W.; Wang, L.; Mao, X.; Ye, Z.; Zhang, H. Intestinal Ultrasound for Differentiating Fibrotic or Inflammatory Stenosis in Crohn’s Disease: A Systematic Review and Meta-analysis. J. Crohns Colitis 2022, 16, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.R.; Stidham, R.W.; Higgins, P.D.; Moons, D.S.; Johnson, L.A.; Keshavarzi, N.R.; Rubin, J.M. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2014, 33, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Muller, H.P.; Grittner, U.; Metzke, D.; Fischer, A.; Guckelberger, O.; Pascher, A.; Sack, I.; Vieth, M.; Rudolph, B. US-based Real-time Elastography for the Detection of Fibrotic Gut Tissue in Patients with Stricturing Crohn Disease. Radiology 2015, 275, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.S.; Fang, Y.; Wan, J.; Zhao, C.K.; Xiang, L.H.; Liu, H.; Pu, H.; Xu, G.; Zhang, K.; Xu, X.R.; et al. Usefulness of Strain Elastography, ARFI Imaging, and Point Shear Wave Elastography for the Assessment of Crohn Disease Strictures. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2019, 38, 2861–2870. [Google Scholar] [CrossRef]
- Kratzer, W.; von Tirpitz, C.; Mason, R.; Reinshagen, M.; Adler, G.; Moller, P.; Rieber, A.; Kachele, V. Contrast-enhanced power Doppler sonography of the intestinal wall in the differentiation of hypervascularized and hypovascularized intestinal obstructions in patients with Crohn’s disease. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2002, 21, 149–157, quiz 158–149. [Google Scholar] [CrossRef]
- Lenze, F.; Wessling, J.; Bremer, J.; Ullerich, H.; Spieker, T.; Weckesser, M.; Gonschorrek, S.; Kannengiesser, K.; Rijcken, E.; Heidemann, J.; et al. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: Prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm. Bowel Dis. 2012, 18, 2252–2260. [Google Scholar] [CrossRef]
- Lu, C.; Gui, X.; Chen, W.; Fung, T.; Novak, K.; Wilson, S.R. Ultrasound Shear Wave Elastography and Contrast Enhancement: Effective Biomarkers in Crohn’s Disease Strictures. Inflamm. Bowel Dis. 2017, 23, 421–430. [Google Scholar] [CrossRef]
- Maconi, G.; Carsana, L.; Fociani, P.; Sampietro, G.M.; Ardizzone, S.; Cristaldi, M.; Parente, F.; Vago, G.L.; Taschieri, A.M.; Bianchi Porro, G. Small bowel stenosis in Crohn’s disease: Clinical, biochemical and ultrasonographic evaluation of histological features. Aliment. Pharmacol. Ther. 2003, 18, 749–756. [Google Scholar] [CrossRef]
- Nylund, K.; Jirik, R.; Mezl, M.; Leh, S.; Hausken, T.; Pfeffer, F.; Odegaard, S.; Taxt, T.; Gilja, O.H. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with Crohn’s disease. Ultrasound Med. Biol. 2013, 39, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Orlando, S.; Fraquelli, M.; Coletta, M.; Branchi, F.; Magarotto, A.; Conti, C.B.; Mazza, S.; Conte, D.; Basilisco, G.; Caprioli, F. Ultrasound Elasticity Imaging Predicts Therapeutic Outcomes of Patients With Crohn’s Disease Treated With Anti-Tumour Necrosis Factor Antibodies. J. Crohns Colitis 2018, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Gennari, A.G.; Cova, M.A.; van Beek, E.J.R. Differentiation of Inflammatory From Fibrotic Ileal Strictures among Patients with Crohn’s Disease Based on Visual Analysis: Feasibility Study Combining Conventional B-Mode Ultrasound, Contrast-Enhanced Ultrasound and Strain Elastography. Ultrasound Med. Biol. 2018, 44, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; De Paoli, L.; Stocca, T.; Cabibbo, B.; Casagrande, F.; Cova, M.A. The value of small bowel wall contrast enhancement after sulfur hexafluoride-filled microbubble injection to differentiate inflammatory from fibrotic strictures in patients with Crohn’s disease. Ultrasound Med. Biol. 2012, 38, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Orlova, L.; Samsonova, T.; Khalif, I. P1018 Strain elastography and differential diagnosis of inflammatory and fibrotic strictures in Crohn’s disease. United Eur. Gastroenterol. J. 2017, 5, A518. [Google Scholar]
- Schirin-Sokhan, R.; Winograd, R.; Tischendorf, S.; Wasmuth, H.E.; Streetz, K.; Tacke, F.; Trautwein, C.; Tischendorf, J.J. Assessment of inflammatory and fibrotic stenoses in patients with Crohn’s disease using contrast-enhanced ultrasound and computerized algorithm: A pilot study. Digestion 2011, 83, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Rizzello, F.; Pratico, C.; Felicani, C.; Fiorini, E.; Brugnera, R.; Mazzotta, E.; Giunchi, F.; Fiorentino, M.; D’Errico, A.; et al. Real-time elastography for the detection of fibrotic and inflammatory tissue in patients with stricturing Crohn’s disease. J. Ultrasound 2017, 20, 273–284. [Google Scholar] [CrossRef]
- Stidham, R.; Dillman, J.; Rubin, J.; Higgins, P. P-111 Using Stiffness Imaging of the Intestine to Predict Response to Medical Therapy in Obstructive Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, S44–S45. [Google Scholar] [CrossRef]
- Wilkens, R.; Hagemann-Madsen, R.H.; Peters, D.A.; Nielsen, A.H.; Norager, C.B.; Glerup, H.; Krogh, K. Validity of Contrast-enhanced Ultrasonography and Dynamic Contrast-enhanced MR Enterography in the Assessment of Transmural Activity and Fibrosis in Crohn’s Disease. J. Crohns Colitis 2018, 12, 48–56. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, D.; Wilkens, R.; Krogh, K.; Glerup, H.; Gregersen, H. Bowel stiffness associated with histopathologic scoring of stenosis in patients with Crohn’s disease. Acta Biomater. 2021, 130, 332–342. [Google Scholar] [CrossRef]
- Nylund, K.; Leh, S.; Immervoll, H.; Matre, K.; Skarstein, A.; Hausken, T.; Gilja, O.H.; Birger Nesje, L.; Odegaard, S. Crohn’s disease: Comparison of in vitro ultrasonographic images and histology. Scand. J. Gastroenterol. 2008, 43, 719–726. [Google Scholar] [CrossRef]
- Sidhu, S.D.; Joseph, S.; Dunn, E.; Cuffari, C. The Utility of Contrast Enhanced Ultrasound and Elastography in the Early Detection of Fibro-Stenotic Ileal Strictures in Children with Crohn’s Disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2023, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Knieling, F.; Cesnjevar, R.; Regensburger, A.P.; Wagner, A.L.; Purbojo, A.; Dittrich, S.; Münch, F.; Neubert, A.; Woelfle, J.; Jüngert, J.; et al. Transfontanellar Contrast-enhanced US for Intraoperative Imaging of Cerebral Perfusion during Neonatal Arterial Switch Operation. Radiology 2022, 304, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Rüffer, A.; Knieling, F.; Cesnjevar, R.; Regensburger, A.; Purbojo, A.; Dittrich, S.; Münch, F.; Wölfle, J.; Jüngert, J. Equal cerebral perfusion during extended aortic coarctation repair. Eur. J. Cardiothorac. Surg. 2022, 61, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Knieling, F.; Rüffer, A.; Cesnjevar, R.; Regensburger, A.P.; Purbojo, A.; Dittrich, S.; Münch, F.; Neubert, A.; Meyer, S.; Strobel, D.; et al. Transfontanellar Contrast-Enhanced Ultrasound for Monitoring Brain Perfusion During Neonatal Heart Surgery. Circ. Cardiovasc. Imaging 2020, 13, e010073. [Google Scholar] [CrossRef] [PubMed]
- Knieling, F.; Schmarz, S.; Denis, L.; Nedoschill, E.; Buehler, A.; Danko, V.; Mandelbaum, H.; Nuñez, F.B.; Dürr, N.; Schlunz-Hendann, M. Ultrasound super-resolution imaging of neonatal cerebral vascular reorganization during neurovascular interventions. Tech. Rep. 2023. preprint. [Google Scholar]
- Civitelli, F.; Di Nardo, G.; Oliva, S.; Nuti, F.; Ferrari, F.; Dilillo, A.; Viola, F.; Pallotta, N.; Cucchiara, S.; Aloi, M. Ultrasonography of the colon in pediatric ulcerative colitis: A prospective, blind, comparative study with colonoscopy. J. Pediatr. 2014, 165, 78–84.e72. [Google Scholar] [CrossRef] [PubMed]
- Van Wassenaer, E.A.; van Rijn, R.R.; Zwetsloot, S.L.M.; de Voogd, F.A.E.; van Schuppen, J.; Kindermann, A.; de Meij, T.G.J.; van Limbergen, J.E.; Gecse, K.B.; D’Haens, G.R.; et al. Intestinal Ultrasound to Assess Ulcerative Colitis Disease Activity in Children: External Validation and Comparison of 2 Intestinal Ultrasound Activity Indices. Inflamm. Bowel Dis. 2023, 29, 1217–1222. [Google Scholar] [CrossRef]
- Van Wassenaer, E.A.; van Rijn, R.R.; de Voogd, F.A.E.; van Schuppen, J.; Kindermann, A.; de Meij, T.G.J.; van Limbergen, J.E.; Gecse, K.B.; D’Haens, G.R.; Benninga, M.A.; et al. Assessing Disease Activity in Pediatric Crohn’s Disease Using Ultrasound: The Pediatric Crohn Disease Intestinal Ultrasound Score. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 582–589. [Google Scholar] [CrossRef]
- Kellar, A.; Wilson, S.; Kaplan, G.; DeBruyn, J.; Tanyingoh, D.; Novak, K.L. The Simple Pediatric Activity Ultrasound Score (SPAUSS) for the Accurate Detection of Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 69, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Fodor, I.; Serban, O.; Serban, D.E.; Farcau, D.; Fufezan, O.; Asavoaie, C.; Man, S.C.; Dumitrascu, D.L. The value of abdominal ultrasonography compared to colonoscopy and faecal calprotectin in following up paediatric patients with ulcerative colitis. Med. Ultrason. 2021, 23, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Curie, P.; Curie, J. Développement par compression de l’électricité polaire dans les cristaux hémièdres à faces inclinées. Bull. Minéralogie 1880, 3, 90–93. [Google Scholar] [CrossRef]
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, s3–s20, 305–324. [Google Scholar] [CrossRef]
- Chen, Q.X.; Dewhurst, R.J.; Payne, P.A.; Davies, A. Photo-acoustic probe for intra-arterial imaging and therapy. Electron. Lett. 1993, 29, 1632–1633. [Google Scholar] [CrossRef]
- Oraevsky, A.J.; Jacques, A.J.; Esenaliev, R.; Tittel, F. Laser-based optoacoustic imaging in biological tissues. In Proceedings of the SPIE, OE/LASE ‘94, 1994, Los Angeles, CA, USA, 23 January 1994. [Google Scholar]
- Razansky, D.; Distel, M.; Vinegoni, C.; Ma, R.; Perrimon, N.; Köster, R.W.; Ntziachristos, V. Multispectral opto-acoustic tomography of deep-seated fluorescent proteins in vivo. Nat. Photon. 2009, 3, 412–417. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Tzoumas, S.; Deliolanis, N.; Morscher, S.; Ntziachristos, V. Unmixing Molecular Agents From Absorbing Tissue in Multispectral Optoacoustic Tomography. IEEE Trans. Med. Imaging 2014, 33, 48–60. [Google Scholar] [CrossRef]
- Cao, Q.; Zhegalova, N.G.; Wang, S.T.; Akers, W.J.; Berezin, M.Y. Multispectral imaging in the extended near-infrared window based on endogenous chromophores. J. Biomed. Opt. 2013, 18, 101318. [Google Scholar] [CrossRef]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Brown, E.; Kronke, G.; Waldner, M.J.; Knieling, F. Optoacoustic Imaging in Inflammation. Biomedicines 2021, 9, 483. [Google Scholar] [CrossRef]
- Karlas, A.; Fasoula, N.A.; Paul-Yuan, K.; Reber, J.; Kallmayer, M.; Bozhko, D.; Seeger, M.; Eckstein, H.H.; Wildgruber, M.; Ntziachristos, V. Cardiovascular optoacoustics: From mice to men—A review. Photoacoustics 2019, 14, 19–30. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, Y.; He, Y.; Shu, C.; Chen, D.; Zhang, J.; Chen, J.; Liu, C.; Sheng, Z.; Liu, H.; et al. In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging. Theranostics 2020, 10, 4694–4704. [Google Scholar] [CrossRef] [PubMed]
- Günther, J.S.; Knieling, F.; Träger, A.P.; Lang, W.; Meyer, A.; Regensburger, A.P.; Wagner, A.L.; Trollmann, R.; Woelfle, J.; Klett, D.; et al. Targeting Muscular Hemoglobin Content for Classification of Peripheral Arterial Disease by Noninvasive Multispectral Optoacoustic Tomography. JACC Cardiovasc. Imaging 2023, 16, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Knieling, F.; Gonzales Menezes, J.; Claussen, J.; Schwarz, M.; Neufert, C.; Fahlbusch, F.B.; Rath, T.; Thoma, O.M.; Kramer, V.; Menchicchi, B.; et al. Raster-Scanning Optoacoustic Mesoscopy for Gastrointestinal Imaging at High Resolution. Gastroenterology 2018, 154, 807–809.e803. [Google Scholar] [CrossRef] [PubMed]
- Bhutiani, N.; Grizzle, W.E.; Galandiuk, S.; Otali, D.; Dryden, G.W.; Egilmez, N.K.; McNally, L.R. Noninvasive Imaging of Colitis Using Multispectral Optoacoustic Tomography. J. Nucl. Med. 2017, 58, 1009–1012. [Google Scholar] [CrossRef]
- Buehler, A.; Brown, E.; Paulus, L.P.; Eckstein, M.; Thoma, O.M.; Oraiopoulou, M.E.; Rother, U.; Hoerning, A.; Hartmann, A.; Neurath, M.F.; et al. Transrectal Absorber Guide Raster-Scanning Optoacoustic Mesoscopy for Label-Free In Vivo Assessment of Colitis. Adv. Sci. 2023, 10, e2300564. [Google Scholar] [CrossRef]
- Lei, H.; Johnson, L.A.; Eaton, K.A.; Liu, S.; Ni, J.; Wang, X.; Higgins, P.D.R.; Xu, G. Characterizing intestinal strictures of Crohn’s disease. Biomed. Opt. Express 2019, 10, 2542–2555. [Google Scholar] [CrossRef]
- Lei, H.; Johnson, L.A.; Liu, S.; Moons, D.S.; Ma, T.; Zhou, Q.; Rice, M.D.; Ni, J.; Wang, X.; Higgins, P.D.; et al. Characterizing intestinal inflammation and fibrosis in Crohn’s disease by photoacoustic imaging: Feasibility study. Biomed. Opt. Express 2016, 7, 2837–2848. [Google Scholar] [CrossRef]
- Waldner, M.J.; Knieling, F.; Egger, C.; Morscher, S.; Claussen, J.; Vetter, M.; Kielisch, C.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography in Crohn’s Disease: Noninvasive Imaging of Disease Activity. Gastroenterology 2016, 151, 238–240. [Google Scholar] [CrossRef]
- Knieling, F.; Neufert, C.; Hartmann, A.; Claussen, J.; Urich, A.; Egger, C.; Vetter, M.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography for Assessment of Crohn’s Disease Activity. N. Engl. J. Med. 2017, 376, 1292–1294. [Google Scholar] [CrossRef] [PubMed]
- Paulus, L.P.; Wagner, A.L.; Buehler, A.; Raming, R.; Jungert, J.; Simon, D.; Tascilar, K.; Schnell, A.; Gunther, J.; Rother, U.; et al. Multispectral optoacoustic tomography of the human intestine—Temporal precision and the influence of postprandial gastrointestinal blood flow. Photoacoustics 2023, 30, 100457. [Google Scholar] [CrossRef] [PubMed]
- Paulus, L.P.; Buehler, A.; Wagner, A.L.; Raming, R.; Jüngert, J.; Simon, D.; Tascilar, K.; Schnell, A.; Rother, U.; Eckstein, M.; et al. Contrast-Enhanced Multispectral Optoacoustic Tomography for Functional Assessment of the Gastrointestinal Tract. Adv. Sci. 2023, 10, e2302562. [Google Scholar] [CrossRef]
- Bhutiani, N.; Samykutty, A.; McMasters, K.M.; Egilmez, N.K.; McNally, L.R. In vivo tracking of orally-administered particles within the gastrointestinal tract of murine models using multispectral optoacoustic tomography. Photoacoustics 2019, 13, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Morscher, S.; Driessen, W.H.; Claussen, J.; Burton, N.C. Semi-quantitative Multispectral Optoacoustic Tomography (MSOT) for volumetric PK imaging of gastric emptying. Photoacoustics 2014, 2, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Pogacnik, J.S.; Salgado, G. Perianal Crohn’s Disease. Clin. Colon. Rectal Surg. 2019, 32, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.; Rimola, J. Perianal fistulizing Crohn’s disease: Pathogenesis, diagnosis and therapy. Nat. Reviews. Gastroenterol. Hepatol. 2017, 14, 652–664. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Fonteyne, L.M.; Jungert, J.; Wagner, A.L.; Gerhalter, T.; Nagel, A.M.; Heiss, R.; Flenkenthaler, F.; Qurashi, M.; Neurath, M.F.; et al. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 1905–1915. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Wagner, A.L.; Danko, V.; Jungert, J.; Federle, A.; Klett, D.; Schuessler, S.; Buehler, A.; Neurath, M.F.; Roos, A.; et al. Multispectral optoacoustic tomography for non-invasive disease phenotyping in pediatric spinal muscular atrophy patients. Photoacoustics 2022, 25, 100315. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, A.P.; Wagner, A.L.; Claussen, J.; Waldner, M.J.; Knieling, F. Shedding light on pediatric diseases: Multispectral optoacoustic tomography at the doorway to clinical applications. Mol. Cell Pediatr. 2020, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, A.P.; Eckstein, M.; Wetzl, M.; Raming, R.; Paulus, L.-P.; Buehler, A.; Nedoschill, E.; Danko, V.; Jüngert, J.; Wagner, A.L.; et al. Multispectral optoacoustic tomography enables assessment of disease activity in paediatric inflammatory bowel disease. Photoacoustics 2024, 35, 100578. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.J.; Bano, S.; Januszewicz, W.; Stoyanov, D.; Fitzgerald, R.C.; di Pietro, M.; Bohndiek, S.E. First-in-human pilot study of snapshot multispectral endoscopy for early detection of Barrett’s-related neoplasia. J. Biomed. Opt. 2021, 26, 106002. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Favazza, C.; Chen, R.; Yao, J.; Cai, X.; Maslov, K.; Zhou, Q.; Shung, K.K.; Wang, L.V. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat. Med. 2012, 18, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Stylogiannis, A.; Afshari, P.; Wiedemann, T.; Steiger, K.; Buehler, A.; Zakian, C.; Ntziachristos, V. Capsule optoacoustic endoscopy for esophageal imaging. J. Biophotonics 2019, 12, e201800439. [Google Scholar] [CrossRef] [PubMed]
- Goodsall, T.M.; Jairath, V.; Feagan, B.G.; Parker, C.E.; Nguyen, T.M.; Guizzetti, L.; Asthana, A.K.; Begun, J.; Christensen, B.; Friedman, A.B.; et al. Standardisation of intestinal ultrasound scoring in clinical trials for luminal Crohn’s disease. Aliment. Pharmacol. Ther. 2021, 53, 873–886. [Google Scholar] [CrossRef]
- Maaser, C.; Kucharzik, T.; Gecse, K. Is Intestinal Ultrasound Ready to be Used as Standard Monitoring Tool in Daily Practice and as Endpoint in Clinical Trials? J. Crohn’s Colitis 2021, 15, 1–2. [Google Scholar] [CrossRef]
- Kucharzik, T.; Wilkens, R.; D’Agostino, M.A.; Maconi, G.; Le Bars, M.; Lahaye, M.; Bravatà, I.; Nazar, M.; Ni, L.; Ercole, E.; et al. Early Ultrasound Response and Progressive Transmural Remission After Treatment With Ustekinumab in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 153–163.e112. [Google Scholar] [CrossRef]
| US Aspect | Anatomic Structure | Microscopic Aspect/Tissue Composition |
|---|---|---|
| Hypoechoic (fluid) or hyperechoic (air) lumen | Intestinal content (stool, air, fluids) | |
| Hyperechoic entrance | Transition lumen/mucosa | |
| Hypoechoic | Mucosa | Epithelial cells |
| Hyperechoic | Submucosa | Connective tissue |
| Hypoechoic | Muscularis propria | Muscle cells |
| Hyperechoic | Transition muscularis propria/serosa, surrounding structures (fat, peritoneal wall) | Epithelial cells, connective tissue, fat |
| Age (Years) | Jejunum (mm) | Ileum (mm) | Cecum (mm) | Asc. Colon (mm) | Tr. Colon (mm) | Desc. Colon (mm) | Jejunum (mm) |
|---|---|---|---|---|---|---|---|
| 0–4 | 1.0 ± 0.4 | 1.3 ± 0.6 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.4 |
| 5–9 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.8 ± 0.1 |
| 10–14 | 0.8 ± 0.1 | 1.0 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.2 | 0.8 ± 0.1 |
| 15–19 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.6 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.2 | 0.9 ± 0.1 |
| Grade | B-Mode | Doppler |
|---|---|---|
| Limberg I | Intestinal wall thickening (hypoechoic, sometimes hyperechoic submucosa, partial loss layers) | No intramural vessels |
| Limberg II | Intestinal wall thickening | Short-stretched vessels detectable |
| Limberg III | Intestinal wall thickening (homogenous, hypoechoic) | long-stretched vessels detectable |
| Limberg IV | Intestinal wall thickening | Long-stretched vessels detectable reaching the adjacent mesentery |
| Reference | Name of Scoring System | Disease | N | Items | Measures of Accuracy |
|---|---|---|---|---|---|
| [98] | Civitelli | UC | 60 | Bowel wall thickness > 3 mm, bowel wall stratification, vascularity, presence of haustra coli, and enlarged mesenteric lymph nodes. | 90% concordance with endoscopy (95% CI: 0.82–0.96) |
| [99] | UC-IUS | UC | 35 | Bowel wall thickness, Doppler signals, colonic haustrations, wall layer stratification, presence of mesenteric fat wrapping | AUROC for detecting Mayo endoscopic score ≥ 2 Asc. Colon: 0.82 Trans. Colon: 0.88 Desc. Colon: 0.84 |
| [100] | PCD-US | CD | 74 | Bowel wall thickness, bowel wall perfusion, mesenteric fat proliferation, visibility of colonic haustrations, visibility of wall layer stratification, peristalsis, presence and size of lymph nodes, presence of complications | AUROC for detecting inflammation Terminal ileum: 0.73 Colon: 0.75 |
| [101] | SPAUSS | IBD | 75 | Bowel wall thickness (BWT), mesenteric inflammatory fat, lymphadenopathy, and hyperemia | AUCROC distinguish active disease from normal condition (absence of disease): 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoerning, A.; Jüngert, J.; Siebenlist, G.; Knieling, F.; Regensburger, A.P. Ultrasound in Pediatric Inflammatory Bowel Disease—A Review of the State of the Art and Future Perspectives. Children 2024, 11, 156. https://doi.org/10.3390/children11020156
Hoerning A, Jüngert J, Siebenlist G, Knieling F, Regensburger AP. Ultrasound in Pediatric Inflammatory Bowel Disease—A Review of the State of the Art and Future Perspectives. Children. 2024; 11(2):156. https://doi.org/10.3390/children11020156
Chicago/Turabian StyleHoerning, André, Jörg Jüngert, Gregor Siebenlist, Ferdinand Knieling, and Adrian P. Regensburger. 2024. "Ultrasound in Pediatric Inflammatory Bowel Disease—A Review of the State of the Art and Future Perspectives" Children 11, no. 2: 156. https://doi.org/10.3390/children11020156
APA StyleHoerning, A., Jüngert, J., Siebenlist, G., Knieling, F., & Regensburger, A. P. (2024). Ultrasound in Pediatric Inflammatory Bowel Disease—A Review of the State of the Art and Future Perspectives. Children, 11(2), 156. https://doi.org/10.3390/children11020156






