Cerebrospinal Fluid Protein Concentrations in Hydrocephalus
Abstract
1. Introduction
2. Methods
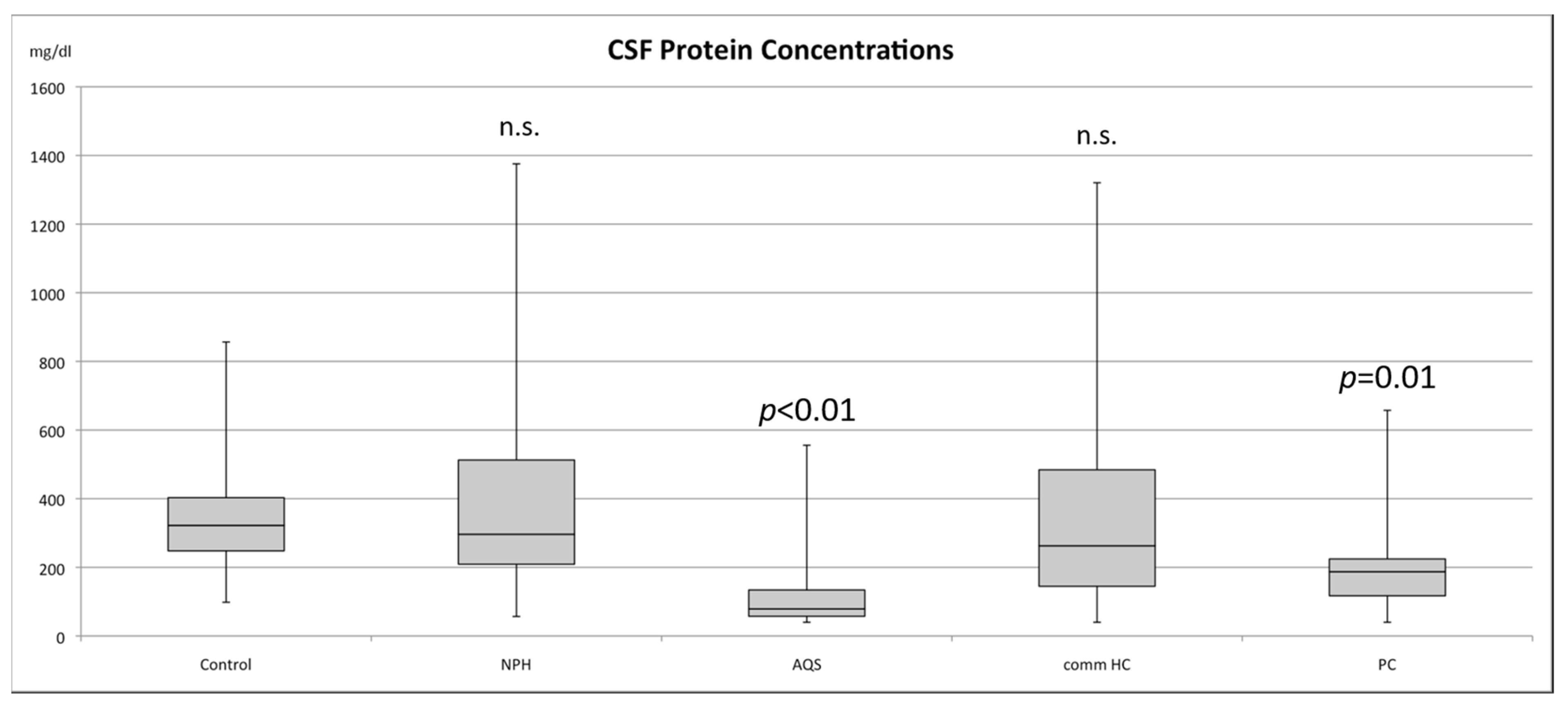
3. Results
4. Discussion
4.1. CSF Ciruculation in Underlying Hydrocephalic Pathologies
4.2. Interpretation of Altered Protein Concentrations in AQS and PC Patients
4.3. Interpretation of Altered Protein Concentrations in NPH and Communicating HC Patients
4.4. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef]
- Johanson, C.E.; Duncan, J.A.; Klinge, P.M.; Brinker, T.; Stopa, E.G.; Silverberg, G.D. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cereb. Fluid Res. 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Guldbrandsen, A.; Vethe, H.; Farag, Y.; Oveland, E.; Garberg, H.; Berle, M.; Myhr, K.-M.; Opsahl, J.A.; Barsnes, H.; Berven, F.S. In-depth characterization of the cerebrospinal fluid (CSF) proteome displayed through the CSF proteome resource (CSF-PR). Mol. Cell. Proteom. 2014, 13, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Angel, T.E.; Jacobs, J.M.; Spudich, S.S.; Gritsenko, M.A.; Fuchs, D.; Liegler, T.; Zetterberg, H.; Camp, D.G.; Price, R.W.; Smith, R.D. The cerebrospinal fluid proteome in HIV infection: Change associated with disease severity. Clin. Proteom. 2012, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Schutzer, S.E.; Liu, T.; Natelson, B.H.; Angel, T.E.; Schepmoes, A.A.; Purvine, S.O.; Hixson, K.K.; Lipton, M.S.; Camp, D.G.; Coyle, P.K.; et al. Establishing the proteome of normal human cerebrospinal fluid. PLoS ONE 2010, 5, e10980. [Google Scholar] [CrossRef] [PubMed]
- Schob, S.; Schicht, M.; Sel, S.; Stiller, D.; Kekulé, A.S.; Paulsen, F.; Maronde, E.; Bräuer, L. The detection of surfactant proteins A, B, C and D in the human brain and their regulation in cerebral infarction, autoimmune conditions and infections of the CNS. PLoS ONE 2013, 8, e74412. [Google Scholar] [CrossRef]
- Reiber, H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor. Neurol. Neurosci. 2003, 21, 79–96. [Google Scholar]
- Dandy, W.E. Experimental Hydrocephalus. Ann. Surg. 1919, 70, 129–142. [Google Scholar] [CrossRef]
- Kimelberg, H.K. Water homeostasis in the brain: Basic concepts. Neuroscience 2004, 129, 851–860. [Google Scholar] [CrossRef]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef]
- Weller, R.O.; Djuanda, E.; Yow, H.-Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, A.; Papaiconomou, C.; Koh, L.; Djenic, J.; Bozanovic-Sosic, R.; Johnston, M. Integrating the roles of extracranial lymphatics and intracranial veins in cerebrospinal fluid absorption in sheep. Microvasc. Res. 2004, 67, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef]
- Abbott, N.J. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochem. Int. 2004, 45, 545–552. [Google Scholar] [CrossRef]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef] [PubMed]
- Klarica, M.; Miše, B.; Vladić, A.; Radoš, M.; Orešković, D. “Compensated hyperosmolarity” of cerebrospinal fluid and the development of hydrocephalus. Neuroscience 2013, 248, 278–289. [Google Scholar] [CrossRef]
- Bothwell, S.W.; Janigro, D.; Patabendige, A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 2019, 16, 9. [Google Scholar] [CrossRef]
- Cinalli, G.; Spennato, P.; Nastro, A.; Aliberti, F.; Trischitta, V.; Ruggiero, C.; Mirone, G.; Cianciulli, E. Hydrocephalus in aqueductal stenosis. Child’s. Nerv. Syst. 2011, 27, 1621–1642. [Google Scholar] [CrossRef]
- Burkett, J.G.; Ailani, J. An Up to Date Review of Pseudotumor Cerebri Syndrome. Curr. Neurol. Neurosci. Rep. 2018, 18, 33. [Google Scholar] [CrossRef]
- Said, H.M.; Kaya, D.; Yavuz, I.; Dost, F.S.; Altun, Z.S.; Isik, A.T. A Comparison of Cerebrospinal Fluid Beta-Amyloid and Tau in Idiopathic Normal Pressure Hydrocephalus and Neurodegenerative Dementias. Clin. Interv. Aging 2022, 17, 467–477. [Google Scholar] [CrossRef]
- Rekate, H.L. A contemporary definition and classification of hydrocephalus. Semin. Pediatr. Neurol. 2009, 16, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Li, J. New concepts in the pathogenesis of hydrocephalus. Transl. Pediatr. 2014, 3, 185–194. [Google Scholar] [CrossRef]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology E-Book, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9781455733286. [Google Scholar]
- Akiyama, Y.; Yokoyama, R.; Takashima, H.; Kawata, Y.; Arihara, M.; Chiba, R.; Kimura, Y.; Mikami, T.; Mikuni, N. Accumulation of Macromolecules in Idiopathic Normal Pressure Hydrocephalus. Neurol. Med. Chir. 2021, 61, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Agren-Wilsson, A.; Roslin, M.; Eklund, A.; Koskinen, L.-O.D.; Bergenheim, A.T.; Malm, J. Intracerebral microdialysis and CSF hydrodynamics in idiopathic adult hydrocephalus syndrome. J. Neurol. Neurosurg. Psychiatry 2003, 74, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Lalou, A.D.; Czosnyka, M.; Placek, M.M.; Smielewski, P.; Nabbanja, E.; Czosnyka, Z. CSF Dynamics for Shunt Prognostication and Revision in Normal Pressure Hydrocephalus. J. Clin. Med. 2021, 10, 1711. [Google Scholar] [CrossRef]
- Preuss, M.; Hoffmann, K.-T.; Reiss-Zimmermann, M.; Hirsch, W.; Merkenschlager, A.; Meixensberger, J.; Dengl, M. Updated physiology and pathophysiology of CSF circulation—The pulsatile vector theory. Child’s Nerv. Syst. 2013, 29, 1811–1825. [Google Scholar] [CrossRef]
| Control | AQS | Communicating HC | NPH | Pseudotumor Cerebri | |
|---|---|---|---|---|---|
| n | 95 | 27 | 25 | 24 | 7 |
| Age (yrs) | 42.7 | 17.3 | 28.3 | 69.7 | 25.7 |
| Sex (m/f) | 50/45 | 18/9 | 13/12 | 19/8 | 1/5 |
| Lac | 1.63 (1.62–1.64) | 1.50 (1.49–1.51) | 1.95 (1.77–2.11) | 1.85 (1.77–1.94) | 1.53 (1.32–1.74) |
| Glu | 3.8 (3.62–3.98) | 3.48 (3.06–3.8) | 3.22 (3.01–3.43) | 4.10 (3.97–4.33) | 3.53 (3.08–3.97) |
| Tot. Protein | 0.34 (0.33–0.35) | 0.13 (0.10–0.16) | 0.35 (0.27–0.43) | 0.37 (0.30–0.44) | 0.18 (0.12–0.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilhelmy, F.; Krause, M.; Schob, S.; Merkenschlager, A.; Wachowiak, R.; Härtig, W.; Meixensberger, J.; Gburek-Augustat, J.; Wende, T. Cerebrospinal Fluid Protein Concentrations in Hydrocephalus. Children 2023, 10, 644. https://doi.org/10.3390/children10040644
Wilhelmy F, Krause M, Schob S, Merkenschlager A, Wachowiak R, Härtig W, Meixensberger J, Gburek-Augustat J, Wende T. Cerebrospinal Fluid Protein Concentrations in Hydrocephalus. Children. 2023; 10(4):644. https://doi.org/10.3390/children10040644
Chicago/Turabian StyleWilhelmy, Florian, Matthias Krause, Stefan Schob, Andreas Merkenschlager, Robin Wachowiak, Wolfgang Härtig, Jürgen Meixensberger, Janina Gburek-Augustat, and Tim Wende. 2023. "Cerebrospinal Fluid Protein Concentrations in Hydrocephalus" Children 10, no. 4: 644. https://doi.org/10.3390/children10040644
APA StyleWilhelmy, F., Krause, M., Schob, S., Merkenschlager, A., Wachowiak, R., Härtig, W., Meixensberger, J., Gburek-Augustat, J., & Wende, T. (2023). Cerebrospinal Fluid Protein Concentrations in Hydrocephalus. Children, 10(4), 644. https://doi.org/10.3390/children10040644






