Abstract
Introduction: Paediatric pulmonary hypertension (PH) represents a heterogeneous illness that is responsible for high morbidity and mortality if left without treatment. Idiopathic pulmonary arterial hypertension (IPAH) is a subtype of PAH rarely seen in paediatrics. Limited long-term data are available. Methods: Over a period of 20 years, 10 paediatric patients were enrolled at two tertiary centres. Their clinical, echocardiographic, and right heart catheterisation (RHC) features and outcome were evaluated. Results: The mean age at first diagnosis was 5.7 ± 5.7 years. The age at the last follow-up was 12.4 ± 6.1 years. The average follow-up was 6.6 ± 0.8 years. There was a female prevalence of 60% (p < 0.05) in this case series. Regarding the NYHA functional class, 80% of IPAH subjects were in class III or IV. The mean saturation was 91 ± 5%. In this regard, 70% of the patients were on a combination of three drugs, with sildenafil (90%) included. On echocardiography, longitudinal right ventricular contractility (TAPSE) was slightly reduced (13.4 ± 2.6 mm), whilst RVSP was severely elevated (101 ± 19 mmHg). The RHC data showed that mPAP was 61.8 ± 23.1 mmHg (p = 0.0017 with RVSP on echocardiography), mRAP was 10.7 ± 3.8 mmHg, CI was 2.6 ± 1 L·min−1·m−2, PVRi was 16.8 ± 12.6 WU·m2, and SVO2 was 63.6 ± 14.8%. Regarding the outcome, two male IPAH patients (20%) died, and 50% underwent lung transplant or were on transplant assessment or already on the waiting list for lung transplantation. One patient underwent a ductus arteriosus stenting (reverse Potts shunt) and another underwent atrial septostomy and stenting. Conclusions: Notwithstanding the progress in medical therapy, IPAH continues to represent a serious challenge, particularly in the paediatric population, with the need for lung transplantation and significant mortality.
1. Introduction
Paediatric pulmonary hypertension (PH) represents a heterogeneous illness that is responsible for high morbidity and mortality if left without treatment [1]. The definition of PH in paediatrics is the same as that used in the adult setting. In fact it is represented by the harmful combination of increased mean pulmonary artery pressure (mPAP) at rest >20 mmHg, pulmonary arterial wedge pressure (PAWP) ≤15 mmHg, and pulmonary vascular resistance index (PVRi) >3 WU·m2 in patients with biventricular physiology being subjected to right heart catheterisation (RHC) [2]. Systemic blood pressure in paediatrics shows fluctuations related to age and height. Hence, also pulmonary-to-systemic pressure ratio >0.4 can suggest the presence of PAH in children [3].
Idiopathic pulmonary arterial hypertension (IPAH) is a subtype of PAH rarely seen in paediatrics. An exact underlying risk factor is unknown. It is classified in group I PH, together with PH triggered by congenital heart disease [4]. IPAH can manifest in newborns or infants in the form of persistent pulmonary hypertension of the newborn (PPHN) [5]. In these patients, lung histopathology changes are very similar to those seen in older patients suffering from IPAH [6].
However, little is known on outcomes of the disease in paediatrics. The aim of this case series is to evaluate the clinical, echocardiographic, and RHC characteristics and outcome in a small group of IPAH children and adolescents who were followed up at two tertiary centres.
2. Materials and Methods
Over a period of 20 years (2002–2022), IPAH was diagnosed in 10 children and adolescents < 18 years old according to the criteria of the 2018 World Symposium on PH, which have tried to conform the PH codification in paediatrics to that used in the adult setting [7].
The exclusion criteria were children with any other aetiologies of PH other than IPAH and people aged over 18 years.
Clinical, echocardiographic, and RHC characteristics and outcome of the enrolled subjects were analysed.
The study was conducted in accordance with the Declaration of Helsinki. Ethics Committee approval was waived since this is a retrospective audit with a small number of patients.
2.1. Echocardiography
The scan was carried out in the left lateral decubitus position with a Philips iE33 ultrasound machine (Koninklijke Philips N.V., Amsterdam, The Netherlands), using a 12–5 MHz probe, in accordance with international recommendations [8]. The right ventricular longitudinal systolic function was recorded in terms of tricuspid annular plane systolic excursion (TAPSE). The right ventricular systolic pressure (RVSP) was calculated from tricuspid valve insufficiency peak velocity (modified Bernoulli equation) plus the estimated right atrial pressure.
Acquisition of the above-stated parameters was from an apical view. Six to eight beats were gathered for evaluation keeping away from images of pre- and post-extrasystolic beats. At least three consecutive measurements were taken on collected images and the average was used for analysis.
2.2. Right Heart Catheterisation
All the patients underwent RHC to measure the right-sided cardiac pressures and vascular resistances as well as to calculate cardiac output invasively [9].
A local anaesthetic was provided subcutaneously at the access site in the femoral, antecubital, or jugular areas. Venous access was obtained through anatomical landmarks or with echographic guidance. Subsequently, an appropriate sheath was inserted into the vein and fixed securely. A pulmonary artery wire was placed through the sheath into the vein. The balloon at the tip of the wire was inflated for an easier advancement to the right cardiac chambers. The wire advancement was detected by fluoroscopy. When it reached the right atrium, the right atrial pressure (mRAP mmHg) was recorded. Afterward, the wire was advanced into the right ventricle, and intraventricular pressure was recorded. The wire was moved ahead to measure the pulmonary capillary wedge pressure. After that, the balloon was deflated and then brought back a few centimetres into the pulmonary artery to measure mean pulmonary artery pressure (mPAP mmHg). All cardiac pressures were collected at end-expiration averaging at least three cardiac cycles. Blood samples were withdrawn in superior vena cava, inferior vena cava, right atrium, right ventricle, and main pulmonary artery (mixed venous oxygen saturation—SVO2) to rule out intracardiac shunts and calculate cardiac output with the Fick principle. Thermodilution was repeated at least three times to obtain an average cardiac output and cardiac index (CI L·min−1 ·m−2, e.g., the cardiac output indexed for body surface area) [10,11].
Finally, the pulmonary vascular resistance index (PVRi WU∗m2) was calculated through the formula: [(Mean pulmonary artery pressure−Pulmonary capillary wedge pressure)/Cardiac index] [11].
3. Statistics
All data are presented as percentage or mean ± standard deviation. The Chi-squared and Mann–Whitney U tests were used to make a comparison between genders. Statistical significance was set at p < 0.05 throughout the study.
4. Results
IPAH patients with their features are summarised in Table S1 (Supplementary Material). Echocardiography and RHC are performed at the last follow-up.
The age range at first diagnosis was between 1 month and 17 years (mean 5.7 ± 5.7 years). Two children (20%) were diagnosed before 1 year of age. The age at the last follow-up was 12.4 ± 6.1 years. The average follow-up was 6.6 ± 0.8 years. There was a female prevalence of 60% in the case series. Regarding the NYHA functional class, most of the IPAH subjects were in class III (40%) or IV (40%). The mean saturation on room air was 91 ± 5%. As to therapy, 70% of the patients were on a combination of three drugs, with sildenafil (90%) included. On echocardiography, right ventricular contractility, expressed as TAPSE, was slightly reduced (13.4 ± 2.6 mm), whilst RVSP was severely elevated (101 ± 19 mmHg). The RHC data showed that mPAP was 61.8 ± 23.1 mmHg, mRAP was 10.7 ± 3.8 mmHg, CI was 2.6 ± 1 L·min−1·m−2, PVRi was 16.8 ± 12.6 WU·m2, and SVO2 63.6 ± 14.8%. The estimated RVSP by Doppler was significantly different compared to right ventricular pressure that was measured on RHC (p = 0.0017). Regarding the outcome, two IPAH patients (20%) died, and 50% underwent lung transplant or were on transplant assessment or already on the waiting list for lung transplantation. One patient underwent a ductus arteriosus stenting (reverse Potts shunt procedure) and another patient underwent atrial septostomy and then atrial stenting.
Regarding gender differences, two male patients died. As such, mortality was significantly higher in males (p < 0.05). No other statistically significant differences were found between genders.
4.1. Case 1
A young male refugee was diagnosed with IPAH in Italy at the age of 11. He was symptomatic for shortness of breath and desaturation on mild exertion (NYHA class III), while the saturation on room air was 90%. Echocardiography displayed severely elevated RVSP (Figure 1).
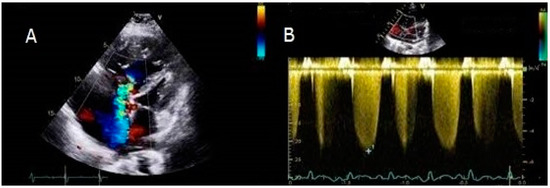
Figure 1.
Apical four chambers view showing a very dilated right ventricle and severe tricuspid valve regurgitation. The interventricular septum bulged toward the left ventricle (panel A). The RVSP was significantly increased (panel B).
Severely increased pressure on the right side of the heart was confirmed on RHC. An aggressive strategy with three drugs (sildenafil, bosentan, and iloprost) was chosen. Following a multidisciplinary meeting, the patient was put on the waiting list for lung transplantation, but died while waiting for a donor because of heart failure.
4.2. Case 2
A male patient was diagnosed with IPAH as an infant. He grew up with the disease and felt quite well with the help of double therapy (sildenafil and macitentan). A small patent of ductus arteriosus acted as a pop-off, thus providing some relief until he reached adolescence. Since then, his condition deteriorated quickly. Atrial septostomy followed by placement of a fenestrated device was decided as a palliative approach. Two years after the procedure the patient is in good clinical condition and still alive (Figure 2 and Figure 3).
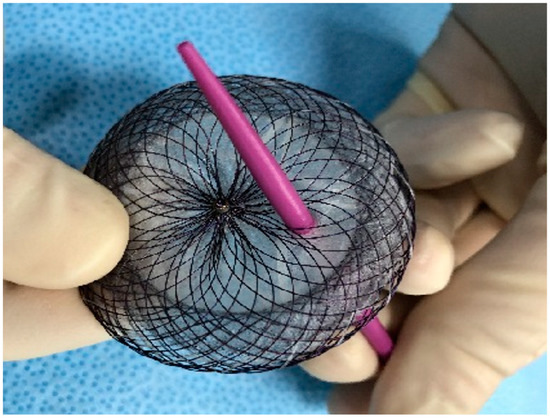
Figure 2.
Fenestrated atrial septal defect.
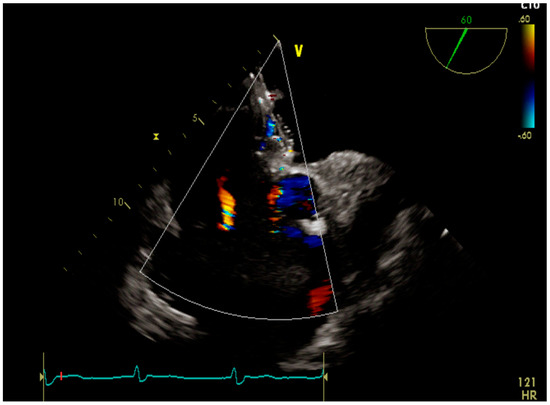
Figure 3.
Transesophageal echocardiography showing the fenestrated device.
4.3. Case 3
A female patient had IPAH since infancy. She was treated with sildenafil, bosentan, and epoprostenol over the years, with a good response. However, her clinical conditions worsened during adolescence. As such, the patient underwent a patent ductus arteriosus stenting (reverse Potts shunt) which was aimed at decompressing the pressure-overloaded right ventricle. It was at the expense of a certain degree of desaturation at the lower half of the body (saturation measured at forefinger was 93%, whereas at foot big toe was 82%). The patent is still alive 3 years following the procedure.
5. Discussion
PAH in paediatrics usually manifests in the form of IPAH, congenital heart disease–related PAH, and PPHN [12]. Notwithstanding the different PH aetiologies, they have in common analogous histopathologic pulmonary vasculature abnormalities. As such, the therapeutic approach is similar [13,14]. IPAH in children is rare (<10:1,000,000) and its incidence is 1–2:1,000,000/year [15]. Many worldwide registries exist including children with PAH of various aetiologies. However, even in these registries, the number of patients recorded with IPAH is limited to a few decades [16,17]. One of them is the American Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) registry. It is a 55-centre, observational study including subjects less than 18 years with group I PAH. More than half of them are suffering from IPAH. There is a slight female predominance (60%) which is consistent with the findings in the presented case series [18]. IPAH is a progressive and lethal disease, as testified by the fact that 80% of the sample size was in NYHA functional class III or IV, and deserves an aggressive treatment provided in specialised centres. The NYHA class is one of the factors predicting survival, with class IV mean survival being less than 6 months [9]. Symptoms in paediatric IPAH include failure to thrive and growth impairment, most of all in children < 5 years, tachycardia, tachypnoea owing to cardiac insufficiency, and cyanosis in the presence of an atrial shunt [19]. According to the current guidelines, PH diagnosis is based on echocardiographic probability followed by RHC [20]. The latter is the “gold standard” in diagnosing and evaluating the degree and prognosis of IPAH. It is considered an undangerous technique when it is carried out at centres with expertise in the field (complication rates and mortality in adulthood are 5.7% and 0.2%, respectively) [21]. Nevertheless, as RHC may be linked with the occurrence of severe adverse events (in 1–3% of cases, mostly in infants and those in worse clinical conditions), it should be executed in paediatric PH centres with a high level of expertise. Risks and benefits should be balanced case by case [22,23]. On echocardiography, average right ventricular contractility, expressed by means of TAPSE, was reduced [24]. TAPSE varies with age and not having used z-scores is a limitation. However, their use is not widely diffused yet [25]. Prognosis in IPAH is strongly linked to right ventricular contractility [26]. Sato and Coll demonstrated that right ventricular systolic function in those with PH is better estimated by TAPSE than by right ventricular fractional change area [27]. With echocardiography, RVSP proved to be severely increased. However, estimated RVSP by Doppler can be significantly different compared with RHC in approximately half of the cases [28]. This is consistent with our findings. The RHC data confirmed that mPAP was severely raised as a consequence of a very elevated PVR, which tends to be progressive in IPAH [29]. The average mRAP was elevated as well, reflecting right ventricular overload. It is an established risk factor for mortality [30]. Despite having high right ventricular afterload, CI was quite preserved. The European Society of Cardiology (ESC) and European Respiratory Society (ERS) guidelines include CI and SVO2 among the haemodynamic parameters that are useful for risk stratification in IPAH patients. Not only that, but a recent study showed that SVO2 is able to predict long-term mortality better than CI [31]. In the studied case series, the average SVO2 was low–normal. Multi-drug therapy is currently suggested for those affected by PAH. This is the reason why the majority of our patients were taking three drugs, which were provided in sequence (e.g., phosphodiesterase inhibitor first, then endothelin receptor antagonist, and finally prostanoid). However, one of the patients in the case series was administered an aggressive upfront combination of three drugs, with an excellent clinical outcome. This is consistent with that previously reported in the literature regarding severe non-reversible PAH [32]. In two patients, a pop-off shunt was created artificially for palliation/bridge to transplant treatment. In the first of the two, a very small ductus arteriosus was stented (reverse Potts shunt) to open a wide interconnection between the descending portion of the aorta and the pulmonary artery resulting in a right-to-left shunt. Potts shunt decompresses the right ventricular chamber and secures enough cardiac output, causing hypoxemia in the lower limbs [33]. In the second patient, an intracardiac right-to-left shunt was created by an atrial septostomy. The artificially created atrial septal defect was then stented to maintain it open. In severe PAH, this palliative procedure decompresses the right ventricle, preserves its function, improves haemodynamics, and increases systemic blood flow [34]. About half of those in the study were subjected to lung transplantation or are waiting for that. IPAH is progressive, and for non-responders to medicines, lung transplantation is the last option [35]. This is not without any risks, because of the extremely high mortality in these patients, owing to the complexity of the treatment and the frequent onset of post-surgical insufficiency of the left ventricle. The other problems are increased mortality, impaired kidney and liver functions, and at times thrombocytopathy related to continuous prostaglandin infusion [36]. Overall, notwithstanding the progress in medical therapy, IPAH continues to represent a serious challenge, particularly in the paediatric population, with the need for lung transplantation and significant mortality in a relatively short time. However, metabolomics, a recent technique, could be of hope, which reveals the complex cascade of metabolic reactions underpinning a number of pathologies including PH/PAH [37]. In the meantime, due to the rarity of the disease, multicentre studies trying to enroll as many patients as possible are needed to shed light on its still obscure characteristics [38].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/children10030518/s1, Table S1: Clinical, echocardiographic, right heart catheterisation features, and outcome of the idiopathic pulmonary hypertension patients who have been studied.
Author Contributions
Conceptualisation, methodology, and data curation, P.P.B.; writing—original draft preparation, P.P.B.; helping in directing and writing the manuscript, P.A.; analysis and record of the clinical data, E.D.; writing—review and editing, C.J.M., K.P.W., M.G.R. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was carried out in accordance with the Declaration of Helsinki. Ethics Committee approval was waived due to the fact that this is a retrospective audit with a small number of patients.
Informed Consent Statement
The patients’ parents gave their informed written consent in the study. Two patients died and it was not possible to find their parents.
Data Availability Statement
Not applicable.
Conflicts of Interest
Pier Paolo Bassareo reports fees from Actelion-Jansen, outside the submitted work. Michele D’Alto reports participation in a monitoring board for Actelion-Janssen, Merck Sharp and Dohme, Dompè, and Ferrer, all outside the submitted work. The other authors declare no conflict of interest.
References
- Hopper, R.K.; Abman, S.H.; Ivy, D.D. Persistent challenges in pediatric pulmonary hypertension. Chest 2016, 150, 226–236. [Google Scholar] [CrossRef]
- Calcaterra, G.; Bassareo, P.P.; Barilla, F.; Martino, F.; Fanos, V.; Fedele, F.; Romeo, F. Pulmonary hypertension in pediatrics. A feasible approach to bridge the gap between real world and guidelines. J. Matern. Fetal Neonatal Med. 2021, 34, 3820–3826. [Google Scholar] [CrossRef]
- Douwes, J.M.; van Loon, R.L.; Hoendermis, E.S.; Vonk-Noordegraaf, A.; Roofthooft, M.T.; Talsma, M.D.; Hillege, H.L.; Berger, R.M. Acute pulmonary vasodilator response in paediatric and adult pulmonary arterial hypertension: Occurrence and prognostic value when comparing three response criteria. Eur. Heart J. 2011, 32, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Calcaterra, G.; Fanos, V.; Bassareo, P.P. Still puzzling about a clear definition of pulmonary arterial hypertension in newborns. Eur. Respir. J. 2019, 53, 1900005. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, S.; Wagenvoort, C.A. Comparison of primary plexogenic arteriopathy in adults and children. A morphometric study in 40 patients. Br. Heart J. 1985, 54, 428–434. [Google Scholar] [CrossRef]
- Rosenzweig, E.B.; Abman, S.H.; Adatia, I.; Beghetti, M.; Bonnet, D.; Haworth, S.; Ivy, D.D.; Berger, R.M.F. Paediatric pulmonary arterial hypertension: Updates on definition, classification, diagnostics and management. Eur. Respir. J. 2019, 53, 1801916. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Chokkalingam Mani, B.; Chaudhari, S.S. Right Heart Cardiac Catheterization; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557404/ (accessed on 3 March 2023).
- Krishnan, A.; Markham, R.; Savage, M.; Wong, Y.W.; Walters, D. Right Heart Catheterisation: How to do it. Heart Lung Circ. 2019, 28, e71–e78. [Google Scholar] [CrossRef]
- Bangalore, S.; Bhatt, D.L. Images in cardiovascular medicine. Right heart catheterization, coronary angiography, and percutaneous coronary intervention. Circulation 2011, 124, e428–e433. [Google Scholar] [CrossRef]
- Saji, T. Update on pediatric pulmonary arterial hypertension. Differences and similarities to adult disease. Circ. J. 2013, 77, 2639–2650. [Google Scholar] [CrossRef]
- Adatia, I.; Kothari, S.S.; Feinstein, J.A. Pulmonary hypertension associated with congenital heart disease: Pulmonary vascular disease: The global perspective. Chest 2010, 137, 52S–61S. [Google Scholar] [CrossRef]
- Levine, D.J. Diagnosis and management of pulmonary arterial hypertension: Implications for respiratory care. Respir. Care 2006, 51, 368–381. [Google Scholar] [PubMed]
- Valencia, G.A.; Krishnan, U. Idiopathic Pulmonary Arterial Hypertension in Children: A Review. Pulm. Ther. 2017, 3, 67–92. [Google Scholar] [CrossRef]
- Berger, R.M.; Beghetti, M.; Humpl, T.; Raskob, G.E.; Ivy, D.D.; Jing, Z.C.; Bonnet, D.; Schulze-Neick, I.; Barst, R.J. Clinical features of paediatric pulmonary hypertension: A registry study. Lancet 2012, 379, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H.; Mullen, M.P.; Sleeper, L.A.; Austin, E.D.; Rosenzweig, E.B.; Kinsella, J.P.; Ivy, D.; Hopper, R.K.; Raj, J.U.; Fineman, J.; et al. Characterisation of paediatric pulmonary hypertensive vascular disease from the PPHNet Registry. Eur. Respir. J. 2021, 59, 2003337. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; McGoon, M.D.; Elliott, C.G.; Foreman, A.J.; Miller, D.P.; Ivy, D.D. Survival in childhood pulmonary arterial hypertension: Insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012, 125, 113–122. [Google Scholar] [CrossRef]
- Ploegstra, M.J.; Ivy, D.D.; Wheeler, J.G.; Brand, M.; Beghetti, M.; Rosenzweig, E.B.; Humpl, T.; Iriart, X.; Rouzic, E.M.; Bonnet, D.; et al. Growth in children with pulmonary arterial hypertension: A longitudinal retrospective multiregistry study. Lancet Respir. Med. 2016, 4, 281–290. [Google Scholar] [CrossRef]
- D’Alto, M.; Di Maio, M.; Romeo, E.; Argiento, P.; Blasi, E.; Di Vilio, A.; Rea, G.; D’Andrea, A.; Golino, P.; Naeije, R. Echocardiographic probability of pulmonary hypertension: A validation study. Eur. Respir. J. 2022, 60, 2102548. [Google Scholar] [CrossRef]
- Zuckerman, W.A.; Turner, M.E.; Kerstein, J.; Torres, A.; Vincent, J.A.; Krishnan, U.; Kerstein, D.; Rosenzweig, E.B. Safety of cardiac catheterization at a center specializing in the care of patients with pulmonary arterial hypertension. Pulm. Circ. 2013, 3, 831–839. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Beghetti, M.; Schulze-Neick, I.; Berger, R.M.; Ivy, D.D.; Bonnet, D.; Weintraub, R.G.; Saji, T.; Yung, D.; Mallory, G.B.; Geiger, R.; et al. Haemodynamic characterisation and heart catheterisation complications in children with pulmonary hypertension: Insights from the Global TOPP Registry (tracking outcomes and practice in paediatric pulmonary hypertension). Int. J. Cardiol. 2016, 203, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, J.; Petersen, C.L.; Kjaer, A.; Schaadt, B.K.; Oh, J.K.; Hassager, C. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur. J. Echocardiogr. 2006, 7, 430–438. [Google Scholar] [CrossRef]
- Koestenberger, M.; Ravekes, W.; Everett, A.D.; Stueger, H.P.; Heinzl, B.; Gamillscheg, A.; Cvirn, G.; Boysen, A.; Fandl, A.; Nagel, B. Right ventricular function in infants, children and adolescents: Reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J. Am. Soc. Echocardiogr. 2009, 22, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, R.; Poscia, R.; Pezzuto, B.; Nocioni, M.; Mezzapesa, M.; Francone, M.; Giannetta, E.; Papa, S.; Gambardella, C.; Sciomer, S.; et al. Right ventricular remodeling in idiopathic pulmonary arterial hypertension: Adaptive versus maladaptive morphology. J. Heart Lung Transplant. 2015, 34, 395–403. [Google Scholar] [CrossRef]
- Sato, T.; Tsujino, I.; Ohira, H.; Oyama-Manabe, N.; Yamada, A.; Ito, Y.M.; Goto, C.; Watanabe, T.; Sakaue, S.; Nishimura, M. Validation study on the accuracy of echocardiographic measurements of right ventricular systolic function in pulmonary hypertension. J. Am. Soc. Echocardiogr. 2012, 25, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.M.; Dwyer, N.; Celermajer, D.; Kritharides, L.; Marwick, T.H. Follow-Up of Pulmonary Hypertension with Echocardiography. JACC Cardiovasc. Imaging 2016, 9, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Lammers, A.E.; Apitz, C.; Zartner, P.; Hager, A.; Dubowy, K.O.; Hansmann, G. Diagnostics, monitoring and outpatient care in children with suspected pulmonary hypertension/paediatric pulmonary hypertensive vascular disease. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016, 102, ii1–ii13. [Google Scholar]
- Raymond, R.J.; Hinderliter, A.L.; Willis, P.W.; Ralph, D.; Caldwell, E.J.; Williams, W.; Ettinger, N.A.; Hill, N.S.; Summer, W.R.; de Boisblanc, B.; et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J. Am. Coll. Cardiol. 2002, 39, 1214–1219. [Google Scholar] [CrossRef]
- Khirfan, G.; Almoushref, A.; Naal, T.; Abuhalimeh, B.; Dweik, R.A.; Heresi, G.A.; Tonelli, A.R. Mixed Venous Oxygen Saturation is a Better Prognosticator than Cardiac Index in Pulmonary Arterial Hypertension. Chest 2020, 158, 2546–2555. [Google Scholar] [CrossRef]
- D’Alto, M.; Badagliacca, R.; Argiento, P.; Romeo, E.; Farro, A.; Papa, S.; Sarubbi, B.; Russo, M.G.; Vizza, C.D.; Golino, P.; et al. Risk Reduction and Right Heart Reverse Remodeling by Upfront Triple Combination Therapy in Pulmonary Arterial Hypertension. Chest 2020, 157, 376–383. [Google Scholar] [CrossRef]
- Boudjemline, Y.; Patel, M.; Malekzadeh-Milani, S.; Szezepanski, I.; Lévy, M.; Bonnet, D. Patent ductus arteriosus stenting (transcatheter Potts shunt) for palliation of suprasystemic pulmonary arterial hypertension: A case series. Circ. Cardiovasc. Interv. 2013, 6, e18–e20. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, F.; Pepke-Zaba, J.; McNeil, K.; Parameshwar, J.; Shapiro, L.M. Atrial septostomy in the treatment of severe pulmonary arterial hypertension. Thorax 2003, 58, 797–800. [Google Scholar] [CrossRef] [PubMed]
- George, M.P.; Champion, H.C.; Pilewski, J.M. Lung transplantation for pulmonary hypertension. Pulm. Circ. 2011, 1, 182–191. [Google Scholar] [CrossRef]
- Moser, B.; Jaksch, P.; Taghavi, S.; Muraközy, G.; Lang, G.; Hager, H.; Krenn, C.; Roth, G.; Faybik, P.; Bacher, A.; et al. Lung transplantation for idiopathic pulmonary arterial hypertension on intraoperative and postoperatively prolonged extracorporeal membrane oxygenation provides optimally controlled reperfusion and excellent outcome. Eur. J. Cardiothorac. Surg. 2018, 53, 178–185. [Google Scholar] [CrossRef]
- Bassareo, P.P.; McMahon, C.J. Metabolomics: A New Tool in Our Understanding of Congenital Heart Disease. Children 2022, 9, 1803. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Chen, H.L.; Liu, Y.C.; Wu, Y.H.; Lo, S.H.; Hsu, J.H.; Yin, H.L.; Hsu, J.S.; Wu, B.N.; Dai, Z.K. Unique Pulmonary Hypertension in Young Children: A Case Series Study. Children 2022, 9, 1064. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).