Evaluation of the Hypotensive Preterm Infant: Evidence-Based Practice at the Bedside?
Abstract
1. Introduction
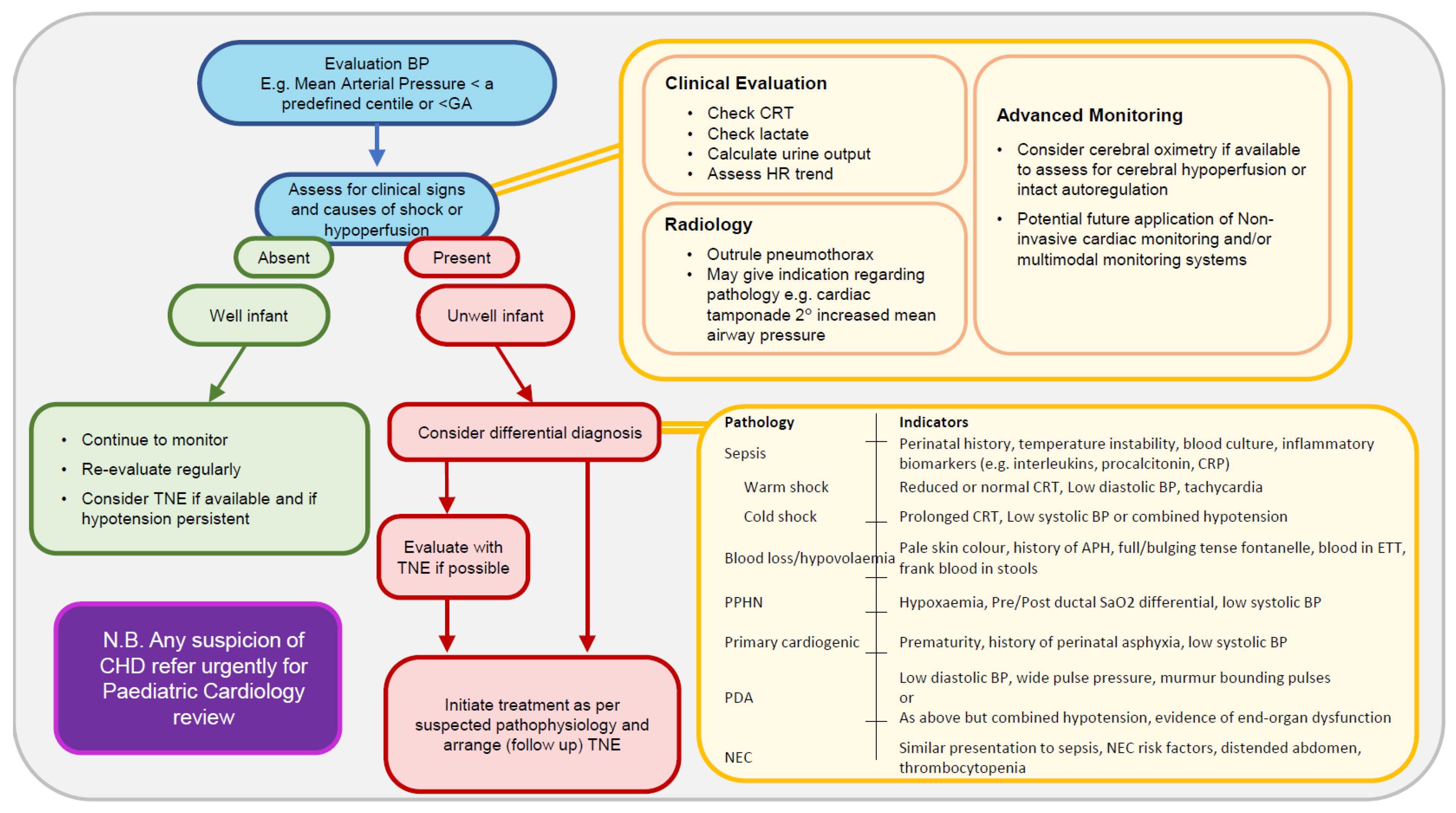
2. Bedside Clinical Assessment
3. Echocardiography
4. Cerebral and Somatic NIRS
5. Noninvasive Cardiac Output Monitoring
6. EEG
7. Multimodal Monitoring in Cardiovascular Assessment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cox, D.J.; Groves, A.M. Inotropes in preterm infants—Evidence for and against. Acta Paediatr. 2012, 101, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.M.; Barrington, K.J. Diagnostic criteria and therapeutic interventions for the hypotensive very low birth weight infant. J. Perinatol. 2006, 26, 677–681. [Google Scholar] [CrossRef]
- Pellicer, A.; Valverde, E.; Elorza, M.D.; Madero, R.; Gaya, F.; Quero, J.; Cabañas, F. Cardiovascular support for low birth weight infants and cerebral hemodynamics: A randomized, blinded, clinical trial. Pediatrics 2005, 115, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.; Evans, N.; Kluckow, M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J. Pediatr. 2002, 140, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.M.; Barrington, K.J.; Marlow, N.; O’Donnell, C.P.F.; Miletin, J.; Naulaers, G.; Cheung, P.Y.; Corcoran, J.D.; El-Khuffash, A.F.; Boylan, G.B.; et al. Hypotension in Preterm Infants (HIP) randomised trial. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Batton, B.; Li, L.; Newman, N.S.; Das, A.; Watterberg, K.L.; Yoder, B.A.; Faix, R.G.; Laughon, M.M.; Stoll, B.J.; Higgins, R.D.; et al. Early blood pressure, antihypotensive therapy and outcomes at 18–22 months’ corrected age in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F201–F206. [Google Scholar] [CrossRef]
- Batton, B.; Li, L.; Newman, N.S.; Das, A.; Watterberg, K.L.; Yoder, B.A.; Faix, R.G.; Laughon, M.M.; Stoll, B.J.; Van Meurs, K.P.; et al. Use of antihypotensive therapies in extremely preterm infants. Pediatrics 2013, 131, e1865–e1873. [Google Scholar] [CrossRef]
- Subhedar, N.V.; Shaw, N.J. Dopamine versus dobutamine for hypotensive preterm infants. Cochrane Database Syst. Rev. 1996, 2010, CD001242. [Google Scholar] [CrossRef]
- Ruelas-Orozco, G.; Vargas-Origel, A. Assessment of therapy for arterial hypotension in critically ill preterm infants. Am. J. Perinatol. 2000, 17, 95–99. [Google Scholar] [CrossRef]
- Bourchier, D.; Weston, P.J. Randomised trial of dopamine compared with hydrocortisone for the treatment of hypotensive very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 76, F174–F178. [Google Scholar] [CrossRef]
- Phillipos, E.; Barrington, K.; Robertson, M. Dopamine versus epinephrine for inotropic support in the neonate: A randomised blinded trial. Pediatr. Res. 1996, 39, 238. [Google Scholar]
- Klarr, J.M.; Faix, R.G.; Pryce, C.J.; Bhatt-Mehta, V. Randomized, blind trial of dopamine versus dobutamine for treatment of hypotension in preterm infants with respiratory distress syndrome. J. Pediatr. 1994, 125, 117–122. [Google Scholar] [CrossRef]
- Roze, J.C.; Tohier, C.; Maingueneau, C.; Lefevre, M.; Mouzard, A. Response to dobutamine and dopamine in the hypotensive very preterm infant. Arch. Dis. Child. 1993, 69, 59–63. [Google Scholar] [CrossRef]
- Cuevas, L.; Yeh, T.F.; John, E.G.; Cuevas, D.; Plides, R.S. The effect of low-dose dopamine infusion on cardiopulmonary and renal status in premature newborns with respiratory distress syndrome. Am. J. Dis. Child. 1991, 145, 799–803. [Google Scholar]
- O’Donnell, C.P.; Kamlin, C.O.; Davis, P.G.; Carlin, J.B.; Morley, C.J. Clinical assessment of infant colour at delivery. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F465–F467. [Google Scholar] [CrossRef]
- De Felice, C.; Flori, M.L.; Pellegrino, M.; Toti, P.; Stanghellini, E.; Molinu, A.; Tosi, P.; Bagnoli, F. Predictive value of skin color for illness severity in the high-risk newborn. Pediatr. Res. 2002, 51, 100–105. [Google Scholar] [CrossRef]
- De Felice, C.; Mazzieri, S.; Pellegrino, M.; Del Pasqua, A.; Toti, P.; Bagnoli, F.; Rosati, E.; Latini, G. Skin reflectance changes in preterm infants with patent ductus arteriosus. Early Hum. Dev. 2004, 78, 45–51. [Google Scholar] [CrossRef]
- Osborn, D.A.; Evans, N.; Kluckow, M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central-peripheral temperature difference. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F168–F173. [Google Scholar] [CrossRef]
- Wodey, E.; Pladys, P.; Betremieux, P.; Kerebel, C.; Ecoffey, C. Capillary refilling time and hemodynamics in neonates: A Doppler echocardiographic evaluation. Crit. Care Med. 1998, 26, 1437–1440. [Google Scholar] [CrossRef]
- Strozik, K.S.; Pieper, C.H.; Cools, F. Capillary refilling time in newborns—Optimal pressing time, sites of testing and normal values. Acta Paediatr. 1998, 87, 310–312. [Google Scholar] [CrossRef]
- Groenendaal, F.; Lindemans, C.; Uiterwaal, C.S.; de Vries, L.S. Early arterial lactate and prediction of outcome in preterm neonates admitted to a neonatal intensive care unit. Neonatology 2003, 83, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.A.; Dewhurst, C.J.; Yoxall, C.W. The prognostic value of initial blood lactate concentration measurements in very low birthweight infants and their use in development of a new disease severity scoring system. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F275–F280. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Clarke, A.; Dempsey, E.M. Day 1 serum lactate values in preterm infants less than 32 weeks gestation. Eur. J. Pediatr. 2010, 169, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Rivers, E.P.; Knoblich, B.P.; Jacobsen, G.; Muzzin, A.; Ressler, J.A.; Tomlanovich, M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004, 32, 1637–1642. [Google Scholar] [CrossRef]
- Bidiwala, K.S.; Lorenz, J.M.; Kleinman, L.I. Renal function correlates of postnatal diuresis in preterm infants. Pediatrics 1988, 82, 50–58. [Google Scholar] [CrossRef]
- Rozycki, H.J.; Baumgart, S. Atrial natriuretic factor and postnatal diuresis in respiratory distress syndrome. Arch. Dis. Child. 1991, 66, 43–47. [Google Scholar] [CrossRef]
- Miletin, J.; Pichova, K.; Dempsey, E.M. Bedside detection of low systemic flow in the very low birth weight infant on day 1 of life. Eur. J. Pediatr. 2009, 168, 809–813. [Google Scholar] [CrossRef]
- De Boode, W.P. Clinical monitoring of systemic hemodynamics in critically ill newborns. Early Hum. Dev. 2010, 86, 137–141. [Google Scholar] [CrossRef]
- Dannevig, I.; Dale, H.C.; Liestol, K.; Lindemann, R. Blood pressure in the neonate: Three non-invasive oscillometric pressure monitors compared with invasively measured blood pressure. Acta Paediatr. 2005, 94, 191–196. [Google Scholar] [CrossRef]
- Dionne, J.M.; Bremner, S.A.; Baygani, S.K.; Batton, B.; Ergenekon, E.; Bhatt-Mehta, V.; Dempsey, E.; Kluckow, M.; Koplowitz, L.P.; Apele-Freimane, D.; et al. Method of Blood Pressure Measurement in Neonates and Infants: A Systematic Review and Analysis. J. Pediatr. 2020, 221, 23–31.e5. [Google Scholar] [CrossRef]
- Lee, J.; Rajadurai, V.S.; Tan, K.W. Blood pressure standards for very low birthweight infants during the first day of life. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 81, F168–F170. [Google Scholar] [CrossRef]
- Spinazzola, R.M.; Harper, R.G.; de Soler, M.; Lesser, M. Blood pressure values in 500- to 750-gram birthweight infants in the first week of life. J. Perinatol. 1991, 11, 147–151. [Google Scholar]
- Watkins, A.M.; West, C.R.; Cooke, R.W. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum. Dev. 1989, 19, 103–110. [Google Scholar] [CrossRef]
- Versmold, H.T.; Kitterman, J.A.; Phibbs, R.H.; Gregory, G.A.; Tooley, W.H. Aortic blood pressure during the first 12 hours of life in infants with birth weight 610 to 4220 grams. Pediatrics 1981, 67, 607–613. [Google Scholar] [CrossRef]
- Miall-Allen, V.M.; de Vries, L.S.; Whitelaw, A.G. Mean arterial blood pressure and neonatal cerebral lesions. Arch. Dis. Child. 1987, 62, 1068–1069. [Google Scholar] [CrossRef]
- Stranak, Z.; Semberova, J.; Barrington, K.; O’Donnell, C.; Marlow, N.; Naulaers, G.; Dempsey, E. International survey on diagnosis and management of hypotension in extremely preterm babies. Eur. J. Pediatr. 2014, 173, 793–798. [Google Scholar] [CrossRef]
- Batton, B.; Li, L.; Newman, N.S.; Das, A.; Watterberg, K.L.; Yoder, B.A.; Faix, R.G.; Laughon, M.M.; Stoll, B.J.; Higgins, R.D.; et al. Evolving blood pressure dynamics for extremely preterm infants. J. Perinatol. 2014, 34, 301–305. [Google Scholar] [CrossRef]
- Batton, B.; Batton, D.; Riggs, T. Blood pressure during the first 7 days in premature infants born at postmenstrual age 23 to 25 weeks. Am. J. Perinatol. 2007, 24, 107–115. [Google Scholar] [CrossRef]
- McNamara, P.; Lai, W. Growth of Neonatal Hemodynamics Programs and Targeted Neonatal Echocardiography Performed by Neonatologists. J. Am. Soc. Echocardiogr. 2020, 33, A15–A16. [Google Scholar] [CrossRef]
- Singh, Y.; Gupta, S.; Groves, A.M.; Gandhi, A.; Thomson, J.; Qureshi, S.; Simpson, J.M. Expert consensus statement ‘Neonatologist-performed Echocardiography (NoPE)’-training and accreditation in U.K. Eur. J. Pediatr. 2016, 175, 281–287. [Google Scholar] [CrossRef]
- Groves, A.M.; Singh, Y.; Dempsey, E.; Molnar, Z.; Austin, T.; El-Khuffash, A.; de Boode, W.P. Introduction to neonatologist-performed echocardiography. Pediatr. Res. 2018, 84, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cleator, A.J.; Subhedar, N.V. Targeted neonatal echocardiography training: A survey of trainees in a region of England. BMJ Paediatr. Open. 2022, 6, e001465. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Porzio, S.; Balestriere, L.; Esposito, P.; Santantonio, A.; Spagnuolo, F.; Giannattasio, A.; Capasso, L.; de Leva, F. Basic-targeted echocardiography for neonatologists: A trainee’s perspective. J. Matern.-Fetal Neonatal Med. 2017, 30, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.R.; Giesinger, R.E.; Rios, D.R.; Mertens, L.; Ashwath, R.; McNamara, P.J. Anatomic Concordance of Neonatologist-Performed Echocardiography as Part of Hemodynamics Consultation and Pediatric Cardiology. J. Am. Soc. Echocardiogr. 2021, 34, 301–307. [Google Scholar] [CrossRef]
- Papadhima, I.; Louis, D.; Purna, J.; Deshpande, P.; Diambomba, Y.; Lee, S.; Shah, P.; Weisz, D.; El-Khuffash, A.; McNamara, P.J.; et al. Targeted neonatal echocardiography (TNE) consult service in a large tertiary perinatal center in Canada. J. Perinatol. 2018, 38, 1039–1045. [Google Scholar] [CrossRef]
- Casani, A.; Tozzi, N.; Cocca, F. The impact of neonatologist performed echocardiography in an Italian neonatal unit. J. Neonatal-Perinat. Med. 2022, 15, 237–242. [Google Scholar] [CrossRef]
- Alammary, D.; Narvey, M.; Soni, R.; Elsayed, Y.; Louis, D. Targeted neonatal echocardiography service in neonatal intensive care in Manitoba, Canada. J. Perinatol. 2022, 42, 655–659. [Google Scholar] [CrossRef]
- Poon, W.B.; Wong, K.Y. Neonatologist-performed point-of-care functional echocardiography in the neonatal intensive care unit. Singapore Med. J. 2017, 58, 230–233. [Google Scholar] [CrossRef]
- Miyata, M.; Toyoshima, K.; Yoda, H.; Murase, M.; Kawato, H.; Yamamoto, K.; Tanaka, K.; Kotani, M.; Kobayashi, M. Extensive use of vasodilator agents and functional echocardiography to monitor extremely-low-birth-weight infants in Japan. J. Neonatal-Perinat. Med. 2016, 9, 261–269. [Google Scholar] [CrossRef]
- King, B.C.; Hagan, J.; Richardson, T.; Berry, J.; Slaughter, J.L. Hospital variation in neonatal echocardiography among very preterm infants at US children’s hospitals. J. Perinatol. 2022, 43, 181–186. [Google Scholar] [CrossRef]
- Van Bel, F.; Mintzer, J.P. Correction: Monitoring cerebral oxygenation of the immature brain: A neuroprotective strategy? Pediatr. Res. 2018, 84, 786. [Google Scholar] [CrossRef]
- Alderliesten, T.; Lemmers, P.M.; van Haastert, I.C.; de Vries, L.S.; Bonestroo, H.J.; Baerts, W.; van Bel, F. Hypotension in preterm neonates: Low blood pressure alone does not affect neurodevelopmental outcome. J. Pediatr. 2014, 164, 986–991. [Google Scholar] [CrossRef]
- Hyttel-Sorensen, S.; Austin, T.; van Bel, F.; Benders, M.; Claris, O.; Dempsey, E.; Fumagalli, M.; Greisen, G.; Grevstad, B.; Hagmann, C.; et al. A phase II randomized clinical trial on cerebral near-infrared spectroscopy plus a treatment guideline versus treatment as usual for extremely preterm infants during the first three days of life (SafeBoosC): Study protocol for a randomized controlled trial. Trials 2013, 14, 120. [Google Scholar] [CrossRef]
- Bonestroo, H.J.; Lemmers, P.M.; Baerts, W.; van Bel, F. Effect of antihypotensive treatment on cerebral oxygenation of preterm infants without PDA. Pediatrics 2011, 128, e1502–e1510. [Google Scholar] [CrossRef]
- Van Bel, F.; Lemmers, P.; Naulaers, G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: Value and pitfalls. Neonatology 2008, 94, 237–244. [Google Scholar] [CrossRef]
- Dotinga, B.M.; Mintzer, J.P.; Moore, J.E.; Hulscher, J.B.F.; Bos, A.F.; Kooi, E.M.W. Maturation of Intestinal Oxygenation: A Review of Mechanisms and Clinical Implications for Preterm Neonates. Front. Pediatr. 2020, 8, 354. [Google Scholar] [CrossRef]
- Wardle, S.P.; Yoxall, C.W.; Weindling, A.M. Peripheral Oxygenation in Hypotensive Preterm Babies. Pediatr. Res. 1999, 45, 343–349. [Google Scholar] [CrossRef]
- Kissack, C.M.; Weindling, A.M. Peripheral Blood Flow and Oxygen Extraction in the Sick, Newborn Very Low Birth Weight Infant Shortly After Birth. Pediatr. Res. 2009, 65, 462–467. [Google Scholar] [CrossRef]
- Binder-Heschl, C.; Urlesberger, B.; Schwaberger, B.; Koestenberger, M.; Pichler, G. Borderline hypotension: How does it influence cerebral regional tissue oxygenation in preterm infants? J. Matern. Fetal Neonatal. Med. 2016, 29, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Pichler, G.; Holler, N.; Baik-Schneditz, N.; Schwaberger, B.; Mileder, L.; Stadler, J.; Avian, A.; Pansy, J.; Urlesberger, B. Avoiding Arterial Hypotension in Preterm Neonates (AHIP)-A Single Center Randomised Controlled Study Investigating Simultaneous Near Infrared Spectroscopy Measurements of Cerebral and Peripheral Regional Tissue Oxygenation and Dedicated Interventions. Front. Pediatr. 2018, 6, 15. [Google Scholar] [CrossRef]
- Garner, R.S.; Burchfield, D.J. Treatment of presumed hypotension in very low birthweight neonates: Effects on regional cerebral oxygenation. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F117–F121. [Google Scholar] [CrossRef] [PubMed]
- Thewissen, L.; Naulaers, G.; Hendrikx, D.; Caicedo, A.; Barrington, K.; Boylan, G.; Cheung, P.Y.; Corcoran, D.; El-Khuffash, A.; Garvey, A.; et al. Cerebral oxygen saturation and autoregulation during hypotension in extremely preterm infants. Pediatr. Res. 2021, 90, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Vesoulis, Z.A.; Liao, S.M.; Trivedi, S.B.; Ters, N.E.; Mathur, A.M. A novel method for assessing cerebral autoregulation in preterm infants using transfer function analysis. Pediatr. Res. 2016, 79, 453–459. [Google Scholar] [CrossRef]
- Garvey, A.; Kooi, E.; Smith, A.; Dempsey, E. Interpretation of Cerebral Oxygenation Changes in the Preterm Infant. Children 2018, 5, 94. [Google Scholar] [CrossRef]
- Elsayed, Y.N.; Fraser, D. Integrated Evaluation of Neonatal Hemodynamics, Part 2: Systematic Bedside Assessment. Neonatal Netw. 2016, 35, 192–203. [Google Scholar] [CrossRef]
- Elsayed, Y.N.; Amer, R.; Seshia, M.M. The impact of integrated evaluation of hemodynamics using targeted neonatal echocardiography with indices of tissue oxygenation: A new approach. J. Perinatol. 2017, 37, 527–535. [Google Scholar] [CrossRef]
- El-Khuffash, A.; James, A.T.; Corcoran, J.D.; Dicker, P.; Franklin, O.; Elsayed, Y.N.; Ting, J.Y.; Sehgal, A.; Malikiwi, A.; Harabor, A.; et al. A Patent Ductus Arteriosus Severity Score Predicts Chronic Lung Disease or Death before Discharge. J. Pediatr. 2015, 167, 1354–1361.e2. [Google Scholar] [CrossRef]
- Beausoleil, T.P.; Janaillac, M.; Barrington, K.J.; Lapointe, A.; Dehaes, M. Cerebral oxygen saturation and peripheral perfusion in the extremely premature infant with intraventricular and/or pulmonary haemorrhage early in life. Sci. Rep. 2018, 8, 6511. [Google Scholar] [CrossRef]
- Lee, A.J.; Cohn, J.H.; Ranasinghe, J.S. Cardiac output assessed by invasive and minimally invasive techniques. Anesthesiol. Res. Pract. 2011, 2011, 475151. [Google Scholar] [CrossRef]
- McGovern, M.; Miletin, J. Cardiac Output Monitoring in Preterm Infants. Front. Pediatr. 2018, 6, 84. [Google Scholar] [CrossRef]
- O’Neill, R.; Dempsey, E.M.; Garvey, A.A.; Schwarz, C.E. Non-invasive Cardiac Output Monitoring in Neonates. Front. Pediatr. 2021, 8, 614585. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, L.; Gupta, S.; Lawrenson, J.; de Boode, W.P. Accuracy and Trending Ability of Electrical Biosensing Technology for Non-invasive Cardiac Output Monitoring in Neonates: A Systematic Qualitative Review. Front. Pediatr. 2022, 10, 851850. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.E.; O’Toole, J.M.; Livingstone, V.; Pavel, A.M.; Dempsey, E.M. Signal Quality of Electrical Cardiometry and Perfusion Index in Very Preterm Infants. Neonatology 2021, 118, 672–677. [Google Scholar] [CrossRef]
- De Boode, W.P.; van der Lee, R.; Horsberg Eriksen, B.; Nestaas, E.; Dempsey, E.; Singh, Y.; Austin, T.; El-Khuffash, A. The role of Neonatologist Performed Echocardiography in the assessment and management of neonatal shock. Pediatr. Res. 2018, 84, 57–67. [Google Scholar] [CrossRef]
- Kobe, J.; Mishra, N.; Arya, V.K.; Al-Moustadi, W.; Nates, W.; Kumar, B. Cardiac output monitoring: Technology and choice. Ann. Card. Anaesth. 2019, 22, 6–17. [Google Scholar]
- Van Wyk, L.; Smith, J.; Lawrenson, J.; Lombard, C.J.; de Boode, W.P. Bioreactance Cardiac Output Trending Ability in Preterm Infants: A Single Centre, Longitudinal Study. Neonatology 2021, 118, 600–608. [Google Scholar] [CrossRef]
- Van Wyk, L.; Smith, J.; Lawrenson, J.; de Boode, W.P. Agreement of Cardiac Output Measurements between Bioreactance and Transthoracic Echocardiography in Preterm Infants during the Transitional Phase: A Single-Centre, Prospective Study. Neonatology 2020, 117, 271–278. [Google Scholar] [CrossRef]
- Schwarz, C.E.; Livingstone, V.; O’Toole, J.M.; Healy, D.B.; Panaviene, J.; Dempsey, E.M. Agreement of Cardiac Output Estimates between Electrical Cardiometry and Transthoracic Echocardiography in Very Preterm Infants. Neonatology 2022, 119, 594–601. [Google Scholar] [CrossRef]
- Weisz, D.E.; Jain, A.; McNamara, P.J.; Afif, E.K. Non-invasive cardiac output monitoring in neonates using bioreactance: A comparison with echocardiography. Neonatology 2012, 102, 61–67. [Google Scholar] [CrossRef]
- Cappelleri, A.; Bussmann, N.; Harvey, S.; Levy, P.T.; Franklin, O.; El-Khuffash, A. Myocardial function in late preterm infants during the transitional period: Comprehensive appraisal with deformation mechanics and non-invasive cardiac output monitoring. Cardiol. Young 2020, 30, 249–255. [Google Scholar] [CrossRef]
- Miletin, J.; Semberova, J.; Martin, A.M.; Janota, J.; Stranak, Z. Low cardiac output measured by bioreactance and adverse outcome in preterm infants with birth weight less than 1250 g. Early Hum. Dev. 2020, 149, 105153. [Google Scholar] [CrossRef]
- Song, R.; Rich, W.; Kim, J.H.; Finer, N.N.; Katheria, A.C. The use of electrical cardiometry for continuous cardiac output monitoring in preterm neonates: A validation study. Am. J. Perinatol. 2014, 31, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, T.; Sato, S.; Nagayama, Y.; Sato, T.; Yamazaki, H. Influence of patent ductus arteriosus and ventilators on electrical velocimetry for measuring cardiac output in very-low/low birth weight infants. J. Perinatol. 2015, 35, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Victor, S.; Marson, A.G.; Appleton, R.E.; Beirne, M.; Weindling, A.M. Relationship between blood pressure, cerebral electrical activity, cerebral fractional oxygen extraction, and peripheral blood flow in very low birth weight newborn infants. Pediatr. Res. 2006, 59, 314–319. [Google Scholar] [CrossRef]
- Victor, S.; Appleton, R.E.; Beirne, M.; Marson, A.G.; Weindling, A.M. The relationship between cardiac output, cerebral electrical activity, cerebral fractional oxygen extraction and peripheral blood flow in premature newborn infants. Pediatr. Res. 2006, 60, 456–460. [Google Scholar] [CrossRef]
- Shah, D.; Paradisis, M.; Bowen, J.R. Relationship between systemic blood flow, blood pressure, inotropes, and aEEG in the first 48 h of life in extremely preterm infants. Pediatr. Res. 2013, 74, 314–320. [Google Scholar] [CrossRef]
- Pereira, S.S.; Sinha, A.K.; Morris, J.K.; Wertheim, D.F.; Shah, D.K.; Kempley, S.T. Blood pressure intervention levels in preterm infants: Pilot randomised trial. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F298–F305. [Google Scholar] [CrossRef]
- Fairchild, K.D. Predictive monitoring for early detection of sepsis in neonatal ICU patients. Curr. Opin. Pediatr. 2013, 25, 172–179. [Google Scholar] [CrossRef]
- Pavel, A.M.; O’Toole, J.M.; Proietti, J.; Livingstone, V.; Mitra, S.; Marnane, W.P.; Finder, M.; Dempsey, E.M.; Murray, D.M.; Boylan, G.B.; et al. Machine learning for the early prediction of infants with electrographic seizures in neonatal hypoxic-ischemic encephalopathy. Epilepsia 2022, 64, 456–468. [Google Scholar] [CrossRef]
- Azhibekov, T.; Soleymani, S.; Lee, B.H.; Noori, S.; Seri, I. Hemodynamic monitoring of the critically ill neonate: An eye on the future. Semin. Fetal Neonatal. Med. 2015, 20, 246–254. [Google Scholar] [CrossRef]
- Da Costa, C.S.; Greisen, G.; Austin, T. Is near-infrared spectroscopy clinically useful in the preterm infant? Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F558–F561. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, E.; Healy, D.B.; Chioma, R.; Dempsey, E.M. Evaluation of the Hypotensive Preterm Infant: Evidence-Based Practice at the Bedside? Children 2023, 10, 519. https://doi.org/10.3390/children10030519
Murphy E, Healy DB, Chioma R, Dempsey EM. Evaluation of the Hypotensive Preterm Infant: Evidence-Based Practice at the Bedside? Children. 2023; 10(3):519. https://doi.org/10.3390/children10030519
Chicago/Turabian StyleMurphy, Elizabeth, David B. Healy, Roberto Chioma, and Eugene M. Dempsey. 2023. "Evaluation of the Hypotensive Preterm Infant: Evidence-Based Practice at the Bedside?" Children 10, no. 3: 519. https://doi.org/10.3390/children10030519
APA StyleMurphy, E., Healy, D. B., Chioma, R., & Dempsey, E. M. (2023). Evaluation of the Hypotensive Preterm Infant: Evidence-Based Practice at the Bedside? Children, 10(3), 519. https://doi.org/10.3390/children10030519




