Accelerated Aging and the Life Course of Individuals Born Preterm
Abstract
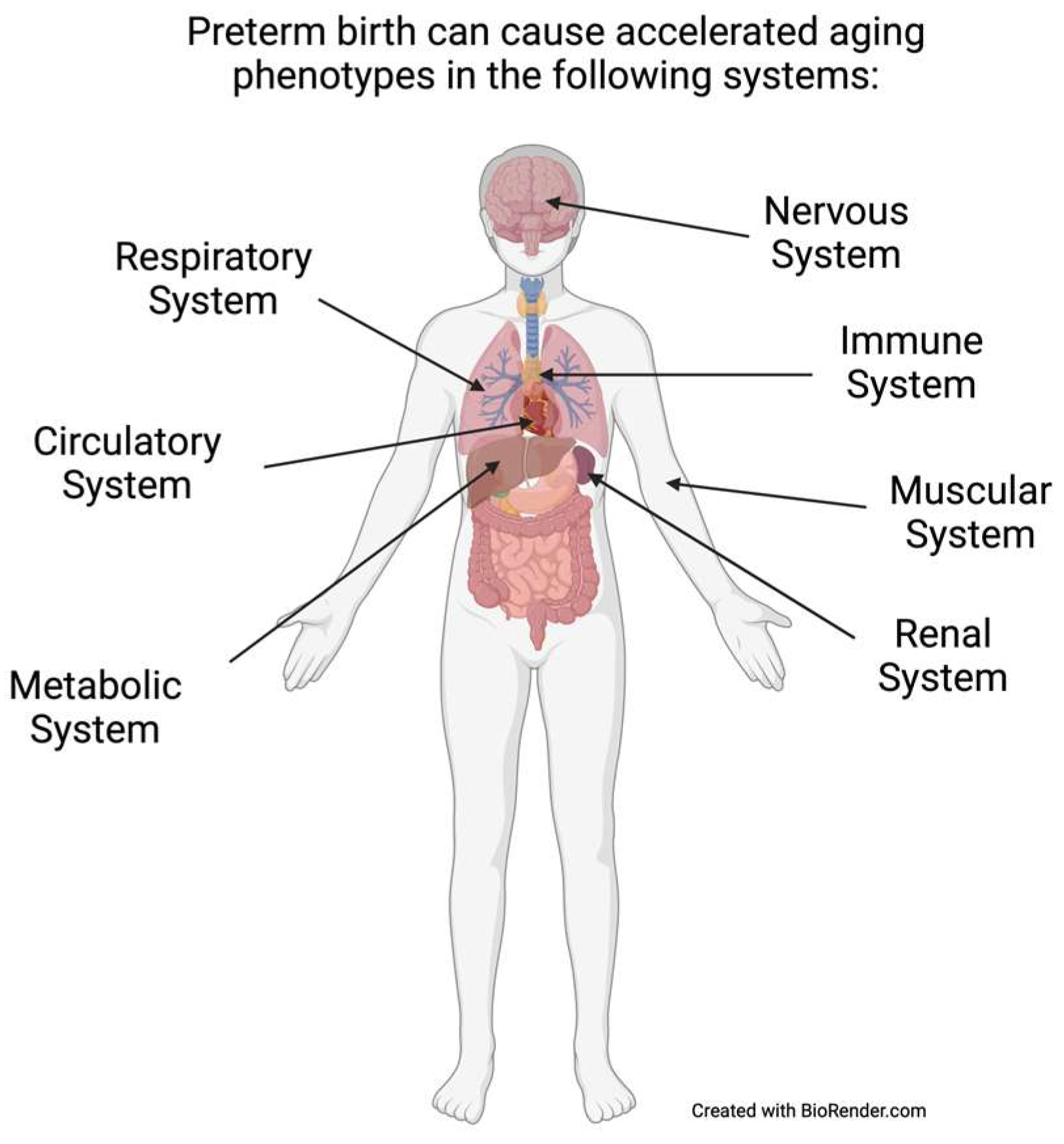
:1. Introduction
2. Results
2.1. Accelerated Aging Phenotypes and Biomarkers
2.1.1. Prematurity or LBW and Cardiovascular Diseases
2.1.2. Prematurity or LBW and Metabolic–Endocrine Diseases
2.1.3. Prematurity or LBW and Brain Disorders
2.1.4. Prematurity or LBW and Lung Diseases
2.1.5. Prematurity or LBW and Sarcopenia
2.1.6. Prematurity or LBW and Kidney Diseases
2.1.7. Immune Function Measures
2.1.8. Hepatic Measures
2.2. Biomarkers of Mechanisms Contributing to Biological Aging
2.2.1. Cellular Aging Biomarkers
2.2.2. Biomarkers of Stress Responses
2.3. Resiliency Factors and Moderators for Accelerated Aging
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hidalgo-Lopezosa, P.; Jiménez-Ruz, A.; Carmona-Torres, J.; Hidalgo-Maestre, M.; Rodríguez-Borrego, M.; López-Soto, P. Sociodemographic factors associated with preterm birth and low birth weight: A cross-sectional study. Women Birth 2019, 32, e538–e543. [Google Scholar] [CrossRef] [PubMed]
- Inder, T.E.; Volpe, J.J.; Anderson, P.J. Defining the Neurologic Consequences of Preterm Birth. N. Engl. J. Med. 2023, 389, 441–453. [Google Scholar] [CrossRef]
- Phillippe, M. Telomeres, oxidative stress, and timing for spontaneous term and preterm labor. Am. J. Obstet. Gynecol. 2022, 227, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Risnes, K.; Bilsteen, J.F.; Brown, P.; Pulakka, A.; Andersen, A.M.N.; Opdahl, S.; Kajantie, E.; Sandin, S. Mortality among young adults born preterm and early term in 4 Nordic nations. JAMA Netw. Open 2021, 4, e2032779. [Google Scholar] [CrossRef]
- Crump, C.; Groves, A.; Sundquist, J.; Sundquist, K. Association of preterm birth with long-term risk of heart failure into adulthood. JAMA Pediatr. 2021, 175, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, J.; Sundquist, K. Preterm or early term birth and long-term risk of asthma into midadulthood: A national cohort and cosibling study. Thorax 2023, 78, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, K.; Winkleby, M.A.; Sundquist, J. Preterm birth and risk of epilepsy in Swedish adults. Neurology 2011, 77, 1376–1382. [Google Scholar] [CrossRef]
- Crump, C.; Winkleby, M.A.; Sundquist, J.; Sundquist, K. Risk of asthma in young adults who were born preterm: A Swedish national cohort study. Pediatrics 2011, 127, e913–e920. [Google Scholar] [CrossRef]
- Crump, C.; Winkleby, M.A.; Sundquist, K.; Sundquist, J. Preterm birth and psychiatric medication prescription in young adulthood: A Swedish national cohort study. Int. J. Epidemiol. 2010, 39, 1522–1530. [Google Scholar] [CrossRef]
- Bury, M. The sociology of chronic illness: A review of research and prospects. Sociol. Health Illn. 1991, 13, 451–468. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Poznyak, A.V.; Nikiforov, N.G.; Sobenin, I.A.; Orekhov, A.N. The link between chronic stress and accelerated aging. Biomedicines 2020, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.N.; Zmora, R.; Schreiner, P.J.; Jacobs, D.R., Jr.; Roger, V.L.; Thorpe, R.J., Jr.; Kiefe, C.I. Accelerated aging: A marker for social factors resulting in cardiovascular events? SSM-Popul. Health 2021, 13, 100733. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, R.J.; McGowan, P.O.; de Vega, W.C.; Savoy, C.D.; Morrison, K.M.; Saigal, S.; Mathewson, K.J.; Schmidt, L.A. Extremely low birth weight and accelerated biological aging. Pediatrics 2021, 147, e2020001230. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, H.; Johnson, S.; Ni, Y.; Wolke, D.; Marlow, N. Neuropsychological Outcomes at 19 Years of Age Following Extremely Preterm Birth. Pediatrics 2020, 145, e20192087. [Google Scholar] [CrossRef] [PubMed]
- Bäuml, J.G.; Meng, C.; Daamen, M.; Baumann, N.; Busch, B.; Bartmann, P.; Wolke, D.; Boecker, H.; Wohlschläger, A.; Sorg, C. The association of children’s mathematic abilities with both adults’ cognitive abilities and intrinsic fronto-parietal networks is altered in preterm-born individuals. Brain Struct. Funct. 2017, 222, 799–812. [Google Scholar] [CrossRef]
- Lapidaire, W.; Clark, C.; Fewtrell, M.S.; Lucas, A.; Leeson, P.; Lewandowski, A.J. The Preterm Heart-Brain Axis in Young Adulthood: The Impact of Birth History and Modifiable Risk Factors. J. Clin. Med. 2021, 10, 1285. [Google Scholar] [CrossRef]
- Sullivan, M.C.; Winchester, S.B.; Msall, M.E. Prematurity and cardiovascular risk at early adulthood. Child Care Health Dev. 2019, 45, 71–78. [Google Scholar] [CrossRef]
- Hennessy, E.M.; Bracewell, M.A.; Wood, N.; Wolke, D.; Costeloe, K.; Gibson, A.; Marlow, N.; Group, E.P.S. Respiratory health in pre-school and school age children following extremely preterm birth. Arch Dis. Child 2008, 93, 1037–1043. [Google Scholar] [CrossRef]
- Hovi, P.; Andersson, S.; Eriksson, J.G.; Jarvenpaa, A.L.; Strang-Karlsson, S.; Makitie, O.; Kajantie, E. Glucose regulation in young adults with very low birth weight. N. Engl. J. Med. 2007, 356, 2053–2063. [Google Scholar] [CrossRef]
- Hovi, P.; Kajantie, E.; Soininen, P.; Kangas, A.J.; Järvenpää, A.-L.; Andersson, S.; Eriksson, J.G.; Ala-Korpela, M.; Wehkalampi, K. Lipoprotein subclass profiles in young adults born preterm at very low birth weight. Lipids Health Dis. 2013, 12, 57. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, J.; Winkleby, M.A.; Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: National cohort study. BMJ 2019, 365, l1346. [Google Scholar] [CrossRef] [PubMed]
- Linsell, L.; Johnson, S.; Wolke, D.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely preterm birth: A prospective cohort study. Eur. Child Adolesc. Psychiatry 2019, 28, 531–542. [Google Scholar] [CrossRef]
- Johnson, S.; O’Reilly, H.; Ni, Y.; Wolke, D.; Marlow, N. Psychiatric Symptoms and Disorders in Extremely Preterm Young Adults at 19 Years of Age and Longitudinal Findings from Middle Childhood. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 820–826.e6. [Google Scholar] [CrossRef]
- Cai, N.; Wu, Y.; Huang, Y. Induction of accelerated aging in a mouse model. Cells 2022, 11, 1418. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.; McGuire, W. Epidemiology of preterm birth. BMJ 2004, 329, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardají, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35, 6492. [Google Scholar]
- de Jong, F.; Monuteaux, M.C.; van Elburg, R.M.; Gillman, M.W.; Belfort, M.B. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012, 59, 226–234. [Google Scholar] [CrossRef]
- Bracewell, M.A.; Hennessy, E.M.; Wolke, D.; Marlow, N. The EPICure study: Growth and blood pressure at 6 years of age following extremely preterm birth. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F108–F114. [Google Scholar] [CrossRef]
- Bonamy, A.-K.E.; Källén, K.; Norman, M. High blood pressure in 2.5-year-old children born extremely preterm. Pediatrics 2012, 129, e1199–e1204. [Google Scholar] [CrossRef]
- Crimmins, E.; Vasunilashorn, S.; Kim, J.K.; Alley, D. Biomarkers related to aging in human populations. Adv. Clin. Chem. 2008, 46, 161–216. [Google Scholar]
- Stamler, J.; Neaton, J.D.; Wentworth, D.N. Blood pressure (systolic and diastolic) and risk of fatal coronary heart disease. Hypertension 1989, 13 (Suppl. S5), I2. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Khan, S.A.; Wong, N.D.; Larson, M.G.; Levy, D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation 1999, 100, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Gillum, R.F.; Makuc, D.M.; Feldman, J.J. Pulse rate, coronary heart disease, and death: The NHANES I Epidemiologic Follow-up Study. Am. Heart J. 1991, 121, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef]
- Faizi, A.K.; Kornmo, D.W.; Agewall, S. Evaluation of endothelial function using finger plethysmography. Clin. Physiol. Funct. Imaging 2009, 29, 372–375. [Google Scholar] [CrossRef]
- Arnesen, E.; Refsum, H.; Bønaa, K.H.; Ueland, P.M.; Førde, O.H.; Nordrehaug, J.E. Serum total homocysteine and coronary heart disease. Int. J. Epidemiol. 1995, 24, 704–709. [Google Scholar] [CrossRef]
- Flahault, A.; Oliveira Fernandes, R.; De Meulemeester, J.; Ravizzoni Dartora, D.; Cloutier, A.; Gyger, G.; El-Jalbout, R.; Bigras, J.-L.; Luu, T.M.; Nuyt, A.M. Arterial structure and stiffness are altered in young adults born preterm. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2548–2556. [Google Scholar] [CrossRef]
- Darlow, B.A.; Horwood, J.; Dhakal, B.; Harris, S.L.; McKelvey, V.A.; Elliott, J.M.; Yang, J.; Mackay, R.J. Biomarkers of ageing in New Zealand VLBW young adults and controls. Pediatr. Res. 2021, 89, 533–539. [Google Scholar] [CrossRef]
- Sipola-Leppänen, M.; Karvonen, R.; Tikanmäki, M.; Matinolli, H.-M.; Martikainen, S.; Pesonen, A.-K.; Räikkönen, K.; Järvelin, M.-R.; Hovi, P.; Eriksson, J.G. Ambulatory blood pressure and its variability in adults born preterm. Hypertension 2015, 65, 615–621. [Google Scholar] [CrossRef]
- Juonala, M.; Cheung, M.M.; Sabin, M.A.; Burgner, D.; Skilton, M.R.; Kähönen, M.; Hutri-Kähönen, N.; Lehtimäki, T.; Jula, A.; Laitinen, T. Effect of birth weight on life-course blood pressure levels among children born premature: The Cardiovascular Risk in Young Finns Study. J. Hypertens. 2015, 33, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Hovi, P.; Vohr, B.; Ment, L.R.; Doyle, L.W.; McGarvey, L.; Morrison, K.M.; Evensen, K.A.I.; van der Pal, S.; Grunau, R.E. Blood pressure in young adults born at very low birth weight: Adults born preterm international collaboration. Hypertension 2016, 68, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A. Preterm heart in adult life: Cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013, 127, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sipola-Leppänen, M.; Vääräsmäki, M.; Tikanmäki, M.; Matinolli, H.-M.; Miettola, S.; Hovi, P.; Wehkalampi, K.; Ruokonen, A.; Sundvall, J.; Pouta, A. Cardiometabolic risk factors in young adults who were born preterm. Am. J. Epidemiol. 2015, 181, 861–873. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Ronalds, G.; Clark, H.; Davey Smith, G.; Leon, D.A. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: Findings from the Aberdeen Children of the 1950s prospective cohort study. Circulation 2005, 112, 1414–1418. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Forsen, T.; Tuomilehto, J.; Winter, P.D.; Osmond, C.; Barker, D.J. Catch-up growth in childhood and death from coronary heart disease: Longitudinal study. BMJ 1999, 318, 427–431. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, J.; Guo, H.; Ji, D.; Yuan, D.; Li, M.; Yan, T.; Xue, C.; Ma, H.; Zhou, X. Effect of metabolic health and obesity phenotype on risk of diabetes mellitus: A population-based longitudinal study. Diabetes Metab. Syndr. Obes. 2021, 14, 3485–3498. [Google Scholar] [CrossRef]
- Akintola, A.A.; van Heemst, D. Insulin, aging, and the brain: Mechanisms and implications. Front. Endocrinol. 2015, 6, 13. [Google Scholar] [CrossRef]
- Burns, J.M.; Honea, R.A.; Vidoni, E.D.; Hutfles, L.J.; Brooks, W.M.; Swerdlow, R.H. Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 333–339. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s disease as type 3 diabetes: Common pathophysiological mechanisms between Alzheimer’s disease and type 2 diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Juster, R.-P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Habes, M.; Jacobson, A.M.; Braffett, B.H.; Rashid, T.; Ryan, C.M.; Shou, H.; Cui, Y.; Davatzikos, C.; Luchsinger, J.A.; Biessels, G.J. Patterns of Regional Brain Atrophy and Brain Aging in Middle-and Older-Aged Adults With Type 1 Diabetes. JAMA Netw. Open 2023, 6, e2316182. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Parkinson, J.R.; Hyde, M.J.; Yap, I.K.; Holmes, E.; Doré, C.J.; Bell, J.D.; Modi, N. Aberrant adiposity and ectopic lipid deposition characterize the adult phenotype of the preterm infant. Pediatr. Res. 2011, 70, 507–512. [Google Scholar] [CrossRef]
- Mathai, S.; Cutfield, W.S.; Derraik, J.G.; Dalziel, S.R.; Harding, J.E.; Robinson, E.; Biggs, J.; Jefferies, C.; Hofman, P.L. Insulin sensitivity and β-cell function in adults born preterm and their children. Diabetes 2012, 61, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.M.; Ramsingh, L.; Gunn, E.; Streiner, D.; Van Lieshout, R.; Boyle, M.; Gerstein, H.; Schmidt, L.; Saigal, S. Cardiometabolic health in adults born premature with extremely low birth weight. Pediatrics 2016, 138, e20160515. [Google Scholar] [CrossRef]
- Crane, J.D.; Yellin, S.A.; Ong, F.J.; Singh, N.P.; Konyer, N.; Noseworthy, M.D.; Schmidt, L.A.; Saigal, S.; Morrison, K.M. ELBW survivors in early adulthood have higher hepatic, pancreatic and subcutaneous fat. Sci. Rep. 2016, 6, 31560. [Google Scholar] [CrossRef]
- Kajantie, E.; Strang-Karlsson, S.; Hovi, P.; Wehkalampi, K.; Lahti, J.; Kaseva, N.; Järvenpää, A.-L.; Räikkönen, K.; Eriksson, J.G.; Andersson, S. Insulin sensitivity and secretory response in adults born preterm: The Helsinki Study of Very Low Birth Weight Adults. J. Clin. Endocrinol. Metab. 2015, 100, 244–250. [Google Scholar] [CrossRef]
- Jackson, W.M.; O’Shea, T.M.; Allred, E.N.; Laughon, M.M.; Gower, W.A.; Leviton, A. Risk factors for chronic lung disease and asthma differ among children born extremely preterm. Pediatr. Pulmonol. 2018, 53, 1533–1540. [Google Scholar] [CrossRef]
- O’Shea, T.M.; McGrath, M.; Aschner, J.L.; Lester, B.; Santos, H.P., Jr.; Marsit, C.; Stroustrup, A.; Emmanuel, C.; Hudak, M.; McGowan, E. Environmental influences on child health outcomes: Cohorts of individuals born very preterm. Pediatr. Res. 2023, 93, 1161–1176. [Google Scholar] [CrossRef]
- Linthavong, O.; O’Shea, T.M.; Allred, E.; Perrin, E.; Bauserman, M.; Joseph, R.M.; Leviton, A.; Heeren, T.C.; Kuban, K.C.; for the Extremely Low Gestational Age Newborn Research Study. Neurocognitive and health correlates of overweight and obesity among ten-year-old children born extremely preterm. J. Pediatr. 2018, 200, 84–90.e84. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Selective review of cognitive aging. J. Int. Neuropsychol. Soc. 2010, 16, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Resnick, S.M.; Pham, D.L.; Kraut, M.A.; Zonderman, A.B.; Davatzikos, C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J. Neurosci. 2003, 23, 3295–3301. [Google Scholar] [CrossRef]
- Cole, J.H.; Franke, K. Predicting age using neuroimaging: Innovative brain ageing biomarkers. Trends Neurosci. 2017, 40, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Ritchie, S.J.; Bastin, M.E.; Hernández, V.; Muñoz Maniega, S.; Royle, N.; Corley, J.; Pattie, A.; Harris, S.E.; Zhang, Q. Brain age predicts mortality. Mol. Psychiatry 2018, 23, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Moster, D.; Lie, R.T.; Markestad, T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 2008, 359, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Hille, E.e.T.; Weisglas-Kuperus, N.; Van Goudoever, J.; Jacobusse, G.W.; Ens-Dokkum, M.H.; de Groot, L.; Wit, J.M.; Geven, W.B.; Kok, J.H.; de Kleine, M.J. Functional outcomes and participation in young adulthood for very preterm and very low birth weight infants: The Dutch Project on Preterm and Small for Gestational Age Infants at 19 years of age. Pediatrics 2007, 120, e587–e595. [Google Scholar] [CrossRef]
- Ericson, A.; Källén, B. Very low birthweight boys at the age of 19. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 78, F171–F174. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment. Contin. Lifelong Learn. Neurol. 2016, 22, 404–418. [Google Scholar] [CrossRef]
- White, T.P.; Symington, I.; Castellanos, N.P.; Brittain, P.J.; Walsh, S.F.; Nam, K.-W.; Sato, J.R.; Allin, M.P.; Shergill, S.S.; Murray, R.M. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. NeuroImage Clin. 2014, 4, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Bjuland, K.J.; Rimol, L.M.; Løhaugen, G.C.; Skranes, J. Brain volumes and cognitive function in very-low-birth-weight (VLBW) young adults. Eur. J. Paediatr. Neurol. 2014, 18, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Bäuml, J.G.; Daamen, M.; Jaekel, J.; Neitzel, J.; Scheef, L.; Busch, B.; Baumann, N.; Boecker, H.; Zimmer, C. Extensive and interrelated subcortical white and gray matter alterations in preterm-born adults. Brain Struct. Funct. 2016, 221, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Brossard-Racine, M.; Du Plessis, A.J.; Limperopoulos, C. Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum 2015, 14, 151–164. [Google Scholar] [CrossRef]
- Parker, J.; Mitchell, A.; Kalpakidou, A.; Walshe, M.; Jung, H.-Y.; Nosarti, C.; Santosh, P.; Rifkin, L.; Wyatt, J.; Murray, R.M. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain 2008, 131, 1344–1351. [Google Scholar]
- Ueda, P.; Cnattingius, S.; Stephansson, O.; Ingelsson, E.; Ludvigsson, J.F.; Bonamy, A.-K.E. Cerebrovascular and ischemic heart disease in young adults born preterm: A population-based Swedish cohort study. Eur. J. Epidemiol. 2014, 29, 253–260. [Google Scholar] [CrossRef]
- Portegies, M.; Koudstaal, P.; Ikram, M. Cerebrovascular disease. Handb. Clin. Neurol. 2016, 138, 239–261. [Google Scholar]
- Daskalakis, G.; Psarris, A.; Koutras, A.; Fasoulakis, Z.; Prokopakis, I.; Varthaliti, A.; Karasmani, C.; Ntounis, T.; Domali, E.; Theodora, M.; et al. Maternal Infection and Preterm Birth: From Molecular Basis to Clinical Implications. Children 2023, 10, 907. [Google Scholar] [CrossRef]
- Fasoulakis, Z.; Koutras, A.; Ntounis, T.; Antsaklis, P.; Theodora, M.; Valsamaki, A.; Daskalakis, G.; Kontomanolis, E.N. Inflammatory Molecules Responsible for Length Shortening and Preterm Birth. Cells 2023, 12, 209. [Google Scholar] [CrossRef]
- Han, P.P.; Han, Y.; Shen, X.Y.; Gao, Z.K.; Bi, X. NLRP3 inflammasome activation after ischemic stroke. Behav. Brain Res. 2023, 452, 114578. [Google Scholar] [CrossRef]
- Libby, P.; Okamoto, Y.; Rocha, V.Z.; Folco, E. Inflammation in atherosclerosis: Transition from theory to practice. Circ. J. 2010, 74, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kuban, K.C.; O’Shea, T.M.; Allred, E.N.; Paneth, N.; Hirtz, D.; Fichorova, R.N.; Leviton, A. Systemic inflammation and cerebral palsy risk in extremely preterm infants. J. Child Neurol. 2014, 29, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Joseph, R.M.; Kuban, K.C.; Allred, E.N.; Ware, J.; Coster, T.; Fichorova, R.N.; Dammann, O.; Leviton, A. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatr. Res. 2014, 75, 781–787. [Google Scholar] [CrossRef]
- Kuban, K.C.; Joseph, R.M.; O’Shea, T.M.; Heeren, T.; Fichorova, R.N.; Douglass, L.; Jara, H.; Frazier, J.A.; Hirtz, D.; Rollins, J.V. Circulating inflammatory-associated proteins in the first month of life and cognitive impairment at age 10 years in children born extremely preterm. J. Pediatr. 2017, 180, 116–123.e111. [Google Scholar] [CrossRef] [PubMed]
- Marable, C.A.; Roell, K.; Kuban, K.; O’Shea, T.M.; Fry, R.C. Placental transcriptional signatures associated with cerebral white matter damage in the neonate. Front. Neurosci. 2022, 16, 1017953. [Google Scholar] [CrossRef] [PubMed]
- Leviton, A.; Dammann, O.; O’Shea, T.M.; Paneth, N. Adult stroke and perinatal brain damage: Like grandparent, like grandchild? Neuropediatrics 2002, 33, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Vollsæter, M.; Clemm, H.H.; Satrell, E.; Eide, G.E.; Røksund, O.D.; Markestad, T.; Halvorsen, T. Adult respiratory outcomes of extreme preterm birth. A regional cohort study. Ann. Am. Thorac. Soc. 2015, 12, 313–322. [Google Scholar] [CrossRef]
- Cannavò, L.; Perrone, S.; Viola, V.; Marseglia, L.; Di Rosa, G.; Gitto, E. Oxidative stress and respiratory diseases in preterm newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 2019, 5, 78. [Google Scholar] [CrossRef]
- Gough, A.; Spence, D.; Linden, M.; Halliday, H.L.; McGarvey, L.P. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: A systematic review. Chest 2012, 141, 1554–1567. [Google Scholar] [CrossRef]
- Lovering, A.T.; Elliott, J.E.; Laurie, S.S.; Beasley, K.M.; Gust, C.E.; Mangum, T.S.; Gladstone, I.M.; Duke, J.W. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann. Am. Thorac. Soc. 2014, 11, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.W.; Elliott, J.E.; Laurie, S.S.; Beasley, K.M.; Mangum, T.S.; Hawn, J.A.; Gladstone, I.M.; Lovering, A.T. Pulmonary gas exchange efficiency during exercise breathing normoxic and hypoxic gas in adults born very preterm with low diffusion capacity. J. Appl. Physiol. 2014, 117, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Broström, E.B.; Akre, O.; Katz-Salamon, M.; Jaraj, D.; Kaijser, M. Obstructive pulmonary disease in old age among individuals born preterm. Eur. J. Epidemiol. 2013, 28, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Saarenpää, H.-K.; Tikanmäki, M.; Sipola-Leppänen, M.; Hovi, P.; Wehkalampi, K.; Siltanen, M.; Vääräsmäki, M.; Järvenpää, A.-L.; Eriksson, J.G.; Andersson, S. Lung function in very low birth weight adults. Pediatrics 2015, 136, 642–650. [Google Scholar] [CrossRef]
- Walter, E.C.; Ehlenbach, W.J.; Hotchkin, D.L.; Chien, J.W.; Koepsell, T.D. Low birth weight and respiratory disease in adulthood: A population-based case-control study. Am. J. Respir. Crit. Care Med. 2009, 180, 176–180. [Google Scholar] [CrossRef]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef]
- Naseeb, M.A.; Volpe, S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017, 40, 1–20. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Bean, J.F.; Kiely, D.K.; LaRose, S.; Alian, J.; Frontera, W.R. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch. Phys. Med. Rehabil. 2007, 88, 604–609. [Google Scholar] [CrossRef]
- Rogeri, P.S.; Zanella, R., Jr.; Martins, G.L.; Garcia, M.D.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P. Strategies to prevent sarcopenia in the aging process: Role of protein intake and exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef]
- Ylihärsilä, H.; Kajantie, E.; Osmond, C.; Forsen, T.; Barker, D.J.; Eriksson, J.G. Birth size, adult body composition and muscle strength in later life. Int. J. Obes. 2007, 31, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Stoskopf, B.; Boyle, M.; Paneth, N.; Pinelli, J.; Streiner, D.; Goddeeris, J. Comparison of current health, functional limitations, and health care use of young adults who were born with extremely low birth weight and normal birth weight. Pediatrics 2007, 119, e562–e573. [Google Scholar] [CrossRef] [PubMed]
- Nyengaard, J.; Bendtsen, T. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat. Rec. 1992, 232, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Takazakura, E.; Sawabu, N.; Handa, A.; Takada, A.; Shinoda, A.; Takeuchi, J. Intrarenal vascular changes with age and disease. Kidney Int. 1972, 2, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Lieske, J.C.; Chakkera, H.A.; Poggio, E.D.; Alexander, M.P.; Singh, P.; Kremers, W.K.; Lerman, L.O.; Rule, A.D. The substantial loss of nephrons in healthy human kidneys with aging. J. Am. Soc. Nephrol. JASN 2017, 28, 313. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Zager, R.A. Plasma and urinary p21: Potential biomarkers of AKI and renal aging. Am. J. Physiol. Ren. Physiol. 2018, 315, F1329–F1335. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Black, M.J.; Sutherland, M.R.; Gubhaju, L.; Kent, A.L.; Dahlstrom, J.E.; Moore, L. When birth comes early: Effects on nephrogenesis. Nephrology 2013, 18, 180–182. [Google Scholar] [CrossRef]
- Sutherland, M.R.; Gubhaju, L.; Moore, L.; Moore, L.; Kent, A.L.; Dahlstrom, J.E.; Horne, R.S.; Hoy, W.E.; Bertram, J.F.; Black, M.J. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J. Am. Soc. Nephrol. JASN 2011, 22, 1365. [Google Scholar] [CrossRef]
- Hill, C.; Duffy, S.; Kettyle, L.M.; McGlynn, L.; Sandholm, N.; Salem, R.M.; Thompson, A.; Swan, E.J.; Kilner, J.; Rossing, P. Differential methylation of telomere-related genes is associated with kidney disease in individuals with type 1 diabetes. Genes 2023, 14, 1029. [Google Scholar] [CrossRef]
- Parkinson, J.R.; Emsley, R.; Adkins, J.L.T.; Longford, N.; Ozanne, S.E.; Holmes, E.; Modi, N. Clinical and molecular evidence of accelerated ageing following very preterm birth. Pediatr. Res. 2020, 87, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.F. Pathophysiological Implications and Therapeutic Approach of Klotho in Chronic Kidney Disease. A Systematic Review. Lab. Investig. 2023, 103, 100178. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Shaltout, H.A.; Gwathmey, T.M.; Jensen, E.T.; Nixon, P.A.; Diz, D.I.; Chappell, M.C.; Washburn, L.K. Lower urinary α-Klotho is associated with lower angiotensin-(1-7) and higher blood pressure in young adults born preterm with very low birthweight. J. Clin. Hypertens. 2020, 22, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, J.H.; Hilgers, K.F.; Steinbach, M.P.; Hartner, A.; Klanke, B.; Amann, K.; Melk, A. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16 INK4a. Hypertension 2008, 52, 123–129. [Google Scholar] [CrossRef]
- South, A.M.; Nixon, P.A.; Chappell, M.C.; Diz, D.I.; Russell, G.B.; Jensen, E.T.; Shaltout, H.A.; O’Shea, T.M.; Washburn, L.K. Renal function and blood pressure are altered in adolescents born preterm. Pediatr. Nephrol. 2019, 34, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Du, P.Y.; Gandhi, A.; Bawa, M.; Gromala, J. The ageing immune system as a potential target of senolytics. Oxf. Open Immunol. 2023, 4, iqad004. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- Fard, M.T.; Savage, K.M.; Stough, C.K. Peripheral inflammation marker relationships to cognition in healthy older adults-A systematic review. Psychoneuroendocrinology 2022, 144, 105870. [Google Scholar] [CrossRef]
- Zeeh, J.; Platt, D. The aging liver: Structural and functional changes and their consequences for drug treatment in old age. Gerontology 2002, 48, 121–127. [Google Scholar] [CrossRef]
- Elliott, M.L.; Belsky, D.W.; Knodt, A.R.; Ireland, D.; Melzer, T.R.; Poulton, R.; Ramrakha, S.; Caspi, A.; Moffitt, T.E.; Hariri, A.R. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol. Psychiatry 2021, 26, 3829–3838. [Google Scholar] [CrossRef]
- Baker, G.T., III; Sprott, R.L. Biomarkers of aging. Exp. Gerontol. 1988, 23, 223–239. [Google Scholar] [CrossRef]
- Consortium, A.B.; Bao, H.; Cao, J.; Chen, M.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Y. Biomarkers of aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar]
- Levine, M.E. Assessment of Epigenetic Clocks as Biomarkers of Aging in Basic and Population Research; Oxford University Press: Cary, NC, USA, 2020; Volume 75, pp. 463–465. [Google Scholar]
- Ryan, C.P. “Epigenetic clocks”: Theory and applications in human biology. Am. J. Hum. Biol. 2021, 33, e23488. [Google Scholar] [CrossRef] [PubMed]
- Bocklandt, S.; Lin, W.; Sehl, M.E.; Sánchez, F.J.; Sinsheimer, J.S.; Horvath, S.; Vilain, E. Epigenetic predictor of age. PLoS ONE 2011, 6, e14821. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choufani, S.; Weksberg, R.; Wilson, S.L.; Yuan, V.; Burt, A.; Marsit, C.; Lu, A.T.; Ritz, B.; Bohlin, J. Placental epigenetic clocks: Estimating gestational age using placental DNA methylation levels. Aging 2019, 11, 4238. [Google Scholar] [CrossRef] [PubMed]
- Oeseburg, H.; de Boer, R.A.; Van Gilst, W.H.; van der Harst, P. Telomere biology in healthy aging and disease. Pflügers Arch. Eur. J. Physiol. 2010, 459, 259–268. [Google Scholar] [CrossRef]
- Joosten, S.A.; van Ham, V.; Nolan, C.E.; Borrias, M.C.; Jardine, A.G.; Shiels, P.G.; van Kooten, C.; Paul, L.C. Telomere shortening and cellular senescence in a model of chronic renal allograft rejection. Am. J. Pathol. 2003, 162, 1305–1312. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Booth, T.; Starr, J.M.; Deary, I. Modeling multisystem biological risk in later life: Allostatic load in the Lothian birth cohort study 1936. Am. J. Hum. Biol. 2013, 25, 538–543. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic load and its impact on health: A systematic review. Psychother. Psychosom. 2020, 90, 11–27. [Google Scholar] [CrossRef]
- McEwen, B.S.; Stellar, E. Stress and the individual: Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.E.; Singer, B.H.; Rowe, J.W.; Horwitz, R.I.; McEwen, B.S. Price of adaptation—Allostatic load and its health consequences: MacArthur studies of successful aging. Arch. Intern. Med. 1997, 157, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.E.; McEwen, B.S.; Rowe, J.W.; Singer, B.H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl. Acad. Sci. USA 2001, 98, 4770–4775. [Google Scholar] [CrossRef]
- Casavant, S.G.; Cong, X.; Fitch, R.H.; Moore, J.; Rosenkrantz, T.; Starkweather, A. Allostatic load and biomarkers of stress in the preterm infant: An integrative review. Biol. Res. Nurs. 2019, 21, 210–223. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.R. Redefining chronic inflammation in aging and age-related diseases: Proposal of the senoinflammation concept. Aging Dis. 2019, 10, 367. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Göpel, W.; Härtel, C.; German Center for Lung Research and Priming Immunity at the beginning of life (PRIMAL) Consortium. Preterm birth and sustained inflammation: Consequences for the neonate. Semin. Immunopathol. 2020, 42, 451–468. [Google Scholar] [CrossRef]
- Oldenburg, K.S.; O’Shea, T.M.; Fry, R.C. Genetic and epigenetic factors and early life inflammation as predictors of neurodevelopmental outcomes. Semin. Fetal Neonatal Med. 2020, 25, 101115. [Google Scholar] [CrossRef]
- Srivastava, K.K.; Kumar, R. Stress, oxidative injury and disease. Indian J. Clin. Biochem. 2015, 30, 3–10. [Google Scholar] [CrossRef]
- Lee, J.W.; Davis, J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011, 23, 161. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Grassi, F.; Caporilli, C.; Boscarino, G.; Carbone, G.; Petrolini, C.; Gambini, L.M.; Di Peri, A.; Moretti, S.; Buonocore, G.; et al. Brain Damage in Preterm and Full-Term Neonates: Serum Biomarkers for the Early Diagnosis and Intervention. Antioxidants 2023, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Katsu, Y.; Baker, M.E. Cortisol. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2021; pp. 947–949. [Google Scholar]
- Stalder, T.; Kirschbaum, C.; Kudielka, B.M.; Adam, E.K.; Pruessner, J.C.; Wüst, S.; Dockray, S.; Smyth, N.; Evans, P.; Hellhammer, D.H. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef]
- Inder, W.J.; Dimeski, G.; Russell, A. Measurement of salivary cortisol in 2012—Laboratory techniques and clinical indications. Clin. Endocrinol. 2012, 77, 645–651. [Google Scholar] [CrossRef]
- Dorn, L.D.; Lucke, J.F.; Loucks, T.L.; Berga, S.L. Salivary cortisol reflects serum cortisol: Ana#lysis of circadian profiles. Ann. Clin. Biochem. 2007, 44, 281–284. [Google Scholar]
- Mörelius, E.; He, H.-G.; Shorey, S. Salivary cortisol reactivity in preterm infants in neonatal intensive care: An integrative review. Int. J. Environ. Res. Public Health 2016, 13, 337. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The hypothalamic-pituitary-adrenal axis: Development, programming actions of hormones, and maternal-fetal interactions. Front. Behav. Neurosci. 2021, 14, 256. [Google Scholar] [CrossRef]
- Brummelte, S.; Chau, C.M.; Cepeda, I.L.; Degenhardt, A.; Weinberg, J.; Synnes, A.R.; Grunau, R.E. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology 2015, 51, 151–163. [Google Scholar] [CrossRef]
- Zyriax, B.-C.; Windler, E. Lifestyle changes to prevent cardio-and cerebrovascular disease at midlife: A systematic review. Maturitas 2023, 167, 60–65. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I. The future of aging interventions: Aging intervention, prevention, and therapy through hormesis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, B705–B709. [Google Scholar] [CrossRef] [PubMed]
- Mogic, L.; Rutter, E.C.; Tyas, S.L.; Maxwell, C.J.; O’Connell, M.E.; Oremus, M. Functional social support and cognitive function in middle-and older-aged adults: A systematic review of cross-sectional and cohort studies. Syst. Rev. 2023, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, P.; Oremus, M. Examining the association between functional social support, marital status, and memory: A systematic review. BMC Geriatr. 2023, 23, 290. [Google Scholar] [CrossRef]
- Yoo, S.S.; Tyas, S.L.; Maxwell, C.J.; Oremus, M. The association between functional social support and memory in middle-aged and older adults: A Prospective Analysis of the Canadian Longitudinal Study on Aging’s Comprehensive Cohort. Arch. Gerontol. Geriatr. 2023, 114, 105076. [Google Scholar] [CrossRef]
- Duffner, L.A.; DeJong, N.; Jansen, J.F.; Backes, W.; de Vugt, M.; Deckers, K.; Köhler, S. Associations between social health factors, cognitive activity and neurostructural markers for brain health—A systematic literature review and meta-analysis. Ageing Res. Rev. 2023, 89, 101986. [Google Scholar] [CrossRef]
- Hieber, C.; Grabbe, S.; Bros, M. Counteracting Immunosenescence—Which Therapeutic Strategies Are Promising? Biomolecules 2023, 13, 1085. [Google Scholar] [CrossRef]
- Belloc, N.B.; Breslow, L. Relationship of physical health status and health practices. Prev. Med. 1972, 1, 409–421. [Google Scholar] [CrossRef]
- Bortz, W.M. Effect of exercise on aging—Effect of aging on exercise. J. Am. Geriatr. Soc. 1980, 28, 49–51. [Google Scholar] [CrossRef]
- Wolkove, N.; Elkholy, O.; Baltzan, M.; Palayew, M. Sleep and aging: 1. Sleep disorders commonly found in older people. Cmaj 2007, 176, 1299–1304. [Google Scholar] [CrossRef]
- Hutchinson, M.L. Nutrition and Aging; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Ames, B.N. Micronutrients prevent cancer and delay aging. Toxicol. Lett. 1998, 102, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R.; Sohal, R.S. Caloric intake and aging. N. Engl. J. Med. 1997, 337, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. Exercise, nutrition, and aging. Clin. Geriatr. Med. 1995, 11, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Tzankoff, S.P.; Norris, A.H. Longitudinal changes in basal metabolism in man. J. Appl. Physiol. 1978, 45, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Martens, D.S.; Cox, B.; Janssen, B.G.; Clemente, D.B.; Gasparrini, A.; Vanpoucke, C.; Lefebvre, W.; Roels, H.A.; Plusquin, M.; Nawrot, T.S. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr. 2017, 171, 1160–1167. [Google Scholar] [CrossRef]
- Sarkar, S.M.; Dhar, B.K.; Fahlevi, M.; Ahmed, S.; Hossain, M.J.; Rahman, M.M.; Gazi, M.A.I.; Rajamani, R. Climate Change and Aging Health in Developing Countries. Glob. Chall. 2023, 7, 2200246. [Google Scholar] [CrossRef]
- Suda, M.; Paul, K.H.; Minamino, T.; Miller, J.D.; Lerman, A.; Ellison-Hughes, G.M.; Tchkonia, T.; Kirkland, J.L. Senescent Cells: A Therapeutic Target in Cardiovascular Diseases. Cells 2023, 12, 1296. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Palmer, A.K.; Jensen, M.D. Metabolic changes in aging humans: Current evidence and therapeutic strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousquet, A.; Sanderson, K.; O’Shea, T.M.; Fry, R.C. Accelerated Aging and the Life Course of Individuals Born Preterm. Children 2023, 10, 1683. https://doi.org/10.3390/children10101683
Bousquet A, Sanderson K, O’Shea TM, Fry RC. Accelerated Aging and the Life Course of Individuals Born Preterm. Children. 2023; 10(10):1683. https://doi.org/10.3390/children10101683
Chicago/Turabian StyleBousquet, Audrey, Keia Sanderson, T. Michael O’Shea, and Rebecca C. Fry. 2023. "Accelerated Aging and the Life Course of Individuals Born Preterm" Children 10, no. 10: 1683. https://doi.org/10.3390/children10101683
APA StyleBousquet, A., Sanderson, K., O’Shea, T. M., & Fry, R. C. (2023). Accelerated Aging and the Life Course of Individuals Born Preterm. Children, 10(10), 1683. https://doi.org/10.3390/children10101683





