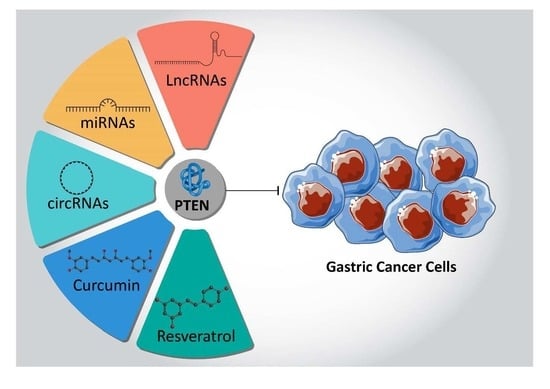
PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation
Abstract
1. Introduction
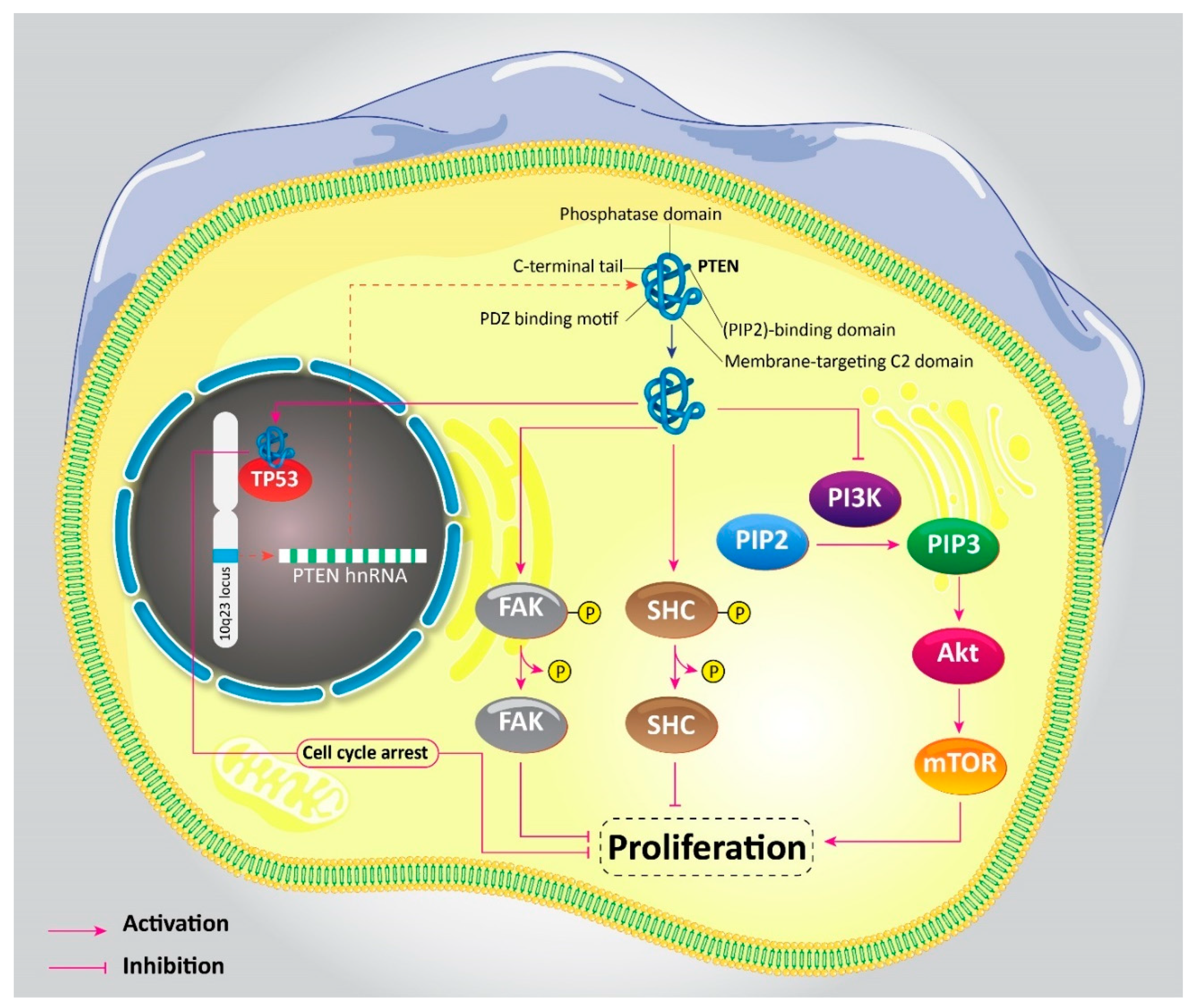
2. PTEN Signaling: An Overview
3. PTEN Pathway in Oncology
4. PTEN in Clinical Studies
5. PTEN and Gastric Cancer
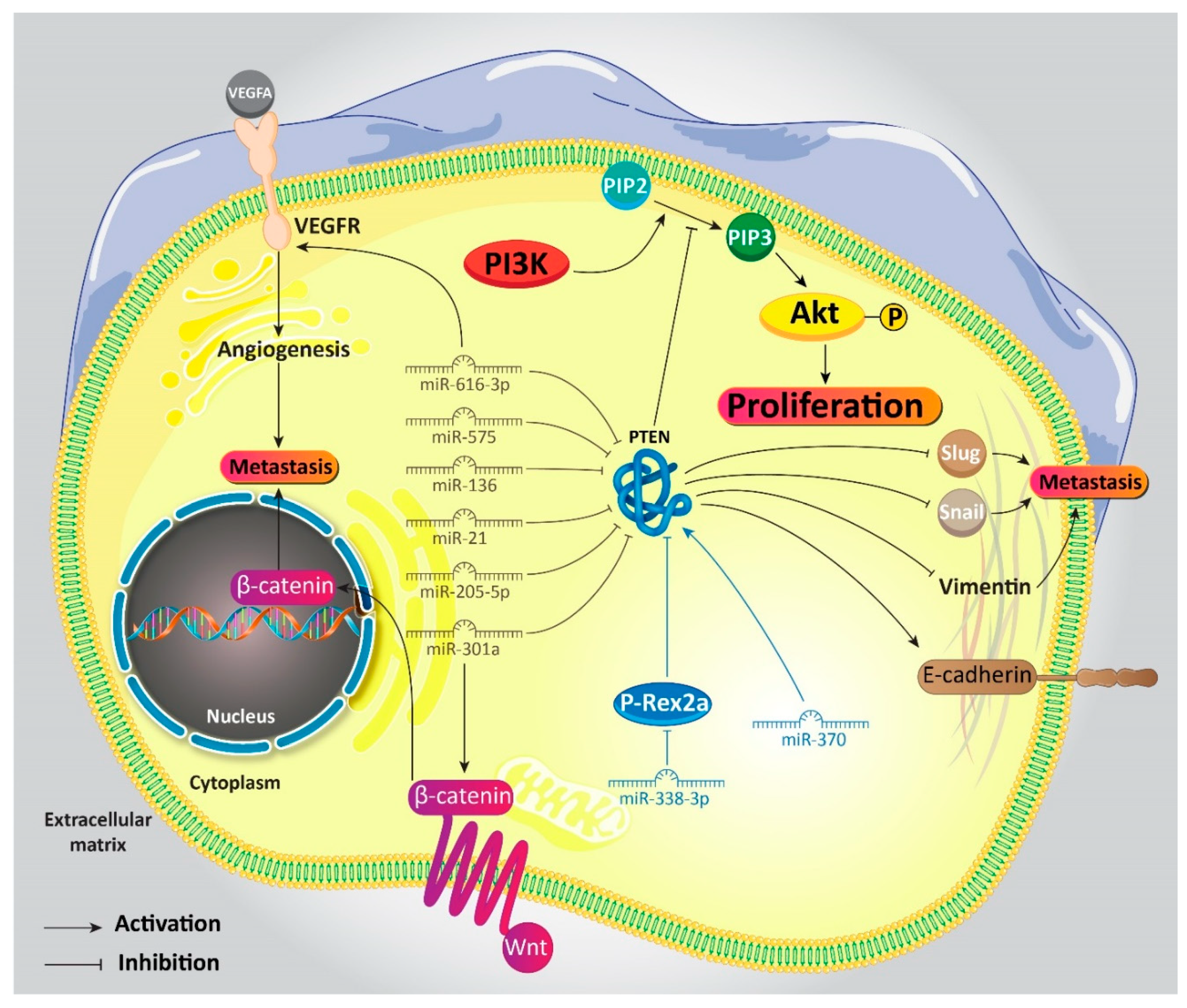
5.1. MicroRNAs Target PTEN
5.1.1. MicroRNAs and PTEN Induction
5.1.2. MicroRNAs and PTEN Inhibition
5.2. MicroRNAs, PTEN, Drug-Therapy, and Radio-Therapy
5.3. CircularRNAs Target PTEN
5.4. LncRNAs Target PTEN
6. Antitumor Compounds Target PTEN
- The antitumor compounds are able to affect PTEN in a time- and dose-dependent manners,
- They suppress the PI3K/Akt signaling pathway [204],
- They are capable of regulating upstream mediators of PTEN, such as HDAC1 [205],
- They inhibit the development of chemoresistance in GC cells [206],
- And, finally, they interfere with proliferation and invasion of GC cells via targeting PTEN [207].
7. Upstream Modulators of PTEN
7.1. Proliferation of Gastric Cancer Cells
7.2. Metastasis of Gastric Cancer Cells
7.3. Drug Resistance of Gastric Cancer Cells
8. Activation and Deactivation of PTEN in Gastric Cancer
9. Conclusions and Remarks
Funding
Conflicts of Interest
Abbreviations
| PTEN | phosphatase and tensin homolog |
| GC | gastric cancer |
| PIP2 | phosphatidylinositol 4-5-diphosphate |
| TRPV4 | transient receptor potential vanilloid type 4 |
| NF-B | nuclear factor-kappaB |
| lncRNA | long non-coding RNA |
| pCR | pathologic complete response |
| ncRNAs | non-coding RNAs |
| EMT | epithelial-to-mesenchymal transition |
| GEF | guanine nucleotide exchange factor |
| circRNAs | circular RNAs |
| MMPs | matrix metalloproteinases |
| ceRMAs | competing endogenous RNAs |
| Res | resveratrol |
| CagA | cytotoxin-associated gene A |
| H. pylori | Helicobacter pylori |
| RSRC1 | arginine/serine-rich coiled coil-1 |
| KLFs | Krüppel-like factors |
| TET1 | Ten-Eleven Translocation 1 |
References
- Islami, F.; Siegel, R.L.; Jemal, A. The changing landscape of cancer in the USA—Opportunities for advancing prevention and treatment. Nat. Rev. Clin. Oncol. 2020, 1–19. [Google Scholar] [CrossRef]
- Yue, B.; Song, C.; Yang, L.; Cui, R.; Cheng, X.; Zhang, Z.; Zhao, G. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol. Cancer 2019, 18, 142. [Google Scholar] [CrossRef]
- Fang, X.; Wen, J.; Sun, M.; Yuan, Y.; Xu, Q. CircRNAs and its relationship with gastric cancer. J. Cancer 2019, 10, 6105–6113. [Google Scholar] [CrossRef]
- Jeong, M.H.; Park, S.Y.; Lee, S.H.; Seo, J.; Yoo, J.Y.; Park, S.H.; Kim, M.J.; Lee, S.; Jang, S.; Choi, H.K.; et al. EPB41L5 Mediates TGFβ-Induced Metastasis of Gastric Cancer. Clin. Cancer Res. 2019, 25, 3617–3629. [Google Scholar] [CrossRef]
- Yang, F.; Hu, A.; Li, D.; Wang, J.; Guo, Y.; Liu, Y.; Li, H.; Chen, Y.; Wang, X.; Huang, K.; et al. Circ-HuR suppresses HuR expression and gastric cancer progression by inhibiting CNBP transactivation. Mol. Cancer 2019, 18, 158. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Wang, T.; Su, S.; Shen, L.T.; Zhu, G.X.; Liu, Q.; Zhang, L.; Liu, K.W.; Zhang, Y.; Zhou, Z.H.; et al. BRD4 Promotes Gastric Cancer Progression and Metastasis through Acetylation-Dependent Stabilization of Snail. Cancer Res. 2019, 79, 4869–4881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Huang, S.; Deng, C.; Zhou, S.; Yang, J.; Cao, Y.; Xu, L.; Yuan, Y.; Yang, J.; et al. SIRT1 inhibits gastric cancer proliferation and metastasis via STAT3/MMP-13 signaling. J. Cell. Physiol. 2019, 234, 15395–15406. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ding, Y.; Zong, W.; Ju, S. Non-coding RNAs in regulating gastric cancer metastasis. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 496, 125–133. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, S.; Piao, H.Y.; Wang, Y.; Wu, Y.; Meng, X.Y.; Yang, D.; Zheng, Z.C.; Zhao, Y. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J. Physiol. Biochem. 2019, 75, 379–389. [Google Scholar] [CrossRef]
- Chen, E.B.; Qin, X.; Peng, K.; Li, Q.; Tang, C.; Wei, Y.C.; Yu, S.; Gan, L.; Liu, T.S. HnRNPR-CCNB1/CENPF axis contributes to gastric cancer proliferation and metastasis. Aging 2019, 11, 7473–7491. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhao, Y.; Liu, Y.; Fan, L.; Jia, N.; Zhao, Q. ILF3 promotes gastric cancer proliferation and may be used as a prognostic marker. Mol. Med. Rep. 2019, 20, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, H.Y.; Wu, Y.; Zheng, Z.C.; Guo, S.; Wang, Y.; Yang, D.; Meng, X.Y.; Xu, X.; Zhao, Y. Hypoxia-induced LncRNA PCGEM1 promotes invasion and metastasis of gastric cancer through regulating SNAI1. Clin. Transl. Oncol. 2019, 21, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Lv, J.; Liu, S.; Sun, L.; Wang, Y.; Li, H.; Qi, W.; Qiu, W. TSPAN9 and EMILIN1 synergistically inhibit the migration and invasion of gastric cancer cells by increasing TSPAN9 expression. BMC Cancer 2019, 19, 630. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Q.; Jiang, M.; Li, S.; Zhang, J.; Xu, Z.; Guo, D.; Gu, T.; Wang, B.; Xiao, L.; et al. KLF9 suppresses gastric cancer cell invasion and metastasis through transcriptional inhibition of MMP28. FASEB J. 2019, 33, 7915–7928. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Samarghandian, S.; Najafi, M. PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur. J. Pharmacol. 2020, 173226. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Calegari, M.A.; Loupakis, F.; Fassan, M.; Di Stefano, B.; Bensi, M.; Bria, E.; Tortora, G. PTEN in Colorectal Cancer: Shedding Light on Its Role as Predictor and Target. Cancers 2019, 11, 1765. [Google Scholar] [CrossRef]
- Luongo, F.; Colonna, F.; Calapà, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN tumor-suppressor: The dam of stemness in cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Hao, Y.; Lin, H.; Dong, M.; Ye, J.; Song, L.; Wang, Y.; Li, Q.; Shan, B.; et al. Myeloid PTEN promotes chemotherapy-induced NLRP3-inflammasome activation and antitumour immunity. Nat. Cell Biol. 2020, 22, 716–727. [Google Scholar] [CrossRef]
- Lee, Y.R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.Y.; Fu, T.M.; Chen, H.; Ishikawa, T.; Chiang, S.Y.; Katon, J.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sun, L.; Han, J.; Shi, S.; Dai, Y.; Liu, W. RILPL2 regulates breast cancer proliferation, metastasis, and chemoresistance via the TUBB3/PTEN pathway. Am. J. Cancer Res. 2019, 9, 1583–1606. [Google Scholar]
- Gkountakos, A.; Sartori, G.; Falcone, I.; Piro, G.; Ciuffreda, L.; Carbone, C.; Tortora, G.; Scarpa, A.; Bria, E.; Milella, M. PTEN in lung cancer: Dealing with the problem, building on new knowledge and turning the game around. Cancers 2019, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Alimonti, A.; Carracedo, A.; Clohessy, J.G.; Trotman, L.C.; Nardella, C.; Egia, A.; Salmena, L.; Sampieri, K.; Haveman, W.J.; Brogi, E. Subtle variations in Pten dose determine cancer susceptibility. Nat. Genet. 2010, 42, 454. [Google Scholar] [CrossRef] [PubMed]
- Shen-Li, H.; Koujak, S.; Szablocs, M.; Parsons, R. Reduction of Pten dose leads to neoplastic development in multiple organs of PtenshRNA mice. Cancer Biol. Ther. 2010, 10, 1194–1200. [Google Scholar] [CrossRef]
- Furnari, F.B.; Huang, H.S.; Cavenee, W.K. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998, 58, 5002–5008. [Google Scholar]
- Kotelevets, L.; van Hengel, J.; Bruyneel, E.; Mareel, M.; Van Roy, F.; Chastre, E. The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J. Cell Biol. 2001, 155, 1129–1136. [Google Scholar] [CrossRef]
- Morais, C.E.; Gurgel, D.C.; Teixeira, A.C.; Mattos, T.V.A.; Silva, A.; Tavora, F. Prevalence of ERG expression and PTEN loss in a Brazilian prostate cancer cohort. Braz. J. Med. Biol. Res. 2019, 52, e8483. [Google Scholar] [CrossRef]
- Carbognin, L.; Miglietta, F.; Paris, I.; Dieci, M.V. Prognostic and Predictive Implications of PTEN in Breast Cancer: Unfulfilled Promises but Intriguing Perspectives. Cancers 2019, 11, 1401. [Google Scholar] [CrossRef]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, J.P.; Guo, W.; Wang, D.Q.; Yao, A.; Zhang, H.M.; Huang, F.; Li, H.H.; Dai, Z.T.; Zhang, Z.J.; et al. Novel interactions between ERα-36 and STAT3 mediate breast cancer cell migration. Cell Commun. Signal. 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Jayamohan, S.; Moorthy, R.K.; Chabattula, S.C.; Ganeshan, M.; Arockiam, A.J.V. AEG-1/miR-221 Axis Cooperatively Regulates the Progression of Hepatocellular Carcinoma by Targeting PTEN/PI3K/AKT Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 5526. [Google Scholar] [CrossRef]
- Ling, C.; Wang, X.; Zhu, J.; Tang, H.; Du, W.; Zeng, Y.; Sun, L.; Huang, J.A.; Liu, Z. MicroRNA-4286 promotes cell proliferation, migration, and invasion via PTEN regulation of the PI3K/Akt pathway in non-small cell lung cancer. Cancer Med. 2019, 8, 3520–3531. [Google Scholar] [CrossRef]
- Pang, Y.; Wu, J.; Li, X.; Wang, C.; Wang, M.; Liu, J.; Yang, G. NEAT1/miR-124/STAT3 feedback loop promotes breast cancer progression. Int. J. Oncol. 2019, 55, 745–754. [Google Scholar] [CrossRef]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef]
- Ko, J.H.; Nam, D.; Um, J.Y.; Jung, S.H.; Sethi, G.; Ahn, K.S. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Molecules 2018, 23, 1601. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3, 4, 5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Monisha, J.; Roy, N.K.; Padmavathi, G.; Banik, K.; Bordoloi, D.; Khwairakpam, A.D.; Arfuso, F.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. NGAL is Downregulated in Oral Squamous Cell Carcinoma and Leads to Increased Survival, Proliferation, Migration and Chemoresistance. Cancers 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar] [CrossRef]
- Yang, M.H.; Lee, J.H.; Ko, J.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules 2019, 24, 1584. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef]
- Shen, W.H.; Balajee, A.S.; Wang, J.; Wu, H.; Eng, C.; Pandolfi, P.P.; Yin, Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 2007, 128, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Kumagai, T.; Vora, J.; Bose, N.; Sehgal, I.; Koeffler, P.H.; Bose, S. PTEN promoter is methylated in a proportion of invasive breast cancers. Int. J. Cancer 2004, 112, 407–410. [Google Scholar] [CrossRef]
- Mirmohammadsadegh, A.; Marini, A.; Nambiar, S.; Hassan, M.; Tannapfel, A.; Ruzicka, T.; Hengge, U.R. Epigenetic silencing of the PTEN gene in melanoma. Cancer Res. 2006, 66, 6546–6552. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, H.B.; MacDonald, N.; Ryan, A.; Jacobs, I.J.; Lynch, E.D.; Akslen, L.A.; Das, S. PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int. J. Cancer 2001, 91, 22–26. [Google Scholar] [CrossRef]
- Soria, J.-C.; Lee, H.-Y.; Lee, J.I.; Wang, L.; Issa, J.-P.; Kemp, B.L.; Liu, D.D.; Kurie, J.M.; Mao, L.; Khuri, F.R. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin. Cancer Res. 2002, 8, 1178–1184. [Google Scholar]
- Zhang, J.; Lee, Y.-R.; Dang, F.; Gan, W.; Menon, A.V.; Katon, J.M.; Hsu, C.-H.; Asara, J.M.; Tibarewal, P.; Leslie, N.R. PTEN Methylation by NSD2 Controls Cellular Sensitivity to DNA Damage. Cancer Discov. 2019, 9, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, D. PI (3) king apart PTEN’s role in cancer. Clin. Cancer Res. 2010, 16, 4325–4330. [Google Scholar] [CrossRef] [PubMed]
- Bazzichetto, C.; Conciatori, F.; Pallocca, M.; Falcone, I.; Fanciulli, M.; Cognetti, F.; Milella, M.; Ciuffreda, L. PTEN as a prognostic/predictive biomarker in cancer: An unfulfilled promise? Cancers 2019, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Alimonti, A.; Pandolfi, P.P. PTEN level in tumor suppression: How much is too little? Cancer Res. 2011, 71, 629–633. [Google Scholar] [CrossRef]
- Trotman, L.C.; Niki, M.; Dotan, Z.A.; Koutcher, J.A.; Di Cristofano, A.; Xiao, A.; Khoo, A.S.; Roy-Burman, P.; Greenberg, N.M.; Van Dyke, T. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003, 1. [Google Scholar] [CrossRef]
- Todorova, P.K.; Fletcher-Sananikone, E.; Mukherjee, B.; Kollipara, R.; Vemireddy, V.; Xie, X.J.; Guida, P.M.; Story, M.D.; Hatanpaa, K.; Habib, A.A.; et al. Radiation-Induced DNA Damage Cooperates with Heterozygosity of TP53 and PTEN to Generate High-Grade Gliomas. Cancer Res. 2019, 79, 3749–3761. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xie, C.; Sham, K.W.Y.; Ng, S.S.M.; Chen, Y.; Cheng, C.H.K. Activation of PTEN by inhibition of TRPV4 suppresses colon cancer development. Cell Death Dis. 2019, 10, 460. [Google Scholar] [CrossRef]
- Bowen, C.; Ostrowski, M.C.; Leone, G.; Gelmann, E.P. Loss of PTEN Accelerates NKX3.1 Degradation to Promote Prostate Cancer Progression. Cancer Res. 2019, 79, 4124–4134. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, M.; Rohatgi, N.; Kaur, J.; Shishodia, S.; Sethi, G.; Gupta, S.D.; Deo, S.V.; Shukla, N.K.; Aggarwal, B.B.; Ralhan, R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer 2007, 120, 2545–2556. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor-kappaB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008, 7, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Man, X.; Piao, C.; Lin, X.; Kong, C.; Cui, X.; Jiang, Y. USP13 functions as a tumor suppressor by blocking the NF-kB-mediated PTEN downregulation in human bladder cancer. J. Exp. Clin. Cancer Res. 2019, 38, 259. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, B.; Zhang, P.; Zhang, J.; Wang, L. LncRNA TUSC8 inhibits the invasion and migration of cervical cancer cells via miR-641/PTEN axis. Cell Biol. Int. 2019, 43, 781–788. [Google Scholar] [CrossRef]
- Ma, J.; Sun, X.; Wang, Y.; Chen, B.; Qian, L.; Wang, Y. Fibroblast-derived CXCL12 regulates PTEN expression and is associated with the proliferation and invasion of colon cancer cells via PI3k/Akt signaling. Cell Commun. Signal. 2019, 17, 119. [Google Scholar] [CrossRef]
- Hamid, A.A.; Gray, K.P.; Huang, Y.; Bowden, M.; Pomerantz, M.; Loda, M.; Sweeney, C.J. Loss of PTEN Expression Detected by Fluorescence Immunohistochemistry Predicts Lethal Prostate Cancer in Men Treated with Prostatectomy. Eur. Urol. Oncol. 2019, 2, 475–482. [Google Scholar] [CrossRef]
- Li, G.; Guo, X.; Chen, M.; Tang, L.; Jiang, H.; Day, J.X.; Xie, Y.; Peng, L.; Xu, X.; Li, J.; et al. Prevalence and spectrum of AKT1, PIK3CA, PTEN and TP53 somatic mutations in Chinese breast cancer patients. PLoS ONE 2018, 13, e0203495. [Google Scholar] [CrossRef]
- Rimawi, M.F.; De Angelis, C.; Contreras, A.; Pareja, F.; Geyer, F.C.; Burke, K.A.; Herrera, S.; Wang, T.; Mayer, I.A.; Forero, A.; et al. Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer. Breast Cancer Res. Treat. 2018, 167, 731–740. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, J.K.; Lin, H.H.; Lan, Y.T.; Lin, C.C.; Yang, S.H.; Chen, W.S.; Liang, W.Y.; Jiang, J.K.; Chang, S.C. A comprehensive analysis of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) loss in colorectal cancer. World J. Surg. Oncol. 2015, 13, 186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snietura, M.; Jaworska, M.; Mlynarczyk-Liszka, J.; Goraj-Zajac, A.; Piglowski, W.; Lange, D.; Wozniak, G.; Nowara, E.; Suwinski, R. PTEN as a prognostic and predictive marker in postoperative radiotherapy for squamous cell cancer of the head and neck. PLoS ONE 2012, 7, e33396. [Google Scholar] [CrossRef] [PubMed]
- Stern, H.M.; Gardner, H.; Burzykowski, T.; Elatre, W.; O’Brien, C.; Lackner, M.R.; Pestano, G.A.; Santiago, A.; Villalobos, I.; Eiermann, W.; et al. PTEN Loss Is Associated with Worse Outcome in HER2-Amplified Breast Cancer Patients but Is Not Associated with Trastuzumab Resistance. Clin. Cancer Res. 2015, 21, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sarkissyan, M.; Elshimali, Y.; Vadgama, J.V. Triple negative breast tumors in African-American and Hispanic/Latina women are high in CD44+, low in CD24+, and have loss of PTEN. PLoS ONE 2013, 8, e78259. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Martin, N.E.; Fiorentino, M.; Flavin, R.; Lis, R.T.; Sinnott, J.A.; Finn, S.; Penney, K.L.; Ma, J.; Fazli, L.; et al. Protein expression of PTEN, insulin-like growth factor I receptor (IGF-IR), and lethal prostate cancer: A prospective study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Taghavipour, M.; Sadoughi, F.; Mirzaei, H.; Yousefi, B.; Moazzami, B.; Chaichian, S.; Mansournia, M.A.; Asemi, Z. Apoptotic functions of microRNAs in pathogenesis, diagnosis, and treatment of endometriosis. Cell Biosci. 2020, 10, 12. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Mahjoubin-Tehran, M.; Karimzadeh, M.R.; Mirzaei, H.R.; Razavi, Z.S.; Sahebkar, A.; Hosseini, N.; Mirzaei, H.; Hamblin, M.R. Autophagy in cancers including brain tumors: Role of MicroRNAs. Cell Commun. Signal. 2020, 18, 88. [Google Scholar] [CrossRef]
- Yousefi, F.; Shabaninejad, Z.; Vakili, S.; Derakhshan, M.; Movahedpour, A.; Dabiri, H.; Ghasemi, Y.; Mahjoubin-Tehran, M.; Nikoozadeh, A.; Savardashtaki, A.; et al. TGF-β and WNT signaling pathways in cardiac fibrosis: Non-coding RNAs come into focus. Cell Commun. Signal. 2020, 18, 87. [Google Scholar] [CrossRef]
- Hallajzadeh, J.; Amirani, E.; Mirzaei, H.; Shafabakhsh, R.; Mirhashemi, S.M.; Sharifi, M.; Yousefi, B.; Mansournia, M.A.; Asemi, Z. Circular RNAs: New genetic tools in melanoma. Biomark. Med. 2020, 14, 7. [Google Scholar] [CrossRef]
- Nahand, J.S.; Karimzadeh, M.R.; Nezamnia, M.; Fatemipour, M.; Khatami, A.; Jamshidi, S.; Moghoofei, M.; Taghizadieh, M.; Hajighadimi, S.; Shafiee, A.; et al. The role of miR-146a in viral infection. Iubmb Life 2020, 72, 343–360. [Google Scholar] [CrossRef]
- Nahand, J.S.; Mahjoubin-Tehran, M.; Moghoofei, M.; Pourhanifeh, M.H.; Mirzaei, H.R.; Asemi, Z.; Khatami, A.; Bokharaei-Salim, F.; Mirzaei, H.; Hamblin, M.R. Exosomal miRNAs: Novel players in viral infection. Epigenomics 2020, 12, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Naeli, P.; Yousefi, F.; Ghasemi, Y.; Savardashtaki, A.; Mirzaei, H. The Role of MicroRNAs in Lung Cancer: Implications for Diagnosis and Therapy. Curr. Mol. Med. 2020, 20, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Javandoost, E.; Firoozi-Majd, E.; Rostamian, H.; Khakpoor- Koosheh, M.; Mirzaei, H.R. Role of microRNAs in Chronic Lymphocytic Leukemia Pathogenesis. Curr. Med. Chem. 2020, 27, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Mahjoubin-Tehran, M.; Aghaee-Bakhtiari, S.H.; Jalili, A.; Movahedpour, A.; Khan, H.; Moghoofei, M.; Shojaei, Z.; Hamblin, M.R.; Mirzaei, H. Autophagy-related MicroRNAs in Chronic Lung Diseases and Lung Cancer. Crit. Rev. Oncol./Hematol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Taheri-Anganeh, M.; Shabaninejad, Z.; Keshavarzi, A.; Taghizadeh, H.; Razavi, Z.S.; Mottaghi, R.; Abolhassan, M.; Movahedpour, A.; Mirzaei, H. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. Iubmb Life 2020, 72, 1306–1321. [Google Scholar] [CrossRef]
- Varghese, E.; Liskova, A.; Kubatka, P.; Samuel, S.M.; Büsselberg, D. Anti-Angiogenic Effects of Phytochemicals on miRNA Regulating Breast Cancer Progression. Biomolecules 2020, 10, 191. [Google Scholar] [CrossRef]
- Ramírez-Moya, J.; Wert-Lamas, L.; Santisteban, P. MicroRNA-146b promotes PI3K/AKT pathway hyperactivation and thyroid cancer progression by targeting PTEN. Oncogene 2018, 37, 3369–3383. [Google Scholar] [CrossRef]
- Jiang, J.H.; Lv, Q.Y.; Yi, Y.X.; Liao, J.; Wang, X.W.; Zhang, W. MicroRNA-200a promotes proliferation and invasion of ovarian cancer cells by targeting PTEN. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6260–6267. [Google Scholar] [CrossRef]
- He, T.; Sun, Y.; Zhang, Y.; Zhao, S.; Zheng, Y.; Hao, G.; Shi, Y. MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Reprod. Biol. Endocrinol. 2019, 17, 68. [Google Scholar] [CrossRef]
- Shi, J.; Han, Y.; Lin, D.; Wang, K.; Liu, B.; Gao, C. MicroRNA-19a promotes proliferative and migratory abilities of NSCLC cells by inhibiting PTEN expression. JBUON 2019, 24, 955–962. [Google Scholar]
- Bao, L.; Li, X. MicroRNA-32 targeting PTEN enhances M2 macrophage polarization in the glioma microenvironment and further promotes the progression of glioma. Mol. Cell. Biochem. 2019, 460, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-T.; Wang, L.; Wang, H.; Tang, F.-R.; Cai, W.-Q.; Sethi, G.; Xin, H.-W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Madamsetty, V.S.; Paul, M.K.; Mukherjee, S. Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. Int. J. Mol. Sci. 2020, 21, 455. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Ghiyami-Hour, F.; Jahangiri, S.; Negahdari, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. Nanoparticles as new tools for inhibition of cancer angiogenesis. J. Cell. Physiol. 2018, 233, 2902–2910. [Google Scholar] [CrossRef]
- Mashreghi, M.; Azarpara, H.; Bazaz, M.R.; Jafari, A.; Masoudifar, A.; Mirzaei, H.; Jaafari, M.R. Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J. Cell. Physiol. 2018, 233, 2949–2965. [Google Scholar] [CrossRef]
- Zang, C.; Sun, J.; Liu, W.; Chu, C.; Jiang, L.; Ge, R. miRNA-21 promotes cell proliferation and invasion via VHL/PI3K/AKT in papillary thyroid carcinoma. Hum. Cell 2019, 32, 428–436. [Google Scholar] [CrossRef]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The PI3k/Akt pathway is associated with angiogenesis, oxidative stress and survival of mesenchymal stem cells in pathophysiologic condition in ischemia. Physiol. Res. 2019, 68. [Google Scholar] [CrossRef]
- Wu, Z.H.; Lin, C.; Liu, C.C.; Jiang, W.W.; Huang, M.Z.; Liu, X.; Guo, W.J. MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem. Biophys. Res. Commun. 2018, 501, 1068–1073. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, G.Q.; Li, D.C. MiR-136 triggers apoptosis in human gastric cancer cells by targeting AEG-1 and BCL2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7251–7256. [Google Scholar] [CrossRef]
- Zheng, J.; Ge, P.; Liu, X.; Wei, J.; Wu, G.; Li, X. MiR-136 inhibits gastric cancer-specific peritoneal metastasis by targeting HOXC10. Tumour Biol. 2017, 39, 1010428317706207. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Z.; Chen, R. Microrna-136 promotes proliferation and invasion ingastric cancer cells through Pten/Akt/P-Akt signaling pathway. Oncol. Lett. 2018, 15, 4683–4689. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, H.; Jiang, M.; Zhang, S.; Chen, J.; Fan, X. Targeting MicroRNA-21 Suppresses Gastric Cancer Cell Proliferation and Migration via PTEN/Akt Signaling Axis. Cell Transplant. 2019, 28, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Shi, W.; Gu, J. Micro-RNA 205-5p is Involved in the Progression of Gastric Cancer and Targets Phosphatase and Tensin Homolog (PTEN) in SGC-7901 Human Gastric Cancer Cells. Med. Sci. Monit. 2019, 25, 6367–6377. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.S.; Yang, X.H.; Wang, X.D.; Wang, Y.L.; Zhou, B.; Song, Z.S. MiR-214 regulate gastric cancer cell proliferation, migration and invasion by targeting PTEN. Cancer Cell Int. 2013, 13, 68. [Google Scholar] [CrossRef]
- Li, L.; Zhu, X.; Shou, T.; Yang, L.; Cheng, X.; Wang, J.; Deng, L.; Zheng, Y. MicroRNA-28 promotes cell proliferation and invasion in gastric cancer via the PTEN/PI3K/AKT signalling pathway. Mol. Med. Rep. 2018, 17, 4003–4010. [Google Scholar] [CrossRef]
- Wang, P.; Guan, Q.; Zhou, D.; Yu, Z.; Song, Y.; Qiu, W. miR-21 Inhibitors Modulate Biological Functions of Gastric Cancer Cells via PTEN/PI3K/mTOR Pathway. DNA Cell Biol. 2018, 37, 38–45. [Google Scholar] [CrossRef]
- Zhang, B.G.; Li, J.F.; Yu, B.Q.; Zhu, Z.G.; Liu, B.Y.; Yan, M. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol. Rep. 2012, 27, 1019–1026. [Google Scholar] [CrossRef]
- Ding, K.; Wu, Z.; Wang, N.; Wang, X.; Wang, Y.; Qian, P.; Meng, G.; Tan, S. MiR-26a performs converse roles in proliferation and metastasis of different gastric cancer cells via regulating of PTEN expression. Pathol. Res. Pract. 2017, 213, 467–475. [Google Scholar] [CrossRef]
- Yang, T.S.; Yang, X.H.; Chen, X.; Wang, X.D.; Hua, J.; Zhou, D.L.; Zhou, B.; Song, Z.S. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. Febs Lett. 2014, 588, 2162–2169. [Google Scholar] [CrossRef]
- Xin, R.; Bai, F.; Feng, Y.; Jiu, M.; Liu, X.; Bai, F.; Nie, Y.; Fan, D. MicroRNA-214 promotes peritoneal metastasis through regulating PTEN negatively in gastric cancer. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 748–754. [Google Scholar] [CrossRef]
- Li, C.; Song, L.; Zhang, Z.; Bai, X.X.; Cui, M.F.; Ma, L.J. MicroRNA-21 promotes TGF-β1-induced epithelial-mesenchymal transition in gastric cancer through up-regulating PTEN expression. Oncotarget 2016, 7, 66989–67003. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Ren, H.Z.; Li, M.H.; Mei, J.H.; Wen, J.F.; Zheng, C.L. Down-regulated miRNA-214 induces a cell cycle G1 arrest in gastric cancer cells by up-regulating the PTEN protein. Pathol. Oncol. Res. 2011, 17, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, J.; Zhong, L.; Wang, L.; Liu, Y.; Wang, Y.; Peng, L.; Guo, B. Upregulated microRNA-301a in breast cancer promotes tumor metastasis by targeting PTEN and activating Wnt/β-catenin signaling. Gene 2014, 535, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fine, B.; Hodakoski, C.; Koujak, S.; Su, T.; Saal, L.H.; Maurer, M.; Hopkins, B.; Keniry, M.; Sulis, M.L.; Mense, S. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science 2009, 325, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, L.; Yao, J.; Ma, R.; Chang, D.; Li, Z.; Song, T.; Huang, C. miR-338-3p suppresses gastric cancer progression through a PTEN-AKT axis by targeting P-REX2a. Mol. Cancer Res. 2014, 12, 313–321. [Google Scholar] [CrossRef]
- Zeng, Y.; Fu, M.; Wu, G.W.; Zhang, A.Z.; Chen, J.P.; Lin, H.Y.; Fu, Y.A.; Jia, J.; Cai, Z.D.; Wu, X.J.; et al. Upregulation of microRNA-370 promotes cell apoptosis and inhibits proliferation by targeting PTEN in human gastric cancer. Int. J. Oncol. 2016, 49, 1589–1599. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capecitabine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li, F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T.; et al. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J. Biol. Chem. 2012, 287, 38028–38040. [Google Scholar] [CrossRef]
- Hwang, S.T.; Kim, C.; Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Cycloastragenol can negate constitutive STAT3 activation and promote paclitaxel-induced apoptosis in human gastric cancer cells. Phytomedicine 2019, 59, 152907. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, X.; Zhu, Y.; Wang, A.; Xiong, Y.; Wang, L.; Fei, Y.; Wang, Y.; Wang, W.; Lin, F.; et al. Cathepsin L-mediated resistance of paclitaxel and cisplatin is mediated by distinct regulatory mechanisms. J. Exp. Clin. Cancer Res. 2019, 38, 333. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tie, J.; Wang, R.; Hu, F.; Gao, L.; Wang, W.; Wang, L.; Li, Z.; Hu, S.; Tang, S.; et al. SOX2, a predictor of survival in gastric cancer, inhibits cell proliferation and metastasis by regulating PTEN. Cancer Lett. 2015, 358, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.M.; Li, X.; Qiu, C.F.; Zhu, T.; Hu, C.P.; Yin, J.Y.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. CLEC4M is associated with poor prognosis and promotes cisplatin resistance in NSCLC patients. J. Cancer 2019, 10, 6374–6383. [Google Scholar] [CrossRef]
- Hou, S.; Jin, W.; Xiao, W.; Deng, B.; Wu, D.; Zhi, J.; Wu, K.; Cao, X.; Chen, S.; Ding, Y.; et al. Integrin α5 promotes migration and cisplatin resistance in esophageal squamous cell carcinoma cells. Am. J. Cancer Res. 2019, 9, 2774–2788. [Google Scholar]
- Yang, S.M.; Huang, C.; Li, X.F.; Yu, M.Z.; He, Y.; Li, J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology 2013, 306, 162–168. [Google Scholar] [CrossRef]
- Shen, J.; Niu, W.; Zhang, H.; Jun, M.; Zhang, H. Downregulation of MicroRNA-147 Inhibits Cell Proliferation and Increases the Chemosensitivity of Gastric Cancer Cells to 5-Fluorouracil by Directly Targeting PTEN. Oncol. Res. 2018, 26, 901–911. [Google Scholar] [CrossRef]
- Ralph, S.J.; Nozuhur, S.; RA, A.L.; Rodríguez-Enríquez, S.; Moreno-Sánchez, R. Repurposing drugs as pro-oxidant redox modifiers to eliminate cancer stem cells and improve the treatment of advanced stage cancers. Med. Res. Rev. 2019, 39, 2397–2426. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, W. A Review of the Molecular Pathways Involved in Resistance to BRAF Inhibitors in Patients with Advanced-Stage Melanoma. Med. Sci. Monit. 2020, 26, e920957. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, C.; Li, J.; Xiang, X.; Zhang, L.; Deng, J.; Xiong, J. miR-21-5p confers doxorubicin resistance in gastric cancer cells by targeting PTEN and TIMP3. Int. J. Mol. Med. 2018, 41, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Li, Z.; Xiao, D.; He, G.; Bai, L.; Yang, Q. Downregulation of microRNA-193-3p inhibits tumor proliferation migration and chemoresistance in human gastric cancer by regulating PTEN gene. Tumour Biol. 2016, 37, 8941–8949. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, T.; Zhang, B.; Li, H.; Wu, Q.; Yang, L.; Nie, Y.; Wu, K.; Shi, Y.; Fan, D. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem. Biophys. Res. Commun. 2013, 434, 688–694. [Google Scholar] [CrossRef]
- Eto, K.; Iwatsuki, M.; Watanabe, M.; Ida, S.; Ishimoto, T.; Iwagami, S.; Baba, Y.; Sakamoto, Y.; Miyamoto, Y.; Yoshida, N.; et al. The microRNA-21/PTEN pathway regulates the sensitivity of HER2-positive gastric cancer cells to trastuzumab. Ann. Surg. Oncol. 2014, 21, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Shen, H.; Li, H.; Cao, Y.; Qin, R.; Long, L.; Zhu, X.; Xie, C.; Xu, W. miR-106a confers cisplatin resistance by regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim. Biophys. Sin. 2013, 45, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Zakka, K.M.; Landry, J.C.; Shaib, W.L.; Lesinski, G.B.; El-Rayes, B.F. Inhibition of HSP90 overcomes resistance to chemotherapy and radiotherapy in pancreatic cancer. Int. J. Cancer 2019, 145, 1529–1537. [Google Scholar] [CrossRef]
- Chun-Zhi, Z.; Lei, H.; An-Ling, Z.; Yan-Chao, F.; Xiao, Y.; Guang-Xiu, W.; Zhi-Fan, J.; Pei-Yu, P.; Qing-Yu, Z.; Chun-Sheng, K. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer 2010, 10, 367. [Google Scholar] [CrossRef]
- Yin, Y.; Long, J.; He, Q.; Li, Y.; Liao, Y.; He, P.; Zhu, W. Emerging roles of circRNA in formation and progression of cancer. J. Cancer 2019, 10, 5015–5021. [Google Scholar] [CrossRef]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef]
- Cocquerelle, C.; Mascrez, B.; Hétuin, D.; Bailleul, B. Mis-splicing yields circular RNA molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Danan, M.; Schwartz, S.; Edelheit, S.; Sorek, R. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res. 2012, 40, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Xu, A.; Zhang, Z.; Gao, F.; Zhao, L.; Chen, X.; Gao, J.; Kong, X. CircRNA hsa_circRNA_101996 increases cervical cancer proliferation and invasion through activating TPX2 expression by restraining miR-8075. J. Cell. Physiol. 2019, 234, 14296–14305. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Chen, B.; Song, X.; Li, Y.; Liang, Y.; Han, D.; Zhang, N.; Zhang, H.; Liu, Y.; Chen, T.; et al. circRNA_0025202 Regulates Tamoxifen Sensitivity and Tumor Progression via Regulating the miR-182-5p/FOXO3a Axis in Breast Cancer. Mol. Ther. 2019, 27, 1638–1652. [Google Scholar] [CrossRef]
- Yang, C.Y.; Zhang, F.X.; He, J.N.; Wang, S.Q. CircRNA_100876 promote proliferation and metastasis of breast cancer cells through adsorbing microRNA-361-3p in a sponge form. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6962–6970. [Google Scholar] [CrossRef]
- Ju, H.Q.; Zhao, Q.; Wang, F.; Lan, P.; Wang, Z.; Zuo, Z.X.; Wu, Q.N.; Fan, X.J.; Mo, H.Y.; Chen, L.; et al. A circRNA signature predicts postoperative recurrence in stage II/III colon cancer. Embo Mol. Med. 2019, 11, e10168. [Google Scholar] [CrossRef]
- Yang, C.; Yuan, W.; Yang, X.; Li, P.; Wang, J.; Han, J.; Tao, J.; Li, P.; Yang, H.; Lv, Q.; et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol. Cancer 2018, 17, 19. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.H.; Xu, H.Y.; Pan, H.S.; Liu, Y.; He, L. Circ_ORC2 enhances the regulatory effect of miR-19a on its target gene PTEN to affect osteosarcoma cell growth. Biochem. Biophys. Res. Commun. 2019, 514, 1172–1178. [Google Scholar] [CrossRef]
- Ren, C.; Liu, J.; Zheng, B.; Yan, P.; Sun, Y.; Yue, B. The circular RNA circ-ITCH acts as a tumour suppressor in osteosarcoma via regulating miR-22. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3359–3367. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Hu, B.; Zhang, F.; Wei, H.; Li, L. Circular RNA ZFR accelerates non-small cell lung cancer progression by acting as a miR-101-3p sponge to enhance CUL4B expression. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3410–3416. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Xu, Y.; Shu, R.; Wang, F.; Chen, C.; Zeng, Y.; Luo, H. Circular RNA-ZFR Inhibited Cell Proliferation and Promoted Apoptosis in Gastric Cancer by Sponging miR-130a/miR-107 and Modulating PTEN. Cancer Res. Treat. 2018, 50, 1396–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Ke, S.; Meng, F.K.; Lu, J.; Zou, X.J.; He, Z.G.; Wang, W.F.; Fang, M.H. CiRS-7 promotes growth and metastasis of esophageal squamous cell carcinoma via regulation of miR-7/HOXB13. Cell Death Dis. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Meng, L.; Liu, S.; Ding, P.; Chang, S.; Ju, Y.; Liu, F.; Gu, L.; Lian, Y.; Geng, C. Circular RNA ciRS-7 Maintains Metastatic Phenotypes as a ceRNA of miR-1299 to Target MMPs. Mol. Cancer Res. 2018, 16, 1665–1675. [Google Scholar] [CrossRef]
- Pan, H.; Li, T.; Jiang, Y.; Pan, C.; Ding, Y.; Huang, Z.; Yu, H.; Kong, D. Overexpression of Circular RNA ciRS-7 Abrogates the Tumor Suppressive Effect of miR-7 on Gastric Cancer via PTEN/PI3K/AKT Signaling Pathway. J. Cell. Biochem. 2018, 119, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Hou, L.; Wang, G.; Zhang, R.; Huang, Y.; Chen, X.; Zhu, J. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol. Cancer 2017, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Wang, P.L.; Gao, Y.; Liang, W.T. Circular RNA_LARP4 is lower expressed and serves as a potential biomarker of ovarian cancer prognosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7178–7182. [Google Scholar] [CrossRef]
- Lin, W.; Ye, H.; You, K.; Chen, L. Up-regulation of circ_LARP4 suppresses cell proliferation and migration in ovarian cancer by regulating miR-513b-5p/LARP4 axis. Cancer Cell Int. 2020, 20, 5. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, N.; Gu, H.; Song, Y.; Ye, Z.; Li, L.; Lu, P.; Shao, Q. Circular RNA_LARP4 Sponges miR-1323 and Hampers Progression of Esophageal Squamous Cell Carcinoma Through Modulating PTEN/PI3K/AKT Pathway. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef]
- Dai, X.; Guo, X.; Liu, J.; Cheng, A.; Peng, X.; Zha, L.; Wang, Z. Circular RNA circGRAMD1B inhibits gastric cancer progression by sponging miR-130a-3p and regulating PTEN and p21 expression. Aging 2019, 11, 9689–9708. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Mohammadi, S.; Fathullahzadeh, S.; Mirzaei, H.R.; Namdar, A.; Savardashtaki, A.; Mirzaei, H. Long Non-Coding RNAs As Epigenetic Regulators in Cancer. Curr. Pharm. Des. 2019, 25, 3563–3577. [Google Scholar] [CrossRef]
- Pal, S.; Garg, M.; Pandey, A.K. Deciphering the Mounting Complexity of the p53 Regulatory Network in Correlation to Long Non-Coding RNAs (lncRNAs) in Ovarian Cancer. Cells 2020, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Kansara, S.; Pandey, V.; Lobie, P.E.; Sethi, G.; Garg, M.; Pandey, A.K. Mechanistic Involvement of Long Non-Coding RNAs in Oncotherapeutics Resistance in Triple-Negative Breast Cancer. Cells 2020, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; He, Y.; Xia, Q.; Liu, S. LncRNA MEG3 impacts proliferation, invasion, and migration of ovarian cancer cells through regulating PTEN. Inflamm. Res. 2018, 67, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wan, Y.; Qiu, M.; Wang, S.; Pan, C.; Wang, Y.; Ou, J. lncRNA MEG3 Suppresses the Tumorigenesis of Hemangioma by Sponging miR-494 and Regulating PTEN/ PI3K/AKT Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 2872–2886. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, Y.; Ma, Z.; Ma, G.; Xue, Q.; Li, F.; Liu, L. Long non-coding RNA PTENP1 functions as a ceRNA to modulate PTEN level by decoying miR-106b and miR-93 in gastric cancer. Oncotarget 2017, 8, 26079–26089. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, F.; Zhang, T.; Xu, H.; Zhang, Y.; Shan, Z.; Wu, S.; Fan, Q.; Sun, Y. miR-516a-3p inhibits breast cancer cell growth and EMT by blocking the Pygo2/Wnt signalling pathway. J. Cell. Mol. Med. 2019, 23, 6295–6307. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Z.; Song, C.; Deng, H.; Yang, R.; Zhou, L.; Sun, Y.; Zhang, Q. Glaucocalyxin A reverses EMT and TGF-β1-induced EMT by inhibiting TGF-β1/Smad2/3 signaling pathway in osteosarcoma. Chem. Biol. Interact. 2019, 307, 158–166. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Wang, B.; Ye, C.; Lai, Z.; Jiang, H.; Wang, Z.; Jiang, K.; Ye, Y.; Wang, S. Long non-coding RNA GPR65-1 is up-regulated in gastric cancer and promotes tumor growth through the PTEN-AKT-slug signaling pathway. Cell Cycle 2018, 17, 759–765. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, X.; Wang, H.; Zheng, M.; Wang, Q.; Chang, W. The Down-Regulation of lncRNA PCAT18 Promotes the Progression of Gastric Cancer via MiR-107/PTEN/PI3K/AKT Signaling Pathway. Oncotargets Ther. 2019, 12, 11017–11031. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Duan, H.; Yuan, L. LncRNA TUBA4B functions as a competitive endogenous RNA to inhibit gastric cancer progression by elevating PTEN via sponging miR-214 and miR-216a/b. Cancer Cell Int. 2019, 19, 156. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Zou, C.; Gong, Z.; Wang, S.; Liu, J.; Ma, G.; Liu, X.; Zhang, W.; Jiang, P. Long noncoding RNA DGCR5 suppresses gastric cancer progression by acting as a competing endogenous RNA of PTEN and BTG1. J. Cell. Physiol. 2019, 234, 11999–12010. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, X.; Zhang, X.; Chen, Z.; Ye, C.; Xiang, W.; Huang, Z. LncRNA LINC00470 promotes the degradation of PTEN mRNA to facilitate malignant behavior in gastric cancer cells. Biochem. Biophys. Res. Commun. 2020, 521, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Q.; Li, S.J.; Guo, G.X. Long Noncoding RNA AFAP1-AS1 Promotes Cell Proliferation and Apoptosis of Gastric Cancer Cells via PTEN/p-AKT Pathway. Dig. Dis. Sci. 2017, 62, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, G.; Yang, Y.; Jiang, Z.; Cai, J.; Zhang, Z.; Hu, H. Long noncoding RNA HOTAIRM1 inhibits cell progression by regulating miR-17-5p/ PTEN axis in gastric cancer. J. Cell. Biochem. 2019, 120, 4952–4965. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Chen, S.; Jiang, Z.; Shao, Y.; Jiang, X.; Li, P.; Xiao, B.; Guo, J. Long noncoding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression. Sci. Rep. 2015, 5, 13445. [Google Scholar] [CrossRef]
- Li, H.; Ma, X.; Yang, D.; Suo, Z.; Dai, R.; Liu, C. PCAT-1 contributes to cisplatin resistance in gastric cancer through epigenetically silencing PTEN via recruiting EZH2. J. Cell. Biochem. 2020, 121, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, F.; Liao, W.; Yu, L.; Hu, Z.; Li, M.; Xia, H. Curcumin suppress glioblastoma cell proliferation by p-AKT/mTOR pathway and increased the PTEN expression. Arch. Biochem. Biophys. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, S.; Wang, M.; Chen, J.; Huang, S.; Wei, Z.; Cheng, Z.; Wang, H.; Long, M.; Li, P. Curcumin inhibits zearalenone-induced apoptosis and oxidative stress in Leydig cells via modulation of the PTEN/Nrf2/Bip signaling pathway. Food Chem. Toxicol. 2020, 141, 111385. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Bishayee, A. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2018, 128, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, L.; Sun, D.; Yin, D. Curcumin ameliorates atherosclerosis through upregulation of miR-126. J. Cell. Physiol. 2019, 234, 21049–21059. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, H.; Razmovski-Naumovski, V.; Chang, D.; Nammi, S. Chronic treatment of curcumin improves hepatic lipid metabolism and alleviates the renal damage in adenine-induced chronic kidney disease in Sprague-Dawley rats. BMC Nephrol. 2019, 20, 431. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Biswas, S.; Chen, S.; Liang, G.; Feng, B.; Cai, L.; Khan, Z.A.; Chakrabarti, S. Curcumin Analogs Reduce Stress and Inflammation Indices in Experimental Models of Diabetes. Front. Endocrinol. 2019, 10, 887. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Ba, P.; Xu, M.; Yu, M.; Li, L.; Duan, X.; Lv, S.; Fu, G.; Yang, J.; Yang, P.; Yang, C.; et al. Curcumin suppresses the proliferation and tumorigenicity of Cal27 by modulating cancer associated fibroblasts of TSCC. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Qiang, Z.; Meng, L.; Yi, C.; Yu, L.; Chen, W.; Sha, W. Curcumin regulates the miR-21/PTEN/Akt pathway and acts in synergy with PD98059 to induce apoptosis of human gastric cancer MGC-803 cells. J. Int. Med. Res. 2019, 47, 1288–1297. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Sethi, G.; Ahn, K.S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 2015, 6, 6386–6405. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Amiri, A.; Asemi, Z.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; Mirzaei, H.R.; Mirzaei, H. Role of resveratrol in modulating microRNAs in human diseases: From cancer to inflammatory disorder. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Guo, X.; Zhang, L.; Ma, Y.; Wang, L.; Liu, Z.; Ji, H.; Xiong, Y. Resveratrol inhibits proliferation, migration and invasion of multiple myeloma cells via NEAT1-mediated Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2018, 107, 484–494. [Google Scholar] [CrossRef]
- Wu, F.; Cui, L. Resveratrol suppresses melanoma by inhibiting NF-κB/miR-221 and inducing TFG expression. Arch. Dermatol. Res. 2017, 309, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Karimi Dermani, F.; Saidijam, M.; Amini, R.; Mahdavinezhad, A.; Heydari, K.; Najafi, R. Resveratrol Inhibits Proliferation, Invasion, and Epithelial-Mesenchymal Transition by Increasing miR-200c Expression in HCT-116 Colorectal Cancer Cells. J. Cell. Biochem. 2017, 118, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Cheng, W.; Wang, S.; Li, P.; He, L. Resveratrol induces cell cycle arrest in human gastric cancer MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway. Oncol. Rep. 2016, 35, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020. [Google Scholar] [CrossRef]
- Ko, J.-H.; Yang, M.H.; Baek, S.H.; Nam, D.; Jung, S.H.; Ahn, K.S. Theacrine attenuates epithelial mesenchymal transition in human breast cancer MDA-MB-231 cells. Phytother. Res. 2019, 33, 1934–1942. [Google Scholar] [CrossRef]
- Guo, Q.; Jing, F.J.; Xu, W.; Li, X.; Li, X.; Sun, J.L.; Xing, X.M.; Zhou, C.K.; Jing, F.B. Ubenimex induces autophagy inhibition and EMT suppression to overcome cisplatin resistance in GC cells by perturbing the CD13/EMP3/PI3K/AKT/NF-κB axis. Aging 2019, 12, 80–105. [Google Scholar] [CrossRef]
- Yu, J.L.; Gao, X. MicroRNA 1301 inhibits cisplatin resistance in human ovarian cancer cells by regulating EMT and autophagy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1688–1696. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D.; Niu, H.; Zhu, G.; Xu, Y.; Ye, D.; Li, J.; Zhang, Q. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 19. [Google Scholar] [CrossRef]
- Ma, J.; Hu, X.; Li, J.; Wu, D.; Lan, Q.; Wang, Q.; Tian, S.; Dong, W. Enhancing conventional chemotherapy drug cisplatin-induced anti-tumor effects on human gastric cancer cells both in vitro and in vivo by Thymoquinone targeting PTEN gene. Oncotarget 2017, 8, 85926–85939. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, L.; Chen, J.; Tang, X.; Liao, C.; Xie, Y.; Xiao, Q. Fentanyl inhibits progression of human gastric cancer MGC-803 cells by NF-κB downregulation and PTEN upregulation in vitro. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2012, 20, 61–69. [Google Scholar] [CrossRef]
- Pan, L.; Matloob, A.F.; Du, J.; Pan, H.; Dong, Z.; Zhao, J.; Feng, Y.; Zhong, Y.; Huang, B.; Lu, J. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A/sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN upregulation. Febs J. 2010, 277, 989–999. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Z.; Ye, Y.; Wang, S. Sodium butyrate induces differentiation of gastric cancer cells to intestinal cells via the PTEN/phosphoinositide 3-kinase pathway. Cell Biol. Int. 2010, 34, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Piao, J.; Li, N.; Yang, Y.; Kim, K.Y.; Lin, Z. Valproic acid targets HDAC1/2 and HDAC1/PTEN/Akt signalling to inhibit cell proliferation via the induction of autophagy in gastric cancer. FEBS J. 2020, 287, 2118–2133. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Sun, J.; Tao, J.; Yuan, Y.X.; Ni, Z.H.; Tang, Q.F. Zuo Jin Wan Reverses DDP Resistance in Gastric Cancer through ROCK/PTEN/PI3K Signaling Pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 4278568. [Google Scholar] [CrossRef]
- He, J.Q.; Zhang, S.R.; Li, D.F.; Tang, J.Y.; Wang, Y.Q.; He, X.; Li, Y.M.; Wu, H.; Zhou, M.; Jiao, J.; et al. Experimental Study on the Effect of a Weifufang on Human Gastric Adenocarcinoma Cell Line BGC-823 Xenografts and PTEN Gene Expression in Nude Mice. Cancer Biother. Radiopharm. 2020, 35, 199–207. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wang, C.; Chang, L.M.; Zhou, K.R.; Wang, J.W.; Ke, Y.; Yang, D.X.; Shi, H.G.; Wang, R.; Shi, X.L.; et al. Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget 2016, 7, 72990–73002. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Zhang, X.H.; Chen, H.H.; Liu, Y.D. Ursolic acid promotes apoptosis of SGC-7901 gastric cancer cells through ROCK/PTEN mediated mitochondrial translocation of cofilin-1. Asian Pac. J. Cancer Prev. 2014, 15, 9593–9597. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, M.; Zhong, C.; Peng, J.; Wang, X.; Li, J.; Chen, Z.; Huang, Y. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol. Rep. 2015, 33, 457–463. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhang, G.-Q.; Yang, Y.; Zhang, C.-Y.; Fu, R.-X.; Yang, Y.-M. Genistein induces G2/M arrest in gastric cancer cells by increasing the tumor suppressor PTEN expression. Nutr. Cancer 2013, 65, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, J.; Zhou, Y.; Cao, L.; Gong, Y.; Wei, Y.; Yang, H.; Tang, L. Methylxanthine derivatives promote autophagy in gastric cancer cells targeting PTEN. Anti Cancer Drugs 2019, 30, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Jeyamani, L.; Jayarajan, J.; Leelakrishnan, V.; Swaminathan, M. CagA and VacA genes of Helicobacter pylori and their clinical relevance. Indian J. Pathol. Microbiol. 2018, 61, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Weel, J.F.; van der Hulst, R.W.; Gerrits, Y.; Roorda, P.; Feller, M.; Dankert, J.; Tytgat, G.N.; van der Ende, A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J. Infect. Dis. 1996, 173, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, X.; Jin, M.; Hu, L.; Zang, M.; Qiu, W.; Wang, S.; Liu, B.; Liu, S.; Guo, D. CagA increases DNA methylation and decreases PTEN expression in human gastric cancer. Mol. Med. Rep. 2019, 19, 309–319. [Google Scholar] [CrossRef]
- McDaniel, L.D.; Conkrite, K.L.; Chang, X.; Capasso, M.; Vaksman, Z.; Oldridge, D.A.; Zachariou, A.; Horn, M.; Diamond, M.; Hou, C.; et al. Common variants upstream of MLF1 at 3q25 and within CPZ at 4p16 associated with neuroblastoma. PLoS Genet. 2017, 13, e1006787. [Google Scholar] [CrossRef]
- Perez, Y.; Menascu, S.; Cohen, I.; Kadir, R.; Basha, O.; Shorer, Z.; Romi, H.; Meiri, G.; Rabinski, T.; Ofir, R.; et al. RSRC1 mutation affects intellect and behaviour through aberrant splicing and transcription, downregulating IGFBP3. Brain 2018, 141, 961–970. [Google Scholar] [CrossRef]
- Yu, S.; Gautam, N.; Quan, M.; Gao, Y. RSRC1 suppresses gastric cancer cell proliferation and migration by regulating PTEN expression. Mol. Med. Rep. 2019, 20, 1747–1753. [Google Scholar] [CrossRef]
- Zhao, C.; Tao, T.; Yang, L.; Qin, Q.; Wang, Y.; Liu, H.; Song, R.; Yang, X.; Wang, Q.; Gu, S.; et al. Loss of PDZK1 expression activates PI3K/AKT signaling via PTEN phosphorylation in gastric cancer. Cancer Lett. 2019, 453, 107–121. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Luo, W.; Luo, Z.; Shi, S. WWP2 Is One Promising Novel Oncogene. Pathol. Oncol. Res. 2019, 25, 443–446. [Google Scholar] [CrossRef]
- Chen, A.; Gao, B.; Zhang, J.; McEwen, T.; Shui, Q.Y.; Zhang, D.; Fang, D. The HECT-type E3 ubiquitin ligase AIP2 inhibits activation-induced T-cell death by catalyzing EGR2 ubiquitination. Mol. Cell. Biol. 2009, 29, 5348–5356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Zhang, Z.; Wang, B.; Zhang, J.; Zhao, Y.; Jin, Y. Wwp2-mediated ubiquitination of the RNA polymerase II large subunit in mouse embryonic pluripotent stem cells. Mol. Cell. Biol. 2007, 27, 5296–5305. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, X.; Shim, J.-H.; Huang, Z.; Brady, N.; Hu, D.; Drapp, R.; Sigrist, K.; Glimcher, L.H.; Jones, D. The E3 ubiquitin ligase Wwp2 regulates craniofacial development through mono-ubiquitylation of Goosecoid. Nat. Cell Biol. 2011, 13, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Maddika, S.; Kavela, S.; Rani, N.; Palicharla, V.R.; Pokorny, J.L.; Sarkaria, J.N.; Chen, J. WWP2 is an E3 ubiquitin ligase for PTEN. Nat. Cell Biol. 2011, 13, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, P.; Zhang, Q.; Li, C.; Zou, W.; Chang, Z.; Cui, C.-P.; Zhang, L. WWP2 is a physiological ubiquitin ligase for phosphatase and tensin homolog (PTEN) in mice. J. Biol. Chem. 2018, 293, 8886–8899. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, Y.; Peng, W.; Ji, D.; Zhang, Z.; Qian, W.; Li, J.; Gu, Q.; Zhang, D.; Tang, J. Long noncoding RNA Linc02023 regulates PTEN stability and suppresses tumorigenesis of colorectal cancer in a PTEN-dependent pathway. Cancer Lett. 2019, 451, 68–78. [Google Scholar] [CrossRef]
- Fukumoto, C.; Nakashima, D.; Kasamatsu, A.; Unozawa, M.; Shida-Sakazume, T.; Higo, M.; Ogawara, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H. WWP2 is overexpressed in human oral cancer, determining tumor size and poor prognosis in patients: Downregulation of WWP2 inhibits the AKT signaling and tumor growth in mice. Oncoscience 2014, 1, 807. [Google Scholar] [CrossRef]
- Yang, R.; He, Y.; Chen, S.; Lu, X.; Huang, C.; Zhang, G. Elevated expression of WWP2 in human lung adenocarcinoma and its effect on migration and invasion. Biochem. Biophys. Res. Commun. 2016, 479, 146–151. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.; Zhao, X.; Li, H.; Luo, G.; Yu, Y.; Guo, Y.; Zhang, L.; Zhu, J.; Wang, S.; et al. WWP2 regulates proliferation of gastric cancer cells in a PTEN-dependent manner. Biochem. Biophys. Res. Commun. 2020, 521, 652–659. [Google Scholar] [CrossRef]
- McConnell, B.B.; Yang, V.W. Mammalian Krüppel-like factors in health and diseases. Physiol. Rev. 2010, 90, 1337–1381. [Google Scholar] [CrossRef]
- Yamanaka, S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 2007, 1, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chan, Y.S.; Loh, Y.H.; Cai, J.; Tong, G.Q.; Lim, C.A.; Robson, P.; Zhong, S.; Ng, H.H. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 2008, 10, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, M.P.; Yang, Y.; Katz, J.P. Krüppel-like factors in cancer. Nat. Rev. Cancer 2013, 13, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, L.; Duan, Q.; Wang, Q.; Chen, J. Krüppel-like factor 2 suppresses human gastric tumorigenesis through inhibiting PTEN/AKT signaling. Oncotarget 2017, 8, 100358–100370. [Google Scholar] [CrossRef]
- Guo, X.; Deng, L.; Deng, K.; Wang, H.; Shan, T.; Zhou, H.; Liang, Z.; Xia, J.; Li, C. Pseudogene PTENP1 Suppresses Gastric Cancer Progression by Modulating PTEN. Anti Cancer Agents Med. Chem. 2016, 16, 456–464. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, H.W.; Baek, J.H.; Cho, Y.H.; Kang, H.G.; Jeong, J.S.; Song, J.; Park, H.S.; Chun, K.H. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene 2016, 35, 251–260. [Google Scholar] [CrossRef]
- Ma, X.M.; Liu, Y.; Guo, J.W.; Liu, J.H.; Zuo, L.F. Relation of overexpression of S phase kinase-associated protein 2 with reduced expression of p27 and PTEN in human gastric carcinoma. World J. Gastroenterol. 2005, 11, 6716–6721. [Google Scholar] [CrossRef]
- Guo, S.L.; Ye, H.; Teng, Y.; Wang, Y.L.; Yang, G.; Li, X.B.; Zhang, C.; Yang, X.; Yang, Z.Z.; Yang, X. Akt-p53-miR-365-cyclin D1/cdc25A axis contributes to gastric tumorigenesis induced by PTEN deficiency. Nat. Commun. 2013, 4, 2544. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.; Kumar, A.P.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int. J. Cancer 2011, 129, 1552–1563. [Google Scholar] [CrossRef]
- Shin, E.M.; Hay, H.S.; Lee, M.H.; Goh, J.N.; Tan, T.Z.; Sen, Y.P.; Lim, S.W.; Yousef, E.M.; Ong, H.T.; Thike, A.A.; et al. DEAD-box helicase DP103 defines metastatic potential of human breast cancers. J. Clin. Investig. 2014, 124, 3807–3824. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, D.; Jiang, Z.; Li, C.; Chen, L.; Xia, Y.; Liu, D.; Yao, Q.; Wang, D. Chrysin Induced Cell Apoptosis and Inhibited Invasion Through Regulation of TET1 Expression in Gastric Cancer Cells. Oncotargets Ther. 2020, 13, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Su, P.H.; Hsu, Y.W.; Huang, R.L.; Chen, L.Y.; Chao, T.K.; Liao, C.C.; Chen, C.W.; Wu, T.I.; Mao, S.P.; Balch, C.; et al. TET1 promotes 5hmC-dependent stemness, and inhibits a 5hmC-independent epithelial-mesenchymal transition, in cervical precancerous lesions. Cancer Lett. 2019, 450, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Collignon, E.; Canale, A.; Al Wardi, C.; Bizet, M.; Calonne, E.; Dedeurwaerder, S.; Garaud, S.; Naveaux, C.; Barham, W.; Wilson, A.; et al. Immunity drives TET1 regulation in cancer through NF-κB. Sci. Adv. 2018, 4, eaap7309. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Xu, C.; Wu, G.; Liu, B.; Wu, D. CircRNA-UCK2 Increased TET1 Inhibits Proliferation and Invasion of Prostate Cancer Cells Via Sponge MiRNA-767-5p. Open Med. 2019, 14, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yan, Z.; Chen, W.; Wu, Y.; Han, J.; Guo, H.; Qiao, J. TET1 inhibits EMT of ovarian cancer cells through activating Wnt/β-catenin signaling inhibitors DKK1 and SFRP2. Gynecol. Oncol. 2017, 147, 408–417. [Google Scholar] [CrossRef]
- Pei, Y.F.; Tao, R.; Li, J.F.; Su, L.P.; Yu, B.Q.; Wu, X.Y.; Yan, M.; Gu, Q.L.; Zhu, Z.G.; Liu, B.Y. TET1 inhibits gastric cancer growth and metastasis by PTEN demethylation and re-expression. Oncotarget 2016, 7, 31322–31335. [Google Scholar] [CrossRef]
- Gan, L.; Xu, M.; Hua, R.; Tan, C.; Zhang, J.; Gong, Y.; Wu, Z.; Weng, W.; Sheng, W.; Guo, W. The polycomb group protein EZH2 induces epithelial-mesenchymal transition and pluripotent phenotype of gastric cancer cells by binding to PTEN promoter. J. Hematol. Oncol. 2018, 11, 9. [Google Scholar] [CrossRef]
- Jackstadt, R.; van Hooff, S.R.; Leach, J.D.; Cortes-Lavaud, X.; Lohuis, J.O.; Ridgway, R.A.; Wouters, V.M.; Roper, J.; Kendall, T.J.; Roxburgh, C.S.; et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 2019, 36, 319–336.e317. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Z.Y.; Guo, X.T.; Xiao, X.H.; Xiong, L.X. Notch and breast cancer metastasis: Current knowledge, new sights and targeted therapy. Oncol. Lett. 2019, 18, 2743–2755. [Google Scholar] [CrossRef]
- Guo, J.; Li, P.; Liu, X.; Li, Y. NOTCH signaling pathway and non-coding RNAs in cancer. Pathol. Res. Pract. 2019, 215, 152620. [Google Scholar] [CrossRef]
- Kim, H.; Yu, Y.; Choi, S.; Lee, H.; Yu, J.; Lee, J.H.; Kim, W.Y. Evodiamine Eliminates Colon Cancer Stem Cells via Suppressing Notch and Wnt Signaling. Molecular 2019, 24, 4520. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Aslam, H.; Flasza, M.; Fostier, M.; Higgs, J.E.; Mazaleyrat, S.L.; Wilkin, M.B. Multiple levels of Notch signal regulation (review). Mol. Membr. Biol. 2002, 19, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Grishina, I.B. Mini-review: Does Notch promote or suppress cancer? New findings and old controversies. Am. J. Clin. Exp. Urol. 2015, 3, 24–27. [Google Scholar]
- Yap, L.F.; Lee, D.; Khairuddin, A.; Pairan, M.F.; Puspita, B.; Siar, C.H.; Paterson, I.C. The opposing roles of NOTCH signalling in head and neck cancer: A mini review. Oral Dis. 2015, 21, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Hu, Y.H.; Gao, H.Y.; Lan, X.W.; Xue, Y.W. Downregulation of Notch1 inhibits the invasion and metastasis of human gastric cancer cells SGC7901 and MKN74 in vitro through PTEN activation and dephosphorylation of Akt and FAK. Mol. Med. Rep. 2017, 16, 2318–2324. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Z.; Zhang, Y.; Li, D.; Zhang, G.; Luo, X.; Jie, Z.; Liu, Y.; Cao, Y.; Le, Z.; et al. PRL-3 promotes the peritoneal metastasis of gastric cancer through the PI3K/Akt signaling pathway by regulating PTEN. Oncol. Rep. 2016, 36, 1819–1828. [Google Scholar] [CrossRef]
- Wendel, H.G.; De Stanchina, E.; Fridman, J.S.; Malina, A.; Ray, S.; Kogan, S.; Cordon-Cardo, C.; Pelletier, J.; Lowe, S.W. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 2004, 428, 332–337. [Google Scholar] [CrossRef]
- West, K.A.; Castillo, S.S.; Dennis, P.A. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist. Updates 2002, 5, 234–248. [Google Scholar] [CrossRef]
- Knuefermann, C.; Lu, Y.; Liu, B.; Jin, W.; Liang, K.; Wu, L.; Schmidt, M.; Mills, G.B.; Mendelsohn, J.; Fan, Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003, 22, 3205–3212. [Google Scholar] [CrossRef]
- Oki, E.; Baba, H.; Tokunaga, E.; Nakamura, T.; Ueda, N.; Futatsugi, M.; Mashino, K.; Yamamoto, M.; Ikebe, M.; Kakeji, Y.; et al. Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int. J. Cancer 2005, 117, 376–380. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Xie, C.; Zhu, Y.; Shu, X.; Zhang, Z.; Li, N.; Chai, N.; Zhang, S.; Wu, K.; et al. PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J. Exp. Clin. Cancer Res. 2018, 37, 198. [Google Scholar] [CrossRef] [PubMed]
- Mina, S.; Bohn, B.A.; Simon, R.; Krohn, A.; Reeh, M.; Arnold, D.; Bokemeyer, C.; Sauter, G.; Izbicki, J.R.; Marx, A.; et al. PTEN deletion is rare but often homogeneous in gastric cancer. J. Clin. Pathol. 2012, 65, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Han, S.N.; Wang, Z.J.; Wang, S.H.; Zhao, G.Q.; Zhang, X.F.; Wang, J.X. Concomitant modulation of PTEN and Livin in gastric cancer treatment. Int. J. Mol. Med. 2018, 41, 2901–2908. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liu, J.; Lei, S.; Zhang, J.; Zhou, W.; Yu, H.G. PTEN inhibits the invasion and metastasis of gastric cancer via downregulation of FAK expression. Cell. Signal. 2014, 26, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qin, X.; Fei, M.; Hou, W.; Greshock, J.; Bachman, K.E.; Kang, J.; Qin, C.Y. Loss and reduced expression of PTEN correlate with advanced-stage gastric carcinoma. Exp. Ther. Med. 2013, 5, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Zheng, Y.C.; Cao, Y.; Li, Q.S.; Sui, Y.J. Suppression of gastric cancer growth by adenovirus-mediated transfer of the PTEN gene. World J. Gastroenterol. 2005, 11, 2224–2229. [Google Scholar] [CrossRef]
- Canbay, E.; Kahraman, O.T.; Bugra, D.; Caykara, B.; Seyhan, M.F.; Bulut, T.; Yamaner, S.; Ozturk, O. Increased gastric cancer risk with PTEN IVS4 polymorphism in a Turkish population. Genet. Test. Mol. Biomark. 2013, 17, 249–253. [Google Scholar] [CrossRef]
| MiR | Molecular Signaling | Results | References |
|---|---|---|---|
| MiR-28 | PTEN/PI3K/Akt | Downregulation of PTEN Stimulation of PI3K/Akt Promoting invasion and proliferation | [106] |
| MiR-21 | PTEN/Akt | Inhibition of PTEN Stimulation of Akt Enhancing Bcl-2/Bax ratio | [107,108] |
| MiR-26a MiR-106b MiR-214 | PTEN | Decreasing PTEN expression Poor prognosis Low survival | [109,110,111] |
| MiR-21 | PTEN/TGF-β/EMT | Downregulation of PTEN Induction of TGF-1/EMT axis Enhancing E-cadherin levels Reducing N-cadherin and vimentin levels Increasing migration of cancer cells | [112] |
| MiR-214 | PTEN | Downregulation of PTEN Reducing cell cycle arrest | [113] |
| MiR-301a | PTEN/Wnt/β-catenin | Suppressing PTEN Activation of Wnt signaling Promoting metastasis | [114] |
| LncRNA | Signaling Network | Results | References |
|---|---|---|---|
| PCAT18 | MiR-107/PTEN/PI3K/Akt | Downregulation of miR-107 by PCAT18 Stimulation of PTEN signaling Inhibition of PI3K/Akt Inducing tumor growth inhibition | [169] |
| TUBA4B | MiR-214/PTEN MiR-216a/b-PTEN | Downregulation of miR-214 and miR-216a/b Stimulation of PTEN Suppressing proliferation and migration | [170] |
| DGCR5 | MiR-23b/PTEN | Inhibition of miR-23b Stimulation of PTEN Induction of apoptosis | [171] |
| LINC00470 | PTEN | Reducing PTEN stability and directing it into degradation Enhancing proliferation and invasion | [172] |
| AFAP1-AS1 | PTEN/Akt | Reducing PTEN expression Stimulation of Akt Downregulation of pro-apoptotic factors PARP1, capase-3 and caspase-9 | [173] |
| HOTAIRM1 | MiR-17-5p/PTEN | Inhibition of miR-17-5p expression via sponging Upregulation of PTEN Decreasing viability and proliferation | [174] |
| FER1L4 | MiR-106a-5p/PTEN | Inhibition of miR-106a-5p Stimulation of PTEN Disrupting proliferation | [175] |
| HOTAIR | MiR-17-5p/PTEN | Upregulation of miR-17-5p by HOTAIR Inhibition of PTEN Enhancing chemoresistance | [167] |
| PCAT1 | EZH2/PTEN | Increasing EZH2 expression Downregulation of PTEN Stimulation of cisplatin resistance | [176] |
| Antitumor Agent | Concentration and Time | In Vitro/In Vivo | Results | References |
|---|---|---|---|---|
| Geridonin | 10 μM for 24 h | In vitro (MGC 803 cell line) | Stimulation of PTEN Downregulation of PI3K/Akt signaling Accumulation of p53 Stimulation of apoptosis | [208] |
| Ursolic acid | 0–35 μM for 24 h | In vitro (SGC-7901 cells) | PTEN activation Translocation of cofilin-1 from cytoplasm to mitochondria Stimulation of apoptosis | [209] |
| Baicalein | 0–80 μM for 24 and 48 h | In vitro (AGS cells) | Activation of PTEN Suppressing hypoxia mediated Akt Inhibition of glycolysis | [210] |
| Genistein | 10–80 μmoL/L for 24 h | In vitro (SGC-7901 and BGC-823 cells) | Upregulation of PTEN Stimulation of G2/M arrest | [211] |
| Methylxanthine | Different doses (in vitro) for 12 and 24 h 4 and 8 mmoL/L for 24 days | In vitro (MGC-803 cells) In vivo (model of GC) | Stimulation of PTEN Inhibition of PI3K/Akt/mTOR signaling Suppressing proliferation and migration | [212] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafizadeh, M.; Najafi, M.; Ang, H.L.; Moghadam, E.R.; Mahabady, M.K.; Zabolian, A.; Jafaripour, L.; Bejandi, A.K.; Hushmandi, K.; Saleki, H.; et al. PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation. Biomedicines 2020, 8, 264. https://doi.org/10.3390/biomedicines8080264
Ashrafizadeh M, Najafi M, Ang HL, Moghadam ER, Mahabady MK, Zabolian A, Jafaripour L, Bejandi AK, Hushmandi K, Saleki H, et al. PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation. Biomedicines. 2020; 8(8):264. https://doi.org/10.3390/biomedicines8080264
Chicago/Turabian StyleAshrafizadeh, Milad, Masoud Najafi, Hui Li Ang, Ebrahim Rahmani Moghadam, Mahmood Khaksary Mahabady, Amirhossein Zabolian, Leila Jafaripour, Atefe Kazemzade Bejandi, Kiavash Hushmandi, Hossein Saleki, and et al. 2020. "PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation" Biomedicines 8, no. 8: 264. https://doi.org/10.3390/biomedicines8080264
APA StyleAshrafizadeh, M., Najafi, M., Ang, H. L., Moghadam, E. R., Mahabady, M. K., Zabolian, A., Jafaripour, L., Bejandi, A. K., Hushmandi, K., Saleki, H., Zarrabi, A., & Kumar, A. P. (2020). PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation. Biomedicines, 8(8), 264. https://doi.org/10.3390/biomedicines8080264