Involvement of STAT5 in Oncogenesis
Abstract
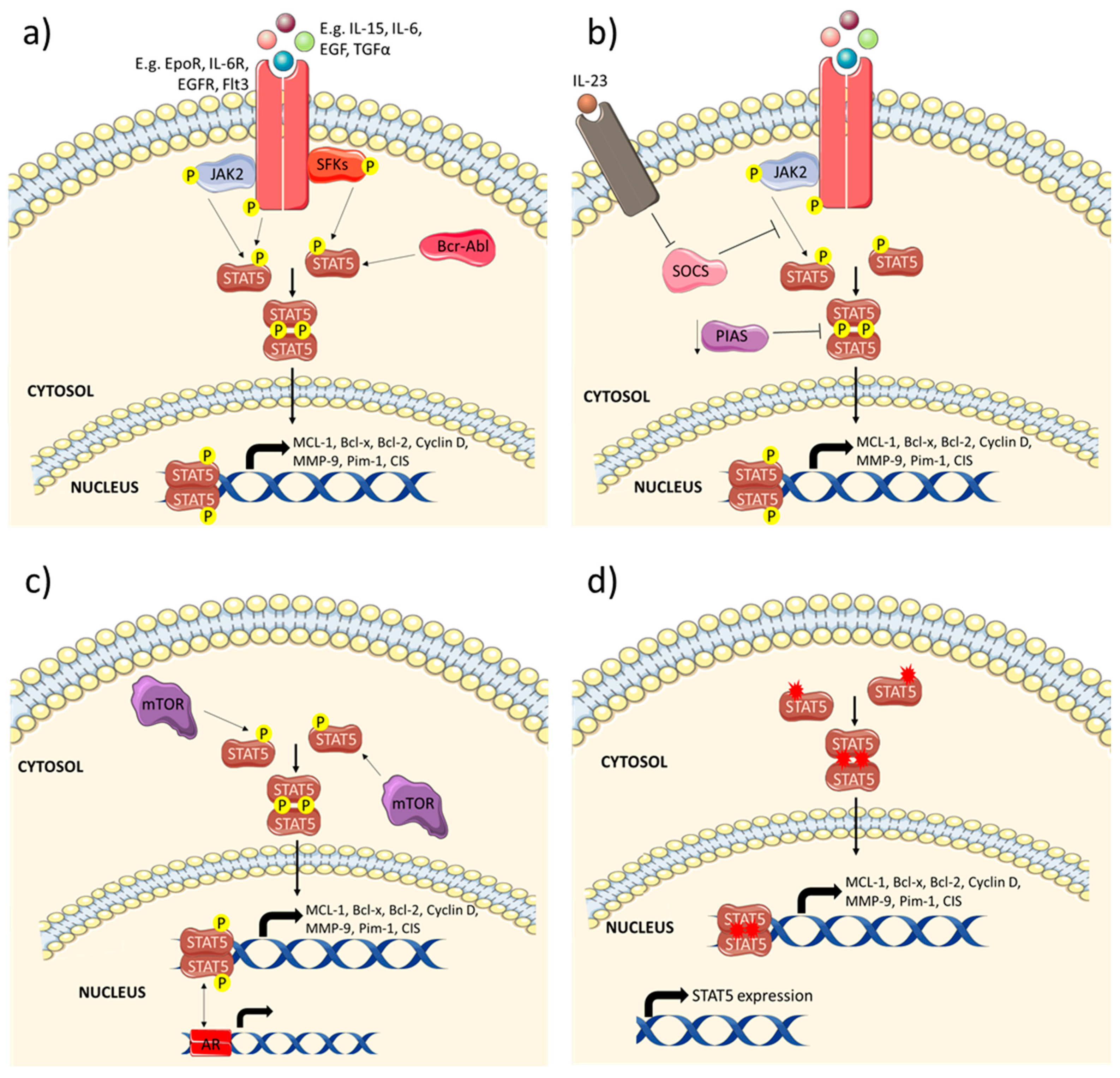
1. Introduction
2. STAT5 and Its Isoforms
3. Roles of STAT5 in Physiology
4. Roles of STAT5 in Various Cancers
4.1. Breast Cancer
4.2. Colorectal Cancer
4.3. Lung Cancer
4.4. Prostate Cancer
4.5. Hepatocellular Carcinoma (HCC)
4.6. Haematological Malignancies
5. Targeting STAT5 in Cancer
5.1. STAT5 Inhibitors
5.1.1. STAT5 pY Inhibitor
5.1.2. SH2 Domain Inhibitors
5.1.3. STAT5 Transcriptional Activity Inhibitors
5.2. Tyrosine Kinase Inhibitors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ihle, J.N. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 2001, 13, 211–217. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Kim, C.; Sikka, S.; Siveen, K.S.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Abrogation of STAT3 signaling cascade by zerumbone inhibits proliferation and induces apoptosis in renal cell carcinoma xenograft mouse model. Mol. Carcinog. 2015, 54, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.K.; Wang, L.; et al. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid. Redox Signal 2016, 24, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Arora, L.; Kumar, A.P.; Arfuso, F.; Chng, W.-J.; Sethi, G. The Role of Signal Transducer and Activator of Transcription 3 (STAT3) and Its Targeted Inhibition in Hematological Malignancies. Cancers 2018, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J.; John, S. Signal transducer and activator of transcription (STAT) signalling and T-cell lymphomas. Immunology 2005, 114, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.; Garcia, R.; Turkson, J.; Jove, R. STATs in oncogenesis. Oncogene 2000, 19, 2474–2488. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.P.; Cao, X. Structure, function, and regulation of STAT proteins. Mol. BioSyst. 2006, 2, 536–550. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Kim, C.; Siveen, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Shi, J.; Kumar, A.P.; Wang, L.Z.; et al. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol. Oncol. 2015, 9, 818–833. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Sethi, G.; Ahn, K.S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 2015, 6, 6386–6405. [Google Scholar] [CrossRef]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.H.; Kim, S.-H.; Sethi, G.; Ahn, K.S. Artesunate suppresses tumor growth and induces apoptosis through the modulation of multiple oncogenic cascades in a chronic myeloid leukemia xenograft mouse model. Oncotarget 2015, 6, 4020–4035. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, K.B.; Rui, L. STAT3 Activation and Oncogenesis in Lymphoma. Cancers 2019, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial–Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; A Harrison, D. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Verdeil, G.; Lawrence, T.; Schmitt-Verhulst, A.-M.; Auphan-Anezin, N.; Verhulst, S.-; Anezin, A.-. Targeting STAT3 and STAT5 in Tumor-Associated Immune Cells to Improve Immunotherapy. Cancers 2019, 11, 1832. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Bharathkumar, H.; Bulusu, K.C.; Pandey, V.; Rangappa, S.; Fuchs, J.E.; Shanmugam, M.K.; Dai, X.; Li, F.; Deivasigamani, A.; et al. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 34296–34307. [Google Scholar] [CrossRef]
- Bromberg, J. Stat proteins and oncogenesis. J. Clin. Investig. 2002, 109, 1139–1142. [Google Scholar] [CrossRef]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.-F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef]
- Lee, M.; Hirpara, J.L.; Eu, J.-Q.; Sethi, G.; Wang, L.; Goh, B.C.; Wong, A.L.-A. Targeting STAT3 and oxidative phosphorylation in oncogene-addicted tumors. Redox Boil. 2019, 25, 101073. [Google Scholar] [CrossRef]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.Q.; Sethi, G.; Goh, B.C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef]
- Orlova, A.; Wagner, C.; De Araujo, E.D.; Bajusz, D.; Neubauer, H.A.; Herling, M.; Gunning, P.T.; Keserű, G.M.; Moriggl, R. Direct Targeting Options for STAT3 and STAT5 in Cancer. Cancers 2019, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta BBA Bioenerg. 2014, 1845, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. Beta-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Baek, S.H.; Ko, J.-H.; Lee, S.G.; Yang, W.M.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 2016, 8, 17700–17711. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Ko, J.-H.; Jung, Y.Y.; Jung, S.H.; Kim, E.; Kong, M.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. Casticin inhibits growth and enhances ionizing radiation–induced apoptosis through the suppression of STAT3 signaling cascade. J. Cell. Biochem. 2018, 120, 9787–9798. [Google Scholar] [CrossRef]
- Chai, E.Z.P.; Shanmugam, M.K.; Arfuso, F.; Dharmarajan, A.; Wang, C.; Kumar, A.P.; Samy, R.P.; Lim, L.H.K.; Wang, L.; Goh, B.C.; et al. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol. Ther. 2016, 162, 86–97. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef]
- Thilakasiri, P.S.; Dmello, R.S.; Nero, T.L.; Parker, M.W.; Ernst, M.; Chand, A.L. Repurposing of drugs as STAT3 inhibitors for cancer therapy. Semin. Cancer Boil. 2019, 1044. [Google Scholar] [CrossRef]
- Wakao, H.; Gouilleux, F.; Groner, B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994, 13, 2182–2191. [Google Scholar] [CrossRef]
- Gouilleux, F.; Wakao, H.; Mundt, M.; Groner, B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994, 13, 4361–4369. [Google Scholar] [CrossRef]
- Grimley, P. Stat5a and Stat5b: Fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 1999, 10, 131–157. [Google Scholar] [CrossRef]
- Kanai, T.; Seki, S.; Jenks, J.A.; Kohli, A.; Kawli, T.; Martin, D.P.; Snyder, M.; Bacchetta, R.; Nadeau, K.C. Identification of STAT5A and STAT5B Target Genes in Human T Cells. PLoS ONE 2014, 9, e86790. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002, 285, 1–24. [Google Scholar] [CrossRef]
- Wingelhofer, B.; Neubauer, H.A.; Valent, P.; Han, X.; Constantinescu, S.N.; Gunning, P.T.; Müller, M.; Moriggl, R. Implications of STAT3 and STAT5 signaling on gene regulation and chromatin remodeling in hematopoietic cancer. Leukemia 2018, 32, 1713–1726. [Google Scholar] [CrossRef]
- Hennighausen, L.; Robinson, G.W. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008, 22, 711–721. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Gadina, M.; Schreiber, R.D. Cytokine Signaling in 2002. Cell 2002, 109, S121–S131. [Google Scholar] [CrossRef]
- Peng, B.; Sutherland, K.D.; Sum, E.Y.M.; Olayioye, M.; Wittlin, S.; Tang, T.K.; Lindeman, G.J.; Visvader, J.E. CPAP Is a Novel Stat5-Interacting Cofactor that Augments Stat5-Mediated Transcriptional Activity. Mol. Endocrinol. 2002, 16, 2019–2033. [Google Scholar] [CrossRef]
- Liu, S.; Walker, S.R.; Nelson, E.A.; Cerulli, R.; Xiang, M.; Toniolo, P.A.; Qi, J.; Stone, R.M.; Wadleigh, M.; Bradner, J.E.; et al. Targeting STAT5 in hematologic malignancies through inhibition of the bromodomain and extra-terminal (BET) bromodomain protein BRD2. Mol. Cancer Ther. 2014, 13, 1194–1205. [Google Scholar] [CrossRef]
- Hennighausen, L.; Robinson, G.W.; Wagner, K.-U.; Liu, X. Developing a Mammary Gland is a Stāt Affair. J. Mammary Gland. Boil. Neoplasia 1997, 2, 365–372. [Google Scholar] [CrossRef]
- Humphreys, R.C.; Hennighausen, L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ. Mol. Boil. J. Am. Assoc. Cancer Res. 1999, 10, 685–694. [Google Scholar]
- Nosaka, T.; Kawashima, T.; Misawa, K.; Ikuta, K.; Mui, A.L.; Kitamura, T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999, 18, 4754–4765. [Google Scholar] [CrossRef] [PubMed]
- Laurence, A.; Tato, C.M.; Davidson, T.S.; Kanno, Y.; Chen, Z.; Yao, Z.; Blank, R.B.; Meylan, F.; Siegel, R.M.; Hennighausen, L.; et al. Interleukin-2 Signaling via STAT5 Constrains T Helper 17 Cell Generation. Immunity 2007, 26, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Kanno, Y.; Kerenyi, M.; Stephens, G.; Durant, L.; Watford, W.T.; Laurence, A.; Robinson, G.W.; Shevach, E.M.; Moriggl, R.; et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 2007, 109, 4368–4375. [Google Scholar] [CrossRef] [PubMed]
- Majri, S.S.; Fritz, J.M.; Villarino, A.V.; Zheng, L.; Kanellopoulou, C.; Chaigne-Delalande, B.; Grönholm, J.; Niemela, J.E.; Afzali, B.; Biancalana, M.; et al. STAT5B: A differential regulator of the life and death of CD4+ effector memory T cells. J. Immunol. 2017, 200, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Oestreich, K.J.; Mohn, S.E.; Weinmann, A.S. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol. 2012, 13, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.R.; Nelson, E.A.; Yeh, J.E.; Pinello, L.; Yuan, G.-C.; Frank, D.A. STAT5 Outcompetes STAT3 To Regulate the Expression of the Oncogenic Transcriptional Modulator BCL6. Mol. Cell. Boil. 2013, 33, 2879–2890. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, G.W.; Wagner, K.-U.; Garrett, L.; Wynshaw-Boris, A.; Hennighausen, L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997, 11, 179–186. [Google Scholar] [CrossRef]
- Udy, G.B.; Towers, R.P.; Snell, R.; Wilkins, R.J.; Park, S.-H.; Ram, P.A.; Waxman, D.J.; Davey, H.W. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. USA 1997, 94, 7239–7244. [Google Scholar] [CrossRef]
- Teglund, S.; McKay, C.; Schuetz, E.; Van Deursen, J.M.; Stravopodis, D.J.; Wang, D.; Brown, M.P.; Bodner, S.; Grosveld, G.; Ihle, J.N. Stat5a and Stat5b Proteins Have Essential and Nonessential, or Redundant, Roles in Cytokine Responses. Cell 1998, 93, 841–850. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Walker, S.R.; Nelson, E.A.; Zou, L.; Chaudhury, M.; Signoretti, S.; Richardson, A.; Frank, D.A. Reciprocal Effects of STAT5 and STAT3 in Breast Cancer. Mol. Cancer Res. 2009, 7, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.J.; Peck, A.R.; Liu, C.; Tran, T.H.; Utama, F.E.; Sjolund, A.B.; Schaber, J.D.; Witkiewicz, A.K.; Rui, H. PTP1B Suppresses Prolactin Activation of Stat5 in Breast Cancer Cells. Am. J. Pathol. 2010, 177, 2971–2983. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Xie, J.; LeBaron, M.J.; Ealley, E.L.; Nevalainen, M.T.; Rui, H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 2004, 24, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Gutzman, J.H.; E Rugowski, D.; E Nikolai, S.; Schuler, L.A. Stat5 activation inhibits prolactin-induced AP-1 activity: Distinct prolactin-initiated signals in tumorigenesis dependent on cell context. Oncogene 2007, 26, 6341–6348. [Google Scholar] [CrossRef]
- Barash, I. Stat5 in breast cancer: Potential oncogenic activity coincides with positive prognosis for the disease. Carcinogenesis 2012, 33, 2320–2325. [Google Scholar] [CrossRef]
- Arendt, L.M.; Rugowski, D.E.; Grafwallner-Huseth, T.A.; Garcia-Barchino, M.J.; Rui, H.; Schuler, L.A. Prolactin-induced mouse mammary carcinomas model estrogen resistant luminal breast cancer. Breast Cancer Res. 2011, 13, R11. [Google Scholar] [CrossRef]
- Britschgi, A.; Andraos, R.; Brinkhaus, H.; Klebba, I.; Romanet, V.; Muller, U.; Murakami, M.; Radimerski, T.; Bentires-Alj, M. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: A rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell 2012, 22, 796–811. [Google Scholar] [CrossRef]
- Wang, J.; Rouse, C.; Jasper, J.; Pendergast, A.M. ABL kinases promote breast cancer osteolytic metastasis by modulating tumor-bone interactions through TAZ and STAT5 signaling. Sci. Signal. 2016, 9, ra12. [Google Scholar] [CrossRef]
- Lee, G.H.; Yoo, K.C.; An, Y.; Lee, H.J.; Lee, M.; Uddin, N.; Kim, M.J.; Kim, I.G.; Suh, Y.; Lee, S.J. FYN promotes mesenchymal phenotypes of basal type breast cancer cells through STAT5/NOTCH2 signaling node. Oncogene 2018, 37, 1857–1868. [Google Scholar] [CrossRef]
- Ikeda, O.; Mizushima, A.; Yamamoto, C.; Muromoto, R.; Nanbo, A.; Oritani, K.; Sekine, Y.; Yoshimura, A.; Matsuda, T. Involvement of STAP-2 in Brk-mediated phosphorylation and activation of STAT5 in breast cancer cells. Cancer Sci. 2011, 102, 756–761. [Google Scholar] [CrossRef]
- Perotti, C.; Liu, R.; Parusel, C.T.; Böcher, N.; Schultz, J.; Bork, P.; Pfitzner, E.; Groner, B.; Shemanko, C.S. Heat shock protein-90-alpha, a prolactin-STAT5 target gene identified in breast cancer cells, is involved in apoptosis regulation. Breast Cancer Res. 2008, 10, R94. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Su, W.-Y.; Liang, Q.-C.; Zhang, Z.-G.; Chen, H.-M.; Du, W.; Chen, Y.-X.; Fang, J.-Y. Inhibition of STAT5 induces G1 cell cycle arrest and reduces tumor cell invasion in human colorectal cancer cells. Lab. Investig. 2009, 89, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, Y.-C.; Hong, J.; Su, W.-Y.; Lin, Y.-W.; Lu, R.; Xiong, H.; Fang, J.-Y. STAT5 isoforms regulate colorectal cancer cell apoptosis via reduction of mitochondrial membrane potential and generation of reactive oxygen species. J. Cell. Physiol. 2012, 227, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-L.; Li, Z.-W.; Lou, C.-J.; Pang, D.; Zhang, Y. PHOSPHO-STAT5 Expression is Associated with Poor Prognosis of Human Colonic Adenocarcinoma. Pathol. Oncol. Res. 2011, 17, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Li, L.; Zhang, J.; Wang, X.; Yang, C.; Li, Y.; Lan, F.; Lin, P. IL-23 selectively promotes the metastasis of colorectal carcinoma cells with impaired Socs3 expression via the STAT5 pathway. Carcinogenesis 2014, 35, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Li, Z.; Lou, C.; Zhang, Y. Expression of phosphorylated Stat5 predicts expression of cyclin D1 and correlates with poor prognosis of colonic adenocarcinoma. Int. J. Color. Dis. 2010, 26, 29–35. [Google Scholar] [CrossRef]
- Weng, Y.-R.; Yu, Y.-N.; Ren, L.-L.; Cui, Y.; Lu, Y.; Chen, H.; Ma, X.; Qin, W.-X.; Cao, W.; Hong, J.; et al. Role of C9orf140 in the promotion of colorectal cancer progression and mechanisms of its upregulation via activation of STAT5, ?-catenin and EZH2. Carcinogenesis 2014, 35, 1389–1398. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, X.; Chen, G.; Wang, M.; Lou, C.; Mao, Y.; Li, Z. STAT5a-targeting miRNA enhances chemosensitivity to cisplatin and 5-fluorouracil in human colorectal cancer cells. Mol. Med. Rep. 2012, 5, 1215–1219. [Google Scholar] [CrossRef]
- Hexner, E.O.; Serdikoff, C.; Jan, M.; Swider, C.R.; Robinson, C.; Yang, S.; Angeles, T.; Emerson, S.G.; Carroll, M.; Ruggeri, B.; et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood 2008, 111, 5663–5671. [Google Scholar] [CrossRef]
- Sánchez-Ceja, S.; Reyes-Maldonado, E.; Vázquez-Manríquez, M.; López-Luna, J.; Belmont, A.; Gutiérrez-Castellanos, S. Differential expression of STAT5 and Bcl-xL, and high expression of Neu and STAT3 in non-small-cell lung carcinoma. Lung Cancer 2006, 54, 163–168. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Shanmugam, M.K.; Narula, A.S.; Kim, C.; Lee, J.H.; Namjoshi, O.A.; Blough, B.E.; Sethi, G.; Ahn, K.S. Oxymatrine Attenuates Tumor Growth and Deactivates STAT5 Signaling in a Lung Cancer Xenograft Model. Cancers 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, T.J.; Xie, J.; LeBaron, M.J.; Zhu, J.; Nurmi, M.; Alanen, K.; Rui, H.; Nevalainen, M.T. Inhibition of Transcription Factor Stat5 Induces Cell Death of Human Prostate Cancer Cells. J. Boil. Chem. 2003, 278, 27287–27292. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liao, Z.; Hoang, D.T.; Dagvadorj, A.; Gupta, S.; Blackmon, S.; Ellsworth, E.; Talati, P.; Leiby, B.; Zinda, M.; et al. Pharmacologic inhibition of Jak2-Stat5 signaling By Jak2 inhibitor AZD1480 potently suppresses growth of both primary and castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5658–5674. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Vogiatzi, P.; Puhr, M.; Dagvadorj, A.; Lutz, J.; Ryder, A.; Addya, S.; Fortina, P.; Cooper, C.; Leiby, B.; et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr. -Relat. Cancer 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Hosui, A.; Kimura, A.; Yamaji, D.; Zhu, B.M.; Na, R.; Hennighausen, L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation. J. Exp. Med. 2009, 206, 819–831. [Google Scholar] [CrossRef]
- Yu, J.H.; Zhu, B.-M.; Riedlinger, G.; Kang, K.; Hennighausen, L. The liver-specific tumor suppressor STAT5 controls expression of the reactive oxygen species-generating enzyme NOX4 and the proapoptotic proteins PUMA and BIM in mice. Hepatology 2012, 56, 2375–2386. [Google Scholar] [CrossRef]
- Fu, B.; Meng, W.; Zhao, H.; Zhang, B.; Tang, H.; Zou, Y.; Yao, J.; Li, H.; Zhang, T. GRAM domain-containing protein 1A (GRAMD1A) promotes the expansion of hepatocellular carcinoma stem cell and hepatocellular carcinoma growth through STAT5. Sci. Rep. 2016, 6, 31963. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Wang, B.; Sun, Q.; He, Z.; Hong, J.; Kuehn, F.; Liu, E.; Zhang, Z. IGF-1 induces the epithelial-mesenchymal transition via Stat5 in hepatocellular carcinoma. Oncotarget 2017, 8, 111922–111930. [Google Scholar] [CrossRef]
- Lee, T.K.; Man, K.; Poon, R.T.; Lo, C.M.; Yuen, A.P.; Ng, I.O.; Ng, K.T.; Leonard, W.; Fan, S.T.; Lee, T.K. Signal Transducers and Activators of Transcription 5b Activation Enhances Hepatocellular Carcinoma Aggressiveness through Induction of Epithelial-Mesenchymal Transition. Cancer Res. 2006, 66, 9948–9956. [Google Scholar] [CrossRef]
- Birkenkamp, K.U.; Geugien, M.; Lemmink, H.H.; Kruijer, W.; Vellenga, E. Regulation of constitutive STAT5 phosphorylation in acute myeloid leukemia blasts. Leukemia 2001, 15, 1923–1931. [Google Scholar] [CrossRef]
- Kollmann, S.; Grundschober, E.; Maurer, B.; Warsch, W.; Grausenburger, R.; Edlinger, L.; Huuhtanen, J.; Lagger, S.; Hennighausen, L.; Valent, P.; et al. Twins with different personalities: STAT5B—but not STAT5A—has a key role in BCR/ABL-induced leukemia. Leukemia 2019, 33, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.P.; Raaijmakers, J.A.; Lammers, J.-W.J.; Jove, R.; Koenderman, L. STAT5 Activation by BCR-Abl Contributes to Transformation of K562 Leukemia Cells. Blood 1999, 94, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Warsch, W.; Kollmann, K.; Eckelhart, E.; Fajmann, S.; Cerny-Reiterer, S.; Hölbl, A.; Gleixner, K.V.; Dworzak, M.; Mayerhofer, M.; Hoermann, G.; et al. High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood 2011, 117, 3409–3420. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nishio, M.; Ando, Y.; Zhang, Z.; Hamaguchi, M.; Mita, K.; Kobayashi, S.; Fujii, Y.; Iwase, H. Stat5 expression predicts response to endocrine therapy and improves survival in estrogen receptor-positive breast cancer. Endocr. -Relat. Cancer 2006, 13, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, M.T.; Xie, J.; Torhorst, J.; Bubendorf, L.; Haas, P.; Kononen, J.; Sauter, G.; Rui, H. Signal Transducer and Activator of Transcription-5 Activation and Breast Cancer Prognosis. J. Clin. Oncol. 2004, 22, 2053–2060. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, G.W.; Hennighausen, L. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 1996, 10, 1496–1506. [Google Scholar]
- Russo, J.; Moral, R.; Balogh, G.; Mailo, D.; Russo, I.H. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005, 7, 131. [Google Scholar] [CrossRef]
- Iavnilovitch, E.; Groner, B.; Barash, I. Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol. Cancer Res. 2002, 1, 32–47. [Google Scholar]
- Pastuszak-Lewandoska, D.; Domanska-Senderowska, D.; Kordiak, J.; Antczak, A.; Czarnecka, K.H.; Migdalska-Sek, M.; Nawrot, E.; Kiszalkiewicz, J.M.; Brzezianska-Lasota, E. Immunoexpression analysis of selected JAK/STAT pathway molecules in patients with non- small-cell lung cancer. Pol. Arch. Intern. Med. 2017, 127, 758–764. [Google Scholar] [CrossRef]
- Cui, J.; Cao, S.; Yan, Y.; Zhang, X.-Y.; Zhang, K.; Liu, C.; Zhao, G.; Han, J.; Dong, Q.; Shen, B.; et al. EGF stimulates cyclooxygenase-2 expression through the STAT5 signaling pathway in human lung adenocarcinoma A549 cells. Int. J. Oncol. 2011, 39, 383–391. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Glass, A.; Zellweger, T.; Gehan, E.; Bubendorf, L.; Gelmann, E.P.; Nevalainen, M.T. Activation of Signal Transducer and Activator of Transcription-5 in Prostate Cancer Predicts Early Recurrence. Clin. Cancer Res. 2005, 11, 5863–5868. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-H.; Dagvadorj, A.; Shen, F.; Gu, L.; Liao, Z.; Abdulghani, J.; Zhang, Y.; Gelmann, E.P.; Zellweger, T.; Culig, Z.; et al. Transcription Factor Stat5 Synergizes with Androgen Receptor in Prostate Cancer Cells. Cancer Res. 2008, 68, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.; Wang, Y.; Rhim, J.S.; Bhattacharya, N.; Loda, M.; Sytkowski, A.J. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate 2006, 66, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Zoubeidi, A.; Kuruma, H.; Fazli, L.; Lamoureux, F.; Beraldi, E.; Monia, B.P.; MacLeod, A.R.; Thüroff, J.W.; Gleave, M.E. Transcription Factor Stat5 Knockdown Enhances Androgen Receptor Degradation and Delays Castration-Resistant Prostate Cancer Progression In Vivo. Mol. Cancer Ther. 2011, 10, 347–359. [Google Scholar] [CrossRef]
- Li, T.; Weng, J.; Zhang, Y.; Liang, K.; Fu, G.; Li, Y.; Bai, X.-C.; Gao, Y. mTOR direct crosstalk with STAT5 promotes de novo lipid synthesis and induces hepatocellular carcinoma. Cell Death Dis. 2019, 10, 619–621. [Google Scholar] [CrossRef]
- Hoelbl, A.; Schuster, C.; Kovacic, B.; Zhu, B.; Wickre, M.; Hoelzl, M.A.; Fajmann, S.; Grebien, F.; Warsch, W.; Stengl, G.; et al. Stat5 is indispensable for the maintenance of bcr/abl -positive leukaemia. EMBO Mol. Med. 2010, 2, 98–110. [Google Scholar] [CrossRef]
- Igelmann, S.; Neubauer, H.A.; Ferbeyre, G. STAT3 and STAT5 Activation in Solid Cancers. Cancers 2019, 11, 1428. [Google Scholar] [CrossRef]
- Bandapalli, O.R.; Schuessele, S.; Kunz, J.B.; Rausch, T.; Stütz, A.M.; Tal, N.; Geron, I.; Gershman, N.; Izraeli, S.; Eilers, J.; et al. The activating STAT5B N642H mutation is a common abnormality in pediatric T-cell acute lymphoblastic leukemia and confers a higher risk of relapse. Haematologica 2014, 99, e188–e192. [Google Scholar] [CrossRef]
- Mizuki, M.; Fenski, R.; Halfter, H.; Matsumura, I.; Schmidt, R.; Müller, C.; Grüning, W.; Kratz-Albers, K.; Serve, S.; Steur, C.; et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 2000, 96, 3907–3914. [Google Scholar] [CrossRef]
- Hantschel, O.; Warsch, W.; Eckelhart, E.; Kaupe, I.; Grebien, F.; Wagner, K.-U.; Superti-Furga, G.; Sexl, V. BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nat. Methods 2012, 8, 285–293. [Google Scholar] [CrossRef]
- Gupta, R.; Li, W.; Yan, X.J.; Barrientos, J.; Kolitz, J.E.; Allen, S.L.; Rai, K.R.; Chiorazzi, N.; Mongini, P.K.A. Mechanism for IL-15-Driven B Cell Chronic Lymphocytic Leukemia Cycling: Roles for AKT and STAT5 in Modulating Cyclin D2 and DNA Damage Response Proteins. J. Immunol. 2019, 202, 2924–2944. [Google Scholar] [CrossRef] [PubMed]
- Klejman, A.; Schreiner, S.J.; Nieborowska-Skorska, M.; Slupianek, A.; Wilson, M.; Smithgall, T.E.; Skórski, T. The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells. EMBO J. 2002, 21, 5766–5774. [Google Scholar] [CrossRef] [PubMed]
- Harir, N.; Pecquet, C.; Kerenyi, M.; Sonneck, K.; Kovacic, B.; Nyga, R.; Brevet, M.; Dhennin, I.; Gouilleux-Gruart, V.; Beug, H.; et al. Constitutive activation of Stat5 promotes its cytoplasmic localization and association with PI3-kinase in myeloid leukemias. Blood 2006, 109, 1678–1686. [Google Scholar] [CrossRef]
- Minieri, V.; De Dominici, M.; Porazzi, P.; Mariani, S.A.; Spinelli, O.; Rambaldi, A.; Peterson, L.F.; Porcu, P.; Nevalainen, M.T.; Calabretta, B. Targeting STAT5 or STAT5-Regulated Pathways Suppresses Leukemogenesis of Ph+ Acute Lymphoblastic Leukemia. Cancer Res. 2018, 78, 5793–5807. [Google Scholar] [CrossRef]
- Nelson, E.A.; Walker, S.R.; Weisberg, E.; Bar-Natan, M.; Barrett, R.; Gashin, L.B.; Terrell, S.; Klitgaard, J.L.; Santo, L.; Addorio, M.R.; et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood 2011, 117, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.A.; Walker, S.R.; Xiang, M.; Weisberg, E.; Bar-Natan, M.; Barrett, R.; Liu, S.; Kharbanda, S.; Christie, A.L.; Nicolais, M.; et al. The STAT5 Inhibitor Pimozide Displays Efficacy in Models of Acute Myelogenous Leukemia Driven by FLT3 Mutations. Genes Cancer 2012, 3, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Nogami, A.; Oshikawa, G.; Okada, K.; Fukutake, S.; Umezawa, Y.; Nagao, T.; Kurosu, T.; Miura, O. FLT3-ITD confers resistance to the PI3K/Akt pathway inhibitors by protecting the mTOR/4EBP1/Mcl-1 pathway through STAT5 activation in acute myeloid leukemia. Oncotarget 2015, 6, 9189–9205. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.M.; Furusawa, A.; Sadashivaiah, K.; Civin, C.I.; Banerjee, A. STAT5 inhibition induces TRAIL/DR4 dependent apoptosis in peripheral T-cell lymphoma. Oncotarget 2018, 9, 16792–16806. [Google Scholar] [CrossRef][Green Version]
- Chen, J.-J.; Cai, N.; Chen, G.-Z.; Jia, C.-C.; Qiu, D.-B.; Du, C.; Liu, W.; Yang, Y.; Long, Z.-J.; Zhang, Q. The neuroleptic drug pimozide inhibits stem-like cell maintenance and tumorigenicity in hepatocellular carcinoma. Oncotarget 2015, 8, 17593–17609. [Google Scholar] [CrossRef]
- Fako, V.; Yu, Z.; Henrich, C.J.; Ransom, T.; Budhu, A.S.; Wang, X.W. Inhibition of wnt/beta-catenin Signaling in Hepatocellular Carcinoma by an Antipsychotic Drug Pimozide. Int. J. Biol. Sci. 2016, 12, 768–775. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.-K.; Yu, H.-T.; Zhong, Z.-H.; Cai, N.; Chen, G.-Z.; Zhang, P.; Chen, J.-J. The antipsychotic drug pimozide inhibits cell growth in prostate cancer through suppression of STAT3 activation. Int. J. Oncol. 2015, 48, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Wingelhofer, B.; Maurer, B.; Heyes, E.C.; Cumaraswamy, A.A.; Berger-Becvar, A.; De Araujo, E.D.; Orlova, A.; Freund, P.; Ruge, F.; Park, J.; et al. Pharmacologic inhibition of STAT5 in acute myeloid leukemia. Leukemia 2018, 32, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Page, B.D.G.; Khoury, H.; Laister, R.C.; Fletcher, S.; Vellozo, M.; Manzoli, A.; Yue, P.; Turkson, J.; Minden, M.D.; Gunning, P.T. Small Molecule STAT5-SH2 Domain Inhibitors Exhibit Potent Antileukemia Activity. J. Med. Chem. 2012, 55, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Sperl, B.; Reindl, W.; Kiessling, A.; Berg, T. Discovery of Chromone-Based Inhibitors of the Transcription Factor STAT5. ChemBioChem 2008, 9, 723–727. [Google Scholar] [CrossRef]
- Schauwecker, S.M.; Kim, J.J.; Licht, J.D.; Clevenger, C.V. Histone H1 and Chromosomal Protein HMGN2 Regulate Prolactin-induced STAT5 Transcription Factor Recruitment and Function in Breast Cancer Cells. J. Boil. Chem. 2016, 292, 2237–2254. [Google Scholar] [CrossRef]
- Cumaraswamy, A.A.; Lewis, A.M.; Geletu, M.; Todic, A.; Diaz, D.B.; Cheng, X.R.; Brown, C.E.; Laister, R.C.; Muench, D.; Kerman, K.; et al. Nanomolar-Potency Small Molecule Inhibitor of STAT5 Protein. ACS Med. Chem. Lett. 2014, 5, 1202–1206. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, J.; Shi, M.; Zhao, S.; Bai, W.; Cao, W.; Tu, Z.; Huang, Z.; Feng, W. Targeted Blockage of Signal Transducer and Activator of Transcription 5 Signaling Pathway with Decoy Oligodeoxynucleotides Suppresses Leukemic K562 Cell Growth. DNA Cell Boil. 2011, 30, 71–78. [Google Scholar] [CrossRef]
- Khalaf, R.; Chakraborty, K. Chronic Myelogenous Leukemia (CML) Treatment Protocols. Available online: https://emedicine.medscape.com/article/2005453-overview (accessed on 27 August 2020).
- Shimada, A. Hematological malignancies and molecular targeting therapy. Eur. J. Pharmacol. 2019, 862, 172641. [Google Scholar] [CrossRef]
- Gallipoli, P.; Cook, A.; Rhodes, S.; Hopcroft, L.; Wheadon, H.; Whetton, A.D.; Jorgensen, H.G.; Bhatia, R.; Holyoake, T.L. JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of CML CD34+ cells in vitro and in vivo. Blood 2014, 124, 1492–1501. [Google Scholar] [CrossRef]
- Nam, S.; Williams, A.; Vultur, A.; List, A.F.; Bhalla, K.; Smith, D.D.; Lee, F.Y.; Jove, R. Dasatinib (BMS-354825) inhibits Stat5 signaling associated with apoptosis in chronic myelogenous leukemia cells. Mol. Cancer Ther. 2007, 6, 1400–1405. [Google Scholar] [CrossRef]
- Huang, M.; Dorsey, J.F.; Epling-Burnette, P.K.; Nimmanapalli, R.; Landowski, T.H.; Mora, L.B.; Niu, G.; Sinibaldi, D.; Bai, F.; Kraker, A.; et al. Inhibition of Bcr–Abl kinase activity by PD180970 blocks constitutive activation of Stat5 and growth of CML cells. Oncogene 2002, 21, 8804–8816. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, A.-M.; Foran, J.M.; Fiedler, W.; Serve, H.; Paquette, R.L.; A Cooper, M.; A Yuen, H.; Louie, S.G.; Kim, H.; Nicholas, S.; et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin. Cancer Res. 2003, 9, 5465–5476. [Google Scholar] [PubMed]


| Types of Cancer | Role of STAT5 | STAT5 Status | Effects of the Status STAT5 in the Various Cancers | Ref. |
|---|---|---|---|---|
| Breast | Tumour suppressor | Inactivated and loss of expression | ↑ STAT3 signalling ↑ Metastasis More aggressive cancer | [51,52,53,54] |
| Oncogene | Constitutively activated | ↑ Tumour initiation ↑ Drug resistance, metastatic capabilities of TNBC | [40,55,56,57,58,59,60,61] | |
| Colorectal | Oncogene | Overexpressed and constitutively activated | ↑ Cell proliferation, survival, metastasis, and drug resistance ↓ Apoptosis | [62,63,64,65,66,67,68,69] |
| Lung | Oncogene | Overexpressed | ↑ Cell proliferation and metastasis | [70,71] |
| Prostate | Oncogene | Constitutively activated | ↑ Cell proliferation and metastasis ↓ Apoptosis | [72,73,74] |
| Liver | Tumour suppressor | Loss of expression | ↑ STAT3 signalling leading to development of HCC. ↓ Apoptosis | [75,76] |
| Oncogene | Increased activation | ↑ Cell proliferation, metastasis, drug resistance, and CSC population | [77,78,79] | |
| Haematological malignancies | Oncogene | Mutated and constitutively activated | ↑ Initiation of cancer ↑ Cell proliferation, survival, and drug resistance ↓ Apoptosis | [80,81,82,83] |
| Target | Drug | Cancer | Effects | Molecular Effects | Ref. |
|---|---|---|---|---|---|
| pY phosphorylation | Pimozide | AML | ↓ Cell viability ↑ Apoptosis in combination with a TKI | [106] | |
| CML | ↑ Apoptosis ↓ Colony formation | ↓ Bcl-x, Pim-1, CIS, cyclin D1, MCL-1 | [105] | ||
| PTCL | ↓ Cell viability ↑ Apoptosis | ↑ Cleaved caspase 8, cleaved caspase 3 | [108] | ||
| STAT5 SH2 domain | Nicotinoyl hydrazine (CAS 285986-31-4) | Breast | ↓ Cell proliferation | [114,115] | |
| BP-1-108 | AML CML | ↓ Cell viability ↑ Apoptosis | ↓ Cyclin D1, cyclin D2, MCL-1, Myc | [113] | |
| 13a | AML CML | ↓ Cell viability ↑ Apoptosis | ↓ Cyclin D1, cyclin D2, MCL-1, Myc ↑ Cleaved caspase 3, cleaved PARP | [116] | |
| AC-4-130 | AML | ↓ Cell viability ↑ Apoptosis ↓ Growth of CSCs | ↓ Cyclin D2, Bcl-2, Myc | [112] | |
| BRD2 / STAT5 transcriptional activity | JQ1 | ALL | ↓ Cell viability especially in combination with imatinib | [38] | |
| STAT5 DBD / transcriptional activity | 21-mer dODN | CML | ↓ Cell viability ↑ G0/G1 cell cycle arrest ↑ Apoptosis | ↓ Bcl-xL, cyclin D1, c-Myc | [117] |
| Drug | Target | Cancer | Ref. |
|---|---|---|---|
| Ruxolitinib in combination with nilotinib | JAK2 | CML | [120] |
| Dasatinib (BMS-354825) | Bcr-Abl SFKs | CML ALL | [121] |
| Lestaurtinib (CEP-701) | JAK2 Flt3 | AML | [69] |
| PD180970 | Bcr-Abl | CML | [122] |
| AZD1480 | JAK2 | Prostate | [73] |
| Sunitinib (SU11248) | Flt3 | AML | [123] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halim, C.E.; Deng, S.; Ong, M.S.; Yap, C.T. Involvement of STAT5 in Oncogenesis. Biomedicines 2020, 8, 316. https://doi.org/10.3390/biomedicines8090316
Halim CE, Deng S, Ong MS, Yap CT. Involvement of STAT5 in Oncogenesis. Biomedicines. 2020; 8(9):316. https://doi.org/10.3390/biomedicines8090316
Chicago/Turabian StyleHalim, Clarissa Esmeralda, Shuo Deng, Mei Shan Ong, and Celestial T. Yap. 2020. "Involvement of STAT5 in Oncogenesis" Biomedicines 8, no. 9: 316. https://doi.org/10.3390/biomedicines8090316
APA StyleHalim, C. E., Deng, S., Ong, M. S., & Yap, C. T. (2020). Involvement of STAT5 in Oncogenesis. Biomedicines, 8(9), 316. https://doi.org/10.3390/biomedicines8090316





