A Gas Sensor Based on Network Nanowire for H2S Monitor in Construction Waste Landfill
Abstract
:1. Introduction
2. Experiments
2.1. Synthesis of the Network Nanowire Material
2.2. Sample Analysis
2.3. Sensor Fabrication
2.4. Sensor Measurement
3. Results and Discussion
3.1. Structural Information
3.2. Gas Sensing Analysis
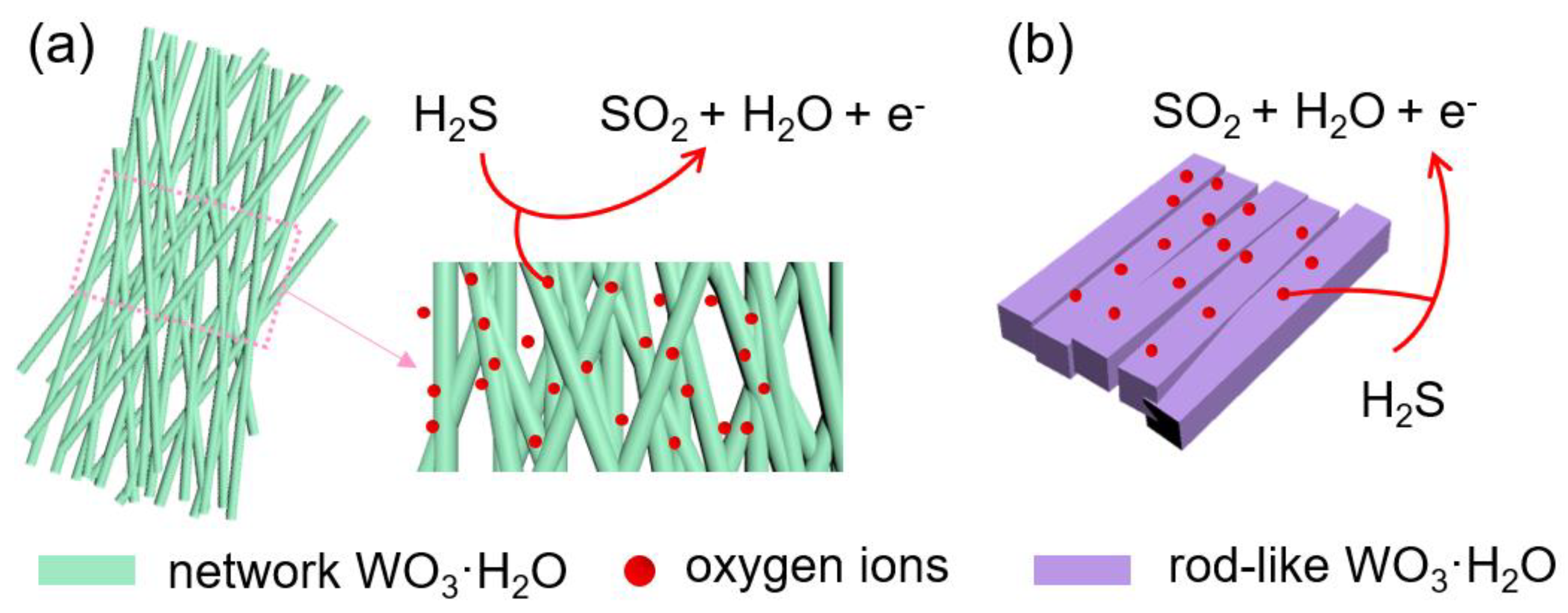
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, K.; Cheng, Z.; Lou, Z.; Wang, L.; Lu, H.; Xu, B.; Jin, N. Chemical and olfactive impacts of organic matters on odor emission patterns from the simulated construction and demolition waste landfills. J. Environ. Sci. 2021, 103, 196–206. [Google Scholar] [CrossRef]
- Liang, X.; Lin, S.; Bi, X.; Lu, E.; Li, Z. Chinese construction industry energy efficiency analysis with undesirable carbon emissions and construction waste outputs. Environ. Sci. Pollut. Res. 2021, 28, 15838–15852. [Google Scholar] [CrossRef]
- Yun, S.; Ryu, D.; Khim, J. Evaluation of the recyclability of construction and demolition waste fines as a garden substrate and soil amendment agent: A case study from the Republic of Korea. J. Mater. Cycles Waste Manag. 2019, 22, 479–487. [Google Scholar] [CrossRef]
- Xu, Q.; Townsend, T. Factors affecting temporal H2S emission at construction and demolition (C&D) debris landfills. Chemosphere 2014, 96, 105–111. [Google Scholar]
- Chung, W.; Jung, S.; Chang, S. The Influence of Waste Composition on Landfill Gas Generation in a Pilot-Scale Lysimeter. Appl. Sci. 2019, 9, 4677. [Google Scholar] [CrossRef] [Green Version]
- Shaha, B.N.; Meeroff, D.E. Prediction of H2S Concentration in Landfill Gas Resulting from Construction and Demolition Debris and the Selection of Treatment Method. J. Environ. Eng. 2020, 146, 04020045. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhang, T. An overview: Facet-dependent metal oxide semiconductor gas sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef]
- Yang, W.; Xiao, X.; Fang, B.; Deng, H. Nanorods-assembled ZnO microflower as a powerful channel for n-butanol sensing. J. Alloys Compd. 2021, 860, 158410. [Google Scholar] [CrossRef]
- Cheng, P.; Lv, L.; Wang, Y.; Zhang, B.; Zhang, Y.; Zhang, Y.; Lei, Z.; Xu, L. SnO2/ZnSnO3 double-shelled hollow microspheres based high-performance acetone gas sensor. Sens. Actuators B Chem. 2021, 332, 129212. [Google Scholar] [CrossRef]
- Ambardekar, V.; Sahoo, S.; Srivastava, D.; Majumder, S.; Bandyopadhyay, P. Plasma sprayed CuO coatings for gas sensing and catalytic conversion applications. Sens. Actuators B Chem. 2021, 331, 129404. [Google Scholar] [CrossRef]
- Americo, S.; Pargoletti, E.; Soave, R.; Cargnoni, F.; Trioni, M.I.; Chiarello, G.L.; Cerrato, G.; Cappelletti, G. Unveiling the acetone sensing mechanism by WO3 chemiresistors through a joint theory-experiment approach. Electrochim. Acta 2021, 371, 137611. [Google Scholar] [CrossRef]
- Wang, C.; Cui, X.; Liu, J.; Zhou, X.; Cheng, X.; Sun, P.; Hu, X.; Li, X.; Zheng, J.; Lu, G. Design of Superior Ethanol Gas Sensor Based on Al-Doped NiO Nanorod-Flowers. ACS Sens. 2016, 1, 131–136. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Jung, J.; Hong, T.; Lee, S.; Kim, H.W.; Lee, C. H2S gas sensing properties of CuO-functionalized WO3 nanowires. Ceram. Int. 2014, 40, 11051–11056. [Google Scholar] [CrossRef]
- Yang, A.; Wang, D.; Lan, T.; Chu, J.; Li, W.; Pan, J.; Liu, Z.; Wang, X.; Rong, M. Single ultrathin WO3 nanowire as a superior gas sensor for SO2 and H2S: Selective adsorption and distinct I-V response. Mater. Chem. Phys. 2020, 240, 122165. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, S.-J.; Cho, H.-J.; Kim, N.-H.; Jang, J.-S.; Choi, S.-J.; Kim, I.-D. WO3 nanofibers functionalized by protein-templated RuO2 nanoparticles as highly sensitive exhaled breath gas sensing layers. Sens. Actuators B Chem. 2017, 241, 1276–1282. [Google Scholar] [CrossRef]
- Choi, M.S.; Mirzaei, A.; Bang, J.H.; Gil Na, H.; Jin, C.; Oum, W.; Han, S.; Kim, S.S.; Kim, H.W. Low-Temperature H2S Sensors Based on Si-Coated SnO2 Nanowires. Korean J. Met. Mater. 2019, 57, 732–740. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Chen, C.; Chen, Z.; Cai, M.; Qu, B.; Wang, T.; Zhang, M. ZnO-carbon nanofibers for stable, high response, and selective H2S sensors. Nanotechnology 2018, 29, 275501. [Google Scholar] [CrossRef]
- Karnati, P.; Akbar, S.; Morris, P.A. Conduction mechanisms in one dimensional core-shell nanostructures for gas sensing: A review. Sens. Actuators B Chem. 2019, 295, 127–143. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]

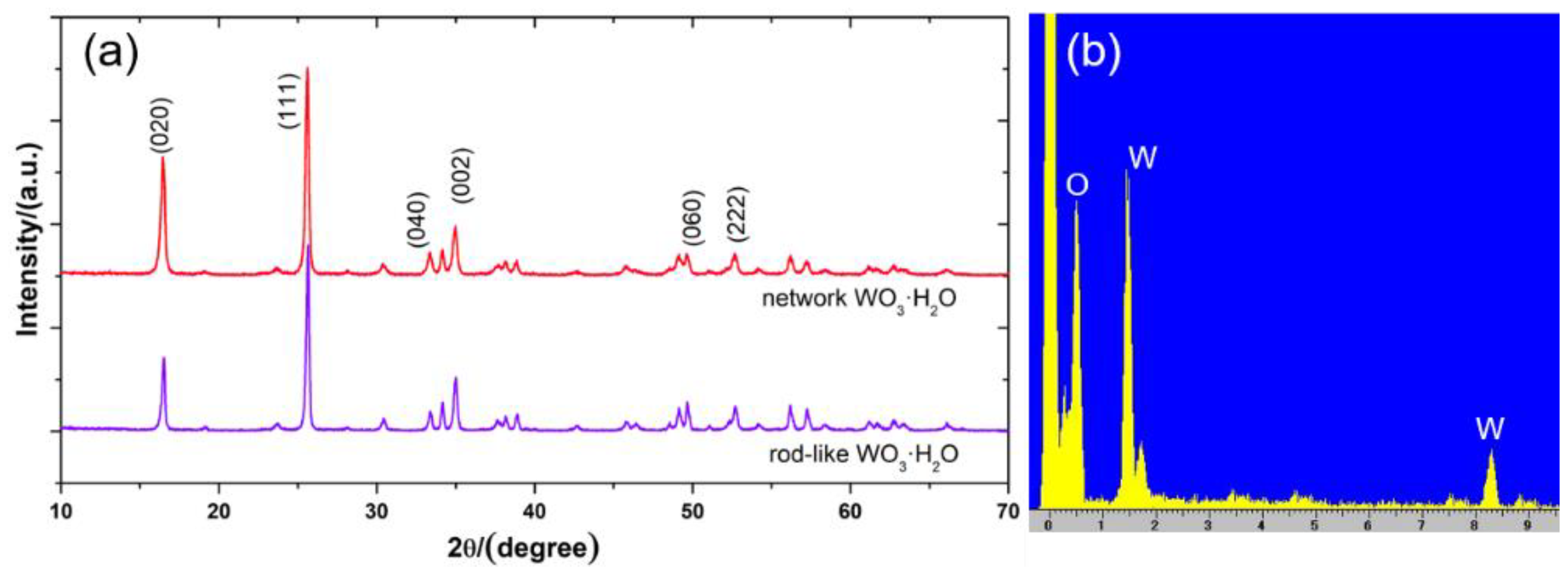
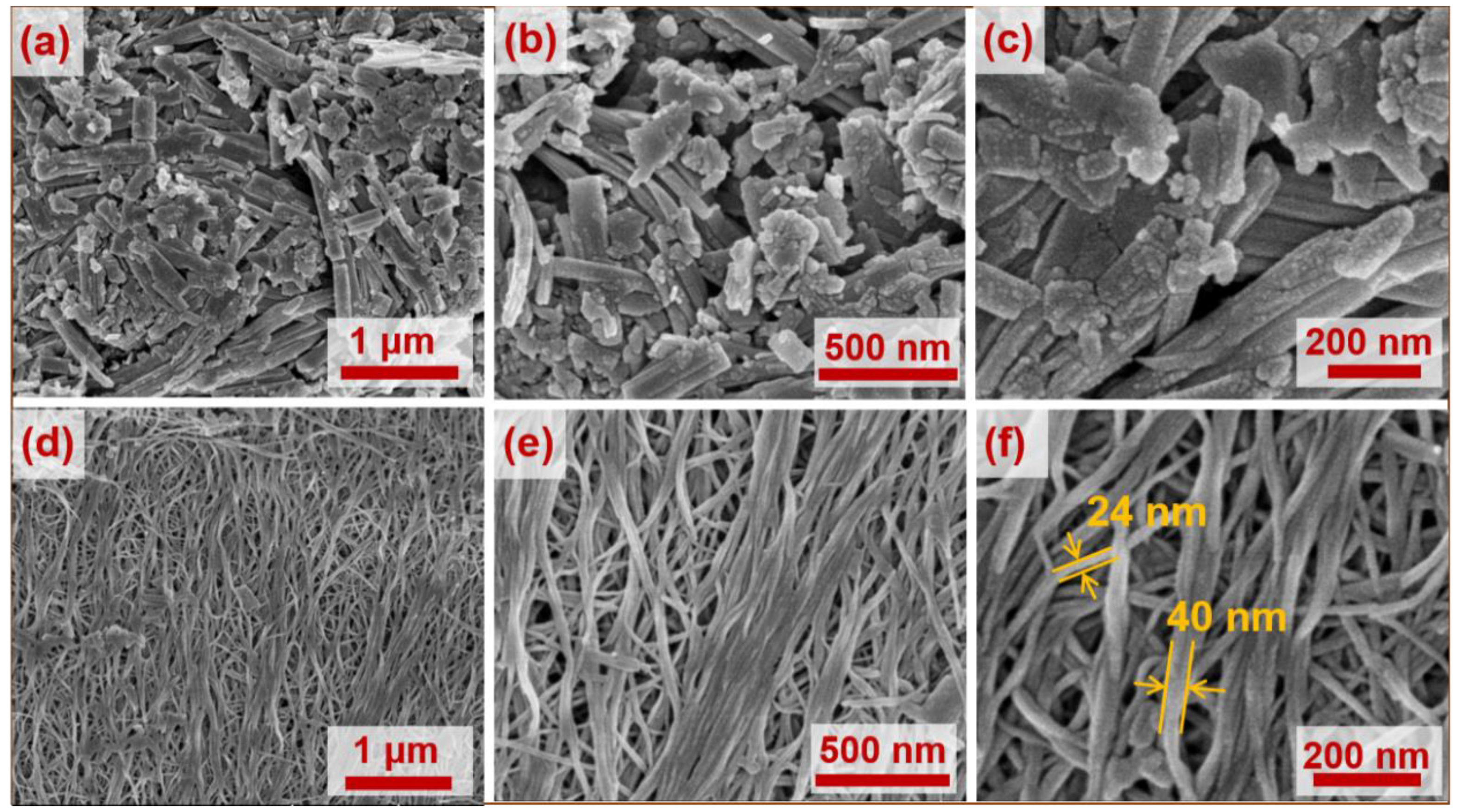
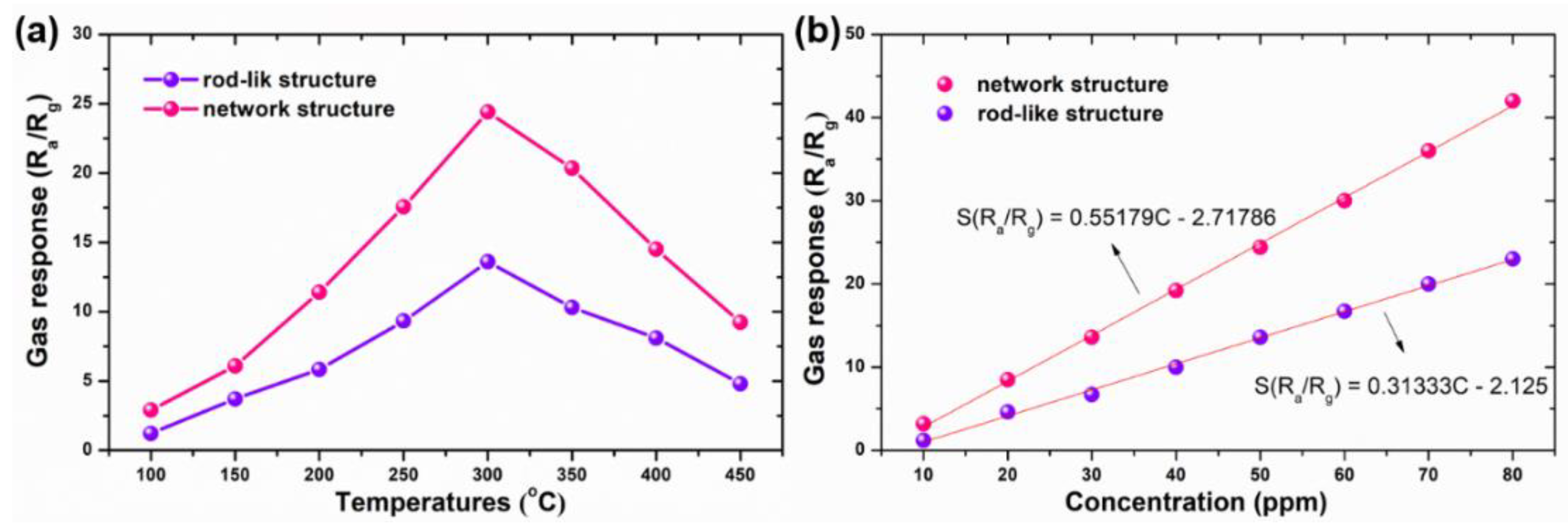

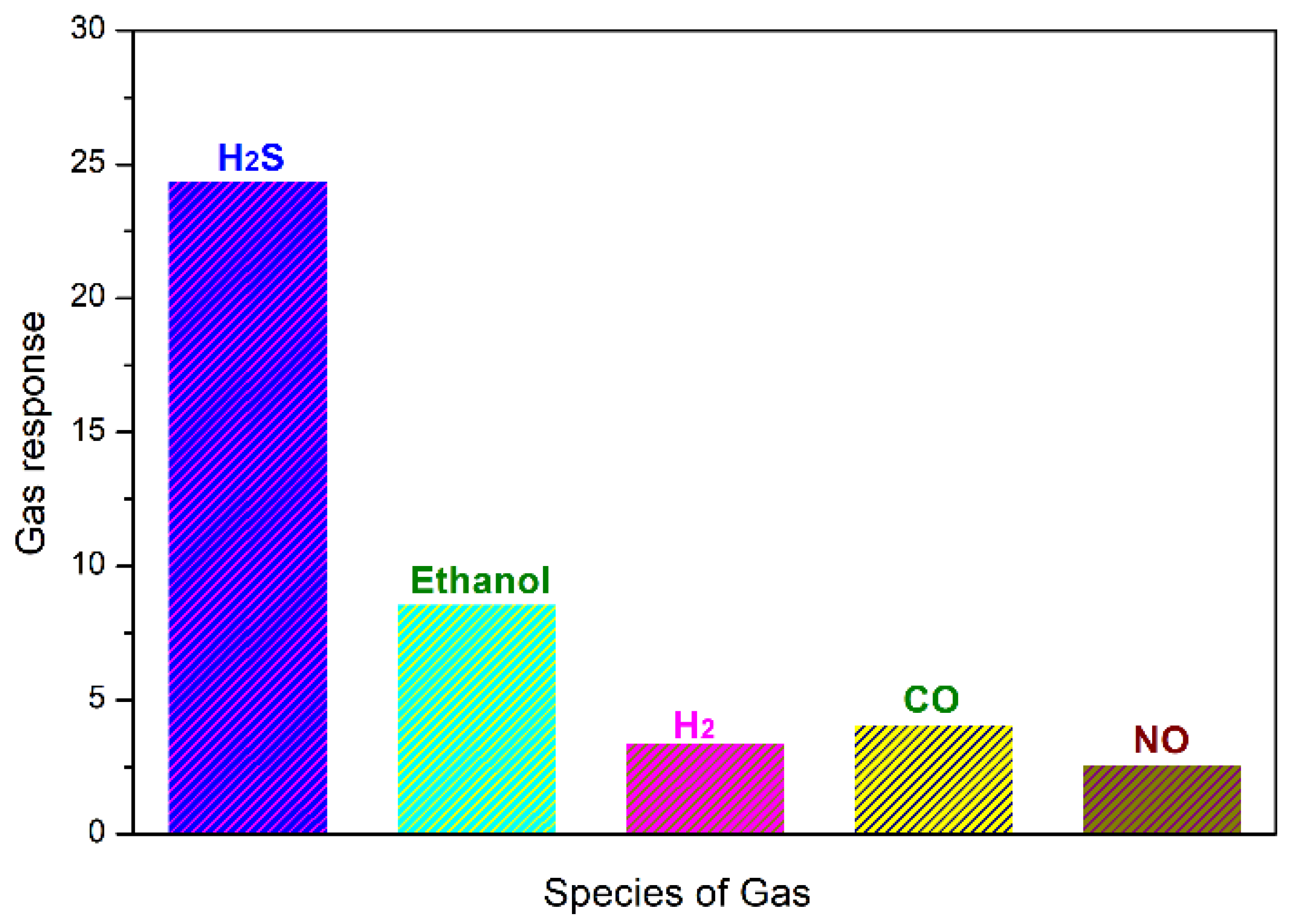
| Sample | BET Surface Area (m2 g−1) | Pore Size (nm) |
|---|---|---|
| Rod-like sample | 25.4 | 11.6 |
| Network nanowire sample | 41.3 | 15.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, P.; Shi, Q.; Qi, L. A Gas Sensor Based on Network Nanowire for H2S Monitor in Construction Waste Landfill. Chemosensors 2021, 9, 156. https://doi.org/10.3390/chemosensors9070156
Ren P, Shi Q, Qi L. A Gas Sensor Based on Network Nanowire for H2S Monitor in Construction Waste Landfill. Chemosensors. 2021; 9(7):156. https://doi.org/10.3390/chemosensors9070156
Chicago/Turabian StyleRen, Pengyu, Qingwei Shi, and Lingling Qi. 2021. "A Gas Sensor Based on Network Nanowire for H2S Monitor in Construction Waste Landfill" Chemosensors 9, no. 7: 156. https://doi.org/10.3390/chemosensors9070156
APA StyleRen, P., Shi, Q., & Qi, L. (2021). A Gas Sensor Based on Network Nanowire for H2S Monitor in Construction Waste Landfill. Chemosensors, 9(7), 156. https://doi.org/10.3390/chemosensors9070156






