Using MoS2/Fe3O4 as Ion-Electron Transduction Layer to Manufacture All-Solid-State Ion-Selective Electrode for Determination of Serum Potassium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the MoS2/Fe3O4 Composites
2.2. Fabrication of All-Solid-State Potassium Selective Electrodes
2.3. Electrochemical Measurements
3. Results and Discussion
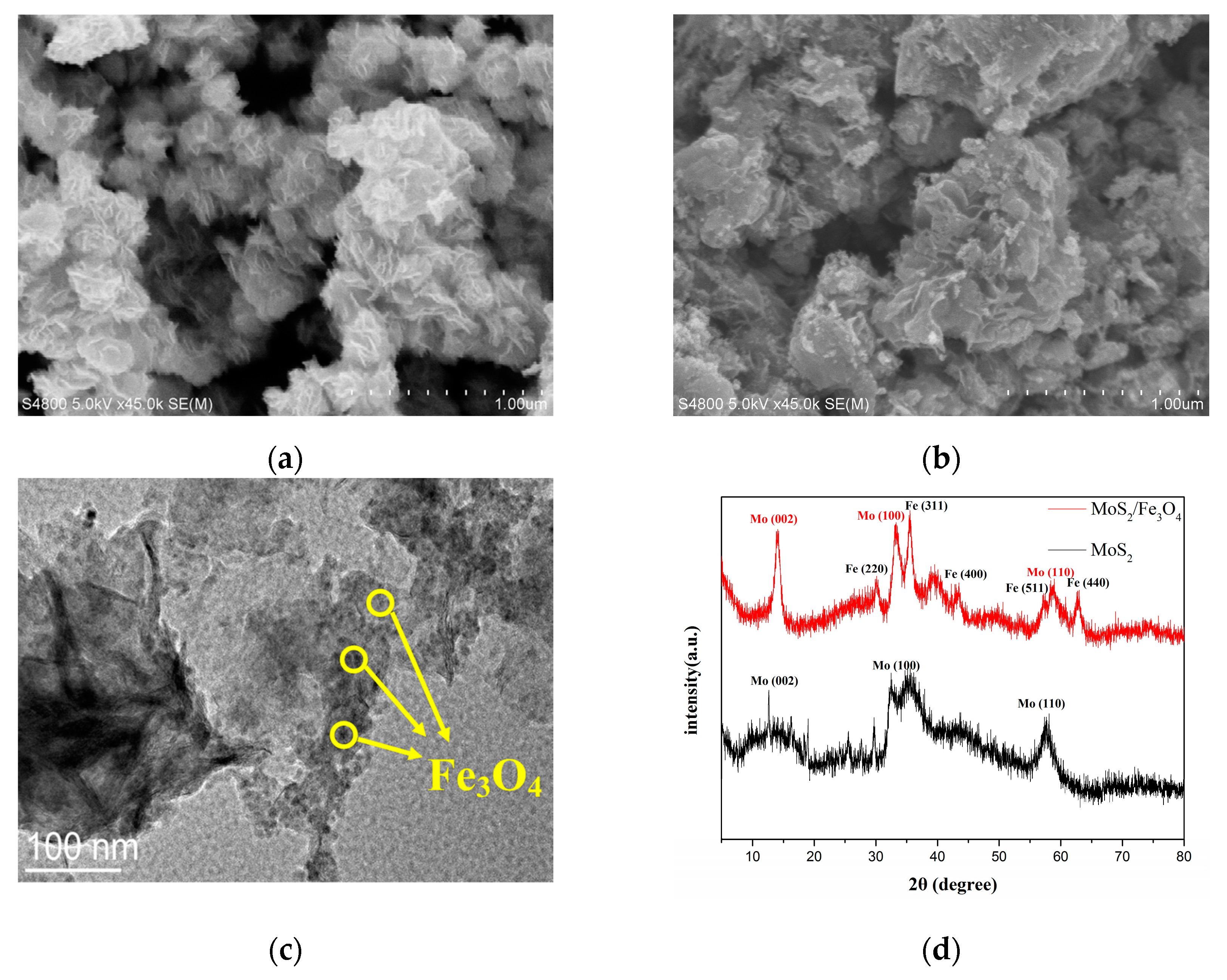
3.1. Characterization of the Synthesized MoS2/Fe3O4 Composites
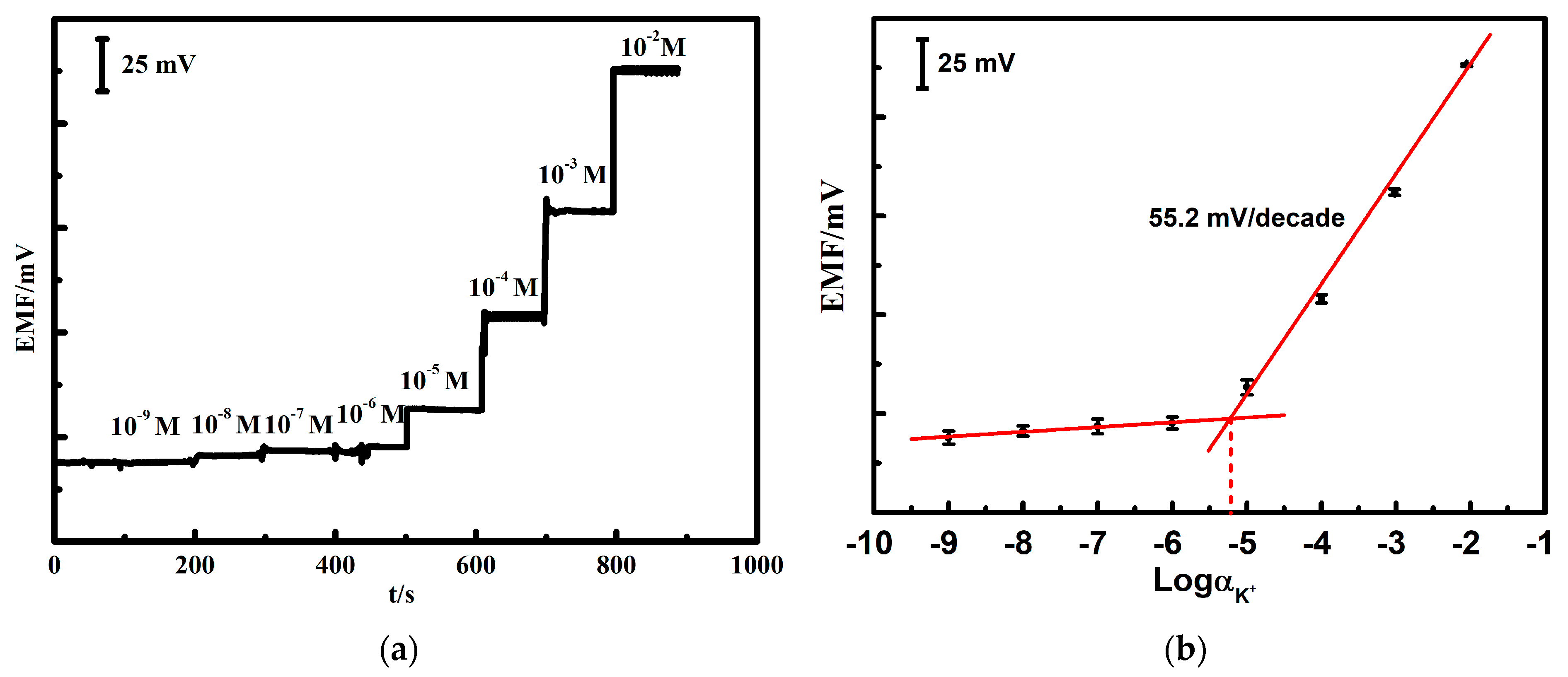
3.2. Potassium Response Test
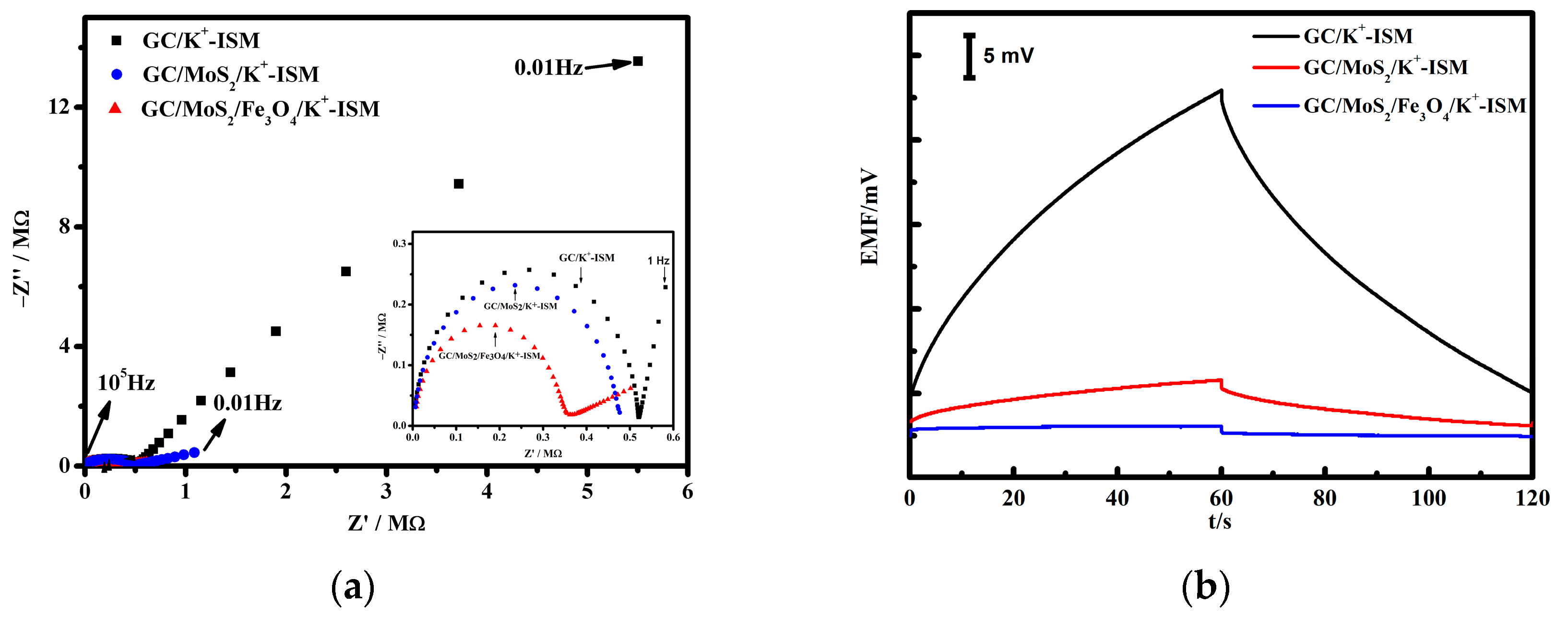
3.3. Electrochemical Impedance and Chronopotentiometry Test
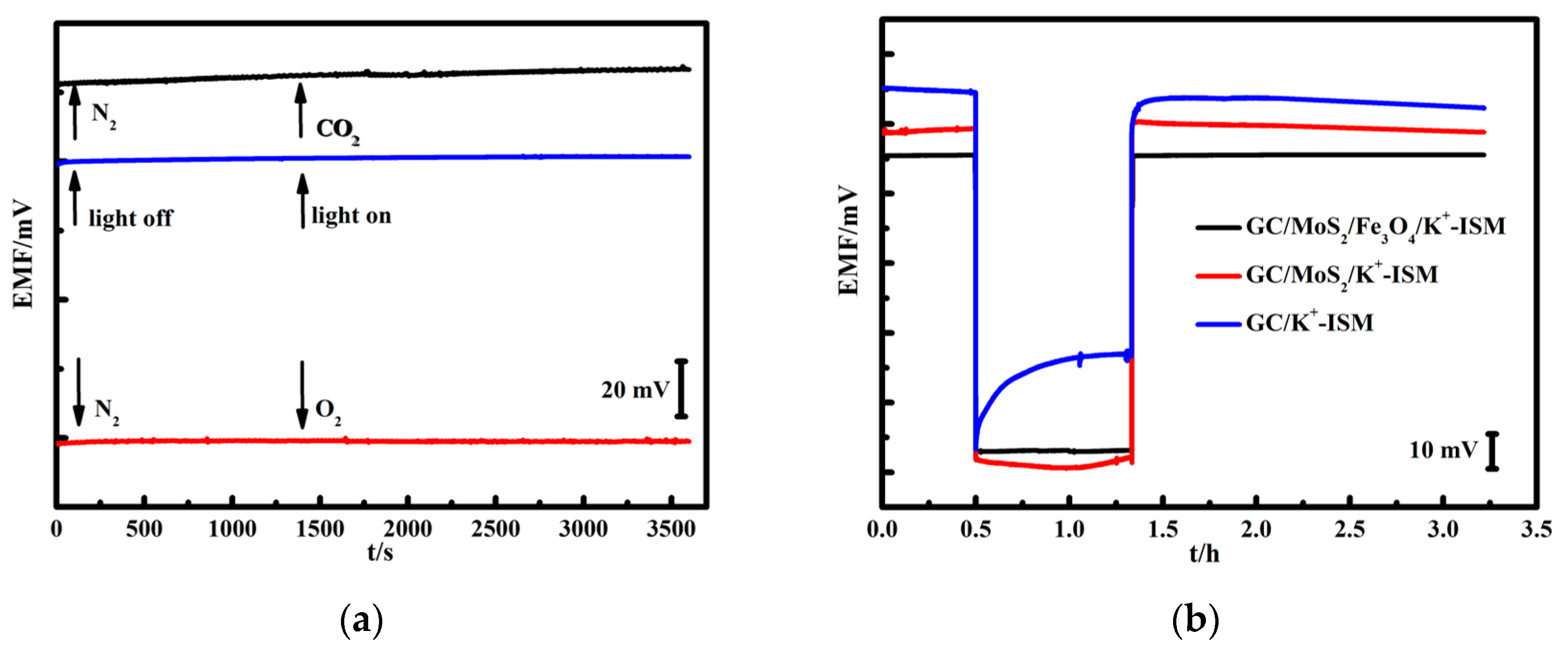
3.4. Anti-Interference and Water Layer Test
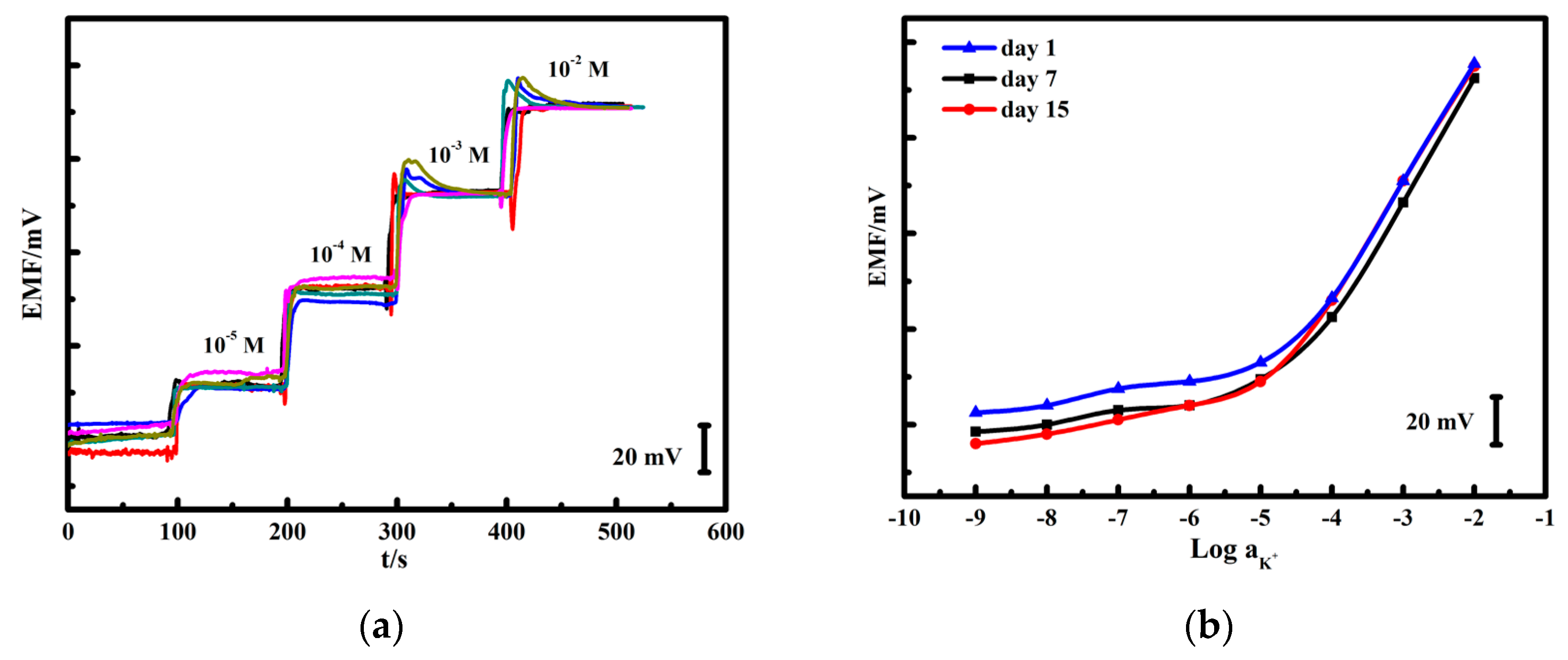
3.5. Potential Reproducibility and Stability Test
3.6. Analytical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, D.L.; Abbate, A. Potassium levels in acute myocardial infarction: Definitely worth paying attention to. Eur. Heart J. Cardiovasc. Pharmacother. 2015, 1, 252–253. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Ruan, W.; Ye, S.; Liu, Y.; Sui, H.; Li, Z.; Sun, X.; He, C.; Zhao, B. Detection of physiological potassium ions level in human serum by Raman scattering spectroscopy. Talanta 2016, 161, 743–747. [Google Scholar] [CrossRef]
- Ahmed, A.; Zannad, F.; Love, T.E.; Tallaj, J.; Gheorghiade, M.; Ekundayo, O.J.; Pitt, B. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur. Heart J. 2007, 28, 1334–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlomai, G.; Berkovitch, A.; Pinchevski-Kadir, S.; Bornstein, G.; Leibowitz, A.; Goldenberg, I.; Grossman, E. The association between normal-range admission potassium levels in Israeli patients with acute coronary syndrome and early and late outcomes. Medicine 2016, 95, e3778. [Google Scholar] [CrossRef] [PubMed]
- Verdian-Doghaei, A.; Housaindokht, M.R.; Abnous, K. A fluorescent aptasensor for potassium ion detection-based triple-helix molecular switch. Anal. Biochem. 2014, 466, 72–75. [Google Scholar] [CrossRef]
- Tounsi, M.; Braiek, M.B.; Barhoumi, H.; Baraket, A.; Lee, M.; Zine, N.; Maaref, A.; Errachid, A. A novel EIS field effect structures coated with TESUD-PPy-PVC-dibromoaza helicene matrix for potassium ions detection. Mater. Sci. Eng. C Mater. 2016, 61, 608–615. [Google Scholar] [CrossRef]
- Anderson, E.L.; Chopade, S.A.; Spindler, B.; Stein, A.; Lodge, T.P.; Hillmyer, M.A.; Bühlmann, P. Solid-Contact Ion-Selective and Reference Electrodes Covalently Attached to Functionalized Poly(ethylene terephthalate). Anal. Chem. 2020, 92, 7621–7629. [Google Scholar] [CrossRef]
- Paut, A.; Prkic, A.; Mitar, I.; Boskovic, P.; Jozic, D.; Jakic, M.; Vukusic, T. Potentiometric Response of Solid-State Sensors Based on Ferric Phosphate for Iron(III) Determination. Sensors 2021, 21, 1612. [Google Scholar] [CrossRef]
- Rousseau, C.R.; Bühlmann, P. Calibration-free potentiometric sensing with solid-contact ion-selective electrodes. TrAC Trends Anal. Chem. 2021, 140, 116277. [Google Scholar] [CrossRef]
- Ivanko, I.; Lindfors, T.; Emanuelsson, R.; Sjödin, M. Conjugated redox polymer with poly(3,4-ethylenedioxythiophene) backbone and hydroquinone pendant groups as the solid contact in potassium-selective electrodes. Sens. Actuators B Chem. 2021, 329, 129231. [Google Scholar] [CrossRef]
- Kamel, A.H.; Amr, A.E.-G.E.; Al-Omar, M.A.; Almehizia, A.A. Solid-State Membrane Sensors Based on Man-Tailored Biomimetic Receptors for Selective Recognition of Isoproturon and Diuron Herbicides. Membranes 2020, 10, 279. [Google Scholar] [CrossRef]
- Cattrall, R.W.; Freiser, H. Coated wire ion-selective electrodes. Anal. Chem. 1971, 13, 1905–1906. [Google Scholar] [CrossRef]
- Rostampour, M.; Bailey, B.; Autrey, C.; Ferrer, K.; Vantoorenburg, B.; Patel, P.K.; Calvo-Marzal, P.; Chumbimuni-Torres, K.Y. Single-Step Integration of Poly(3-Octylthiophene) and Single-Walled Carbon Nanotubes for Highly Reproducible Paper-Based Ion-Selective Electrodes. Anal. Chem. 2021, 93, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Yu, K.; He, N.; Kumar, N.; Wang, N.; Bobacka, J.; Ivaska, A. Electrosynthesized polypyrrole/zeolite composites as solid contact in potassium ion-selective electrode. Electrochim. Acta 2017, 228, 66–75. [Google Scholar] [CrossRef]
- Bobacka, J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalasys 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Gadhari, N.S.; Gholave, J.V.; Patil, S.S.; Patil, V.R.; Upadhyay, S.S. Enantioselective high performance new solid contact ion-selective electrode potentiometric sensor based on sulphated γ-cyclodextrin-carbon nanofiber composite for determination of multichiral drug moxifloxacin. J. Electroanal. Chem. 2021, 882, 114981. [Google Scholar] [CrossRef]
- Kraikaew, P.; Sailapu, S.K.; Bakker, E. Rapid Constant Potential Capacitive Measurements with Solid-Contact Ion-Selective Electrodes Coupled to Electronic Capacitor. Anal. Chem. 2020, 92, 14174–14180. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yu, S.; Yuan, Q.; Qin, W. Solid-contact K+-selective electrode based on three-dimensional molybdenum sulfide nano-flowers as ion-to-electron transducer. Sens. Actuators B Chem. 2016, 234, 80–83. [Google Scholar] [CrossRef]
- Rutkowska, M.; Lindfors, T.; Boeva, Z.; Strawski, M. Low-cost flexible laminated graphene paper solid-contact ion-selective electrodes. Sens. Actuators B Chem. 2021, 337, 129808. [Google Scholar] [CrossRef]
- Li, J.; Qin, W. An integrated all-solid-state screen-printed potentiometric sensor based on a three-dimensional self-assembled graphene aerogel. Microchem. J. 2020, 159, 105453. [Google Scholar] [CrossRef]
- Kozma, J.; Papp, S.; Gyurcsányi, R.E. Solid-contact ion-selective electrodes based on ferrocene-functionalized multi-walled carbon nanotubes. Electrochem. Commun. 2021, 123, 106903. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. High Capacity Nanocomposite Layers Based on Nanoparticles of Carbon Materials and Ruthenium Dioxide for Potassium Sensitive Electrode. Materials 2021, 14, 1308. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Wyrwa, J.; Paczosa-Bator, B. Potassium-Selective Solid-Contact Electrode with High-Capacitance Hydrous Iridium Dioxide in the Transduction Layer. Membranes 2021, 11, 259. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, B.; Gogotsi, Y. Synthesis of Two-Dimensional Materials for Capacitive Energy Storage. Adv. Mater. 2016, 28, 6104–6135. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Quan, X. Two-dimensional MoS2: A promising building block for biosensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Wang, Y.; Yuan, H. Fe3O4 nanoparticles grown on graphene as advanced electrode materials for supercapacitors. J. Power Sources 2014, 245, 101–106. [Google Scholar] [CrossRef]
- Yin, T.; Jiang, X.; Qin, W. A magnetic field-directed self-assembly solid contact for construction of an all-solid-state polymeric membrane Ca2+-selective electrode. Anal. Chim. Acta 2017, 989, 15–20. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Tang, X.; Lu, L.; Xue, J. Ultrasmall Fe3O4 Nanoparticle/MoS2 Nanosheet Composites with Superior Performances for Lithium Ion Batteries. Small 2014, 10, 1536–1543. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Lu, L.; Xue, J. Ultra-small Fe3O4 nanoparticle decorated graphene nanosheets with superior cyclic performance and rate capability. Nanoscale 2013, 5, 6797–6803. [Google Scholar] [CrossRef]
- Liu, J.; Du, J.; Su, Y.; Zhao, H. A facile solvothermal synthesis of 3D magnetic MoS2/Fe3O4 nanocomposites with enhanced peroxidase-mimicking activity and colorimetric detection of perfluorooctane sulfonate. Microchem. J. 2019, 149, 104091. [Google Scholar] [CrossRef]
- Zeng, X.; Qin, W. A solid-contact potassium-selective electrode with MoO2 microspheres as ion-to-electron transducer. Anal. Chim. Acta 2017, 982, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Rius, F.X. Ion-Selective Electrodes Using Carbon Nanotubes as Ion-to-Electron Transducers. Anal. Chem. 2008, 80, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Qin, W. A solid-contact Ca2+-selective electrode based on an inorganic redox buffer of Ag@AgCl/1-tetradecyl-3-methylimidazolium chloride as ion-to-electron transducer. Talanta 2020, 209, 120570. [Google Scholar] [CrossRef]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential Drifts of Solid-Contacted Ion-Selective Electrodes Due to Zero-Current Ion Fluxes Through the Sensor Membrane. Electroanalysis 2010, 12, 1286–1292. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Liu, T.; Song, C.; Fan, A.; Zhu, N.; Sun, B.; Yang, C. Using MoS2/Fe3O4 as Ion-Electron Transduction Layer to Manufacture All-Solid-State Ion-Selective Electrode for Determination of Serum Potassium. Chemosensors 2021, 9, 155. https://doi.org/10.3390/chemosensors9070155
Su Y, Liu T, Song C, Fan A, Zhu N, Sun B, Yang C. Using MoS2/Fe3O4 as Ion-Electron Transduction Layer to Manufacture All-Solid-State Ion-Selective Electrode for Determination of Serum Potassium. Chemosensors. 2021; 9(7):155. https://doi.org/10.3390/chemosensors9070155
Chicago/Turabian StyleSu, Yan, Ting Liu, Caiqiao Song, Aiqiao Fan, Nan Zhu, Bingbing Sun, and Cheng Yang. 2021. "Using MoS2/Fe3O4 as Ion-Electron Transduction Layer to Manufacture All-Solid-State Ion-Selective Electrode for Determination of Serum Potassium" Chemosensors 9, no. 7: 155. https://doi.org/10.3390/chemosensors9070155
APA StyleSu, Y., Liu, T., Song, C., Fan, A., Zhu, N., Sun, B., & Yang, C. (2021). Using MoS2/Fe3O4 as Ion-Electron Transduction Layer to Manufacture All-Solid-State Ion-Selective Electrode for Determination of Serum Potassium. Chemosensors, 9(7), 155. https://doi.org/10.3390/chemosensors9070155







