Additive Manufacturing of a Flexible Carbon Monoxide Sensor Based on a SnO2-Graphene Nanoink
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of SnO2-Decorated Reduced Graphene Oxide (SnO2-rGO) Aerogel and Ink Formulation
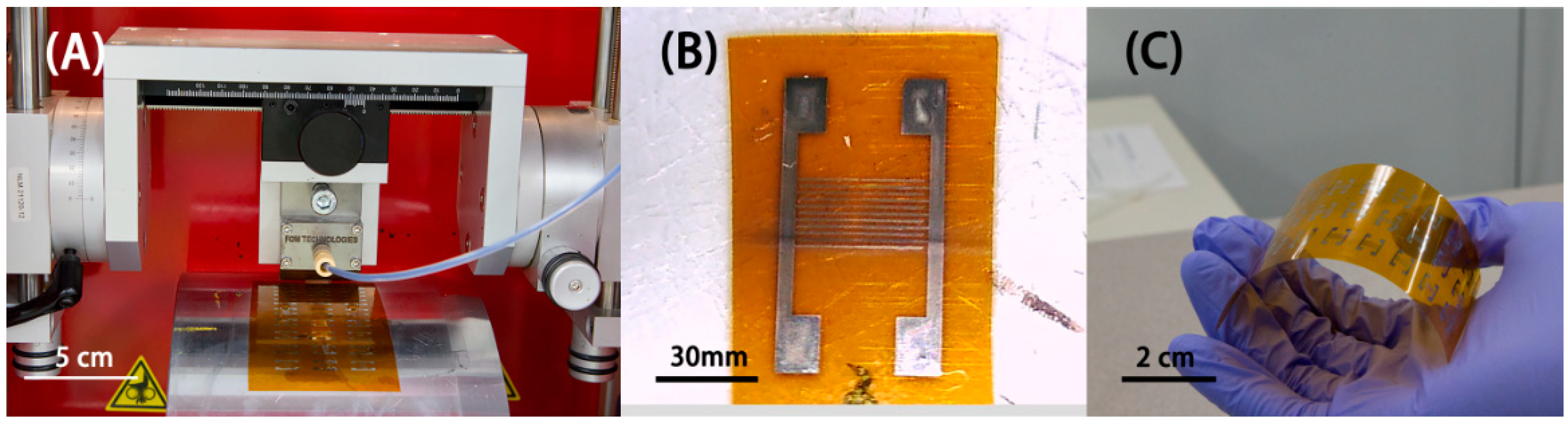
2.2. Device Fabrication
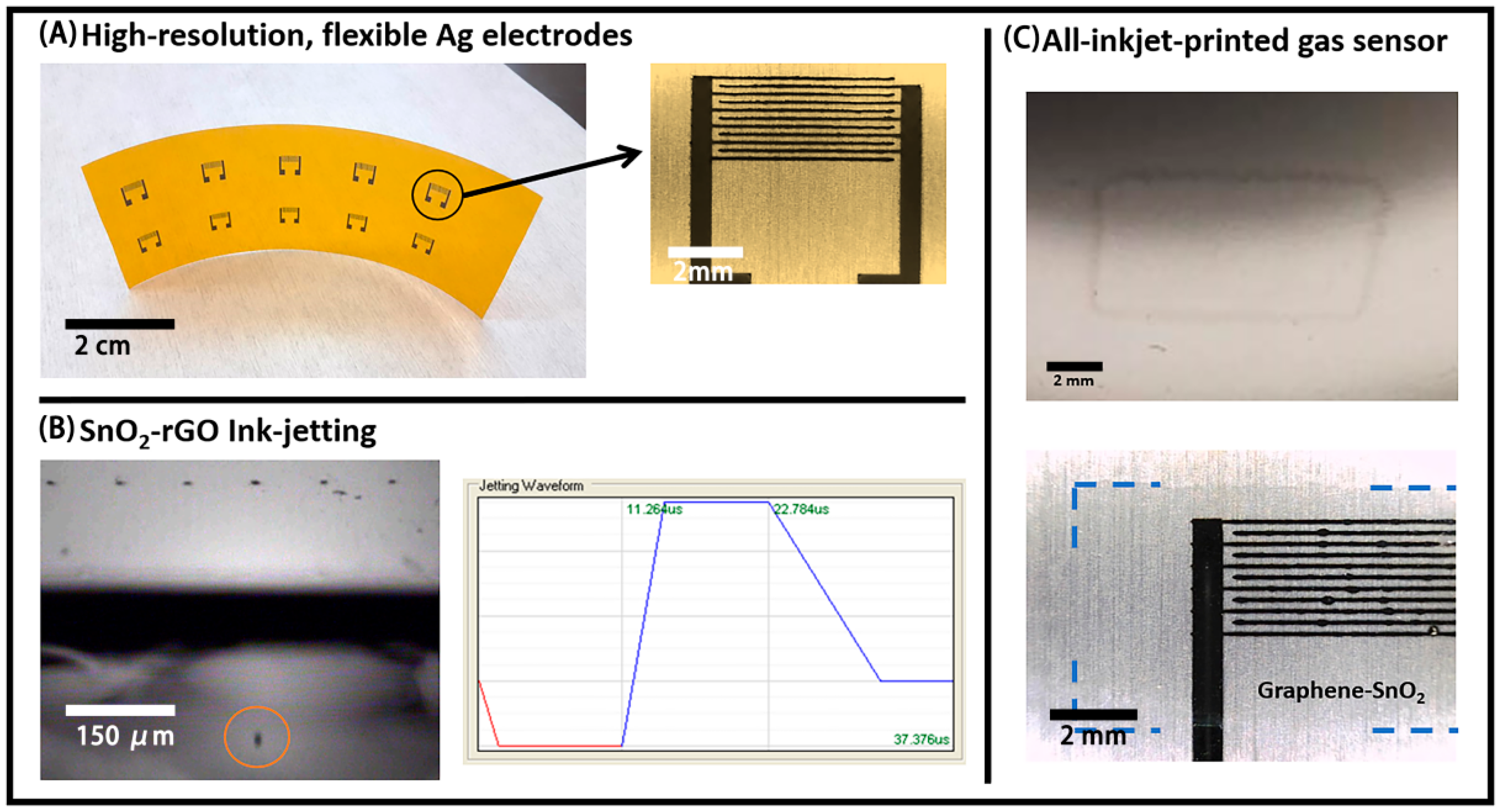
2.2.1. Inkjet-Printed Ag Electrodes
2.2.2. SnO2-rGO Active Layer Printing and Coating
2.3. CO Sensing Measurements
3. Results
3.1. SnO2-rGO Composition and Manostructure
3.2. Inkjet-Printed Electrodes and Active Layer
3.3. Slot-Die Coated SnO2-rGO Film
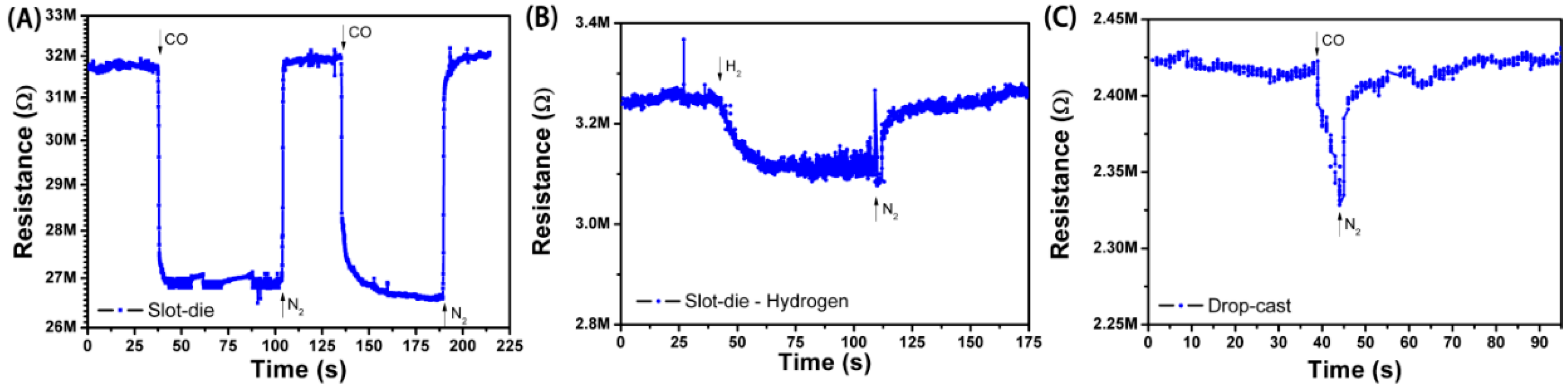
3.4. CO Sensing
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hawley, C. Toxic Gas Sensors. In Hazardous Materials Air Monitoring and Detection Devices, 2nd ed.; Hawley, C., Ed.; Delmar, Clifton Park, Jones and Bartlett Learning: Burlington, MA, USA, 2007; p. 43. [Google Scholar]
- Smith, R.L.; Hobbs, B.S.; Watson, J. Sensors, Nanoscience, and Instruments. In Sensors, Nonsocial. Biomed. Eng. Instruments; Dorf, R.C., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Harrison, P.G.; Willett, M.J. The mechanism of operation of tin(iv) oxide carbon monoxide sensors. Nature 1988, 332, 337–339. [Google Scholar] [CrossRef]
- Li, C.; Lv, M.; Zuo, J.; Huang, X. SnO2 Highly Sensitive CO Gas Sensor Based on Quasi-Molecular-Imprinting Mechanism Design. Sensors 2015, 15, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Jain, K. At room temperature graphene/SnO2 is better than MWCNT/SnO2 as NO2 gas sensor. Mater. Lett. 2016, 169, 28–32. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Paschoal, C.W.; Arias, A.C. Printed and flexible biosensor for antioxidants using interdigitated ink-jetted electrodes and gravure-deposited active layer. Biosens. Bioelectron. 2015, 67, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Bakrania, S.D.; Wooldridge, M.S. The Effects of Two Thick Film Deposition Methods on Tin Dioxide Gas Sensor Performance. Sensors 2009, 9, 6853–6868. [Google Scholar] [CrossRef]
- Martinelli, G.; Carotta, M. Influence of additives on the sensing properties of screen-printed SnO2 gas sensors. Sens. Actuators B Chem. 1993, 16, 363–366. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2sensors in the presence of humidity. J. Physics Condens. Matter 2003, 15, R813–R839. [Google Scholar] [CrossRef]
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.-P.; Pijolat, C.; De Rooij, N.F.; Briand, D. Fully inkjet printed SnO2 gas sensor on plastic substrate. Sens Actuators B Chem. 2016, 236, 1091–1097. [Google Scholar] [CrossRef]
- Shen, W. Properties of SnO2 based gas-sensing thin films prepared by ink-jet printing. Sens. Actuators B Chem. 2012, 166, 110–116. [Google Scholar] [CrossRef]
- Shen, W.; Zhao, Y.; Zhang, C. The preparation of ZnO based gas-sensing thin films by ink-jet printing method. Thin Solid Films 2005, 483, 382–387. [Google Scholar] [CrossRef]
- Mao, S.; Cui, S.; Lu, G.; Yu, K.; Wen, Z.; Chen, J. Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J. Mater. Chem. 2012, 22, 11009. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, S.; Chang, J.; Ocola, L.E.; Chen, J. Highly sensitive room temperature carbon monoxide detection using SnO2nanoparticle-decorated semiconducting single-walled carbon nanotubes. Nanotechnology 2012, 24, 025503. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, J.; Sun, J.; Zhang, X. 3D graphene aerogel-supported SnO2 nanoparticles for efficient detection of NO2. RSC Adv. 2014, 4, 22601–22605. [Google Scholar] [CrossRef]
- Lim, M.B.; Hu, M.; Manandhar, S.; Sakshaug, A.; Strong, A.; Riley, L.; Pauzauskie, P.J. Ultrafast sol–gel synthesis of graphene aerogel materials. Carbon 2015, 95, 616–624. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef]
- Wu, J.; Shen, X.; Jiang, L.; Wang, K.; Chen, K. Solvothermal synthesis and characterization of sandwich-like graphene/ZnO nanocomposites. Appl. Surf. Sci. 2010, 256, 2826–2830. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. High reversible capacity of SnO2/graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta. 2011, 56, 4532–4539. [Google Scholar] [CrossRef]
- Liang, J.; Wei, W.; Zhong, D.; Yang, Q.; Li, L.; Guo, L. One-Step In situ Synthesis of SnO2/Graphene Nanocomposites and Its Application As an Anode Material for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 454–459. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Zhao, X.S. Graphene–metal–oxide composites for the degradation of dyes under visible light irradiation. J. Mater. Chem. 2011, 21, 3634. [Google Scholar] [CrossRef]
- Liu, L.; An, M.; Yang, P.; Zhang, J. Superior cycle performance and high reversible capacity of SnO2/graphene composite as an anode material for lithium-ion batteries. Sci. Rep. 2015, 5, 9055. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic XPS Spectra, The Elements of Native Oxides; John Wiley & Sons Ltd.: New York, NY, USA, 2000. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Khan, Y.; Pavinatto, F.J.; Lin, M.C.; Liao, A.; Swisher, S.L.; Mann, K.; Subramanian, V.; Maharbiz, M.M.; Arias, A.C. Inkjet-Printed Flexible Gold Electrode Arrays for Bioelectronic Interfaces. Adv. Funct. Mater. 2015, 26, 1004–1013. [Google Scholar] [CrossRef]
- Singh, J.; Wells, L.T.; Rugman, A.M.; Amsden, A.H.; Williamson, P.; Zeng, M.; McCarthy, D.J.; Puffer, S.M.; Vikhanski, O.S.; Fleury, A.; et al. Emerging Multinationals from Emerging Markets. Emerg. Multinatl. Emerg. Mark. 2009, 34, 538–542. [Google Scholar] [CrossRef]
- Al-Homoudi, I.A.; Thakur, J.; Naik, R.; Auner, G.W.; Newaz, G. Anatase TiO2 films based CO gas sensor: Film thickness, substrate and temperature effects. Appl. Surf. Sci. 2007, 253, 8607–8614. [Google Scholar] [CrossRef]
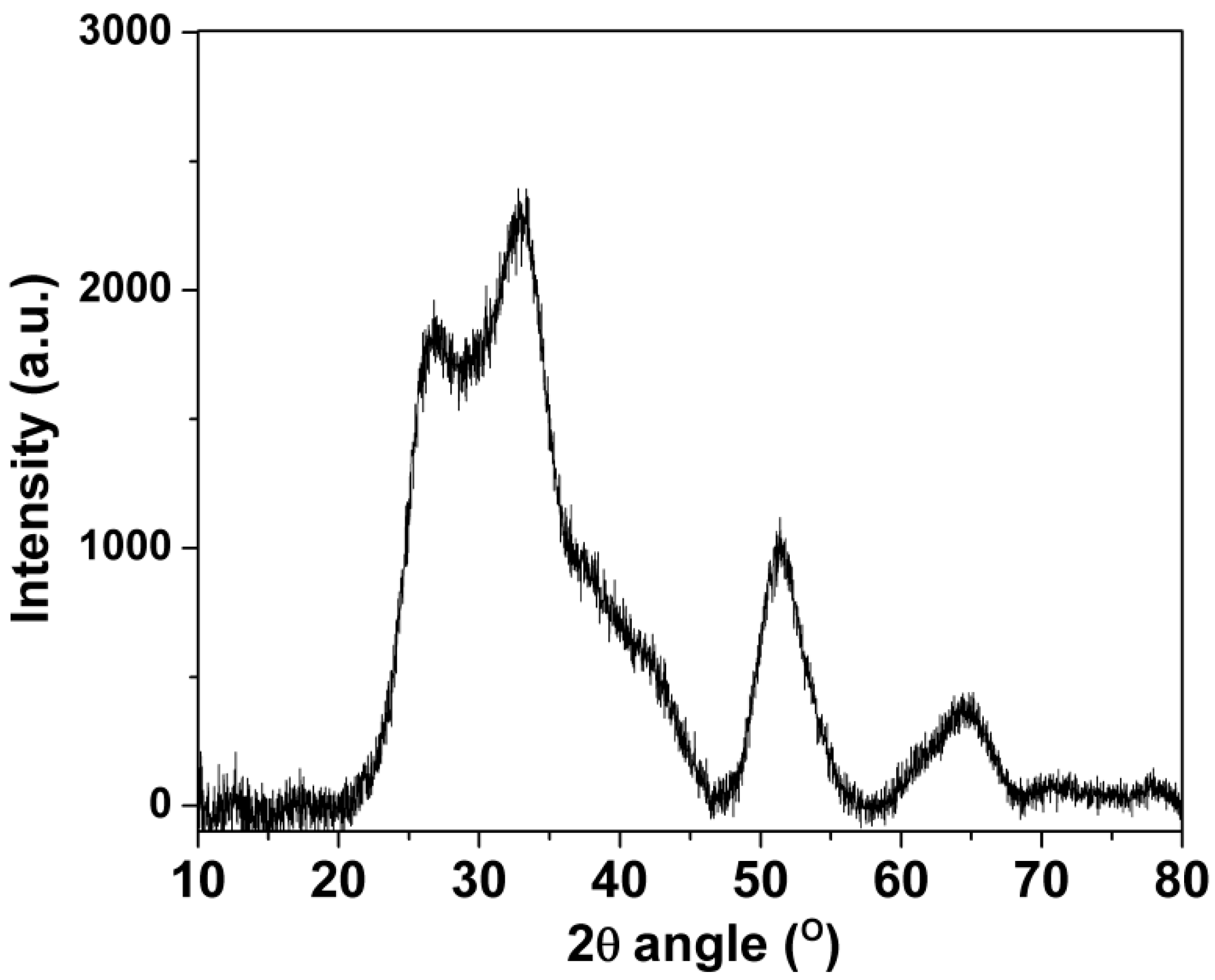
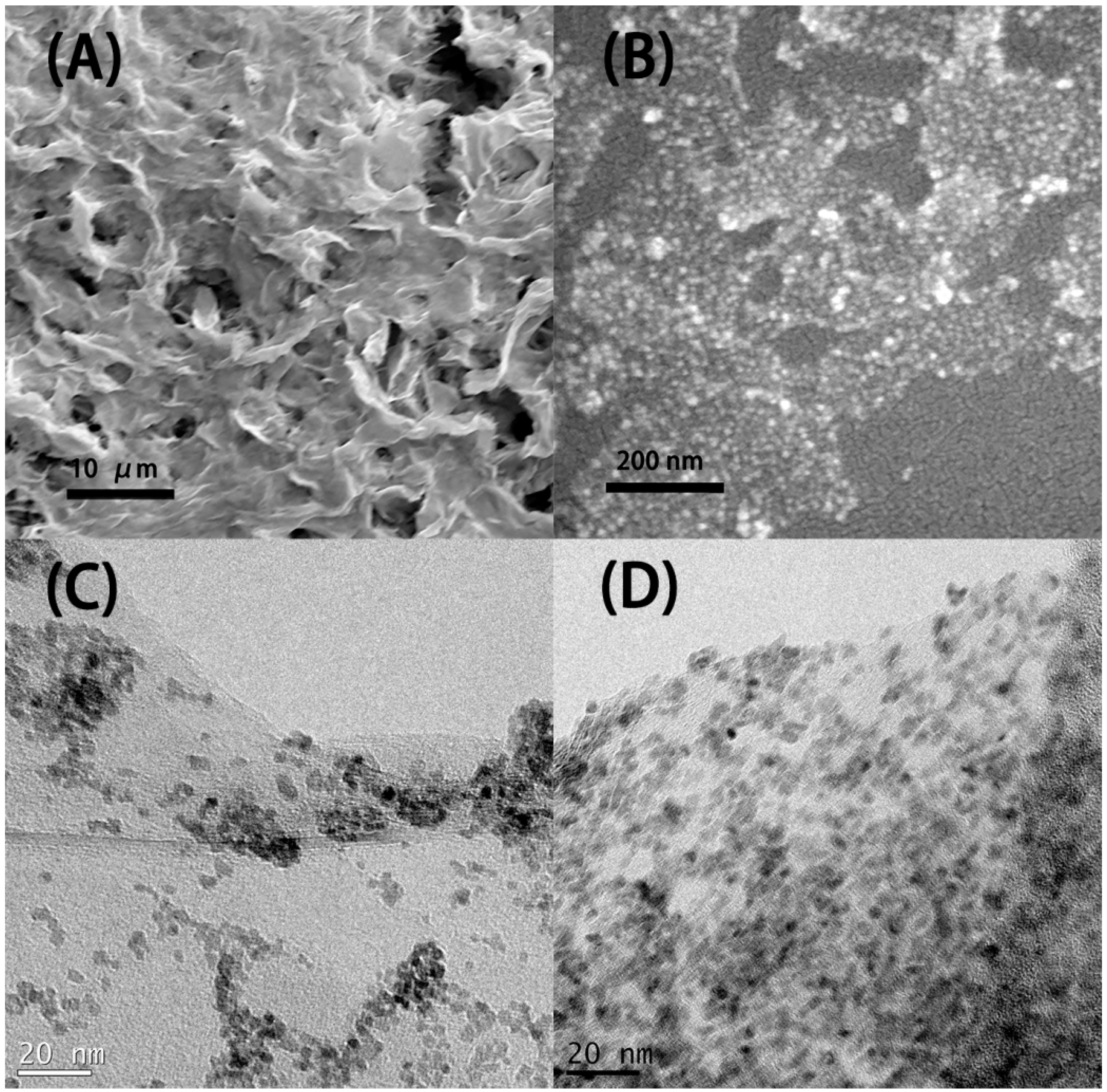
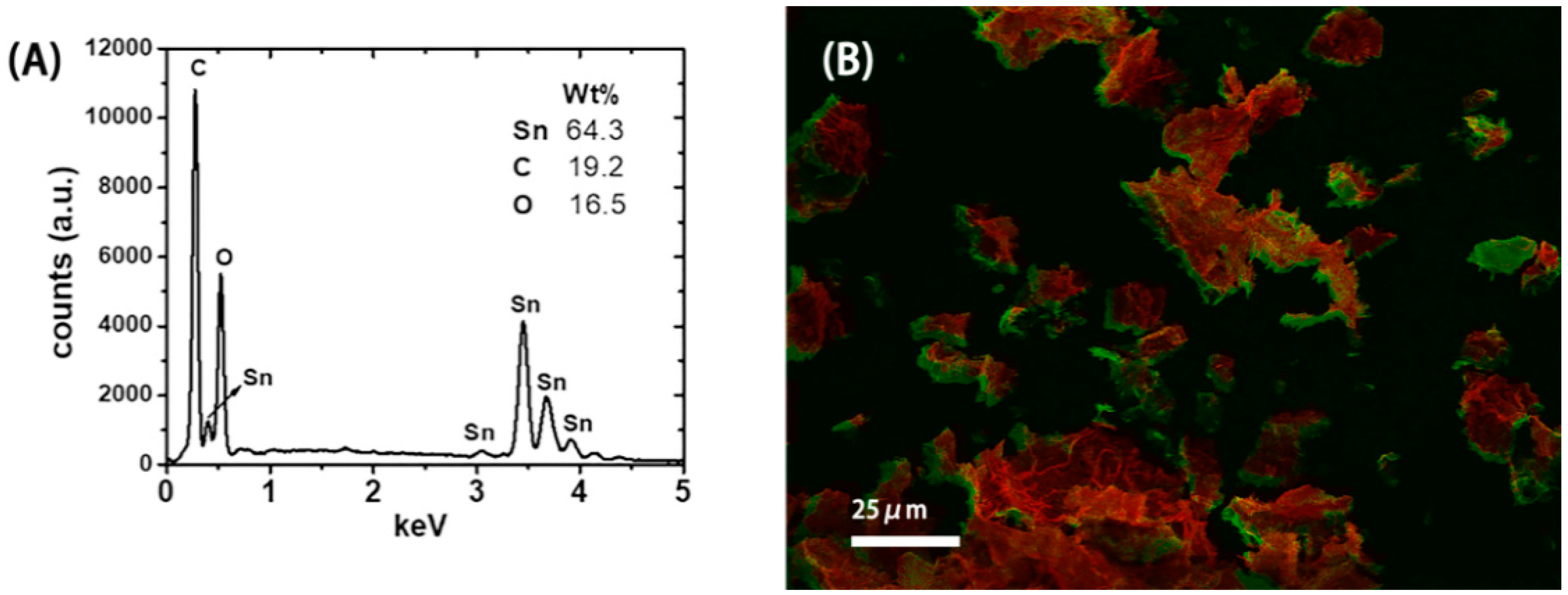
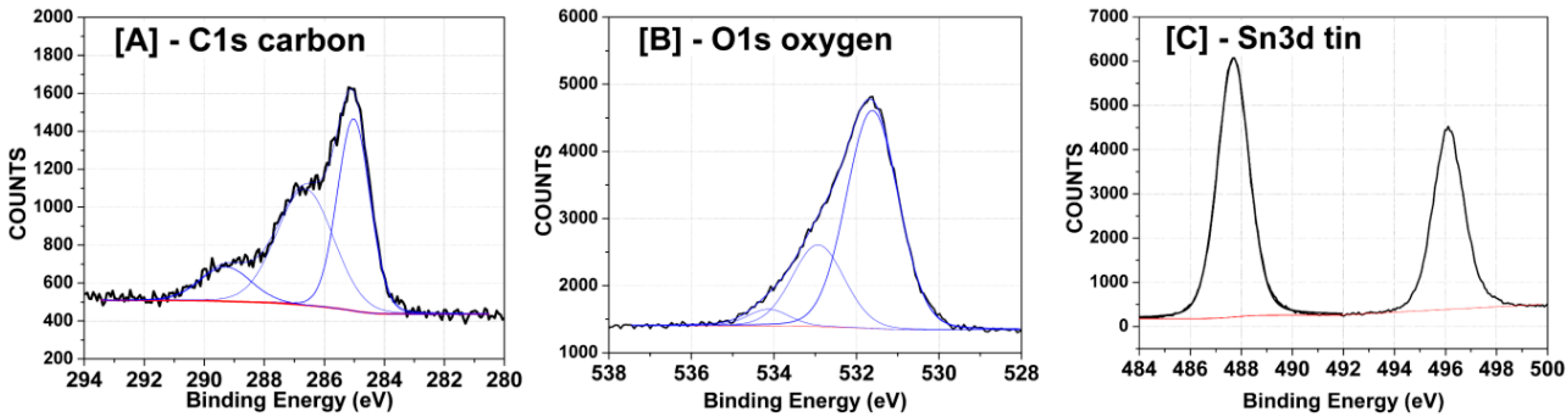
| Sample | Post-Treatment | C:O Ratio * | |
|---|---|---|---|
| 1 | GO | - | 2.4 |
| 2 | r-GO one-step | - | 5.5 |
| 3 | SnO2/r-GO one-step | - | 5.0 |
| 4 | SnO2/r-GO two-step | - | 12.1 |
| 5 | SnO2/r-GO two-step | 120 °C/12 h | 12.5 |
| 6 | SnO2/r-GO two-step | 200 °C/30 min | 16.6 |
| Sample | Response (ΔR) | Response Time/Recovery Time | |
|---|---|---|---|
| 1 | Drop-cast devices | 7% | 6 s/14 s |
| 2 | SnO2/r-GO one-step slot-die coated devices | 12% | 4 s/13 s |
| 3 | SnO2/r-GO two-step slot-die coated devices | 15% | 4.5 s/12 s |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Tavakoli, S.; Mathavakrishnan, D.; Ma, T.; Lim, M.; Rotondo, B.; Pauzauskie, P.; Pavinatto, F.; MacKenzie, D. Additive Manufacturing of a Flexible Carbon Monoxide Sensor Based on a SnO2-Graphene Nanoink. Chemosensors 2020, 8, 36. https://doi.org/10.3390/chemosensors8020036
Zuo J, Tavakoli S, Mathavakrishnan D, Ma T, Lim M, Rotondo B, Pauzauskie P, Pavinatto F, MacKenzie D. Additive Manufacturing of a Flexible Carbon Monoxide Sensor Based on a SnO2-Graphene Nanoink. Chemosensors. 2020; 8(2):36. https://doi.org/10.3390/chemosensors8020036
Chicago/Turabian StyleZuo, Jialin, Sean Tavakoli, Deepakkrishna Mathavakrishnan, Taichong Ma, Matthew Lim, Brandon Rotondo, Peter Pauzauskie, Felippe Pavinatto, and Devin MacKenzie. 2020. "Additive Manufacturing of a Flexible Carbon Monoxide Sensor Based on a SnO2-Graphene Nanoink" Chemosensors 8, no. 2: 36. https://doi.org/10.3390/chemosensors8020036
APA StyleZuo, J., Tavakoli, S., Mathavakrishnan, D., Ma, T., Lim, M., Rotondo, B., Pauzauskie, P., Pavinatto, F., & MacKenzie, D. (2020). Additive Manufacturing of a Flexible Carbon Monoxide Sensor Based on a SnO2-Graphene Nanoink. Chemosensors, 8(2), 36. https://doi.org/10.3390/chemosensors8020036





