Flexible Carbon Electrodes for Electrochemical Detection of Bisphenol-A, Hydroquinone and Catechol in Water Samples
Abstract
1. Introduction
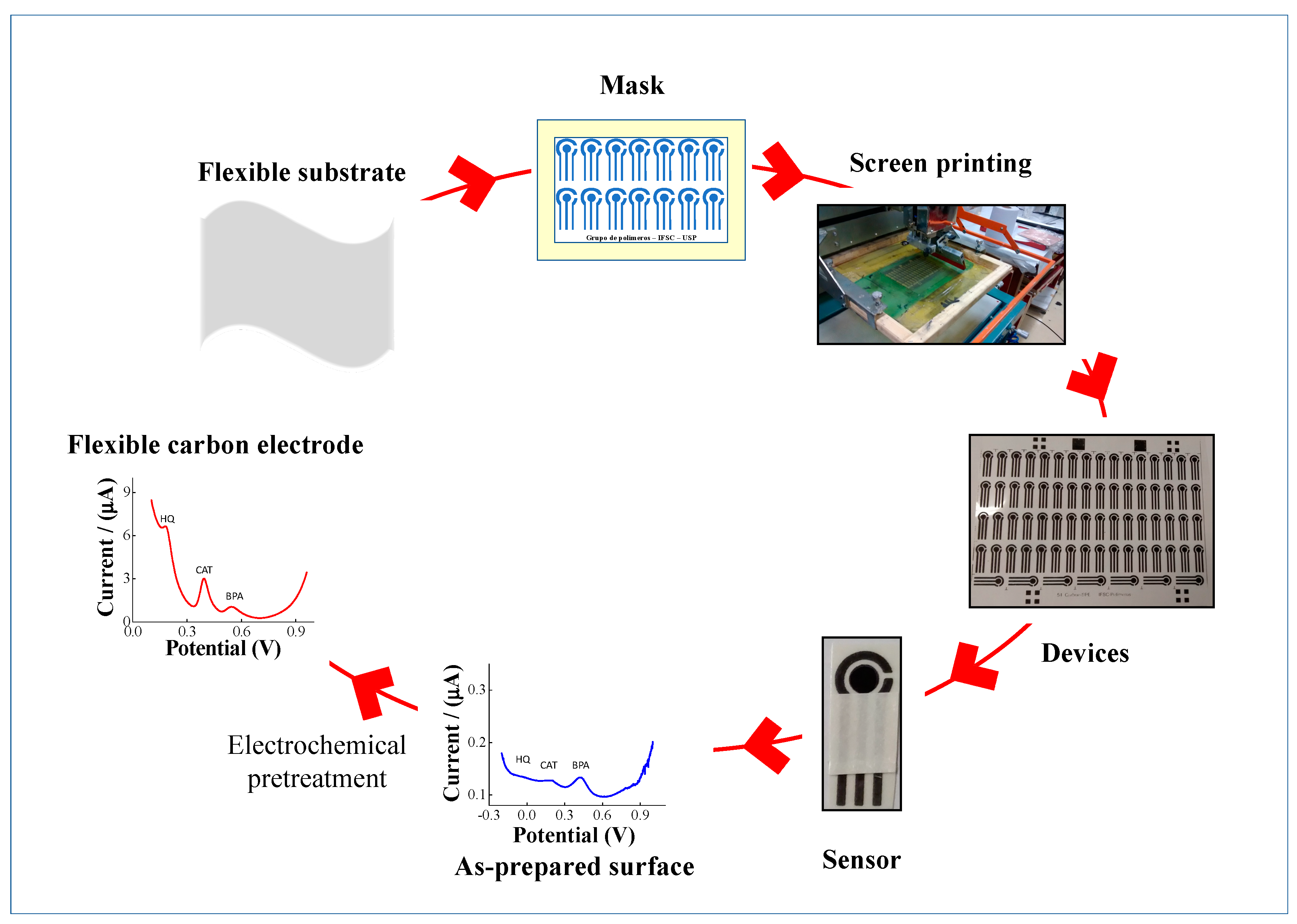
2. Materials and Methods
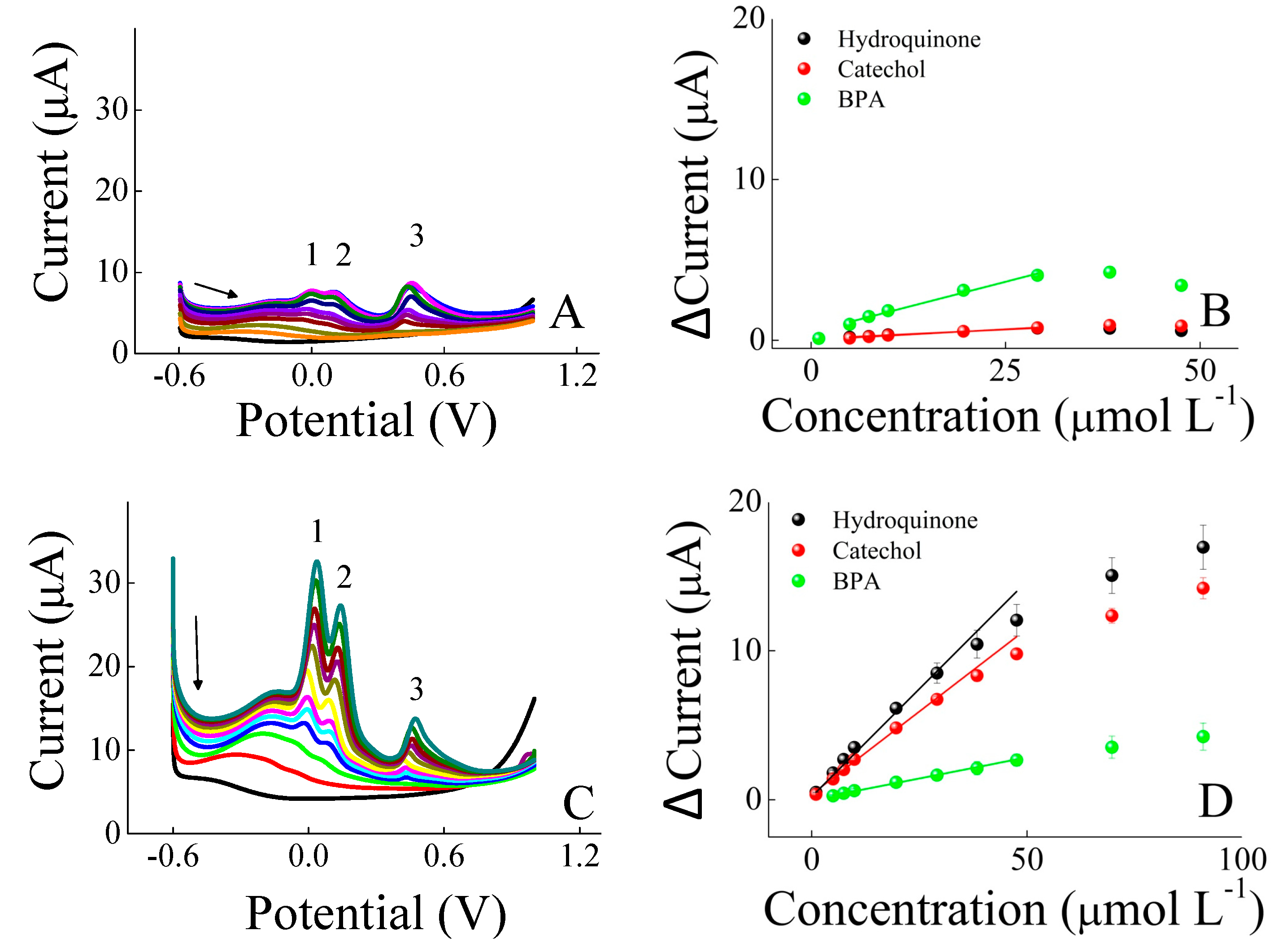
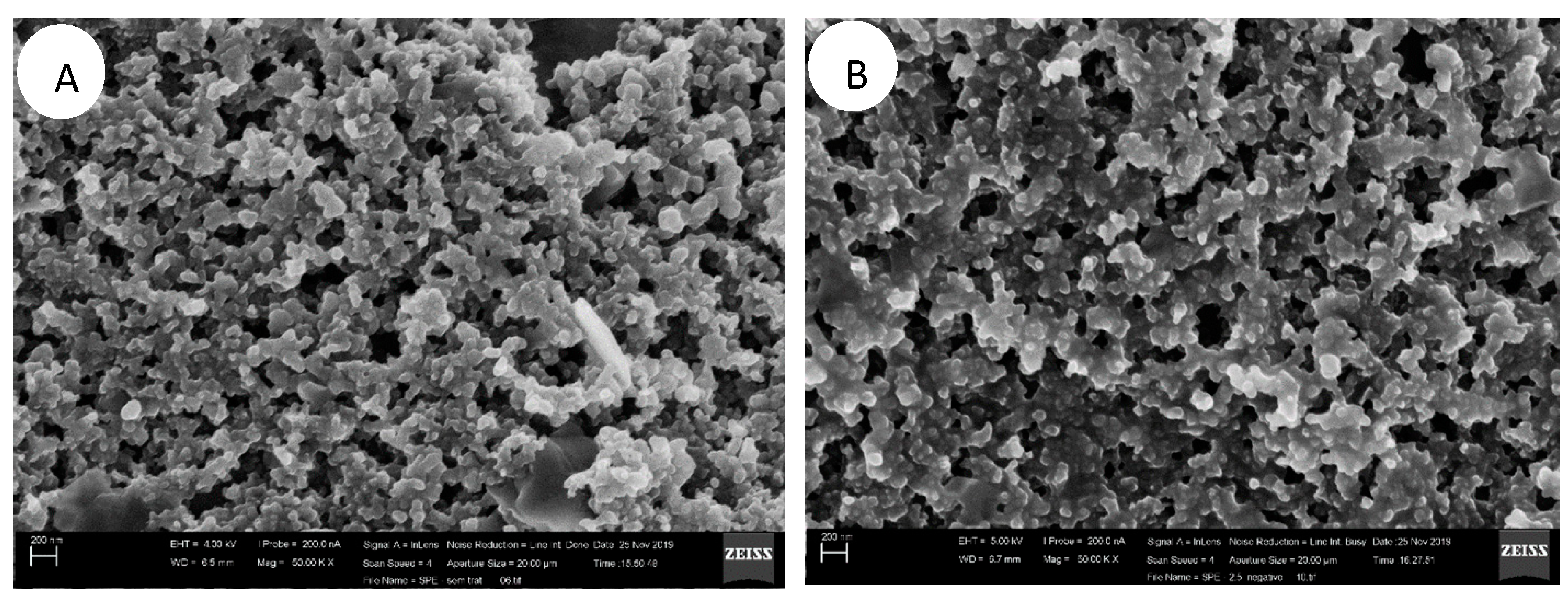
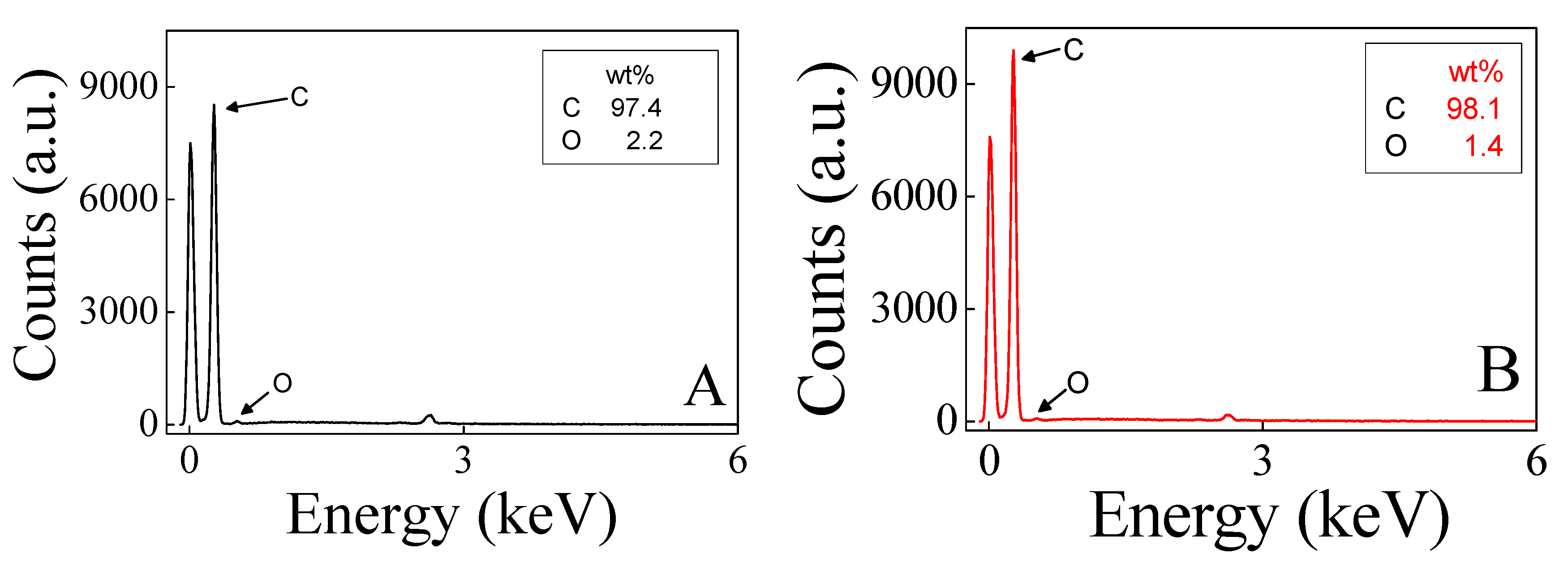
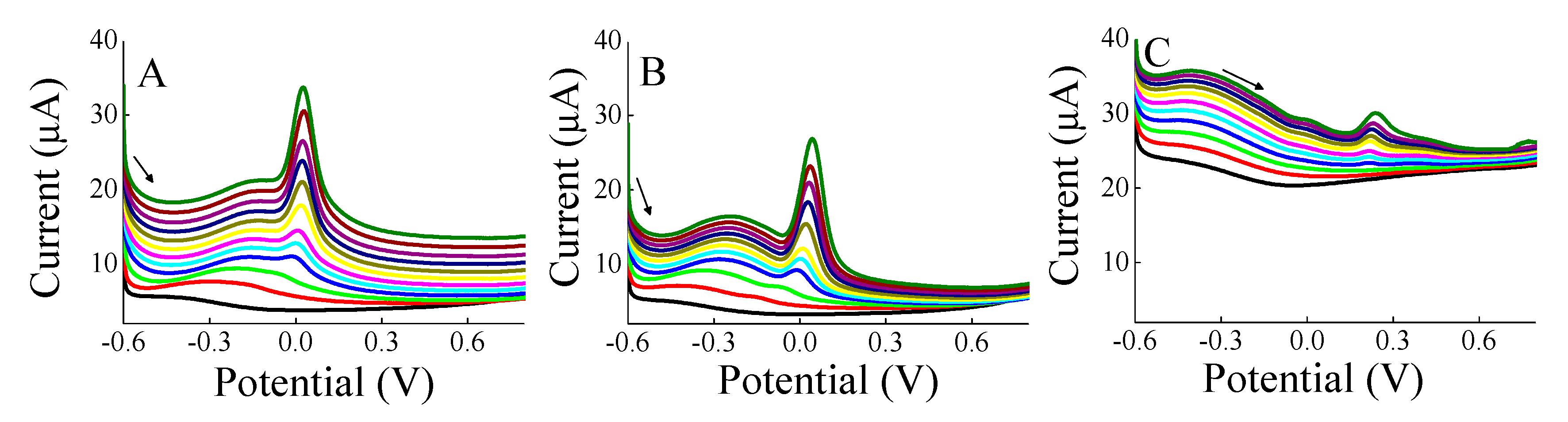
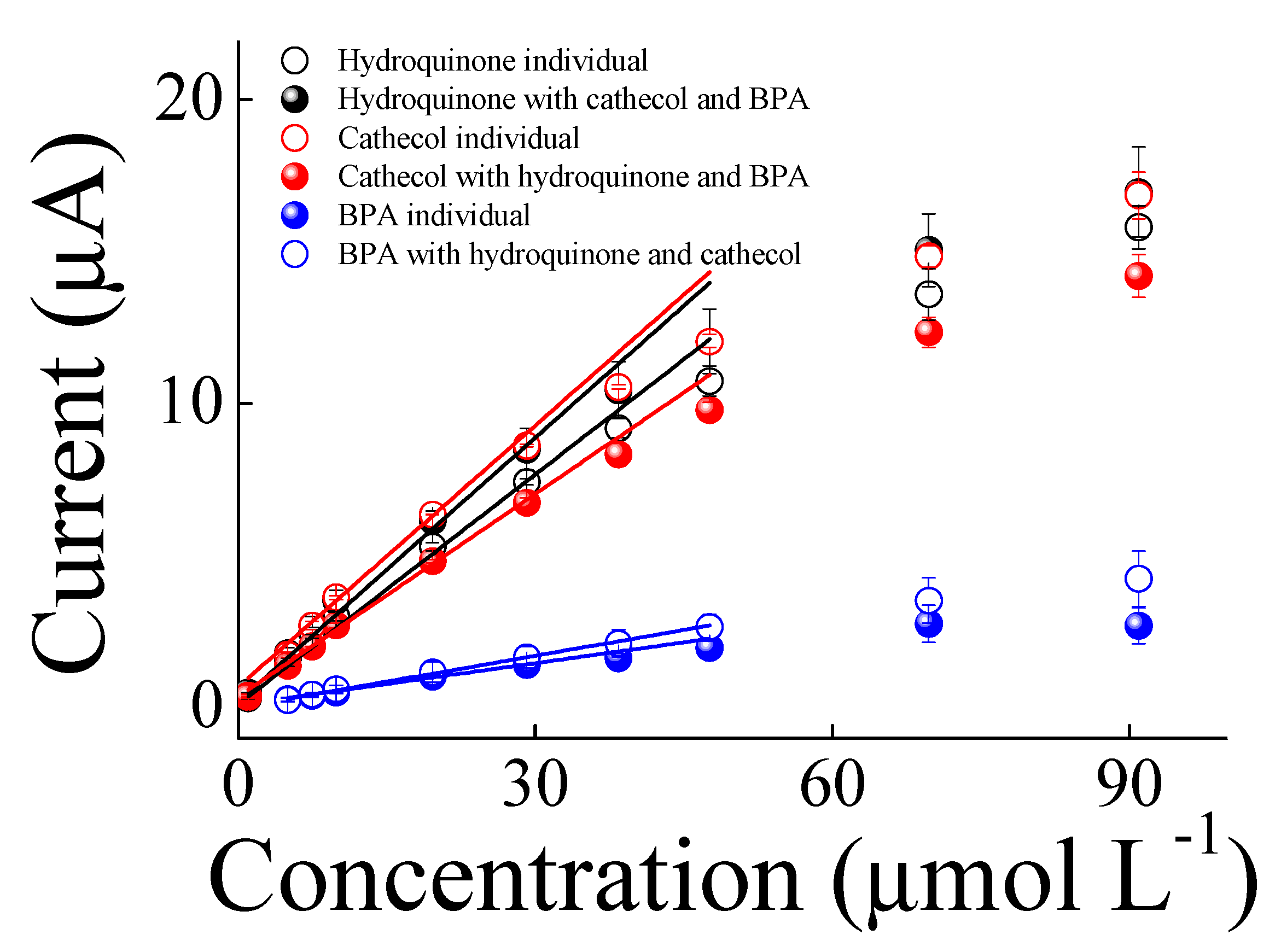
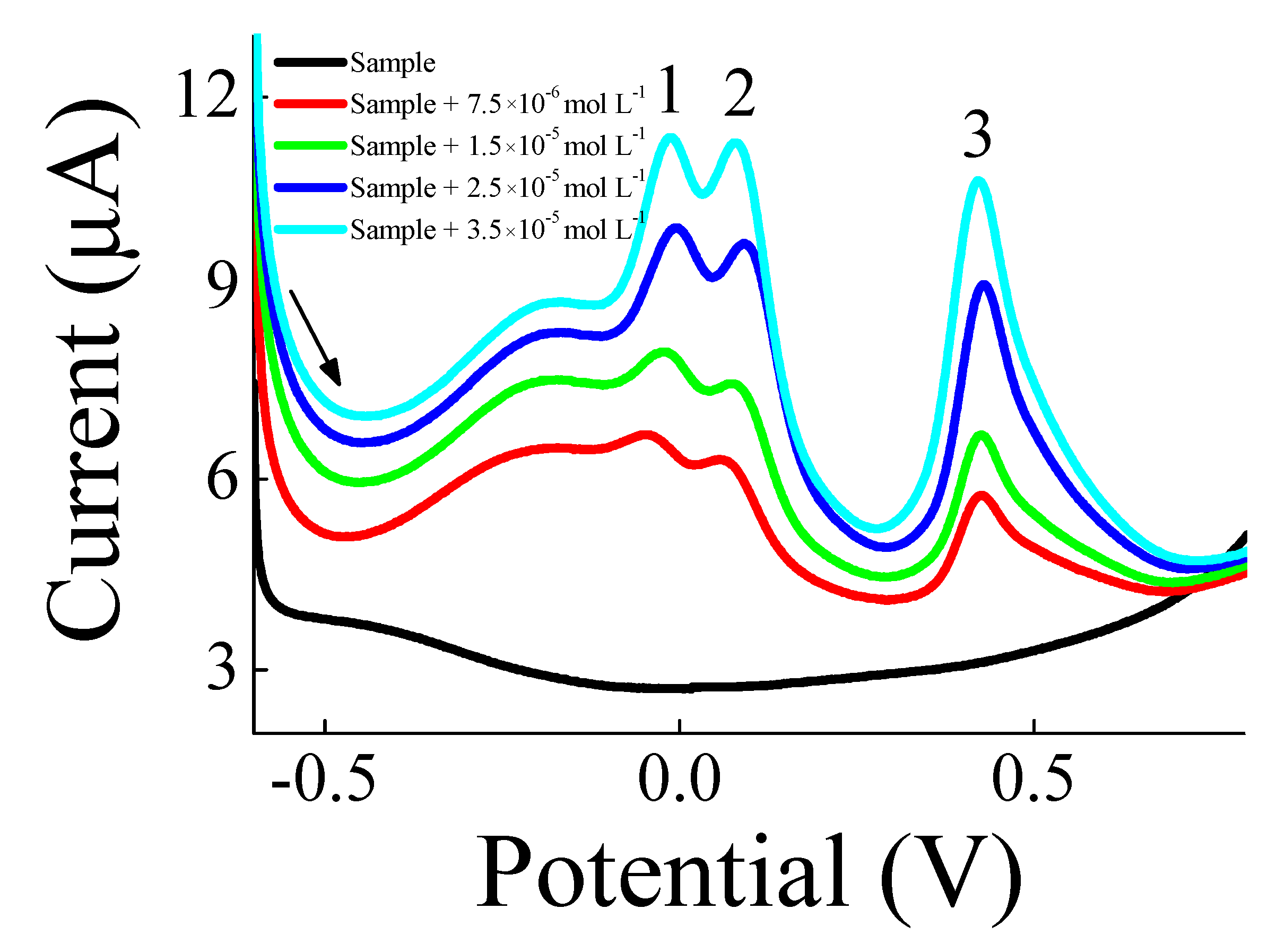
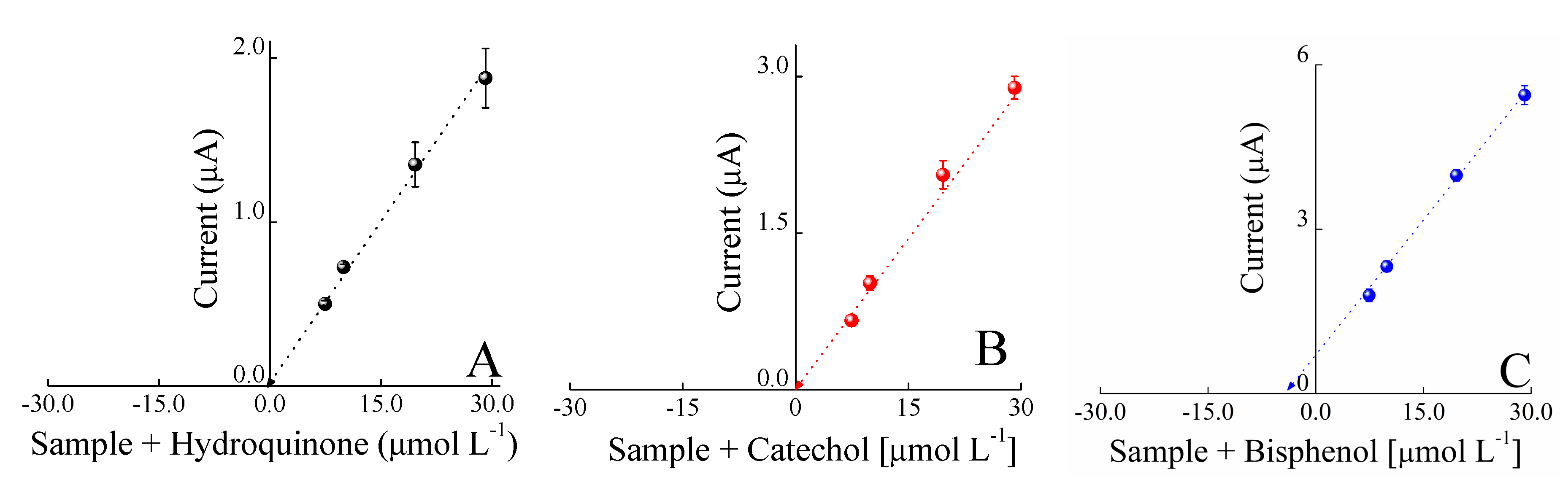
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abegao, L.M.G.; Ribeiro, J.H.F.; Ribeiro, P.A.; Raposo, M. Nano-molar deltamethrin sensor based on electrical impedance of PAH/PAZO layer-by-layer sensing films. Sensors 2013, 13, 10167–10176. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mateus, E.P.; Paz-Garcia, J.M.; Serio, S.; Raposo, M.; Ribeiro, A.B. Electronic tongue coupled to an electrochemical flow reactor for emerging organic contaminants real time monitoring. Sensors 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mateus, E.P.; Raposo, M.; Ribeiro, A.B. Overview of electronic tongue sensing in environmental aqueous matrices: Potential for monitoring emerging organic contaminants. Environ. Rev. 2019, 27, 202–214. [Google Scholar] [CrossRef]
- Marques, I.; Magalhaes-Mota, G.; Pires, F.; Serio, S.; Ribeiro, P.A.; Raposo, M. Detection of traces of triclosan in water. Appl. Surf. Sci. 2017, 421, 142–147. [Google Scholar] [CrossRef]
- Motia, S.; Tudor, I.A.; Ribeiro, P.A.; Raposo, M.; Bouchikhi, B.; El Bari, N. Electrochemical sensor based on molecularly imprinted polymer for sensitive triclosan detection in wastewater and mineral water. Sci. Total. Environ. 2019, 664, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.M.; Raymundo-Pereira, P.A.; Cincotto, F.H.; Canevari, T.C.; Machado, S.A.S. Sensitive determination of the endocrine disruptor bisphenol A at ultrathin film based on nanostructured hybrid material SiO2/GO/AgNP. J. Solid State Electrochem. 2016, 20, 2503–2507. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, C.; Tan, X.M.; Wang, J.X. Determination of bisphenol A in water via inhibition of silver nanoparticles-enhanced chemiluminescence. Anal. Chim. Acta 2011, 689, 92–96. [Google Scholar] [CrossRef]
- Konieczna, A.; Rutkowska, A.; Rachon, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar]
- Reddy, P.V.L.; Kim, K.H.; Kavitha, B.; Kumar, V.; Raza, N.; Kalagara, S. Photocatalytic degradation of bisphenol A in aqueous media: A review. J. Environ. Manag. 2018, 213, 189–205. [Google Scholar] [CrossRef]
- Manimegala, S.; Sendhilvadivu, D.M. Antidiabetic Effect of Ginger (Zingiber officinali) and Oregano (Oregano vulgare) in BPA Induced Catfishes. Int. J. Sci. Res. 2019, 8, 1364–1367. [Google Scholar]
- Vandentorren, S.; Zeman, F.; Morin, L.; Sarter, H.; Bidondo, M.L.; Oleko, A.; Leridon, H. Bisphenol-A and phthalates contamination of urine samples by catheters in the Elfe pilot study: Implications for large-scale biomonitoring studies. Environ. Res. 2011, 111, 761–764. [Google Scholar] [CrossRef]
- Hu, J.Y.; Cheng, S.J.; Aizawa, T.; Terao, Y.; Kunikane, S. Products of aqueous chlorination of 17 beta-estradiol and their estrogenic activities. Environ. Sci. Technol. 2003, 37, 5665–5670. [Google Scholar] [CrossRef]
- Karabiberoglu, S.U. Sensitive voltammetric determination of bisphenol a based on a glassy carbon electrode modified with copper oxide-zinc oxide decorated on graphene oxide. Electroanalysis 2019, 31, 91–102. [Google Scholar] [CrossRef]
- Olea, N.; Arrebola, J.P.; Taoufiki, J.; Fernandez-Valades, R.; Prada, R.; Navea, N.; Molina-Molina, J.M.; Fernandez, M.F. Alkylphenols and bisphenol-A and its chlorinated derivatives in adipose tissue of children. Wit. Trans. Ecol. Environ. 2008, 110, 129–138. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, H.Q.; Yan, B.; Zhang, H.Y. An electrochemical sensor for the determination of bisphenol A using glassy carbon electrode modified with reduced graphene oxide-silver/poly-L-lysine nanocomposites. J. Electroanal. Chem. 2017, 805, 39–46. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Rastogi, N.K.; Thakur, M.S. Sensors and biosensors for analysis of bisphenol-A. TrAC Trends Anal. Chem. 2013, 52, 248–260. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Yang, G.H.; Li, H.; Du, D.; Lin, Y.H. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Baccarin, M.; Ciciliati, M.A.; Oliveira, O.N.; Cavalheiro, E.T.G.; Raymundo-Pereira, P.A. Pen sensor made with silver nanoparticles decorating graphite-polyurethane electrodes to detect bisphenol-A in tap and river water samples. Mater. Sci. Eng. C 2020, 114, 110989. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Campos, A.M.; Prado, T.M.; Furini, L.N.; Boas, N.V.; Calegaro, M.L.; Machado, S.A.S. Synergy between printex nano-carbons and silver nanoparticles for sensitive estimation of antioxidant activity. Anal. Chim. Acta 2016, 926, 88–98. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Carvalho, J.H.S.; Machado, S.A.S.; Oliveira, O.N., Jr.; Janegitz, B.C. Simultaneous detection of quercetin and carbendazim in wine samples using disposable electrochemical sensors. ChemElectroChem 2020, 7, 3074–3081. [Google Scholar] [CrossRef]
- Carr, O.; Raymundo-Pereira, P.A.; Shimizu, F.M.; Sorroche, B.P.; Melendez, M.E.; Pedro, R.D.; Miranda, P.B.; Carvalho, A.L.; Reis, R.M.; Arantes, L.M.R.B.; et al. Genosensor made with a self-assembled monolayer matrix to detect MGMT gene methylation in head and neck cancer cell lines. Talanta 2020, 210. [Google Scholar] [CrossRef] [PubMed]
- Pupin, R.R.; Foguel, M.V.; Goncalves, L.M.; Sotomayor, M.D.T. Magnetic molecularly imprinted polymers obtained by photopolymerization for selective recognition of penicillin G. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Quinto, M.L.; Khan, S.; Picasso, G.; Sotomayor, M.D.T. Synthesis, characterization, and evaluation of a selective molecularly imprinted polymer for quantification of the textile dye acid violet 19 in real water samples. J. Hazard. Mater. 2020, 384, 121374. [Google Scholar] [CrossRef]
- Niu, X.L.; Yang, W.; Wang, G.Y.; Ren, J.; Guo, H.; Gao, J.Z. A novel electrochemical sensor of bisphenol A based on stacked graphene nanofibers/gold nanoparticles composite modified glassy carbon electrode. Electrochim. Acta 2013, 98, 167–175. [Google Scholar] [CrossRef]
- Bishop, G.W.; Ahiadu, B.K.; Smith, J.L.; Patterson, J.D. Use of redox probes for characterization of layer-by-layer gold nanoparticle-modified screen-printed carbon electrodes. J. Electrochem. Soc. 2017, 164, B23–B28. [Google Scholar] [CrossRef]
- Silva, R.R.; Raymundo-Pereira, P.A.; Campos, A.M.; Wilson, D.; Otoni, C.G.; Barud, H.S.; Costa, C.A.R.; Domeneguetti, R.R.; Balogh, D.T.; Ribeiro, S.J.L.; et al. Microbial nanocellulose adherent to human skin used in electrochemical sensors to detect metal ions and biomarkers in sweat. Talanta 2020, 218, 121153. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques (technical report). Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]
- Canevari, T.C.; Raymundo-Pereira, P.A.; Landers, R.; Machado, S.A.S. Direct synthesis of ag nanoparticles incorporated on a mesoporous hybrid material as a sensitive sensor for the simultaneous determination of dihydroxybenzenes isomers. Eur. J. Inorg. Chem. 2013, 2013, 5746–5754. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Campos, A.M.; Mendonca, C.D.; Calegaro, M.L.; Machado, S.A.S.; Oliveira, O.N. Printex 6L carbon nanoballs used in electrochemical sensors for simultaneous detection of emerging pollutants hydroquinone and paracetamol. Sensor Actuat. B Chem. 2017, 252, 165–174. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Electrochemical performance of activated screen printed carbon electrodes for hydrogen peroxide and phenol derivatives sensing. J. Electroanal. Chem. 2019, 839, 75–82. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N. Simultaneous, ultrasensitive detection of hydroquinone, paracetamol and estradiol for quality control of tap water with a simple electrochemical method. J. Electroanal. Chem. 2019, 848. [Google Scholar] [CrossRef]
- Canevari, T.C.; Raymundo-Pereira, P.A.; Landers, R.; Benvenutti, E.V.; Machado, S.A. Sol-gel thin-film based mesoporous silica and carbon nanotubes for the determination of dopamine, uric acid and paracetamol in urine. Talanta 2013, 116, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Beduk, T.; Lahcen, A.A.; Tashkandi, N.; Salama, K.N. One-step electrosynthesized molecularly imprinted polymer on laser scribed graphene bisphenol a sensor. Sensor Actuat. B Chem. 2020, 314. [Google Scholar] [CrossRef]
- Zhan, T.R.; Song, Y.; Li, X.J.; Hou, W.G. Electrochemical sensor for bisphenol A based on ionic liquid functionalized Zn-Al layered double hydroxide modified electrode. Mat. Sci. Eng. C Mater. 2016, 64, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, L.; Altuntas, M.; Buyuksagis, A.; Turk, H.; Yurdakal, S. Electrochemical determination of bisphenol A with pencil graphite electrodes modified with Co(II), Ni(II), Cu(II) and Fe(II) phthalocyaninetetrasulfonates. Anal. Sci. 2016, 32, 881–886. [Google Scholar]
- Silva, T.R.; Merib, J.; Spudeit, D.A.; Carasek, E.; Vieira, I.C. Electrode modified with the ionic liquid [P-6,6,6,14(+)](2)[MnCl42-] for the determination of bisphenol A in plastic samples. J. Brazil. Chem. Soc. 2019, 30, 2076–2084. [Google Scholar] [CrossRef]
- Ntsendwana, B.; Mamba, B.B.; Sampath, S.; Arotiba, O.A. Electrochemical detection of bisphenol A using graphene-modified glassy carbon electrode. Int. J. Electrochem. Sci. 2012, 7, 3501–3512. [Google Scholar]
- Ndlovu, T.; Arotiba, O.A.; Sampath, S.; Krause, R.W.; Mamba, B.B. An exfoliated graphite-based Bisphenol A electrochemical sensor. Sensors 2012, 12, 11601–11611. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.J.; Wu, M.; Shi, Y.R.; Yang, J.J.; Shan, J.J.; Liu, L.F. Simultaneous determination of Bisphenol A and Bisphenol S using multi-walled carbon nanotubes modified electrode. Int. J. Electrochem. Sci. 2018, 13, 11906–11922. [Google Scholar] [CrossRef]
| Sensing Layer | Type of Technique | Linear Range µmol L−1 | Sensitivity µA L µmol−1 | LOD µmol L−1 | Ref. |
|---|---|---|---|---|---|
| NiPCTS | DPV | 0.5–10 | 1.78 | 0.29 | [35] |
| CoPCTS | DPV | 0.5–10 | 1.57 | 0.43 | [35] |
| MIL/CPE | SWV | 2.0–53 | 0.42 | 0.87 | [36] |
| RGO-Ag/PLL/GCE | DPV | 1.0–80 | 0.15 | 0.54 | [15] |
| CGE/graphene | DPV | 0.05–1 | 0.010 | 0.05 | [37] |
| EG | SWV | 1.56–50 | 6.06 | 0.76 | [38] |
| MWCNTs/GCE | DPV | 2–30 | 0.51 | 0.5 | [39] |
| SPEindividual | DPV | 2–50 | 0.046 ± 0.002 | 3.41 | This Work |
| SPEsimultaneous | DPV | 2–50 | 0.055 ± 0.001 | 0.95 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, A.C.d.; Barbosa, S.C.; Raymundo-Pereira, P.A.; Wilson, D.; Shimizu, F.M.; Raposo, M.; Oliveira, O.N., Jr. Flexible Carbon Electrodes for Electrochemical Detection of Bisphenol-A, Hydroquinone and Catechol in Water Samples. Chemosensors 2020, 8, 103. https://doi.org/10.3390/chemosensors8040103
Sá ACd, Barbosa SC, Raymundo-Pereira PA, Wilson D, Shimizu FM, Raposo M, Oliveira ON Jr. Flexible Carbon Electrodes for Electrochemical Detection of Bisphenol-A, Hydroquinone and Catechol in Water Samples. Chemosensors. 2020; 8(4):103. https://doi.org/10.3390/chemosensors8040103
Chicago/Turabian StyleSá, Acelino C. de, Simone C. Barbosa, Paulo A. Raymundo-Pereira, Deivy Wilson, Flávio M. Shimizu, Maria Raposo, and Osvaldo N. Oliveira, Jr. 2020. "Flexible Carbon Electrodes for Electrochemical Detection of Bisphenol-A, Hydroquinone and Catechol in Water Samples" Chemosensors 8, no. 4: 103. https://doi.org/10.3390/chemosensors8040103
APA StyleSá, A. C. d., Barbosa, S. C., Raymundo-Pereira, P. A., Wilson, D., Shimizu, F. M., Raposo, M., & Oliveira, O. N., Jr. (2020). Flexible Carbon Electrodes for Electrochemical Detection of Bisphenol-A, Hydroquinone and Catechol in Water Samples. Chemosensors, 8(4), 103. https://doi.org/10.3390/chemosensors8040103







