Hollow Mesoporous ZnO/ZnCo2O4 Based on Ostwald Ripening for H2S Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO/ZnCo2O4 Hollow Microspheres
2.3. Characterization
2.4. Manufacturing and Measurement of Sensing Elements
3. Results
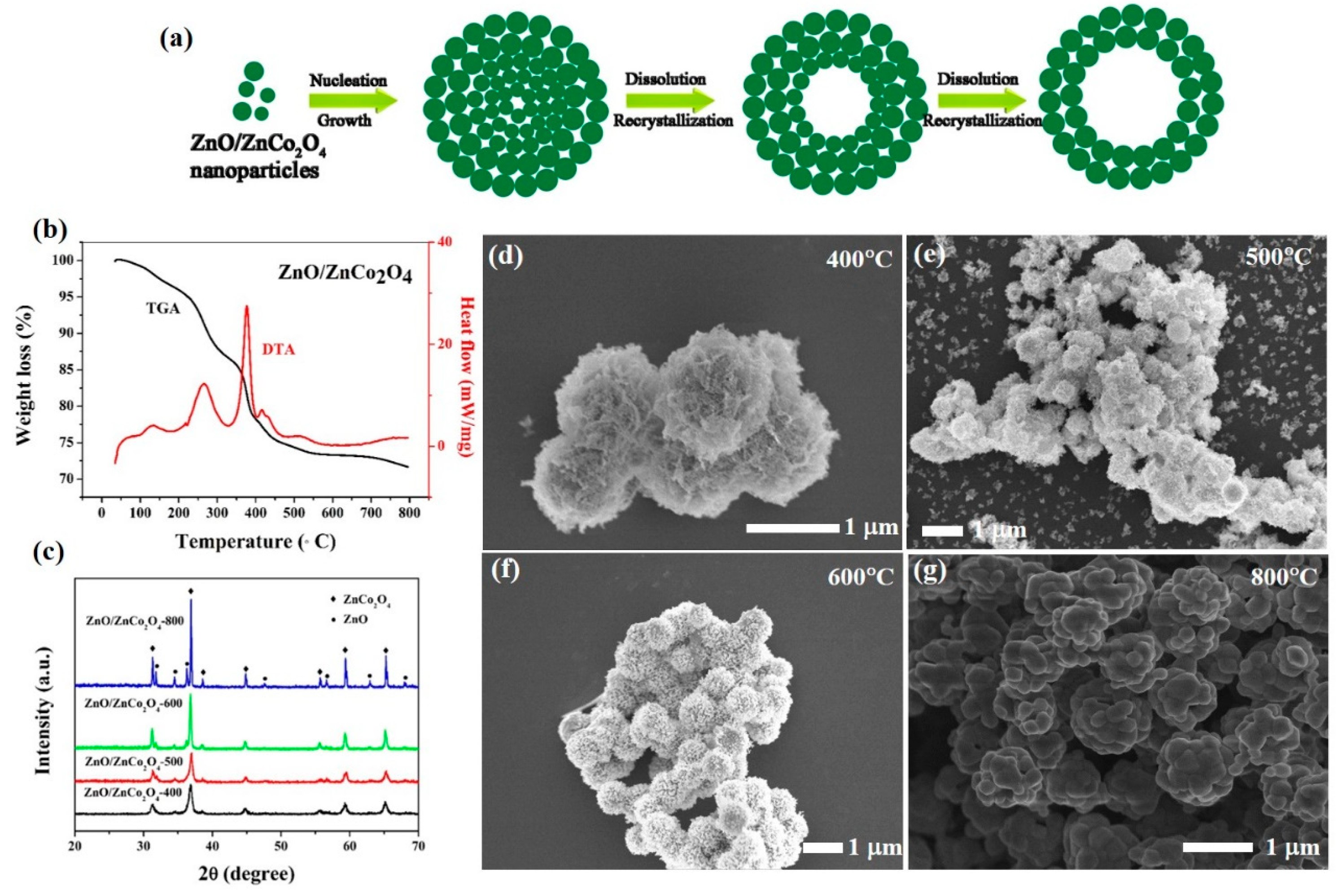
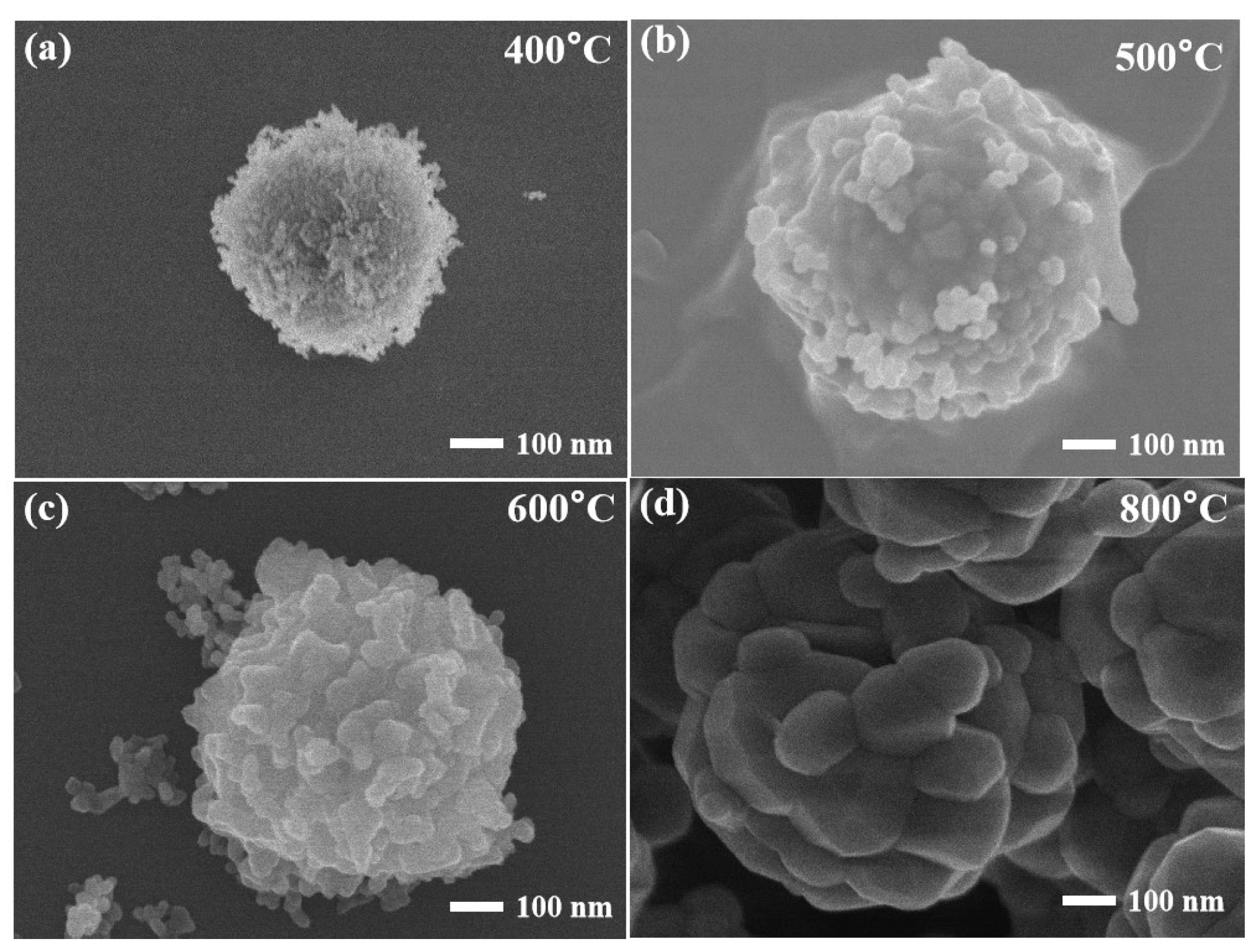
3.1. Characterization of Materials
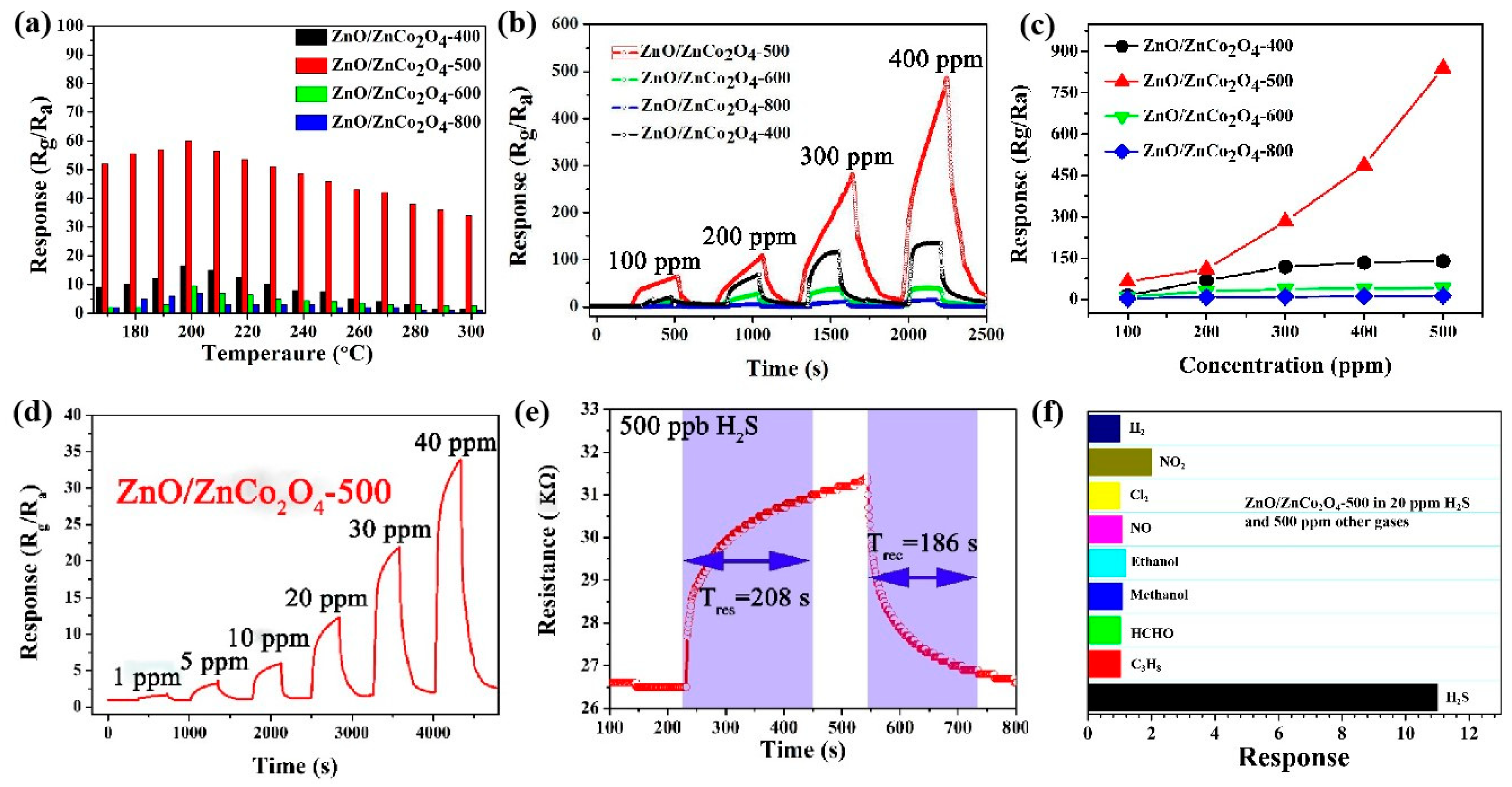
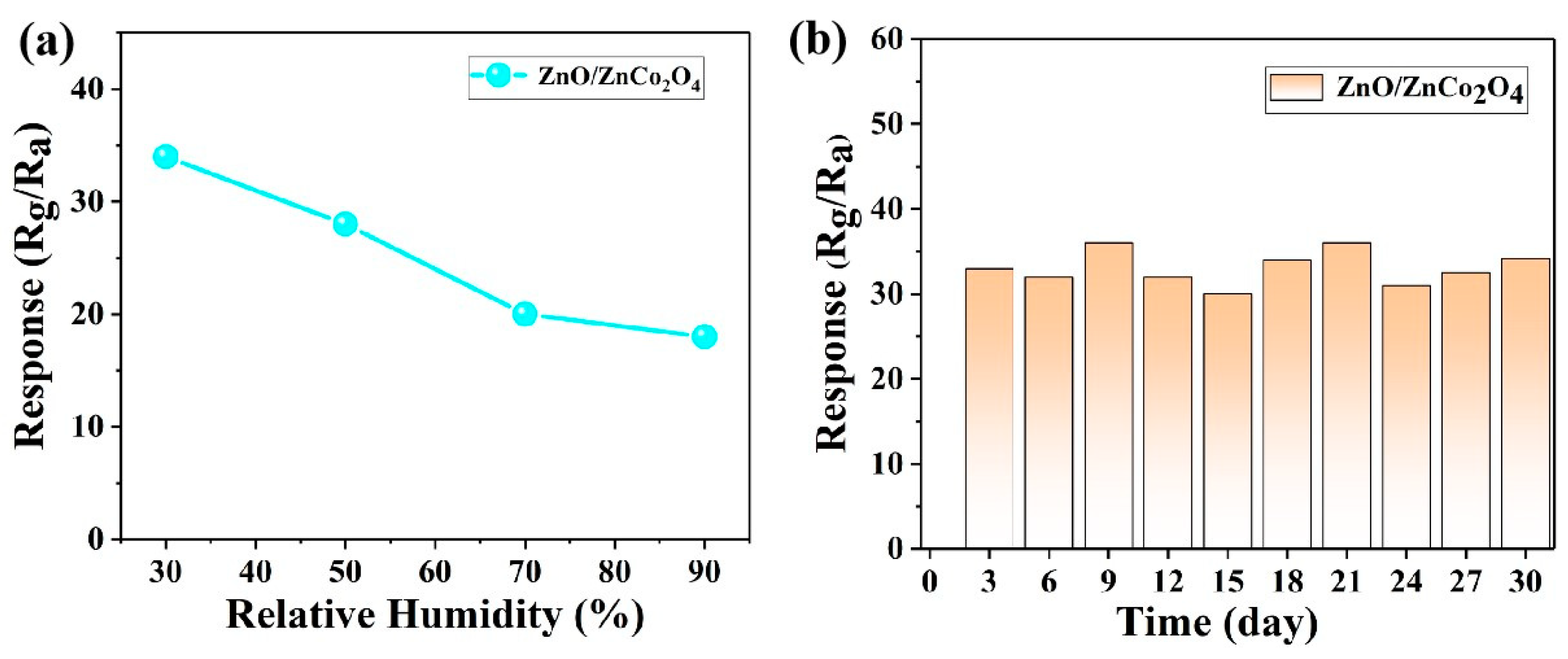
3.2. Gas-Sensing Performance
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, M.; Xu, H.; Dong, C.; Zhang, Z. Toward a Gas Sensor Interface Circuit—A Review. IEEE Sens. J. 2022, 22, 18253–18265. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Y.; Liu, H. Room-Temperature Semiconductor Gas Sensors: Challenges and Opportunities. ACS Sens. 2022, 7, 3582–3597. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Yu, J.-B.; Byun, H.-G.; Kim, H.-J. Chemoresistive Sensor Readout Circuit Design for Detecting Gases with SlowResponse Time Characteristics. Sensors 2022, 22, 1102. [Google Scholar] [CrossRef]
- Korotcenkov, G. Practical Aspects in Design of One-Electrode Semiconductor Gas Sensors: Status Report. Sens. Actuator B Chem. 2007, 121, 664–678. [Google Scholar] [CrossRef]
- Sun, X.; Shi, Y.; Wang, H.; Shao, X.; Yang, L.; Li, X.; Wang, M. Research Progress on Moisture Resistance of Gas Sensors. Clean-Soil Air Water 2024, 52, 2300086. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Li, J.; Zhang, R.; Meng, F. Fast-response xylene gas sensor based on Pt-modified Fe2(MoO4)3 nano-hollow spheres compounded on rGO. Sens. Actuators B Chem. 2025, 439, 137849. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Luo, Y.; Lv, G.; Wang, F.; Zhang, C.; Duan, G. Monolayer self-assembled film of Pd decorated In-doped SnO2 hollow nanospheres for ultrasensitive H2S MEMS gas sensors. Sens. Actuators B Chem. 2025, 440, 137845. [Google Scholar] [CrossRef]
- Pawar, K.; Kim, T.; Mirzaei, A.; Patil, P.; Kim, H.; Kim, S. Hollow CuO/Cu2O octahedrons for selective and stable detection of acetone gas. Sens. Actuators B Chem. 2025, 423, 136783. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Shingange, K.; Mhlongo, G.H. ZnO/ZnFe2O4 Heterostructure for Conductometric Acetone Gas Sensors. Sens. Actuators B Chem. 2023, 377, 133027. [Google Scholar] [CrossRef]
- Park, J.Y.; Kwak, Y.; Lim, H.-R.; Park, S.-W.; Lim, M.S.; Cho, H.-B.; Myung, N.V.; Choa, Y.-H. Tuning the Sensing Responses towards Room-Temperature Hypersensitive Methanol Gas Sensor Using Exfoliated Graphene-Enhanced ZnO Quantum Dot Nanostructures. J. Hazard. Mater. 2022, 438, 129412. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Ahn, S.; Mirzaei, A.; Kim, J.; Park, C. CuO nanoparticles-decorated femtosecond laser-irradiated WS2–WO3 heterojunctions to realize selective H2S gas sensor. Sens. Actuators B Chem. 2025, 427, 137167. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, J.; Zhao, F.; Cong, W.; Wang, G. Synthesis of ZnO/ZnCo2O4 hollow tube clusters by a template method for high-sensitive H2S sensor. Sens. Actuators B Chem. 2023, 394, 134338. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Yang, T.; Deng, M.; Cheng, D.; He, L. Lysosome-specific near-infrared fluorescent probe with large stokes shift for H2S imaging in U87 cells and brain glioma mice. Sens. Actuators B Chem. 2025, 426, 137109. [Google Scholar] [CrossRef]
- Liu, Z.; Song, J.; Wang, T.; Liu, H.; Chen, J.; Sun, Y.; Zhang, P.; Cui, G. Ultrasensitive H2S sensor based on Cu2O/Graphene heterostructures at room temperature. Appl. Surf. Sci. 2025, 702, 163339. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, J.; Fu, S.; Sun, S.; Qian, K.; Luo, Z.; Han, D. Rare Earth-Driven Photogenerated Charge Separation in SnO2@Y2O3Heterojunctions for Enhanced H2S Sensing at Room Temperature. ACS Appl. Mater. Interfaces 2025, 17, 15948–15958. [Google Scholar] [CrossRef]
- Ou, L.; Liu, M.; Zhu, L.; Zhang, D.; Lu, H. Recent Progress on Flexible Room-Temperature Gas Sensors Based on Metal Oxide Semiconductor. Nano-Micro Lett. 2022, 14, 206. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Liu, Y.; Wang, S.; Li, T.; Feng, S.; Qin, S.; Zhang, T. Heteronanostructural metal oxide-based gas microsensors. Microsyst. Nano Eng. 2022, 8, 85. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Tan, C. Hollow Mesoporous SnO2/Zn2SnO4 Heterojunction and RGO Decoration for High Performance Detection of Acetone. ACS Appl. Mater. Interfaces 2022, 14, 55249–55263. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, F.; Liu, L.; Chen, Y.; Wang, P. Highly sensitive H2S sensor based on solvothermally prepared spinel ZnFe2O4 nanoparticles. J. Alloys Compd. 2018, 764, 147–154. [Google Scholar] [CrossRef]
- Runa, A.; Zhang, X.; Wen, G.; Zhang, B.; Fu, W.; Yang, H. Actinomorphic flower-like n-ZnO/p-ZnFe2O4 composite and its improved NO2 gas-sensing property. Mater. Lett. 2018, 255, 73–76. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H.; Chen, J.; Song, M. Gas Sensors Based on Semiconductor Metal Oxides Fabricated by Electrospinning: A Review. Sensors 2024, 24, 2962. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Huang, H.; Wang, T.; Yu, H.; Yang, Y.; Dong, X.; Yang, Y. The MoS2/ZnO p-n heterostructure arrays for ultrasensitive ppb-level self-supporting NO2 gas sensors under UV irradiation. Talanta 2025, 294, 128194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Jin, X.; Sun, G.; Cao, J.; Wang, Y. The updated gas sensing performance of In2O3 porous nanospheres for ppb level formaldehyde by doping with Mo. Vacuum 2025, 238, 114248. [Google Scholar] [CrossRef]
- Guo, R.; Wang, H.; Tian, R.; Shi, D.; Li, H.; Li, Y.; Liu, H. The Enhanced Ethanol Sensing Properties of CNT@ZnSnO3 Hollow Boxes Derived from Zn-MOF(ZIF-8). Ceram. Int. 2020, 46, 7065–7073. [Google Scholar] [CrossRef]
- Cao, H.; Hu, Z.; Wei, X.; Wang, H.; Tian, X.; Ding, S. Conductometric Ethanol Gas Sensor Based on a Bilayer Film Consisting of SnO2 Film and SnO2/ZnSnO3 Porous Film Prepared by Magnetron Sputtering. Sens. Actuators B Chem. 2023, 382, 133562. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Yang, X.W.; Bao, H.M.; Lei, B.; Chen, K.; Wei, Y.; Zhao, Q.; Zhang, H.W.; Cai, W.P. Vortex Engineering on Oxide Bowl-Coated Oxide/Gold Dual-Layer Array for Dual Electrical/Spectroscopic Monitoring of Volatile Organic Compounds. Adv. Funct. Mater. 2024, 34, 2402173. [Google Scholar] [CrossRef]
- Shao, S.F.; Yan, L.W.; Zhang, L.; Zhang, J.; Li, Z.X.; Kim, H.W.; Kim, S.S. Utilizing Data Mining for the Synthesis of Functionalized Tungsten Oxide with Enhanced Oxygen Vacancies for Highly Sensitive Detection of Triethylamine. ACS Appl. Mater. Interfaces 2024, 16, 6098–6112. [Google Scholar] [CrossRef]
- Nakano-Baker, O.; Fong, H.; Shukla, S.; Lee, R.V.; Cai, L.; Godin, D.; Hennig, T.; Rath, S.; Novosselov, I.; Dogan, S.; et al. Data Driven Design of a Multiplexed, Peptide-Sensitized Transistor to Detect Breath VOC Markers of COVID-19. Biosens. Bioelectron. 2023, 229, 115237. [Google Scholar] [CrossRef]
- Inyawilert, K.; Punginsang, M.; Wisitsoraat, A.; Tuantranont, A.; Liewhiran, C. Graphene/Rh-doped SnO2 nanocomposites synthesized by electrochemical exfoliation and flame spray pyrolysis for H2S sensing. J. Alloys Compd. 2022, 916, 165431. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zhao, Y.; Wu, Q.; Nie, H.; Si, H.; Huang, H.; Liu, Y.; Shao, M.; Kang, Z. Highly efficient metal-free catalyst from cellulose for hydrogen peroxide photoproduction instructed by machine learning and transient photovoltage technology. Nano Res. 2022, 15, 4000–4007. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, Y.; Xie, Y.; Ma, J.; Han, D.; Sang, S. Hollow Mesoporous ZnO/ZnCo2O4 Based on Ostwald Ripening for H2S Detection. Chemosensors 2025, 13, 239. https://doi.org/10.3390/chemosensors13070239
Wang H, Liu Y, Xie Y, Ma J, Han D, Sang S. Hollow Mesoporous ZnO/ZnCo2O4 Based on Ostwald Ripening for H2S Detection. Chemosensors. 2025; 13(7):239. https://doi.org/10.3390/chemosensors13070239
Chicago/Turabian StyleWang, Hongtao, Yang Liu, Yuanchao Xie, Jianan Ma, Dan Han, and Shengbo Sang. 2025. "Hollow Mesoporous ZnO/ZnCo2O4 Based on Ostwald Ripening for H2S Detection" Chemosensors 13, no. 7: 239. https://doi.org/10.3390/chemosensors13070239
APA StyleWang, H., Liu, Y., Xie, Y., Ma, J., Han, D., & Sang, S. (2025). Hollow Mesoporous ZnO/ZnCo2O4 Based on Ostwald Ripening for H2S Detection. Chemosensors, 13(7), 239. https://doi.org/10.3390/chemosensors13070239





