Abstract
Highly sensitive real-time detection of hydrogen sulfide (H2S) is important for human health and environmental protection due to its highly toxic properties. The development of high-performance H2S sensors remains challenging for poor selectivity, high limit detection and slow recovery from irreversible sulfidation. To solve these problems, we strategically prepared a layered structure of CuO-sensitized WO3 flower-like hollow spheres with CuO as the sensitizing component. A 15 wt% CuO/WO3 exhibits an ultra-high response (Ra/Rg = 571) to 10 ppm H2S (131-times of pure WO3), excellent selectivity (97-times higher than 100 ppm interference gas), and a low detection limit (100 ppb). Notably, its fast response (4 s) is accompanied by full recovery within 236 s. After 30 days of continuous testing, the response of 15 wt% CuO/WO3 decreased slightly but maintained the initial response of 90.5%. The improved performance is attributed to (1) the p-n heterojunction formed between CuO and WO3 optimizes the energy band structure and enriches the chemisorption sites for H2S; (2) the reaction of H2S with CuO generates highly conductive CuS, which significantly reduces the interfacial resistance; and (3) the hierarchical flowery hollow microsphere structure, heterojunction, and oxygen vacancy synergistically promote the desorption. This work provides a high-performance H2S gas sensor that balances response, selectivity, and response/recovery kinetics.
1. Introduction
Hydrogen sulfide (H2S) is a colorless gas with a strong rotten egg odor [1,2]. It is prevalent in natural sources and is mainly produced by the decomposition of organic substances under anaerobic conditions, such as in swamps, hot springs, and volcanic activity. In addition, H2S also originates from a variety of human activities, including oil and gas extraction and refining, wastewater processing, the pulp and papermaking industry, chemical manufacturing, and livestock and poultry manure handling in agriculture, etc. [3,4,5,6,7]. H2S gas poses a hazard to the natural environment and human health. H2S can react with other chemicals in the atmosphere to form acid rain, which can damage ecosystems [8]. Exposure to low concentrations of hydrogen sulfide (H2S) may induce ocular and respiratory tract irritation in humans, accompanied by symptoms such as headaches and nausea. High concentration exposure to H2S may result in loss of consciousness, coma, and even death [9,10]. Meanwhile, as H2S is heavier than air, it tends to accumulate in lower and enclosed spaces, increasing the risk of poisoning. Given the wide range of sources and potential hazards of H2S, the timely and accurate detection of H2S concentrations in the environment is necessary for preventing health risks and protecting public safety. Therefore, it is of great practical significance to achieve high sensitivity and high selectivity for H2S detection.
Currently, the main techniques for the detection of H2S include colorimetric methods [11,12], fluorescent probes [13,14], electrochemical sensors [15,16], chromatography [17], and metal oxide semiconductor (MOS) sensors [18,19]. In particular, MOS gas sensors have been widely studied for their cost-effectiveness, high response, broad detection scope, and the capability of fast response and real-time monitoring [20,21,22,23,24]. However, for MOS gas sensors, some limitations remain in terms of sensitivity, selectivity and response time. In past studies, researchers have used many oxide semiconductor materials for H2S detection and have obtained some achievements in improving the behavior of H2S sensors [25,26,27,28].
Among various metal oxide semiconductors (MOS), WO3 is an n-type semiconductor with a bandgap of 2.4–2.8 eV [29]. It is widely employed as a promising gas-sensing material due to its excellent chemical stability, low synthesis cost, and tailorable morphology [3,30,31]. However, pristine WO3 usually suffers from low sensor response to gases, slow recovery, and poor selectivity, which limits its practical applications. To address these challenges, doping or compounding other metal oxides (e.g., CuO, ZnO) in hierarchical structure WO3 has been widely explored to improve its sensing performance through synergistic effects. Park et al. [32] used femtosecond laser irradiated WO3 nanosheets to construct a WS2-WO3 heterojunction; meanwhile, CuO was introduced to improve the sensitivity of the H2S gas sensor. The sensor response to 10 ppm H2S at 20 °C is 11.19, and sensor selectivity has also been improved. Zhang et al. [33] reported the use of functionalized nanowires with different contents of FeO for H2S detection. The enhanced interfacial coupling between the oxides gave a sensor response of 140 at 250 °C for 50 ppm H2S with response/recovery times of 40/70 s, respectively. Wang et al. [34] synthesized CuO/WO3 composite hollow microspheres to construct p-n heterojunctions with the hydrothermal method. Evaluation of the sensing performance showed that the composite with a 1:6 molar ratio achieved the best sensing performance, with a response of 1297 at 70 °C for 10 ppm H2S but required a 76 s heating pulse at 200 °C for recovery. This increases energy consumption and reduces the service life of the sensors, and it poses potential safety risks. Therefore, it is particularly necessary to develop an H2S sensor that is easy to operate and can be recovered without pulsed-current heating.
In an earlier study, Tamaki et al. conducted a pioneering exploration of the mechanism of high sensitivity and selectivity for H2S in the CuO-SnO2 system. They found that the introduction of CuO significantly enhanced the performance of the SnO2 sensor, and the mechanism involved the change in electrical resistance due to the sulfidation reaction (CuO → CuS) of CuO in an H2S atmosphere [35]. Although the work of Tamaki et al. focuses on the SnO2 substrate, its mechanism of action on CuO as a sensitizer provides an important basis for subsequent studies. Meanwhile, the construction of p-n heterostructures on the surface of hierarchically structured materials by introducing CuO as a sensitizing component can, on the one hand, increase the defects on the surface of the sensing material and improve the surface activity [36]. On the other hand, the formation of a p-n junction can be utilized to improve the sensitivity of the sensor by modulating the electrons [37]. Semiconductor metal oxide gas sensors using CuO as the sensitive layer still have several limitations: high detection limits limit the ability to detect trace gases; the sulfide reaction product (CuS) is highly chemically stable, which improves the response and shortens the response time while leading to sluggish desorption kinetics and hindering recovery [35].
In this work, to address the necessity of H2S gas detection and the limitations of H2S gas sensors, we constructed a WO3 sensing material matrix with a hollow flower ball structure by a simple hydrothermal synthesis method. On this basis, we have successfully realized the high performance of H2S gas detection by rationally choosing CuO as the sensitizing component. The gas-sensitive performance of WO3 was enhanced by different contents of CuO, where the best comprehensive performance of the sensor for H2S was achieved when the content of CuO was 15 wt%. The introduction of CuO greatly improves the response and selectivity of the sensor to H2S compared to the WO3 pure material, while improving the response and recovery time, enabling the detection of ppb-level H2S. This study elucidates the fundamental gas-sensing mechanism and concurrently provides a viable strategy for high-performance H2S detection.
2. Experimental Section
2.1. Synthesis of WO3 Hollow Flower-like Spherical Materials
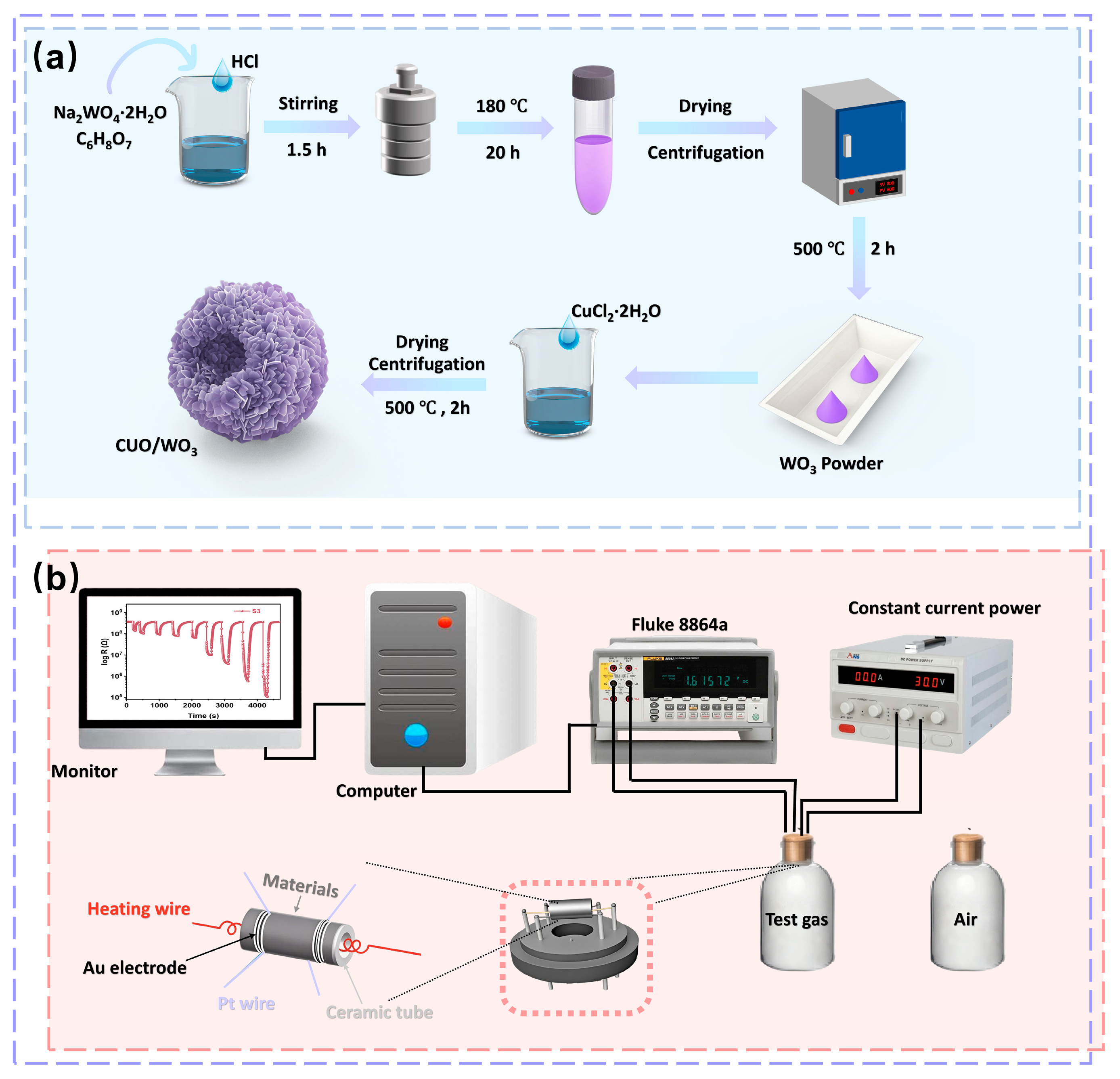
All chemicals used in the preparation of raw materials are of analytical grade and do not require further purification. In this study, WO3 hollow flower-like spherical architectures were synthesized via a hydrothermal method. The detailed procedure is illustrated in Figure 1a: 2 mmol Na2WO4·2H2O and 1 g citric acid were sequentially dissolved in 35 mL deionized water followed by sonication for 3 min to form a homogeneous solution. Subsequently, 4 mL hydrochloric acid (HCl, 4 M) was added dropwise under constant magnetic stirring for 1.5 h. The mixed solution was sealed in a 50 mL Teflon-lined stainless steel autoclave and hydrothermally synthesized at 180 °C for 20 h. After cooling naturally to room temperature, the precipitated products were separated by centrifugation, washed six times alternately with deionized water and anhydrous ethanol, and then dried under vacuum at 60 °C for 10 h. Finally, the precursor was subsequently calcined in a muffle furnace at 500 °C under static atmospheric conditions for 2 h, yielding a light-green WO3 powder designated as S0.
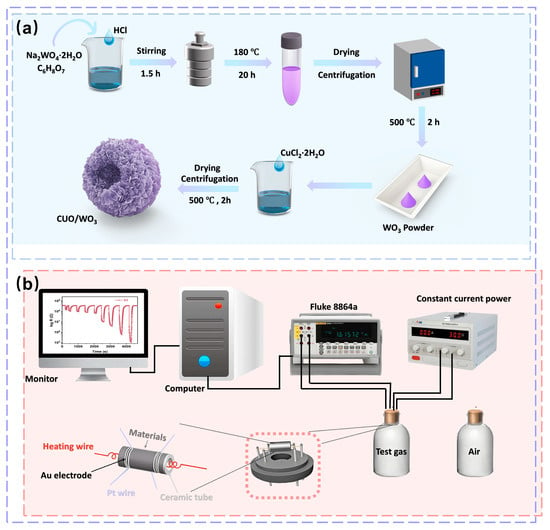
Figure 1.
(a) Synthetic process of WO3 and CuO/WO3 hollow microspheres. (b) Schematic diagram of the sensor performance test system.
2.2. Synthesis of CuO/WO3 Hollow Flower-like Spherical Materials
The preparation process of CuO/WO3 composites is shown in Figure 1a: Firstly, 10 mg of WO3 powder was uniformly dispersed in 20 mL of deionized water, and ultrasonicated for 5 min followed by magnetic stirring. Subsequently, different volumes of CuCl2 solution (concentration: 0.0125 g/mL) were added sequentially, in which 165 μL was added to sample S1, and 207.5, 250.0, 292.5 and 335 μL was added to the remaining samples, S2-S5, respectively, and the solutions were stirred thoroughly for 1.5 h. Next, the precipitated product was acquired by centrifugation, washed with anhydrous ethanol and dried at 60 °C for 10 h, and finally annealed at 500 °C in air for 2 h to obtain a yellow-green powder. During the synthesis process, composite sensing materials with CuO to WO3 mass ratios of 0% (S0, pure WO3), 10% (S1), 12.5% (S2), 15% (S3), 17.5% (S4), and 20% (S5), respectively, were obtained by regulating the amount of CuCl2 added to the solution. The synthesis process was kept the same for all samples except for the different amount of CuCl2 additions.
2.3. Characterization
The crystallographic structure of the sensing materials was analyzed by powder X-ray diffraction (XRD, Bruker D8 Discover, Cu Kα radiation λ = 1.5406 A, Bruker, Billerica, MA, USA) with 2θ scanning from 20° to 80°. Surface morphology and particle size distribution were examined using field-emission scanning electron microscopy (SEM, ZEISS Sigma 500, ZEISS, Oberkochen, German). Microstructural features were further characterized by high-resolution transmission electron microscopy (TEM, JEM2100F, JEOL, Tokyo, Japan). Chemical composition and oxidation states were determined via X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB 250Xi, Thermo, Waltham, MA, USA) with monochromatic Al Kα excitation. The absorbance and energy band structure of the materials were analyzed using a Lambda 1050+ UV-Vis-NIR spectrophotometer.
2.4. Gas Sensor Fabrication and Measurement Process
In this work, the WO3-based gas sensors were prepared in accordance with the method reported in the literature as follows: the prepared material powder (3 mg) was mixed with deionized water (30 µL) and stirred with a fine brush to form a homogeneous paste, which was then coated on the surface of the ceramic tubes (outer diameter of the tubes: 1.2 mm; inner diameter of the tubes: 0.9 mm; length of the tubes: 4.0 mm; spacing of the rings: 1.15 mm; width of the rings: 0.5 mm), and then the tubes were annealed in a muffle furnace at 500 °C for 2 h to optimize the stability of the sensors, and then the ceramic tube was annealed in a muffle furnace at 500 °C for 2 h to optimize the stability of the sensor. Next, the four Pt wires of the ceramic tube were soldered to the four terminals of the base, and the nickel-chromium alloy heating coil (with a resistance of 30–33 Ω at ambient temperature) was inserted into the ceramic tube and fixed to the base (the physical diagram of the sensor is shown in Figure S1), so that the temperature could be modulated by varying the current of the coil in the ceramic tube (Figure 1b).
The sensor performance was tested in the laboratory (25 °C, 30 RH%) using the static test system reported in the literature [38,39]. After the sensor’s resistance stabilizes in air, the device is exposed to a 1 L glass vessel containing the test gas. After the sensor’s resistance stabilizes in the gas to be tested, the device is returned to air to obtain steady-state resistances Ra and Rg. For reducing gases, the response of the sensor to the gas is defined as S = Ra/Rg (Ra and Rg are the resistance of the material in air and in the target gas, respectively). The time required for a 90% change in resistance is considered the response/recovery time [22]. A volume of volatile organic compounds is injected into a 1-liter glass vessel with a micro syringe to obtain a specific concentration of the tested target gas. The formula for calculating volatile organic compound (VOC) gas concentrations is as Equation (1):
where C (ppm) is the target gas concentration, φ, ρ (g/cm3), and V1 (μL) denote the purity, density, and volume of the analyte, respectively, V2 (L) is the volume of the test chamber, M (g/mol) is the molecular weight of the analyte, R (0.082·L·atm·mol−1·K−1) is the ideal gas constant, T (298 K) is the laboratory temperature, P (1 atm) is the atmospheric pressure in the laboratory.
In the moisture resistance test of the sensor, a high and low temperature alternating humidity chamber (BPGHJS-100A) was used as a humidifying device to precisely control the humidity at laboratory temperature of 25 °C.
3. Results and Discussion
3.1. Morphological and Composition Characterization
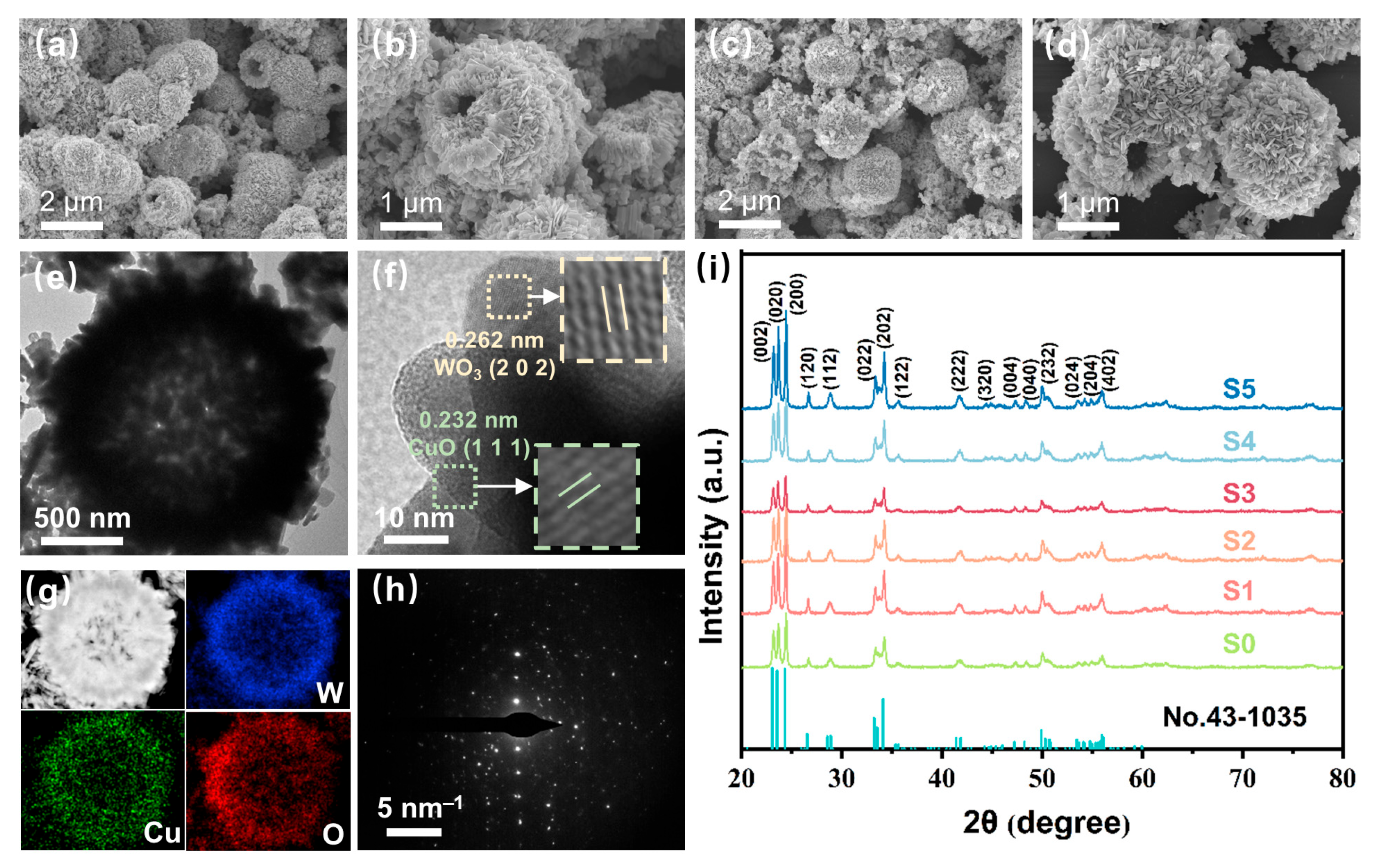
To observe the microstructural and morphological characteristics of the prepared sensing materials, SEM was used to obtain morphological information as shown in Figure 2a–d and Figure S2. The prepared WO3 and CuO/WO3 showed flower-like hollow sphere structures with surfaces assembled by nanosheets. This unique hollow structure can increase the specific surface area of the materials and provide more adsorption sites for gases, thus promoting gas adsorption. The material modified by CuO still maintains the hollow flower-like structure. Transmission electron microscopy (TEM) of S4 in Figure 2e further confirms the existence of the hollow sphere structure. In the high-resolution TEM (HRTEM) image in Figure 2f, obvious lattice stripes can be observed. Line spacing of 0.262 nm and 0.232 nm corresponds to the (202) crystalline surface of WO3 and the (111) crystalline surface of CuO, respectively, which suggests that the CuO has been successfully modified to the WO3 surface. The element mapping in Figure 2g shows the uniform distribution of W, Cu, and O elements in the sensing material, further confirming that CuO has been loaded onto the surface of WO3 hollow microspheres. The Selected Area Electron Diffraction (SAED) pattern (Figure 2h) shows irregular diffraction spots indicating the polycrystalline structure of the sample. The XRD spectra of the sensing materials are shown in Figure 2i. The diffraction peaks of the pristine WO3 and CuO-modified WO3 samples (S0 to S5) corresponded to the JCPDS standard card (No. 43-1035) for WO3. No diffraction peaks of CuO were detected in the XRD spectra for the CuO-modified samples, which may be attributed to the limitation of the detection limit of the XRD, or due to the fact that the theoretical loading of CuO was lower than the actual loading. The mass ratios of W, O, and Cu in the elemental mapping of S3 shown in Figure 2 are presented in Table S1, where Cu has a mass of 1.18%.
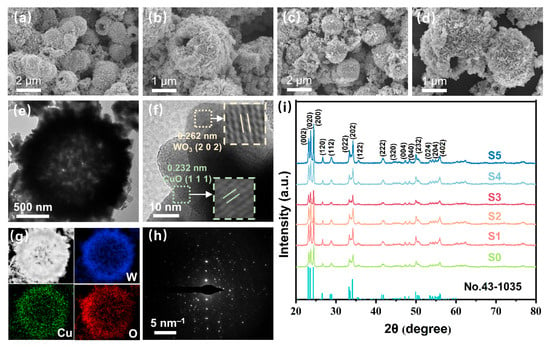
Figure 2.
SEM images of S0 (a,b) and S3 (c,d) samples. (e) TEM image of S3. (f) HRTEM image of S3. (g) TEM image and EDX elemental mapping (W, Cu, O) of S3. (h) SAED image of S3. (i) XRD patterns of the WO3 and CuO/WO3.
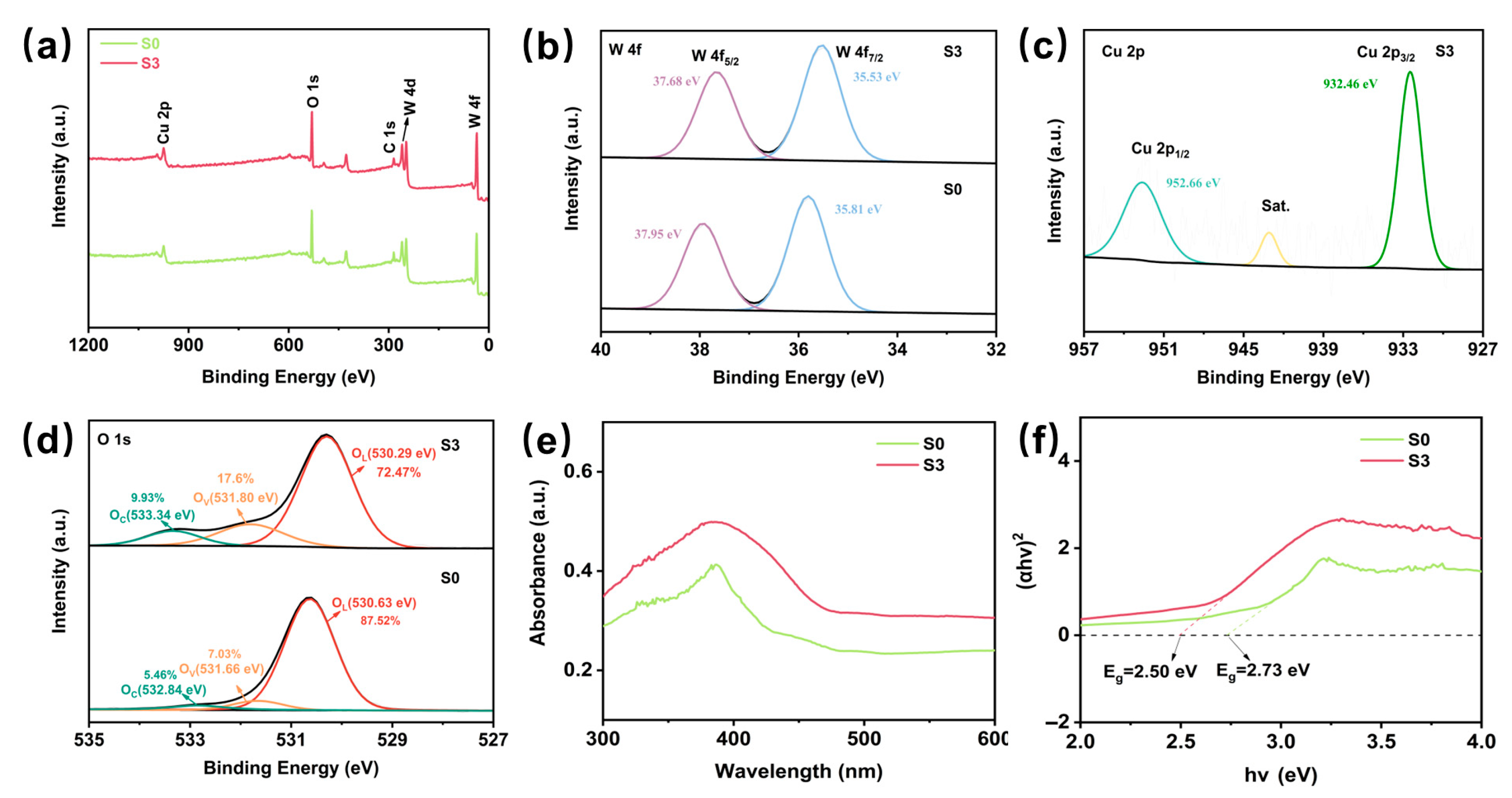
X-ray photoelectron spectroscopy (XPS) was used to investigate the elemental composition and surface chemical states of S0 and S3. As shown in Figure 3a, a clear Cu 2p peak can be identified in S3, indicating the presence of Cu, which confirms the successful introduction of CuO into WO3, compared to S0, where no Cu related peaks were observed. In Figure 3b, the binding energy of W 4f5/2 is 37.68 eV and that of W 4f7/2 is 35.53 eV, whereas in S0, these values are 37.95 eV and 35.81 eV, respectively. These shifts imply that the introduction of the CuO in S3 has altered the electronic environment around the W atom. This change may be due to the formation of a heterogeneous structure between the CuO and WO3, which affects the charge transfer and bonding properties at the interface [34]. The high-resolution Cu 2p spectrum of S3 (Figure 3c) shows two major peaks: Cu 2p1/2 at 952.66 eV and Cu 2p3/2 at 932.46 eV. These positions are characteristic of Cu2+ in CuO, suggesting that the Cu in S3 is predominantly in the +2 oxidation state. Figure 3d shows a high-resolution O 1s spectrum, divided into oxides as OC, OV, and OL by peak fitting [34]. Surface adsorbed oxygen and oxygen vacancies are crucial for sensing materials, and both are involved in the adsorption and reaction processes during gas sensing, the adsorbed oxygen and oxygen vacancy contents of S3 were significantly enhanced by CuO loading, which helped to improve the performance of the sensor for H2S [40]. The optical absorption properties of S0 and S3 were investigated through UV-visible diffuse reflectance spectroscopy (DRS), as shown in Figure 3e. As calculated from the Tauc plot [41] in Figure 3f, S3 exhibits a narrower band gap (2.50 eV) compared to S0, resulting in lower energy requirements for electron transitions [42]. This implies that under identical energy conditions, electrons in S3 are more readily excited, thereby enhancing their high sensitivity towards H2S gas.
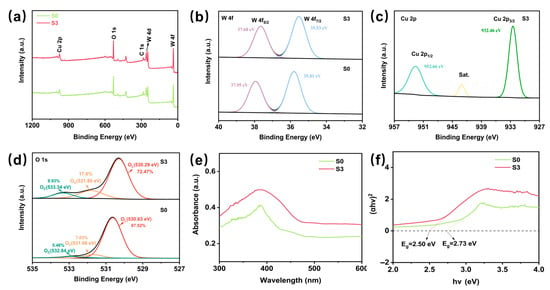
Figure 3.
XPS spectra and the fitted data of (a) Full survey scan XPS spectra of branched S0 and S3. (b) W 4f. (c) Cu 2p. (d) The high-resolution XPS O 1s spectra of S0 and S3. (e) UV-Vis diffuse reflectance spectra of S0 and S3. (f) Tauc plot of S0 and S3.
3.2. Gas Sensing Performance
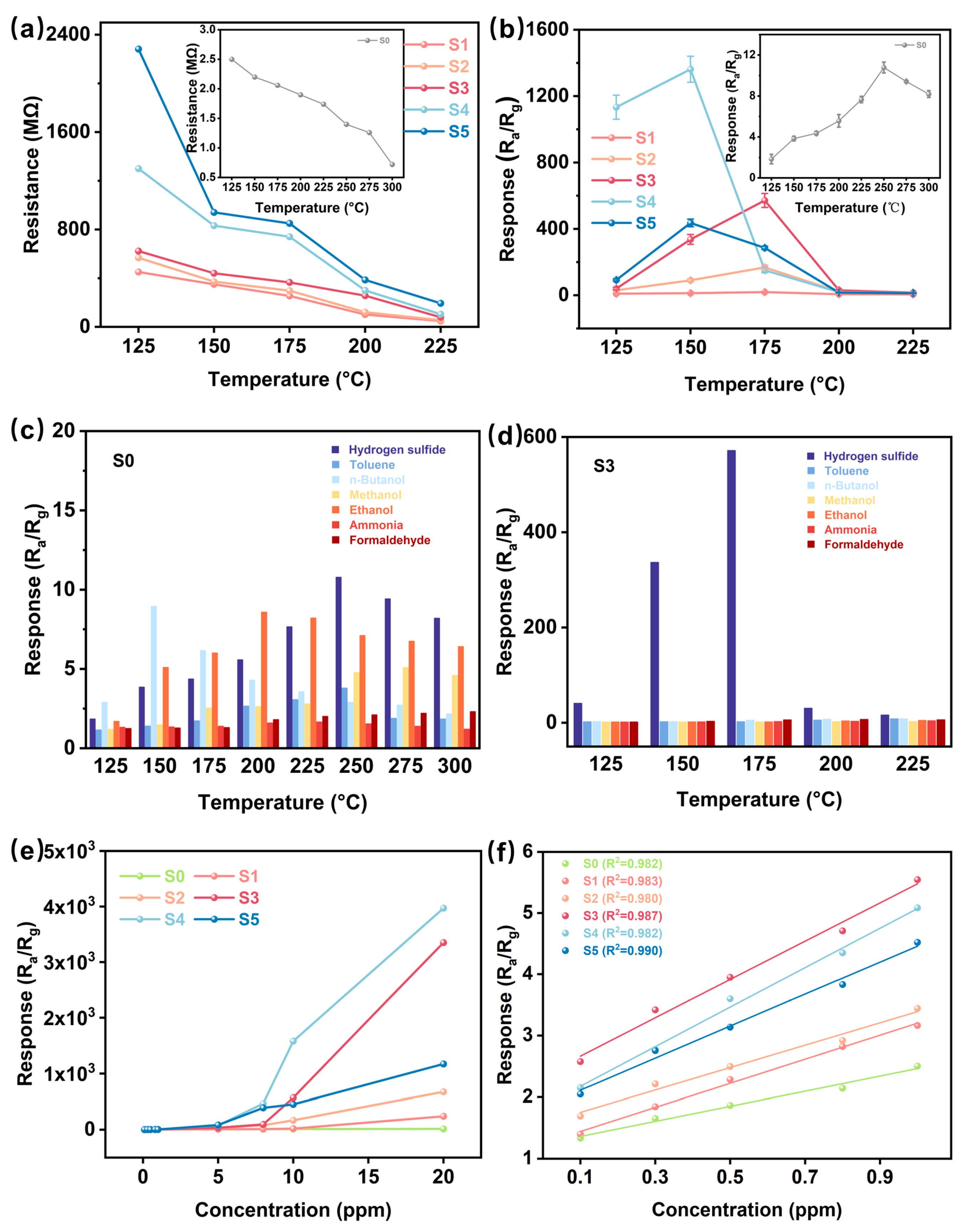
As illustrated in Figure 4a, the temperature-dependent resistance characteristics of S1-S5 sensing materials demonstrate an inverse correlation between electrical resistance and operating temperature. This negative temperature coefficient of resistance represents a fundamental property of metal-oxide-semiconductor gas sensing devices, arising from thermally activated charge carrier migration. It is noteworthy that the resistance of the sensor rises significantly with increasing CuO concentration. This phenomenon is closely related to the conduction mechanism of p-type semiconductor metal oxides. According to the model proposed by Barsan et al. [43], the resistance of p-type materials is mainly determined by the combination of surface depletion layer and bulk phase resistance. Specifically, the energy band bending induced by surface oxygen adsorption leads to the formation of a hole accumulation layer, which reduces the resistance, while the introduction of CuO enhances the interfacial depletion effect of the p-n heterojunction, enlarging the electron depletion region and further increasing the overall resistance. This model is highly consistent with the resistance increase with increasing CuO concentration observed in this study, emphasizing the key role of heterojunction modulation on the charge transport behavior.
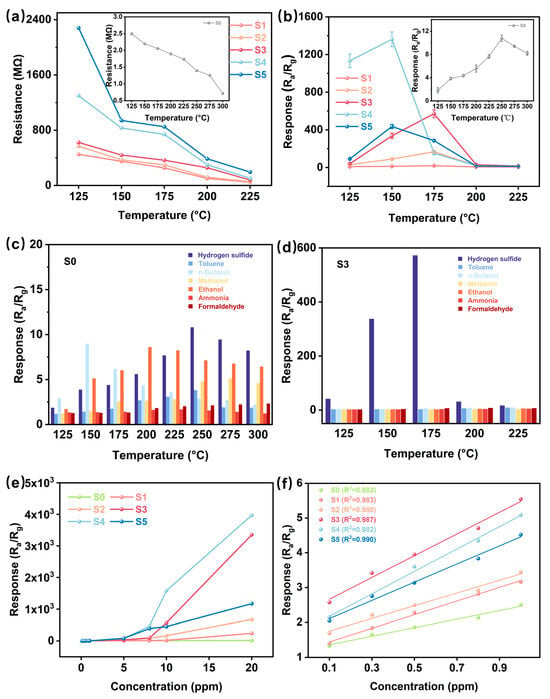
Figure 4.
(a) Resistance of the sensors in air (Ra) at different temperatures. (b) Response of S0-S5 to 10 ppm H2S gas at different temperatures. (c,d) Response of sensors S0 and S3 to different gases at different operating temperatures. (e,f) Response of S0–S5 to different H2S concentrations.
Figure 4b shows the response of these sensors to 10 ppm H2S at different temperatures. It can be observed that the operating temperature of the CuO-modified sensors shifts towards lower thermal conditions. Specifically, the original WO3 sensor achieves a peak response amplitude of 10.7 at 250 °C, while the CuO-incorporated device exhibits superior performance at lower temperatures: the S4 and S5 sensors have maximum responses of 1361.0 and 436.3 at 150 °C, respectively, while the optimal sensitivities of S2 and S3 are 150.7 and 571.5 at 175 °C, respectively. This improvement can be attributed to the formation of a p-n heterojunction between CuO and WO3, which effectively modulates the surface electronic states and creates additional chemically active sites for gas–solid interactions.
Also, the catalytic effect of CuO promotes the adsorption and oxidation of H2S (e.g., the generation of intermediates such as CuS), accelerates the charge transfer, and improves the sensor response to H2S [44]. However, an excessive amount of CuO could increase the resistance of the heterostructure significantly (shown in Figure 4a), limiting the electron transfer and resulting in a decrease in the response [45]. Meanwhile, too much CuO may trigger agglomeration on the WO3 surface, reducing the effective specific surface area of the material and lowering the H2S adsorption efficiency. In this work, although the S4 sensor has the highest response to H2S, we choose the S3 sensor, which has a good overall performance, for the subsequent study due to the difficulty of recovering the S4 sensor, which will be further discussed later.
Gas selectivity is an important measure of sensor performance and is defined as SH2S/Sinterferent, where SH2S and Sinterferent are the sensor response to H2S and to interfering gases, respectively. The gas-selective characteristics of the six sensors are shown in Figure 4c,d and Figure S3, where their response to 10 ppm H2S and 100 ppm concentrations of various interferents (toluene, n-butanol, methanol, ethanol, formaldehyde, ammonia) was evaluated at their respective optimal operating temperatures (S0: 250 °C; S1-S3: 175 °C; S4-S5: 150 °C). As shown in Figure 4c, sensor (S0) prepared from pure WO3 material has a non-specific cross-sensitivity to a wide range of analytes, showing limited target specificity. In contrast, the CuO-modified variants showed significantly improved H2S resolution, with S3 (Figure 4d) showing excellent selectivity over a wide temperature range (97-times higher than 100 ppm interference gas). This improvement may stem from selective surface interactions between the copper oxide phase and the H2S molecules, which is also supported by previous studies on the chemisorption properties of metal oxides [44]. Figure 4e,f show the linear relationship of the response for the S0-S5 sensors versus H2S concentration. In Figure 4e, the response of all devices increased progressively with increasing H2S concentration (0.1–20 ppm), with S4 showing superior response amplitude at increasing analyte concentrations, followed by S3, but the S4 sensor has a slower response and limited repeatability (Figure S4) while the pristine WO3-based sensor has a relatively low response. Figure 4f further shows the linear fit of the response-concentration relationship for each sensor at the lower concentrations, revealing a good linear relationship between the sensor response and the H2S concentration, which facilitates the quantitative detection of H2S. The relatively high sensitivity of the S3 and S4 sensors suggests that appropriate CuO content can improve the sensitivity of the sensors to H2S.
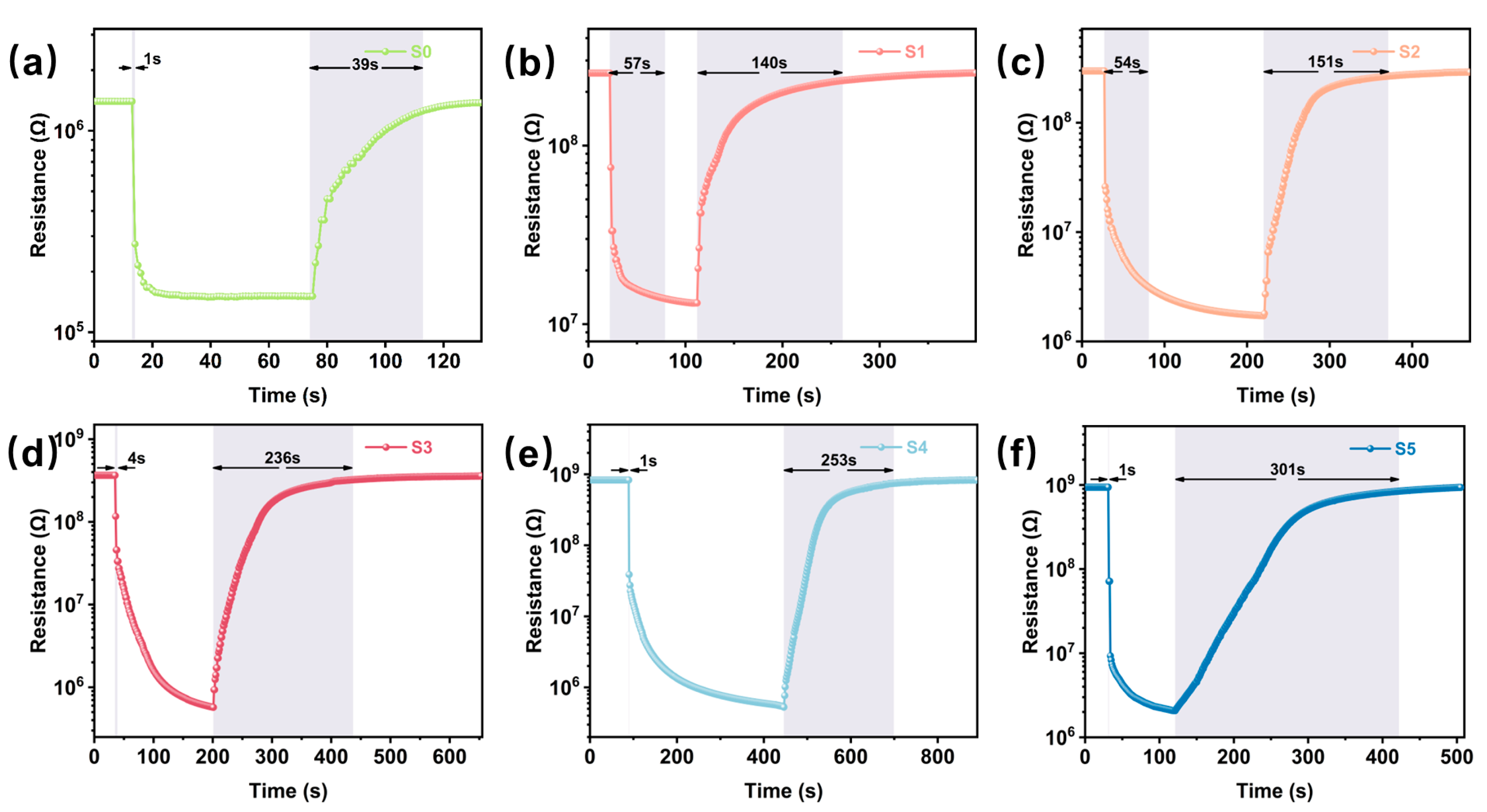
The response recovery curves of the S0-S5 sensors (Figure 5a–f) demonstrate the dynamic response process of the pristine WO3 and CuO/WO3 sensors to 10 ppm H2S. The response time of the S0 sensor (Figure 5a) is 13 s with a recovery time of 39 s. When CuO is introduced, as shown in S1 (Figure 5b) and S2 (Figure 5c) sensor, the response times are 57 s and 54 s, respectively, and the recovery times are 140 s and 151 s, respectively. The resistance of the sensor in H2S gas was greatly reduced and the response increased significantly. This indicates that the addition of an appropriate amount of CuO increases the active sites on the surface of the sensing material, which intensifies the adsorption and reaction process of H2S on the surface of WO3, resulting in a longer response time and increased response. It is noteworthy that the response times of the S4 (Figure 5e) and S5 sensors (Figure 5f) were greatly reduced compared with those of the S0-S3 sensors. This is due to the fact that with the increase in CuO, the active sites on the surface of WO3 also increase, and H2S reacts more rapidly with CuO, e.g., generating CuS intermediates, allowing the sensors to have a lower resistance and a faster response in H2S. However, the recovery time of the sensor also increased, which may be attributed to the higher CuO content that improves the adsorption of H2S by the sensor material (resulting in a fast response) but hinders the desorption process (prolonging the recovery time), which may be due to the stronger chemical interaction between H2S and CuO. The S3 sensor (Figure 5d) had a response time of 4 s and a recovery time of 236 s.
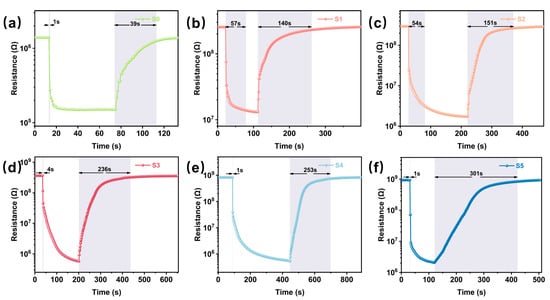
Figure 5.
Dynamic response curves of (a) S0, (b) S1, (c) S2, (d) S3, (e) S4 and (f) S5 to 10 ppm H2S at their respective optimal temperatures.
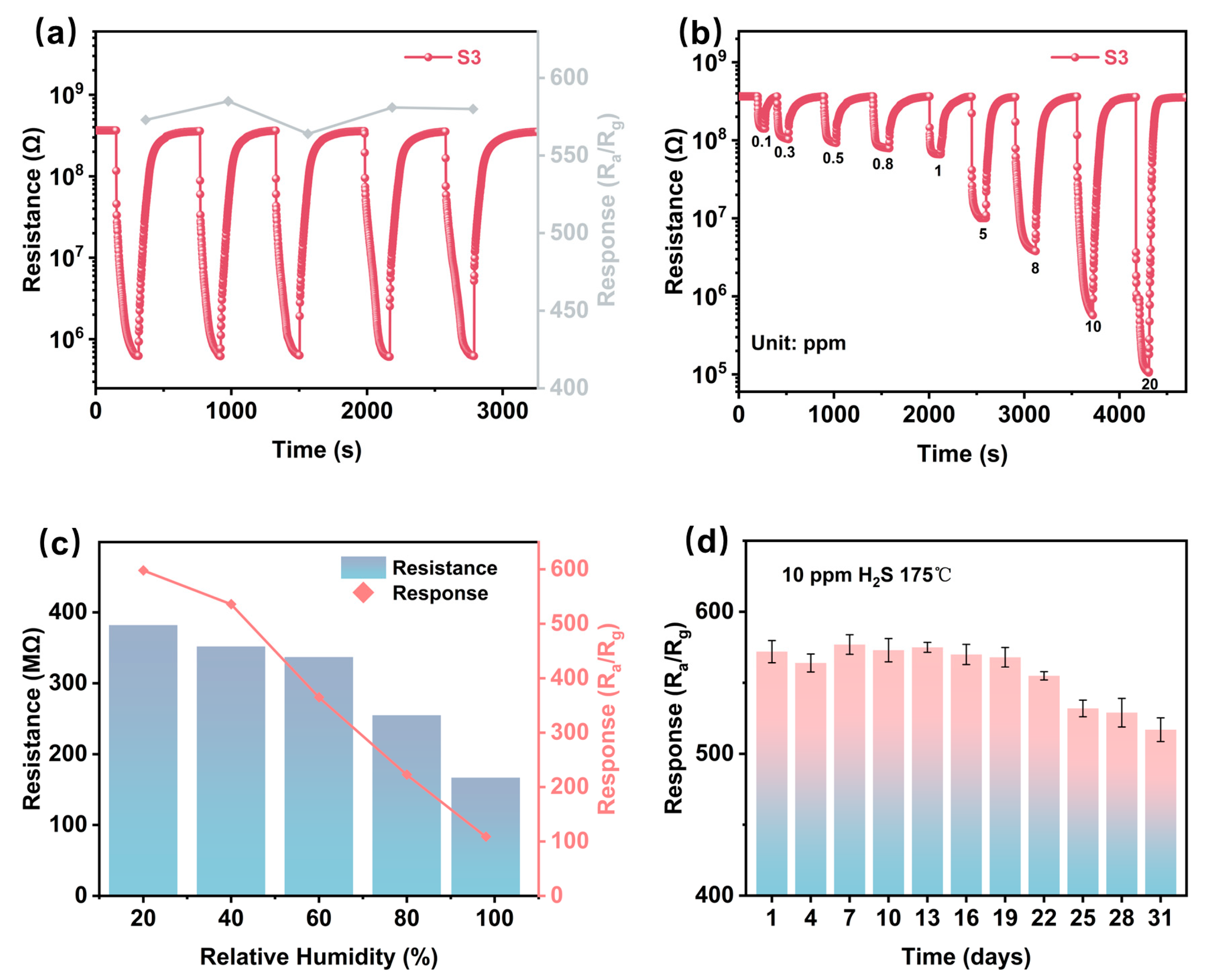
Figure 6a and Figure S4 systematically evaluate the repeatable performance of six sensing devices when exposed to 10 ppm H2S at their respective optimal temperatures. The experimental data show that the repeatability of the sensors is improved after modification of CuO (Figure 6a and Figure S4c,e), but the performance decreases after overdoping. The modified sensors (S4 and S5) exhibit a progressive Rg increase in cyclic testing, a phenomenon that may be related to the inhibition of interfacial charge transport due to the accumulation of CuS by-products during the redox process, which has been documented in a previous study on sulfide detection [46].
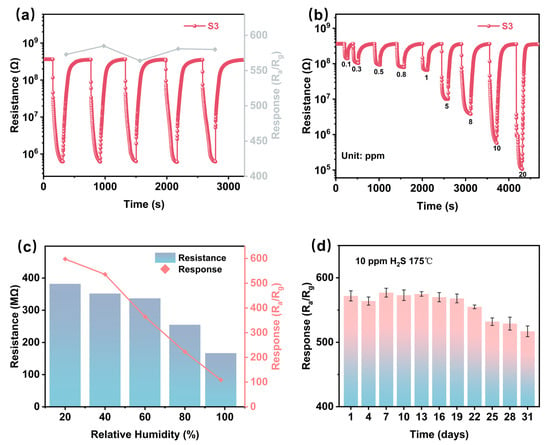
Figure 6.
(a) Five cycles of response and recovery curve to 10 ppm H2S of S3. (b) Dynamic response curves of S3 to various concentrations of H2S at the optimal operating temperature. (c) Baseline resistance and response to 10 ppm H2S of S3 at 175 °C under varying humidity conditions (20%, 40%, 60%, 80%, and 99% RH at 25 °C). (d) Long-term stability of S3 to 10 ppm H2S at 175 °C over a 30-day exposure period.
An environmental disturbance analysis (Figure 6c) showed that the performance of the S3 sensor was closely related to humidity. As the relative humidity increases from 20% to 100% RH, both the baseline resistance and the response amplitude gradually decrease (38.6% and 72.4%, respectively). This decrease in performance suggests a competitive adsorption mechanism between water molecules and H2S at the surface active sites, with hydroxyl groups preferentially occupying the chemisorption centers that are critical for target gas interactions.
Long-term stability is defined as the ability of a sensor to maintain the magnitude of its initial response over an extended period of time under the same operating conditions. In this study, stability was measured by periodically exposing the S3 sensor to 10 ppm H2S at 175 °C for 30 days and recording the response, and the response fluctuated and declined after the 19th day of testing and remained relatively stable at the 25th day of testing (Figure 6d). This is because the sensor operates over a long period of time causing interfering gases (e.g., ambient CO2, volatile organic compounds, etc.) to accumulate on the sensing material, blocking the active sites for H2S adsorption. Meanwhile, during hydrogen-sulfur detection, the reaction between CuO and H2S produces stabilized CuS. Over time, CuS accumulates on the surface of the material, decreasing charge carrier mobility and blocking gas–solid interactions., but the sensor was still able to maintain a high response of 517 on the 30th day (the attenuation rate is about 9.5%.), and it still has the ability to detect H2S.
Finally, to systematically evaluate the overall performance of the developed sensor, this study conducted a comparative analysis between the CuO/WO3 sensor and previously reported H2S sensing devices in the literature (Table 1). The results demonstrate that conventional metal oxide semiconductor-based H2S sensors typically face challenges such as high operating temperatures, low response values, or prolonged recovery times caused by difficult H2S desorption despite high response levels. These devices rarely achieve multi-parameter synergistic optimization, often sacrificing one performance metric to enhance others. In contrast, the gas sensor developed in this work exhibits superior characteristics at 175 °C, including high sensitivity to H2S, short response/recovery times, and a low detection limit, thereby realizing comprehensive performance enhancement without compromising key sensing parameters.

Table 1.
Comparison of H2S gas sensors in this study and the reported literature.
3.3. Gas Sensing Mechanisms
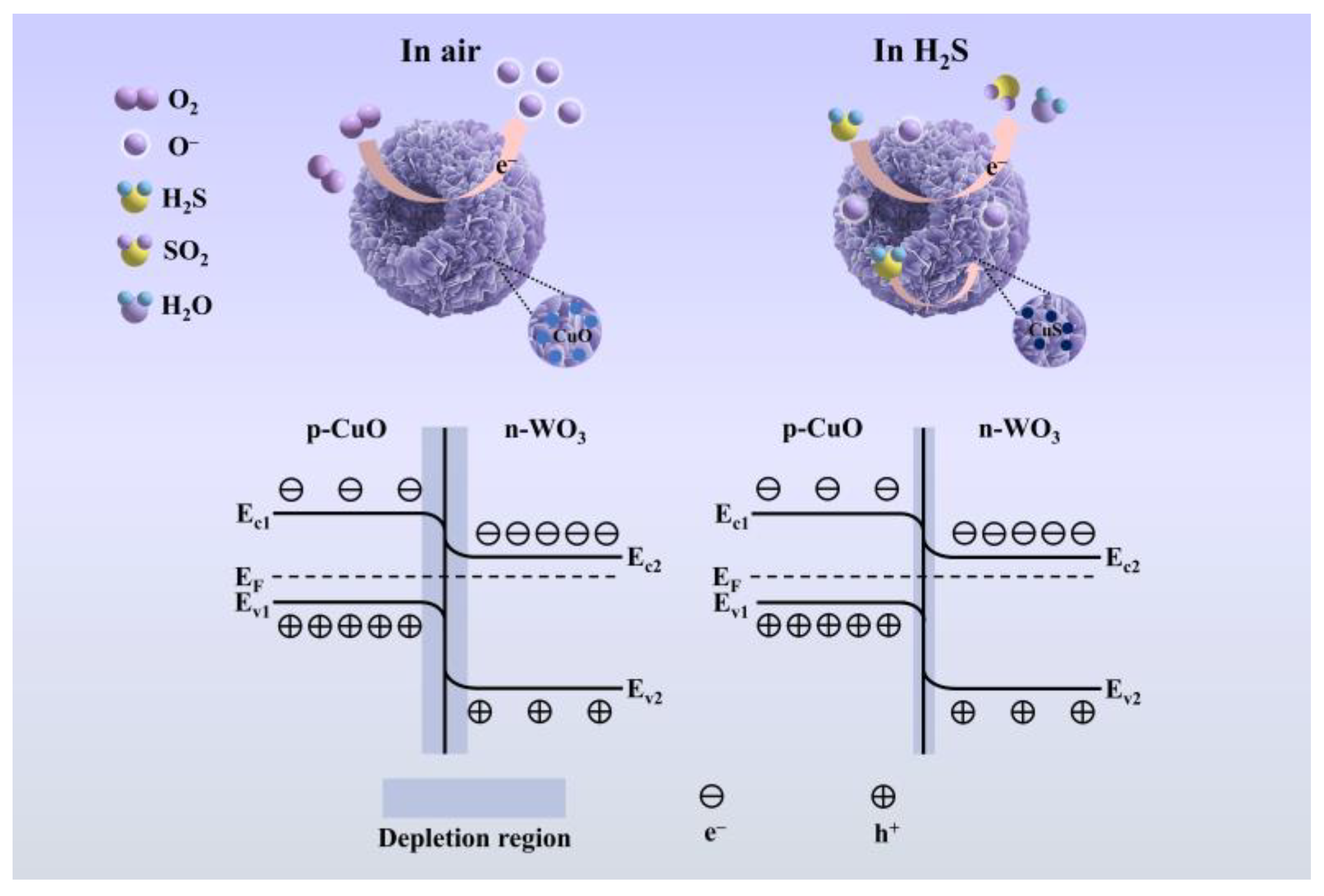
As illustrated in Figure 7, the gas sensing mechanism of CuO/WO3 heterostructures is governed by surface-mediated redox processes and charge carrier modulation within the semiconductor matrix [34]. For the n-type WO3 semiconductor, atmospheric oxygen molecules undergo adsorption on the material surface, forming surface-bound oxygen species (O2−, O−, O2−) through electron extraction from the conduction band. This chemisorption process establishes an electron-depletion layer near the surface region, resulting in elevated baseline resistance (Ra) under ambient conditions [60]. Upon exposure to reducing gases such as H2S, the following surface reaction occurs (Equation (2)) [61]:
H2S + 3O− → H2O + SO2 + 3e−
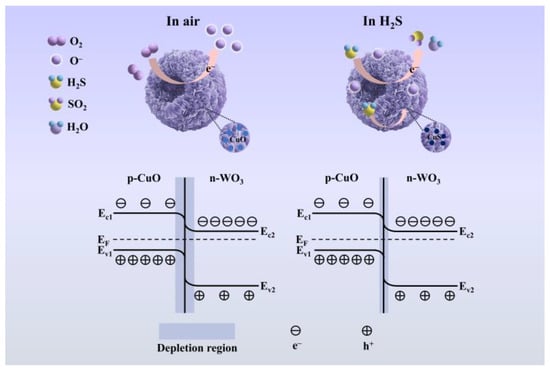
Figure 7.
Schematic diagram of the sensing mechanism.
The liberated electrons recombine with the WO3 conduction band, reducing the depletion layer thickness and consequently decreasing the baseline resistance (Ra) [60]. In CuO/WO3 heterostructures, the p-type CuO establishes a p-n junction with n-type WO3. At the heterointerface, electron transfer occurs from WO3 to CuO, while hole migration proceeds in the reverse direction, creating a built-in electric field that suppresses charge carrier recombination. This interfacial field facilitates rapid transport of electrons generated during H2S oxidation and thereby enhancing the sensing response [61].
There are several factors contributing to the improved performance of the sensing material after the introduction of CuO into the WO3 hollow flower spheres:
- (1)
- Synergistic effect of p-n heterojunctions for sensitization: The formation of CuO/WO3 heterojunctions creates an internal electric field at the heterojunctions to inhibit the electron–hole complexation, which leads to an increase in the baseline resistance of the sensors, which is beneficial for the detection of reducing gases. On the other hand, the heterojunction interface provides additional sites for H2S gas adsorption and redox reactions [62], which also contributes to the sensitivity.
- (2)
- The working temperature in this study was 175 °C. Previous studies have shown that at lower temperatures, H2S undergoes irreversible displacement reactions directly with CuO, producing conducting CuS by Equation (3) [63]:
CuO + H2S → CuS + H2O
The conductive CuS generated by the reaction leads to a significant reduction in the material resistance, which in turn improves the response of the sensor. However, other gases (e.g., NH3, NO2) change the resistance only through the adsorbed oxygen mechanism, with a weak response [64], which makes the sensor selective for H2S enhancement. Henzler et al. achieved the reversal of CuS by high temperature and reconverted it to CuO [63], and the sensors investigated in this work solved the delayed recovery of pure CuO through the coordinated acceleration of desorption by vacancies and oxygen defects in the heterojunction, which is crucial for reducing the power consumption of the sensor in practical applications.
- (3)
- Another reason for the improved sensor performance is linked to the fact that the CuO/WO3 composites have a flower-like hollow sphere morphology. The hierarchical structure enables the WO3 sensing material to have a high specific surface area, which provides a site for subsequent CuO loading. CuO doping introduces oxygen vacancies and lattice defects in WO3, which provide abundant surface sites for gas adsorption. These defects act as additional H2S adsorption sites, enhance the chemisorption of reactive oxygen species and further promote redox reactions [65].
- (4)
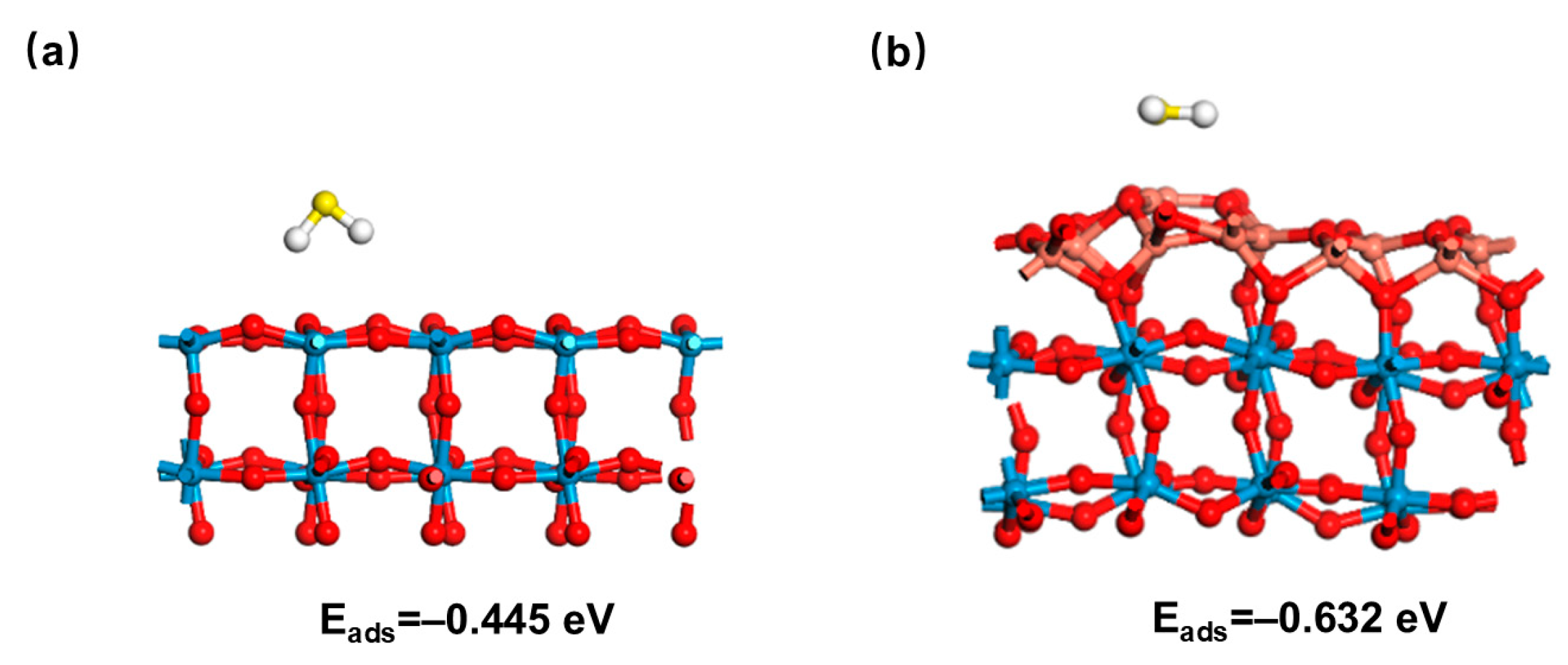
- Improved selectivity of CuO sensitizing component through specific interactions: CuO is more selective for H2S than for other gases (e.g., toluene, methanol) due to a unique reaction pathway: the CuO to CuS generation is highly specific for H2S, while the interfering gases lack similarly strong chemical interactions with the CuO, thus minimizing cross-response. In addition, the formation of heterojunctions alters the surface electronic properties of WO3, making it more favorable for H2S adsorption than neutral or weakly polar gases [66]. DFT calculations reveal an adsorption energy of −0.445 eV for WO3. In contrast, CuO/WO3 demonstrates a lower adsorption energy (−0.632 eV), indicating that H2S molecules adsorb more readily on CuO/WO3 surfaces, consequently promoting reaction processes (Figure 8) [67].
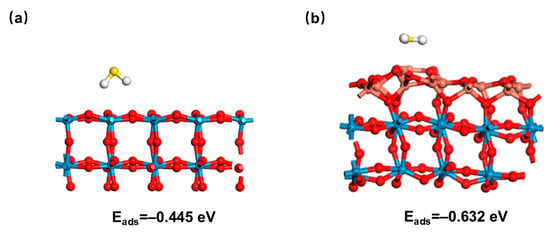 Figure 8. Adsorption model of H2S (a) WO3 and (b) CuO/WO3 heterojunction.
Figure 8. Adsorption model of H2S (a) WO3 and (b) CuO/WO3 heterojunction.
4. Conclusions
In this study, we successfully synthesized CuO/WO3 hollow spheres with flower-like morphology by a facile strategy to demonstrate their excellent performance in ppb-level ultrasensitive H2S detection. Experimental results show that the optimal CuO/WO3 sensing material S3 sensor has a detection limit as low as 100 ppb, a rapid response/recovery time of 4 s/236 s for 10 ppm H2S, a response as high as 547, and excellent selectivity for common interfering gases. The sensitization mechanism originates from the catalytic activity of CuO, which reduces the activation energy for H2S oxidation and generates abundant free electrons through specific redox reactions. Meanwhile, the p-n heterojunction formed between p-type CuO and n-type WO3 generates an internal electric field that promotes charge separation and significantly enhances the initial resistance of the sensor. In addition, the unique hollow spherical morphology provides sufficient sites for CuO loading and enhances the gas surface adsorption. Regarding long-term stability, the sensor retains a response of 517 after 30 days (9.5% attenuation), attributed to gradual CuS accumulation on the surface and adsorption of ambient interfering gases, which block active sites and reduce charge carrier mobility. Future research directions will focus on: inhibiting the aggregation of CuS through engineering surface modifications (such as protective coatings or dopants); optimizing the heterojunction structure to accelerate H2S desorption and reduce irreversible sulfidation; and developing humidity-resistant strategies to minimize environmental interference for practical applications in complex environments. This work provides ideas for engineering metal-oxide semiconductor sensing materials with heterogeneous structures, and it also offers a feasible strategy for developing high-performance sensors for trace H2S monitoring in environmental and industrial applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13070250/s1. Figure S1. The physical diagram of the sensor. Figure S2. SEM images of (a,b) S1, (c,d) S2, (e,f) S4, and (g,h) S5. Table S1. Ratio of W, Cu, O for elemental mapping results. Figure S3. Selectivity of (a) S1, (b) S2, (c) S4 and (d) S5 at different operating temperatures. Figure S4. Five cycles of response and recovery curve to 10 ppm H2S of S0 (a), S1 (c), S2 (e), S4 (g) and S5 (i). Dynamic response curves of S0 (b), S1 (d), S2 (f), S4 (h) and S5 (j) to various concentrations of H2S at the optimal operating temperature. Figure S5. Dynamic response curves of the S3 to 10 ppm H2S for varying humidity conditions (20%, 40%, 60%, 80%, and 99% RH, 25 °C) at an operating temperature of 175 °C.
Author Contributions
Conceptualization, X.Y. and P.W.; methodology, X.Y.; formal analysis, P.W.; investigation, P.W.; data curation, P.W.; writing—original draft preparation, P.W.; writing—review and editing, X.Y.; supervision, X.Y.; funding acquisition, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 62473126, 62003123). Hebei Collaborative Innovation Center of Microelectronic Materials and Technology on Ultra Precision Processing (CIC) and Hebei Engineering Research Center of Microelectronic Materials and Devices (ERC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to thank Yang Ziwen, Yang Shaobin, Sun Zhen and Guo Lanlan for their contributions and support in the investigation of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, Y.; Xu, P.C.; Xu, T.; Zheng, D.; Li, X.X. ZnO-nanowire size effect induced ultra-high sensing response to ppb-level H2S. Sens. Actuators B Chem. 2017, 240, 264–272. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, D.K.; Zhang, D.X.; Liu, Y.M.; Sun, M.L.; Sun, M.J.; Li, J. Construction of Ag2O/WO3-based hollow microsphere p-n heterojunction for continuous detection of ppb-level H2S gas sensor. J. Alloys Compd. 2024, 1003, 175735. [Google Scholar] [CrossRef]
- Li, J.D.; Tang, Y.L.; Ao, D.Y.; Xiang, X.; Wang, S.Y.; Zu, X.T. Ultra-highly sensitive and selective H2S gas sensor based on CuO with sub-ppb detection limit. Int. J. Hydrogen Energy 2019, 44, 3985–3992. [Google Scholar] [CrossRef]
- Li, D.J.; Zu, X.T.; Ao, D.Y.; Tang, Q.B.; Fu, Y.Q.; Guo, Y.J.; Bilawal, K.; Faheem, M.B.; Li, L.; Li, S.; et al. High humidity enhanced surface acoustic wave (SAW) H2S sensors based on sol-gel CuO films. Sens. Actuators B Chem. 2019, 294, 55–61. [Google Scholar] [CrossRef]
- Na, H.B.; Zhang, X.F.; Deng, Z.P.; Xu, Y.M.; Huo, L.H.; Gao, S. Large-Scale Synthesis of Hierarchically Porous ZnO Hollow Tubule for Fast Response to ppb-Level H2S Gas. ACS Appl. Mater. Interfaces 2019, 11, 11627–11635. [Google Scholar] [CrossRef]
- Xiong, B.; Zhou, R.; Hao, J.R.; Jia, Y.H.; He, Y.; Yeung, E.S. Highly sensitive sulphide mapping in live cells by kinetic spectral analysis of single Au-Ag core-shell nanoparticles. Nat. Commun. 2013, 4, 1708. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.F.; Cheng, X.L.; Xu, Y.M.; Gao, S.; Zhao, H.; Huo, L.H. Hierarchical NiO Cube/Nitrogen-Doped Reduced Graphene Oxide Composite with Enhanced H2S Sensing Properties at Low Temperature. ACS Appl. Mater. Interfaces 2017, 9, 26293–26303. [Google Scholar] [CrossRef]
- Beni, A.H.N.; Haghbakhsh, R. Sulfur dioxide capture by deep eutectic solvents: Proposing purely predictive absorption models. J. Hazard Mater. 2025, 490, 137741. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.I.M.; Mahmoud, S.T.; Awwad, F.; Greish, Y.E.; Abu-Hani, A.F.S. Low power consumption and fast response H2S gas sensor based on a chitosan-CuO hybrid nanocomposite thin film. Carbohydr. Polym. 2020, 236, 116064. [Google Scholar] [CrossRef]
- Song, Z.L.; Wei, Z.R.; Wang, B.; Luo, Z.; Xu, S.M.; Zhang, W.K.; Yu, H.X.; Li, M.; Huang, Z.; Zang, J.F.; et al. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Devi, P.; Singh, J.P. A Highly Sensitive Colorimetric Gas Sensor Based on Indium Oxide Nnnostructures for H2S Detection at Room Temperature. IEEE Sens. J. 2021, 21, 18512–18518. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Singh, A.; Jose, D.A. Colorimetric sensor for the detection of H2S and its application in molecular half-subtractor. Anal. Chim. Acta 2018, 1040, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Jothi, D.; Iyer, S.K. A highly sensitive naphthalimide based fluorescent “turn-on” sensor for H2S and its bio-imaging applications. J. Photochem. Photobiol. A-Chem. 2022, 427, 113802. [Google Scholar] [CrossRef]
- Shingole, M.; Banerjee, S.; Modak, B.; Kolay, S.; Mohanty, J.; Sudarsan, V. Cu-MOF-74 as Fluorescent Probe: Selective Optical Sensor for H2S Gas. ChemPhotoChem 2025, 9, e202400300. [Google Scholar] [CrossRef]
- Gu, W.X.; Zheng, W.W.; Liu, H.; Zhao, Y. Electroactive Cu2O nanocubes engineered electrochemical sensor for H2S detection. Anal. Chim. Acta 2021, 1150, 338216. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.X.; Cui, L.Y.; Zheng, F.J.; Song, Q.J. Electroactive Au@Ag nanoparticles driven electrochemical sensor for endogenous H2S detection. Biosens. Bioelectron. 2018, 117, 53–59. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Z.X.; Wang, X.Y.; Xie, J. Hydrogen sulfide in seafood: Formation, hazards, and control. Trends Food Sci. Technol. 2024, 148, 104512. [Google Scholar] [CrossRef]
- Girija, K.G.; Somasundaram, K.; Topkar, A.; Vatsa, R.K. Highly selective H2S gas sensor based on Cu-doped ZnO nanocrystalline films deposited by RF magnetron sputtering of powder target. J. Alloys Compd. 2016, 684, 15–20. [Google Scholar] [CrossRef]
- Shinde, S.D.; Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Synthesis of ZnO nanorods by spray pyrolysis for H2S gas sensor. J. Alloys Compd. 2012, 528, 109–114. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Zheng, J.; Li, Q.; Xu, R.; Xu, J.; Song, Y.-Y.; Song, P.; Gao, Z.; Zhao, C. Integrating Vacancies and Defect Levels in Heterojunctions to Synergistically Enhance the Performance of H2S Chemiresistors for Periodontitis Diagnosis. ACS Sens. 2025, 10, 3072–3080. [Google Scholar] [CrossRef]
- Li, J.Y.; Na, E.T.; Liang, X.D.; Liang, Q.H.; Fan, M.H.; Chen, H.; Li, G.D.; Zou, X.X. Surface oxygen chemistry of metal oxide semiconductors for gas-sensing applications. Inorg. Chem. Front. 2024, 11, 8602–8626. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.Y.; Li, J.; Duan, Z.H.; Yang, Y.J.; Yuan, Z.; Jiang, Y.D.; Tai, H.L. Pd-Decorated ZnO Hexagonal Microdiscs for NH3 Sensor. Chemosensors 2024, 12, 43. [Google Scholar] [CrossRef]
- Zhou, B.D.; Peng, X.Y.; Chu, J.; Malca, C.; Diaz, L.; Zhou, A.F.; Feng, P.X. Type II ZnO-MoS2 Heterostructure-Based Self-Powered UV-MIR Ultra-Broadband p-n Photodetectors. Molecules 2025, 30, 1063. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.J.; Chang, X.T.; Jiang, Y.C.; Gao, W.X.; Sun, S.B. Hydrophobicity-promoted humidity-resistant ethanol sensors based on PTFE/Au/WO3 composite films. Sens. Actuators B Chem. 2025, 430, 137386. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Li, Z.S.; Schierbaum, K. Engineered Porosity ZnO Sensor Enriched with Oxygen Vacancies Enabled Extraordinary Sub-ppm Sensing of Hydrogen Sulfide and Nitrogen Dioxide Air Pollution Gases at Low Temperature in Air. Sensors 2024, 24, 7694. [Google Scholar] [CrossRef]
- Huo, Y.Y.; Qiu, L.M.; Wang, T.Q.; Yu, H.; Yang, W.Y.; Dong, X.T.; Yang, Y. P-N Heterojunction formation: Metal Sulfide@Metal Oxide Chemiresistor for ppb H2S Detection from Exhaled Breath and Food Spoilage at Flexible Room Temperature. ACS Sens. 2024, 9, 3433–3443. [Google Scholar] [CrossRef]
- Yang, Y.N.; Yue, J.W.; Zhang, X.T.; Ren, B.W.; Fu, S.H.; Sun, Y.H.; Luo, Z.X. Accordion-like ZIF-8/MoO3 composite gas sensor for highly selective and sensitive H2S detection. Ceram. Int. 2024, 50, 38253–38262. [Google Scholar]
- Zhang, D.N.; Jiang, J.Y.; Yang, Y.; Li, F.; Yu, H.; Dong, X.T.; Wang, T.Q. SnO2-inserted 2D Layered Ti3C2Tx MXene: From heterostructure construction to ultra-high sensitivity for Ppb-level H2S detection. Sens. Actuators B Chem. 2024, 410, 135727. [Google Scholar] [CrossRef]
- Cai, Z.X.; Li, H.Y.; Ding, J.C.; Guo, X. Hierarchical flowerlike WO3 nanostructures assembled by porous nanoflakes for enhanced NO gas sensing. Sens. Actuators B Chem. 2017, 246, 225–234. [Google Scholar] [CrossRef]
- Can, Y.; Courtois, X.; Duprez, D. Tungsten-Based Catalysts for Environmental Applications. Catalysts 2021, 11, 703. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zhou, W.Q.; Li, D.Q.; Xu, J.K. Multifunctional non-stoichiometric tungsten oxides: Synthesis, properties and application. J. Power Sources 2025, 631, 236222. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Ahn, S.; Mirzaei, A.; Kim, J.H.; Park, C. CuO nanoparticles-decorated femtosecond laser-irradiated WS2-WO3 heterojunctions to realize selective H2S gas sensor. Sens. Actuators B Chem. 2025, 427, 137167. [Google Scholar] [CrossRef]
- Zhang, S.B.; Fang, L.; Cao, Z.M.; Dai, X.Y.; Wang, W.; Geng, Q.; Zhou, M.H.; Zhang, S.H.; Dong, F.; Chen, S. In Situ Generatable and Recyclable Oxygen Vacancy-Modified Fe2O3-Decorated WO3 Nanowires with Super Stability for ppb-Level H2S Sensing. ACS Sens. 2024, 9, 5500–5511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.Y.; Xiao, D.K.; Wang, S.J.; Zhang, T.; Yang, X.; Heng, S.Q.; Sun, M.J. CuO/WO3 hollow microsphere P-N heterojunction sensor for continuous cycle detection of H2S gas. Sens. Actuators B Chem. 2023, 374, 132823. [Google Scholar] [CrossRef]
- Tamaki, T.; Maekawa, N.; Miura, N.; Yamazoe, N. CUO-SNO2 Element for Highly Sensitive and Selective Detection of H2S. Sens. Actuators B-Chem. 1992, 9, 197–203. [Google Scholar] [CrossRef]
- Goyal, C.P.; Goyal, D.; Rajan, S.K.; Ramgir, N.S.; Shimura, Y.; Navaneethan, M.; Hayakawa, Y.; Muthamizhchelvan, C.; Ikeda, H.; Ponnusamy, S. Effect of Zn Doping in CuO Octahedral Crystals towards Structural, Optical, and Gas Sensing Properties. Crystals 2020, 10, 188. [Google Scholar] [CrossRef]
- Joshi, S.; Kumar, C.B.R.; Jones, L.A.; Mayes, E.L.H.; Ippolito, S.J.; Sunkara, M.V. Modulating interleaved ZnO assembly with CuO nanoleaves for multifunctional performance: Perdurable CO2 gas sensor and visible light catalyst. Inorg. Chem. Front. 2017, 4, 1848–1861. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Huang, L.; Zhang, Y.; Hu, Z.; Sun, C.; Yang, X.; Pan, G.; Cheng, Y. AuPd bimetallic functionalized monodisperse In2O3 porous spheres for ultrasensitive trimethylamine detection. Sens. Actuators B Chem. 2023, 381, 133355. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, T.T.; Zhang, L.; Han, W.J.; Yang, J.Q.; Wang, C.; Sun, Y.F.; Liu, F.M.; Sun, P.; Lu, G.Y. Construction of mesoporous In2O3-ZnO hierarchical structure gas sensor for ethanol detection. Sens. Actuators B Chem. 2023, 393, 134203. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Li, Z.S.; Schierbaum, K. Adsorbed Oxygen Ions and Oxygen Vacancies: Their Concentration and Distribution in Metal Oxide Chemical Sensors and Influencing Role in Sensitivity and Sensing Mechanisms. Sensors 2023, 23, 29. [Google Scholar] [CrossRef]
- Sun, L.J.; Su, H.W.; Xu, D.F.; Wang, L.L.; Tang, H.; Liu, Q.Q. Carbon hollow spheres as cocatalyst of Cu-doped TiO2 nanoparticles for improved photocatalytic H2 generation. Rare Met. 2022, 41, 2063–2073. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Chen, J.; Zhang, L.; Zhang, Y.; Pei, X.L. Pd-modified SmFeO3 with hollow tubular structure under light shows extremely high acetone gas sensitivity. Rare Met. 2023, 42, 545–557. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Navale, S.; Shahbaz, M.; Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S. CuxO Nanostructure-Based Gas Sensors for H2S Detection: An Overview. Chemosensors 2021, 9, 127. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, X.T.; Wang, T.Q.; Pei, W.Y.; Yang, Y.; Li, F.; Yin, D.D.; Yu, H.; Dong, X.T. CuO-based gas sensor decorated by polyoxometalates electron acceptors: From constructing heterostructure to improved sensitivity and fast response for ethanol detection. Sens. Actuators B Chem. 2024, 415, 136016. [Google Scholar] [CrossRef]
- Datta, N.; Ramgir, N.S.; Kumar, S.; Veerender, P.; Kaur, M.; Kailasaganapathi, S.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Role of various interfaces of CuO/ZnO random nanowire networks in H2S sensing: An impedance and Kelvin probe analysis. Sens. Actuators B Chem. 2014, 202, 1270–1280. [Google Scholar] [CrossRef]
- He, M.; Xie, L.L.; Zhao, X.L.; Hu, X.B.; Li, S.H.; Zhu, Z.G. Highly sensitive and selective H2S gas sensors based on flower-like WO3/CuO composites operating at low/room temperature. J. Alloys Compd. 2019, 788, 36–43. [Google Scholar] [CrossRef]
- Kim, H.; Jin, C.; Park, S.; Kim, S.; Lee, C. H2S gas sensing properties of bare and Pd-functionalized CuO nanorods. Sens. Actuators B Chem. 2012, 161, 594–599. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Vallejos, S.; Umek, P.; Guttmann, P.; Bittencourt, C.; Llobet, E. Aerosol-Assisted CVD-Grown WO3 Nanoneedles Decorated with Copper Oxide Nanoparticles for the Selective and Humidity-Resilient Detection of H2S. ACS Appl. Mater. Interfaces 2015, 7, 6842–6851. [Google Scholar] [CrossRef]
- Qin, Y.X.; Zhang, Y.Z.; Lei, J.; Lei, S.Y. High-performance gas sensor based on Ag@SnO2-Co3O4 hollow nanocomposite for fast and highly selective H2S detection. Sens. Actuators B Chem. 2025, 428, 137239. [Google Scholar] [CrossRef]
- Xiao, B.X.; Zhao, Q.; Xiao, C.H.; Yang, T.Y.; Wang, P.; Wang, F.; Chen, X.D.; Zhang, M.Z. Low-temperature solvothermal synthesis of hierarchical flower-like WO3 nanostructures and their sensing properties for H2S. CrystEngComm 2015, 17, 5710–5716. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Zhou, X.R.; Ma, J.H.; Zhu, Y.H.; Cheng, X.W.; Luo, W.; Deng, Y. Rational Synthesis and Gas Sensing Performance of Ordered Mesoporous Semiconducting WO3/NiO Composites. ACS Appl. Mater. Interfaces 2019, 11, 26268–26276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Liu, B.; Xiao, S.H.; Wang, X.H.; Sun, L.M.; Li, H.; Xie, W.Y.; Li, Q.H.; Zhang, Q.; Wang, T.H. Low-Temperature H2S Detection with Hierarchical Cr-Doped WO3 Microspheres. ACS Appl. Mater. Interfaces 2016, 8, 9674–9683. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.R.; Zhao, Y.Q.; Zhu, B.L.; Kong, F.H.; Wang, D.; Wu, S.H.; Huang, W.P.; Zhang, S.M. H2S sensing characteristics of Pt-doped α-Fe2O3 thick film. Sens. Actuators B Chem. 2007, 125, 79–84. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Zhang, H.M.; Jiao, W.L. Preparation and sensing performance investigation of ZIF-8-derived ZnO-supported Ag nanoparticle assemblies for trace H2S gas detection. Microchem. J. 2025, 212, 113397. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Yong, K. CuO/ZnO Heterostructured Nanorods: Photochemical Synthesis and the Mechanism of H2S Gas Sensing. J. Phys. Chem. C 2012, 116, 15682–15691. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.W.; Kim, C.; Park, J.; Roh, J.W.; Hwang, J.Y.; Mirzaei, A.; Jin, C.; Choi, M.S. Characterization and H2S gas sensing characteristics of Au-decorated ZnO nanorods prepared by a two-step wet method. J. Alloys Compd. 2025, 1021, 179655. [Google Scholar] [CrossRef]
- Ganapathi, S.K.; Ramgir, N.S.; Datta, N.; Patnaik, V.; Patel, A.; Kaur, M.; Debnath, A.K.; Datta, R.; Saha, T.K.; Aswal, D.K.; et al. Commercial H2S Sensor Based On SnO2: CuO Thin Films. In Proceedings of the 2nd International Symposium on Physics and Technology of Sensors, Pune, India, 7–10 March 2015; IEEE: New York, NY, USA, 2015; pp. 67–69. [Google Scholar]
- Xia, H.; Zhang, D.Z.; Sun, Y.H.; Wang, J.H.; Tang, M.C. Ultrasensitive H2S Gas Sensor Based on SnO2 Nanoparticles Modified WO3 Nanocubes Heterojunction. IEEE Sens. J. 2023, 23, 27031–27037. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.W.; Sun, G.J.; Kim, S.S. An approach to detecting a reducing gas by radial modulation of electron-depleted shells in core-shell nanofibers. J. Mater. Chem. A 2013, 1, 13588–13596. [Google Scholar] [CrossRef]
- Wang, F.G.; Yang, S.; Han, S.M.; Sun, P.; Liu, W.X.; Lu, Q.P.; Cao, W.B. Synthesis of Cu-TiO2/CuS p-n heterojunction via in situ sulfidation for highly efficient photocatalytic NO removal. Prog. Nat. Sci.-Mater. Int. 2022, 32, 561–569. [Google Scholar] [CrossRef]
- Liu, H.C.; Wang, F.P.; Hu, K.L.; Zhang, B.; He, L.; Zhou, Q. Superior Hydrogen Sensing Property of Porous NiO/SnO2 Nanofibers Synthesized via Carbonization. Nanomaterials 2019, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Henzler, K.; Heilemann, A.; Kneer, J.; Guttmann, P.; Jia, H.; Bartsch, E.; Lu, Y.; Palzer, S. Investigation of reactions between trace gases and functional CuO nanospheres and octahedrons using NEXAFS-TXM imaging. Sci. Rep. 2015, 5, 17729. [Google Scholar] [CrossRef] [PubMed]
- Kneer, J.; Knobelspies, S.; Bierer, B.; Wöllenstein, J.; Palzer, S. New method to selectively determine hydrogen sulfide concentrations using CuO layers. Sens. Actuators B Chem. 2016, 222, 625–631. [Google Scholar] [CrossRef]
- Peng, F.; Yu, W.W.; Lu, Y.; Sun, Y.; Fu, X.L.; Hao, J.M.; Chen, X.; Cong, R.; Dai, N. Enhancement of Low-Temperature Gas-Sensing Performance Using Substoichiometric WO3−x Modified with CuO. ACS Appl. Mater. Interfaces 2020, 12, 41230–41238. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, P.; Sharma, G.; Dhiman, P.; Mola, G.T.; Farghali, M.; Rashwan, A.K.; Nasr, M.; Osman, A.I.; Wang, T.T. Simultaneous hydrogen production and photocatalytic pollutant removal: A review. Environ. Chem. Lett. 2024, 22, 2405–2424. [Google Scholar] [CrossRef]
- Li, X.C.; Zhao, G.P.; Xie, K.; Wang, P.T.; Zhang, C.; Lin, L. Cu-decorated HfS2 and Cu-embedded HfS2 for adsorption and gas sensing of lithium-ion thermal runaway gases: A DFT study. Surf. Interfaces 2024, 46, 104028. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).