Platinum-Functionalized Hierarchically Structured Flower-like Nickel Ferrite Sheets for High-Performance Acetone Sensing
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Nickel Ferrite
2.2. Synthesis of Pt-Modified Nickel Ferrite
2.3. Characterization
2.4. Gas Sensor Fabrication and Measurement Process
3. Results and Discussion
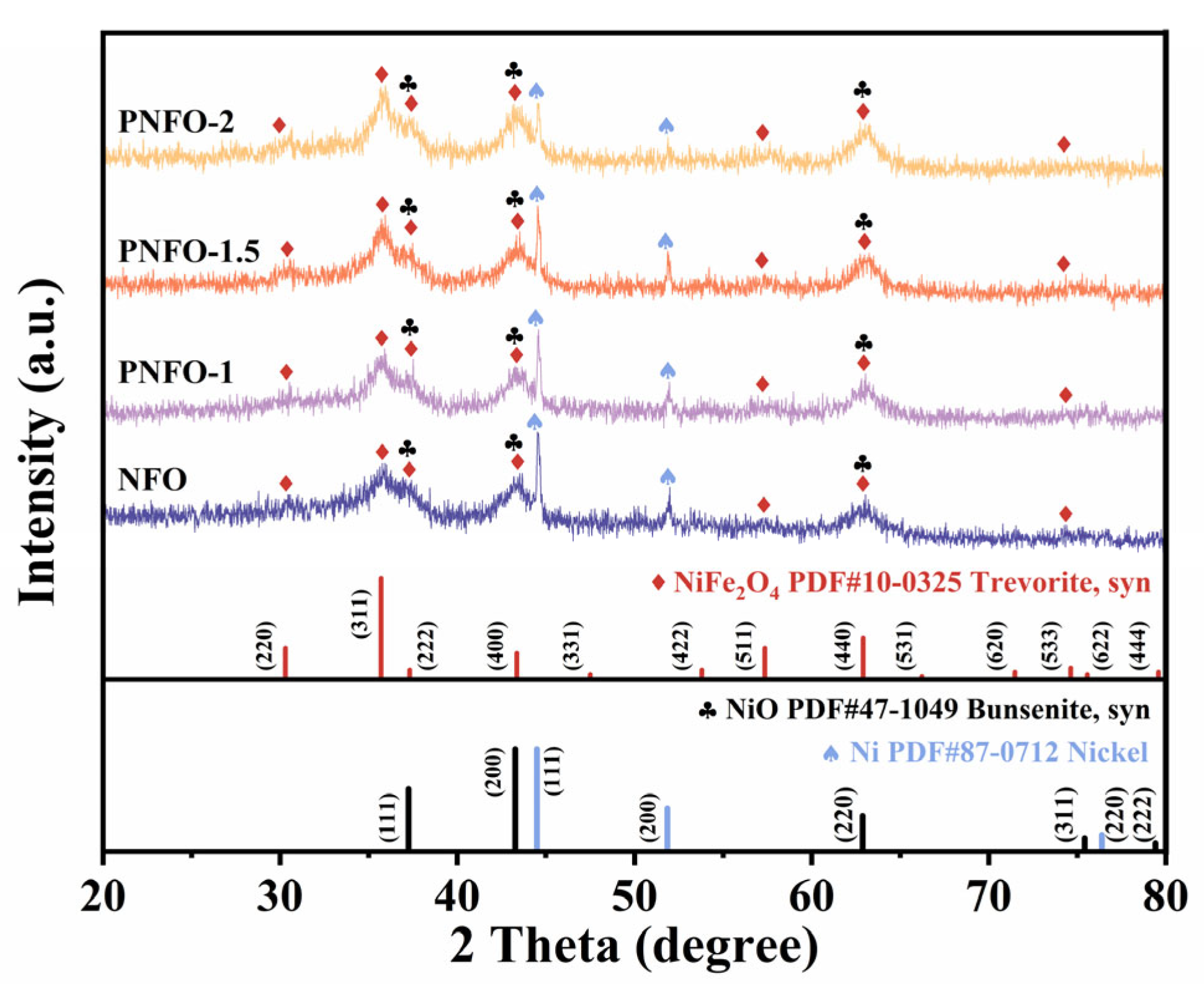
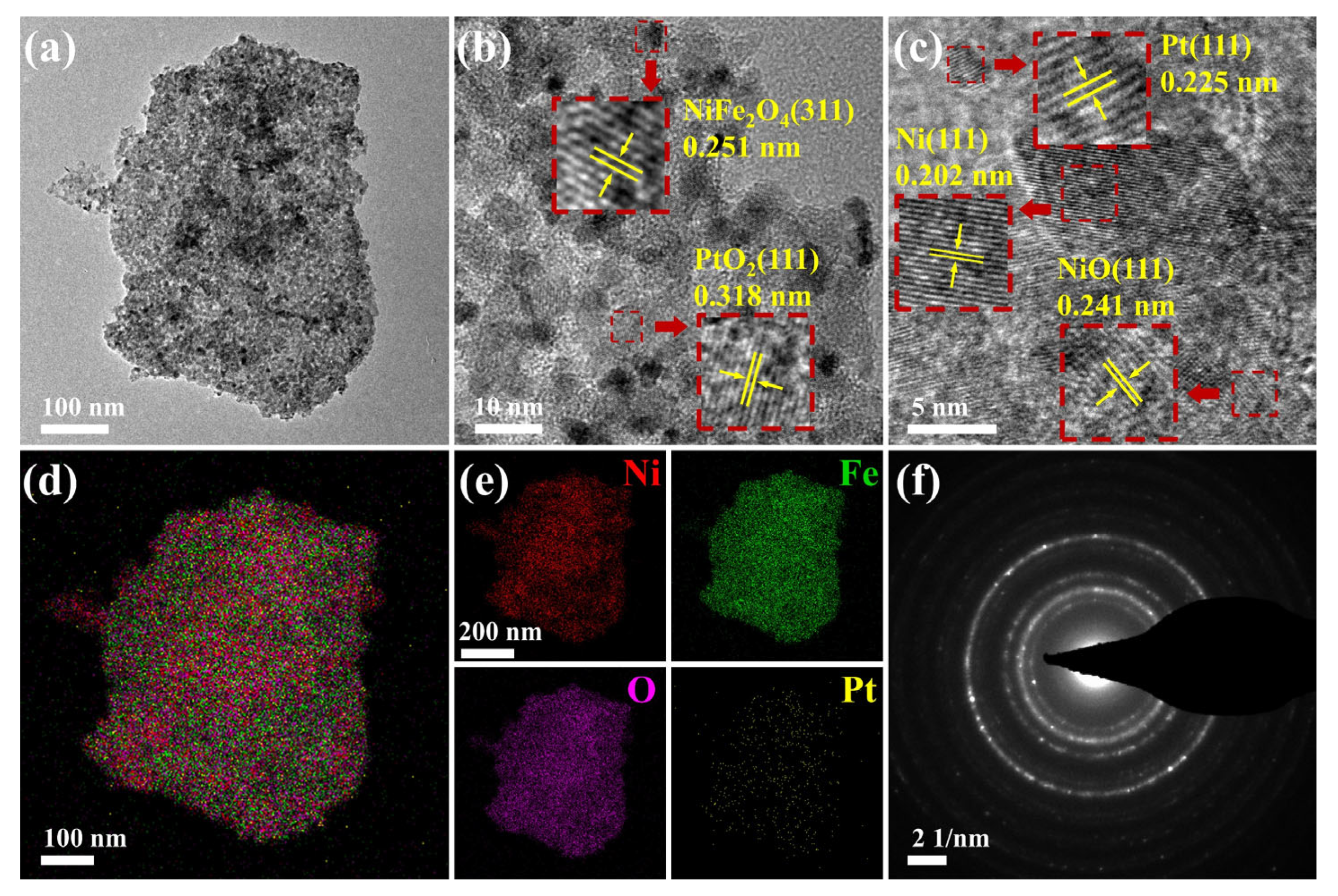
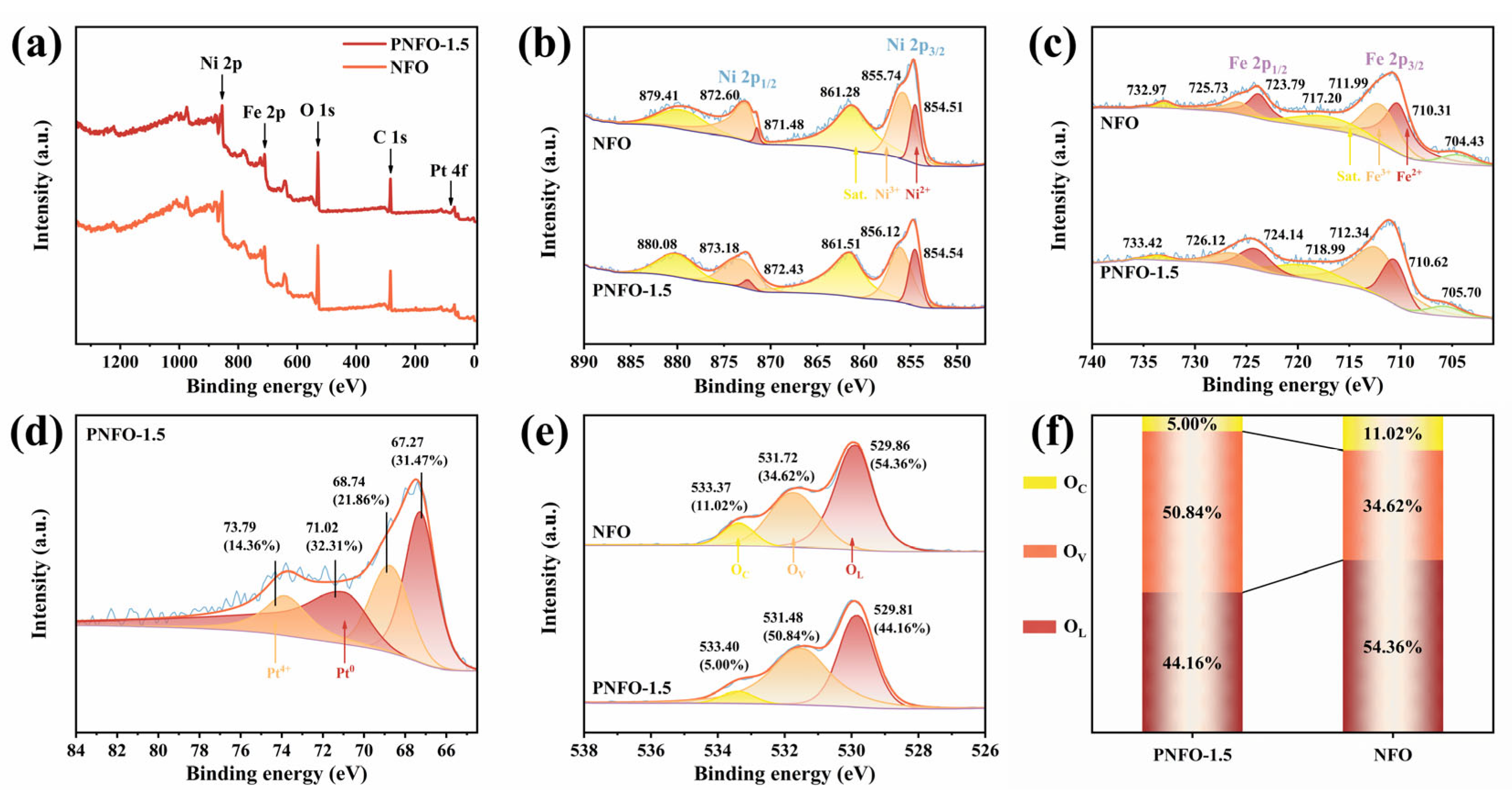
3.1. Material Characterization
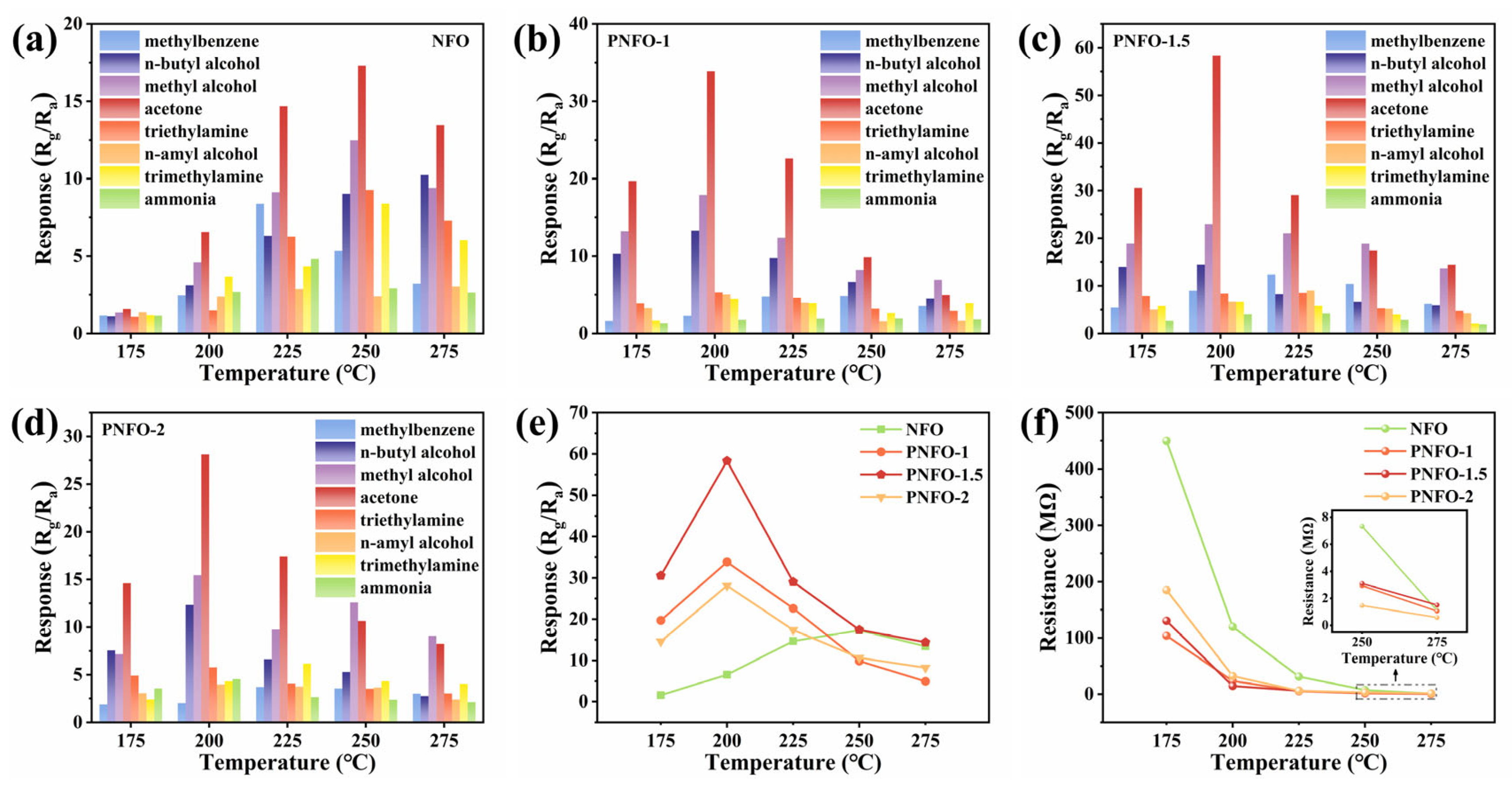
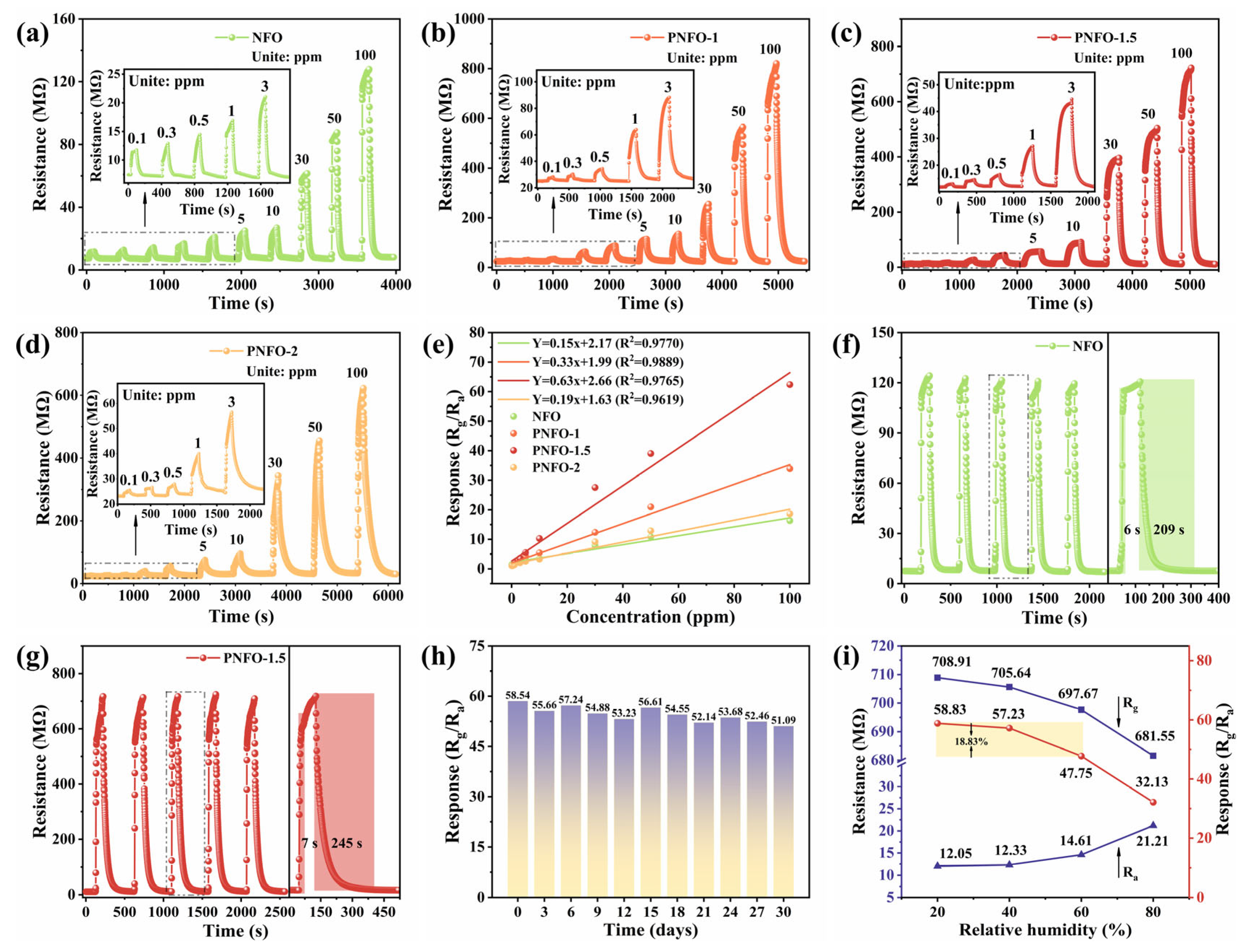
3.2. Gas Sensing Properties
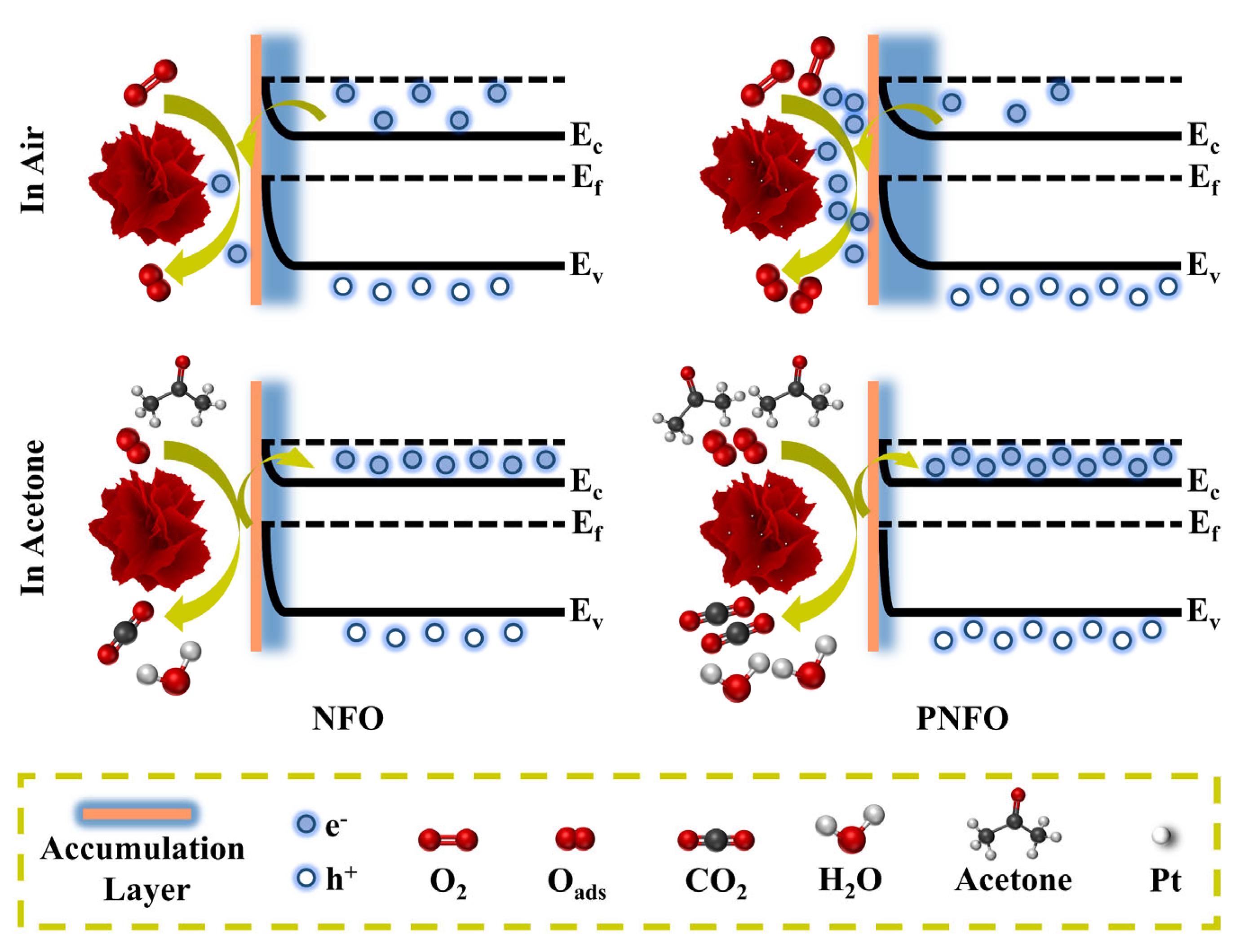
3.3. Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathur, M.; Verma, A.; Singh, A.; Yadav, B.C.; Chaudhary, V. CuMoO4 nanorods-based acetone chemiresistor-enabled non-invasive breathomic-diagnosis of human diabetes and environmental monitoring. Environ. Res. 2023, 229, 115931. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Sinha, R.; Nemade, H.B.; Mandal, T.K. Synthesis of MoS2-CuO nanocomposite for room temperature acetone sensing application. J. Alloys Compd. 2022, 910, 164891. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Chen, J.; Zhang, L.; Zhang, Y.; Pei, X.L. Pd-modified SmFeO3 with hollow tubular structure under light shows extremely high acetone gas sensitivity. Rare Met. 2023, 42, 545–557. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Song, Z.; Yang, X.; Wang, Z.; Zhang, H.; Guo, L.; Sun, C.; Liu, H.; Shao, J.; et al. Influence of nickel doping on ultrahigh toluene sensing performance of core-shell ZnO microsphere gas sensor. Chemosensors 2022, 10, 327. [Google Scholar] [CrossRef]
- Zhang, S.F.; Jiang, W.H.; Li, Y.W.; Yang, X.L.; Sun, P.; Liu, F.M.; Yan, X.; Gao, Y.; Liang, X.H.; Ma, J.; et al. Highly-sensitivity acetone sensors based on spinel-type oxide (NiFe2O4) through optimization of porous structure. Sens. Actuators B-Chem. 2019, 291, 266–274. [Google Scholar] [CrossRef]
- Ama, O.; Sadiq, M.; Johnson, M.; Zhang, Q.; Wang, D. Novel 1D/2D KWO/Ti3C2Tx nanocomposite-based acetone sensor for diabetes prevention and monitoring. Chemosensors 2020, 8, 102. [Google Scholar] [CrossRef]
- Sun, J.; Shi, D.; Wang, L.; Yu, X.; Song, B.; Li, W.; Zhu, J.; Yang, Y.; Cao, B.; Jiang, C. CRDS technology-based integrated breath gas detection system for breath acetone real-time accurate detection application. Chemosensors 2024, 12, 261. [Google Scholar] [CrossRef]
- Fan, X.; Yang, S.; Huang, C.; Lu, Y.; Dai, P. Preparation and enhanced acetone-sensing properties of ZIF-8-derived Co3O4@ZnO microspheres. Chemosensors 2023, 11, 376. [Google Scholar] [CrossRef]
- Cai, L.B.; Dong, X.Q.; Wu, G.G.; Sun, J.P.; Chen, N.; Wei, H.Z.; Zhu, S.; Tian, Q.Y.; Wang, X.Y.; Jing, Q.; et al. Ultrasensitive acetone gas sensor can distinguish the diabetic state of people and its high performance analysis by first-principles calculation. Sens. Actuators B-Chem. 2022, 351, 130863. [Google Scholar] [CrossRef]
- Arakawa, T.; Mizukoshi, N.; Iitani, K.; Toma, K.; Mitsubayashi, K. A bio-fluorometric acetone gas imaging system for the dynamic analysis of lipid metabolism in human breath. Chemosensors 2021, 9, 258. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.X.; Sun, C.X.; Huang, W.Z.; Cui, X.L. A review of non-invasive blood glucose monitoring through breath acetone and body surface. Sens. Actuators A-Phys. 2024, 374, 115500. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si:WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Lei, H.; Zhang, H.Y.; Xue, X.X.; Wang, X.P.; Wang, C.H.; Zhang, Y. High reliable gas sensor based on crystal-facet regulated α-Fe2O3 nanocrystals for rapid detection of exhaled acetone. Rare Met. 2024, 43, 6500–6515. [Google Scholar] [CrossRef]
- Ueda, T.; Torai, S.; Fujita, K.; Shimizu, Y.; Hyodo, T. Effects of Au addition to porous CuO2-added SnO2 gas sensors on their VOC-sensing properties. Chemosensors 2024, 12, 153. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Watanabe, S.; Osada, M.; Tajima, K.; Karashima, A.; Maruo, Y.Y. Small acetone sensor with a porous colorimetric chip for breath acetone detection using the flow–stop method. Chemosensors 2025, 13, 136. [Google Scholar] [CrossRef]
- Koo, A.; Yoo, R.; Woo, S.P.; Lee, H.-S.; Lee, W. Enhanced acetone-sensing properties of Pt-decorated Al-doped ZnO nanoparticles. Sens. Actuators B Chem. 2019, 280, 109–119. [Google Scholar] [CrossRef]
- Maji, B.; Singh, P.; Badhulika, S. A highly sensitive and fully flexible Fe-Co metal-organic framework hydrogel based gas sensor for ppb level detection of acetone. Appl. Surf. Sci. 2024, 678, 161047. [Google Scholar] [CrossRef]
- Wang, S.Y.; Apel, E.C.; Schwantes, R.H.; Bates, K.H.; Jacob, D.J.; Fischer, E.V.; Hornbrook, R.S.; Hills, A.J.; Emmons, L.K.; Pan, L.L.; et al. Global atmospheric budget of acetone: Air-sea exchange and the contribution to hydroxyl radicals. J. Geophys. Res. Atmos. 2020, 125, e2020JD032553. [Google Scholar] [CrossRef]
- Dong, Y.; Du, L.; Jiang, Y.; Wang, Y.; Zhang, J.; Wang, X.; Wei, S.; Sun, M.; Lu, Q.; Yin, G. In2O3-SnO2 hedgehog-like nanostructured heterojunction for acetone detection. Appl. Surf. Sci. 2024, 654, 159543. [Google Scholar] [CrossRef]
- Xing, X.X.; Chen, N.; Yang, Y.; Zhao, R.J.; Wang, Z.Z.; Wang, Z.D.; Zou, T.; Wang, Y.D. Pt-functionalized nanoporous TiO2 nanoparticles with enhanced gas sensing performances toward acetone. Phys. Status Solidi A-Appl. Mater. Sci. 2018, 215, 1800100. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Liu, D.; Lin, G.; Wan, J.W.; Jiang, H.; Lai, X.Y.; Hao, S.J.; Liu, X.H. Lychee-like ZnO/ZnFe2O4 core-shell hollow microsphere for improving acetone gas sensing performance. Ceram. Int. 2020, 46, 5960–5967. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.H.; Li, P.W.; Li, G.; Lian, K.; Zhang, W.D.; Zhuiykov, S.; Hu, J. Pr6O11-functionalized SnO2 flower-like architectures for highly efficient, stable, and selective acetone detection. IEEE Sens. J. 2018, 18, 933–940. [Google Scholar] [CrossRef]
- Jin, S.C.; Wu, D.; Song, W.A.; Hao, H.S.; Gao, W.Y.; Yan, S. Superior acetone sensor based on hetero-interface of SnSe2/SnO2 quasi core shell nanoparticles for previewing diabetes. J. Colloid Interface Sci. 2022, 621, 119–130. [Google Scholar] [CrossRef]
- Narang, S.B.; Pubby, K. Nickel spinel ferrites: A review. J. Magn. Magn. Mater. 2021, 519, 167163. [Google Scholar] [CrossRef]
- Rathore, D.; Kurchania, R.; Pandey, R.K. Nanoferrites gas sensors: A critical review. Sens. Actuators A-Phys. 2024, 379, 115968. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, Y.; Gou, H.; Zhang, W.; Yan, S.; Xu, X. Octahedral NiFe2O4 for high-performance gas sensor with low working temperature. Ceram. Int. 2018, 44, 2620–2625. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, B.; Wang, X.; Wang, C.; Sun, Y.; Zhang, H.; Shimanoe, K.; Lu, G. MOF-derived porous NiO/NiFe2O4 nanocubes for improving the acetone detection. Sens. Actuators B-Chem. 2022, 366, 131985. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Li, Z.; Chen, G.; Mao, Y.; Guan, H.; Dong, C. MOFs-derived NiFe2O4 fusiformis with highly selective response to xylene. J. Alloys Compd. 2019, 784, 102–110. [Google Scholar] [CrossRef]
- Liao, X.; Liu, X.; Wu, Y.; Qian, L.; Chen, Y.; Wang, Y.; Wu, Z.; Huang, A.; Liu, X.; Luo, J. Ni1-xZnxFe2O4 heterojunction composites derived from normal/inverse spinel structure for ppb-level acetone detection. Sens. Actuators B-Chem. 2025, 436, 137733. [Google Scholar] [CrossRef]
- Fu, Q.; Li, J.; Handschuh-Wang, S.; Zhou, X.; Liu, Y. Synergistic effect of NiFe2O4 and MWCNTs for ppb-level acetone detection at 150 °C. Sens. Actuators B-Chem. 2025, 425, 136948. [Google Scholar] [CrossRef]
- Xie, J.; Xie, T.; An, C.; Zhao, Y.; Wu, J.; Wu, X. Amorphous Pt-decorated NiFe2O4 nanorods for acetone sensing. ACS Appl. Nano Mater. 2024, 7, 28478–28485. [Google Scholar] [CrossRef]
- Lv, Y.K.; Yao, B.H.; Liu, Z.Q.; Liang, S.; Liu, Q.C.; Zhai, K.H.; Li, Z.J.; Yao, H.C. Hierarchical Au-loaded WO3 hollow microspheres with high sensitive and selective properties to toluene and xylene. IEEE Sens. J. 2019, 19, 5413–5420. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.Q.; Zeng, W. Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties. Appl. Surf. Sci. 2018, 427, 281–287. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, L.Z.; Zhang, C.S.; Xu, X.M.; Xie, Y.Y.; Chen, W.J.; Wang, J.; Zhang, R.Q. Promoting the generation of active oxygen over Ag-modified nanoflower-like α-MnO2 for soot oxidation: Experimental and DFT studies. Ind. Eng. Chem. Res. 2020, 59, 10407–10417. [Google Scholar] [CrossRef]
- Kim, T.; Lee, T.H.; Park, S.Y.; Eom, T.H.; Cho, I.; Kim, Y.; Kim, C.; Lee, S.A.; Choi, M.-J.; Suh, J.M.; et al. Drastic gas sensing selectivity in 2-dimensional MoS2 nanoflakes by noble metal decoration. ACS Nano 2023, 17, 4404–4413. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, Q.; Xie, Z.X.; Zheng, L.S. The effect of noble metal (Au, Pd and Pt) nanoparticles on the gas sensing performance of SnO2-based sensors: A case study on the {221} high-index faceted SnO2 octahedra. Crystengcomm 2015, 17, 6308–6313. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhao, S.; Bai, J.; Qi, Y.; Han, C.; Wei, D. Effect of noble metal elements on ethanol sensing properties of ZnSnO3 nanocubes. J. Alloys Compd. 2021, 887, 161409. [Google Scholar] [CrossRef]
- Gao, D.H.; Yu, Q.C.; Kebeded, M.A.; Zhuang, Y.Y.; Huang, S.; Jiao, M.Z.; He, X.J. Advances in modification of metal and noble metal nanomaterials for metal oxide gas sensors: A review. Rare Met. 2025, 44, 1443–1496. [Google Scholar] [CrossRef]
- Hanh, N.H.; Duy, L.V.; Hung, C.M.; Xuan, C.T.; Duy, N.V.; Hoa, N.D. High-performance acetone gas sensor based on Pt–Zn2SnO4 hollow octahedra for diabetic diagnosis. J. Alloys Compd. 2021, 886, 161284. [Google Scholar] [CrossRef]
- Chen, X.; Liu, T.; Ouyang, Y.; Huang, S.; Zhang, Z.; Liu, F.; Qiu, L.; Wang, C.; Lin, X.; Chen, J.; et al. Influence of different Pt functionalization modes on the properties of CuO gas-sensing materials. Sensors 2024, 24, 120. [Google Scholar] [CrossRef]
- Jolly Bose, R.; Illyasukutty, N.; Tan, K.S.; Rawat, R.S.; Vadakke Matham, M.; Kohler, H.; Mahadevan Pillai, V.P. Preparation and characterization of Pt loaded WO3 films suitable for gas sensing applications. Appl. Surf. Sci. 2018, 440, 320–330. [Google Scholar] [CrossRef]
- Yang, B.X.; Liu, J.Y.; Qin, H.; Liu, Q.; Jing, X.Y.; Zhang, H.Q.; Li, R.M.; Huang, G.Q.; Wang, J. PtO2-nanoparticles functionalized CuO polyhedrons for n-butanol gas sensor application. Ceram. Int. 2018, 44, 10426–10432. [Google Scholar] [CrossRef]
- Dong, J.Y.; Liu, H.Y.; Sun, C.X.; Shao, J.K.; Wang, M.J.; He, P.; Qi, Y.H.; Pan, G.F.; Yang, X.L. Low-temperature and high-efficient detection of triethylamine based on Pt/PtO2 loaded WO3 gas sensors. J. Alloys Compd. 2023, 966, 171642. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The same chemical state of carbon gives rise to two peaks in X-ray photoelectron spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Tran, P.K.L.; Nguyen, T.H.; Tran, D.T.; Dinh, V.; Ta, T.T.N.; Chung-Li, D.; Kim, N.H.; Lee, J.H. Tunable charge Pt sites-dominated alloy confined by mxene-derived oxycarbide enables ultra-stable ampere-level hydrogen production. Appl. Catal. B-Environ. Energy 2025, 363, 124801. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhang, J.L.; Zhang, W.Z.Z.; Wu, Z.C.; Gao, F. Pt nanoparticles/Fe-doped α-Ni(OH)2 nanosheets array with low Pt loading as a high-performance electrocatalyst for alkaline hydrogen evolution reaction. J. Alloys Compd. 2020, 823, 153790. [Google Scholar] [CrossRef]
- Zhu, G.H.; Wu, K.; Tan, L.; Wang, W.Y.; Huang, Y.P.; Liu, D.Y.; Yang, Y.Q. Liquid phase conversion of phenols into aromatics over magnetic Pt/NiO-Al2O3@Fe3O4 catalysts via a coupling process of hydrodeoxygenation and dehydrogenation. Acs Sustain. Chem. Eng. 2018, 6, 10078–10086. [Google Scholar] [CrossRef]
- Sharma, B.; Karuppasamy, K.; Srivastava, A.K.; Alfantazi, A.; Sharma, A. Highly sensitive and selective nanoengineered PtO2-BNNT heterostructures for ppb level ammonia gas sensing. Sens. Actuators B-Chem. 2024, 400, 134818. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, S.H.; Yang, L.; He, S.F.; Zhou, L.X.; Wang, M.J.; Gao, J.Y.; Hou, M.; Wang, J.; Komarneni, S. Enhanced free-radical generation on MoS2/Pt by light and water vapor co-activation for selective CO detection with high sensitivity. Adv. Mater. 2023, 35, 2303523. [Google Scholar] [CrossRef]
- Xue, L.X.; Ren, Y.; Li, Y.Y.; Xie, W.H.; Chen, K.Y.; Zou, Y.D.; Wu, L.M.; Deng, Y.H. Pt-Pd nanoalloys functionalized mesoporous SnO2 spheres: Tailored synthesis, sensing mechanism, and device integration. Small 2023, 19, 2302327. [Google Scholar] [CrossRef]
- Gandhi, A.C.; Wu, S.Y. Strong deep-level-emission photoluminescence in NiO nanoparticles. Nanomaterials 2017, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Boulahbel, H.; Benamira, M.; Bouremmad, F.; Ahmia, N.; Kiamouche, S.; Lahmar, H.; Souici, A.; Trari, M. Enhanced photodegradation of Congo red dye under sunlight irradiation by p-n NiFe2O4/TiO2 heterostructure. Inorg. Chem. Commun. 2023, 154, 110921. [Google Scholar] [CrossRef]
- Mrabet, C.; Jaballah, R.; Mahdhi, N.; Boukhachem, A.; Amlouk, M. Structural, optical, photoluminescence and sunlight photocatalytic properties of sprayed NiO-ZnO nanocomposite thin films. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2024, 300, 117130. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Miao, J.Y.; Zhang, H.X.; Wang, D.; Sun, J.B. Hollow cubic ZnSnO3 with abundant oxygen vacancies for H2S gas sensing. J. Hazard. Mater. 2020, 391, 122226. [Google Scholar] [CrossRef]
- Liu, L.B.; Tang, Y.F.; Liu, S.; Yu, M.L.; Sun, Y.F.; Fu, X.Z.; Luo, J.L.; Liu, S.B. Unraveling the trade-off between oxygen vacancy concentration and ordering of perovskite oxides for efficient lattice oxygen evolution. Adv. Energy Mater. 2024, 15, 2402967. [Google Scholar] [CrossRef]
- Huang, N.; Qu, Z.P.; Dong, C.; Qin, Y.; Duan, X.X. Superior performance of α@β-MnO2 for the toluene oxidation: Active interface and oxygen vacancy. Appl. Catal. A-Gen. 2018, 560, 195–205. [Google Scholar] [CrossRef]
- Jiayu, Z.; Lin, Y.; Zhihao, Z.; Chunran, Z.; Linjiang, F.; Xin, H.; Hongjun, L.; Leihong, Z.; Yiming, H. Preparation of NaNbO3 microcube with abundant oxygen vacancies and its high photocatalytic N2 fixation activity in the help of Pt nanoparticles. J. Colloid Interface Sci. 2023, 636, 480–491. [Google Scholar] [CrossRef]
- Zhong-Kang, H.; Wen, L.; Yi, G. Advancing the understanding of oxygen vacancies in ceria: Insights into their formation, behavior, and catalytic roles. JACS Au 2025, 5, 1549–1569. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, S.; Liu, Y.; Zhang, A.; Yao, Z.; Tian, Y.; Li, H.; Ning, Y.; Li, F.; Qu, F.; et al. Pt-modified BiVO4 nanosheets for enhanced acetone sensing. Sens. Actuators B-Chem. 2023, 389, 133853. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, H.; Li, Y.S.; Zhang, W.R.; Zhu, P.D.; Zhang, R.Y. Ultrahigh-acetone-sensitivity sensor based on Pt-loaded TiO2 porous nanoparticles synthesized via a facile hydrothermal method. Nanotechnology 2024, 35, 045502. [Google Scholar] [CrossRef]
- Guo, L.; Chen, F.; Xie, N.; Kou, X.; Wang, C.; Sun, Y.; Liu, F.; Liang, X.; Gao, Y.; Yan, X.; et al. Ultra-sensitive sensing platform based on Pt-ZnO-In2O3 nanofibers for detection of acetone. Sens. Actuators B-Chem. 2018, 272, 185–194. [Google Scholar] [CrossRef]
- Liu, C.; Gao, H.; Wang, L.; Wang, T.; Yang, X.; Sun, P.; Gao, Y.; Liang, X.; Liu, F.; Song, H.; et al. Facile synthesis and the enhanced sensing properties of Pt-loaded α-Fe2O3. porous nanospheres. Sens. Actuators B-Chem. 2017, 252, 1153–1162. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, H.; Liu, C.; Chen, C.; Xin, X. Porous NiO-WO3 heterojunction nanofibers fabricated by electrospinning with enhanced gas sensing properties. RSC Adv. 2017, 7, 40499–40509. [Google Scholar] [CrossRef]
- Jiang, L.; Tu, S.; Xue, K.; Yu, H.; Hou, X. Preparation and gas-sensing performance of GO/SnO2/NiO gas-sensitive composite materials. Ceram. Int. 2021, 47, 7528–7538. [Google Scholar] [CrossRef]
- Zhou, C.; Meng, F.; Chen, K.; Yang, X.; Wang, T.; Sun, P.; Liu, F.; Yan, X.; Shimanoe, K.; Lu, G. High sensitivity and low detection limit of acetone sensor based on NiO/Zn2SnO4 p-n heterojunction octahedrons. Sens. Actuators B-Chem. 2021, 339, 129912. [Google Scholar] [CrossRef]
- Lekshmi, M.S.; Suja, K.J. Enhanced acetone detection using ZnS-NiO heterojunction sensor for diabetes detection. Ceram. Int. 2024, 50, 23577–23585. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T.; Zeng, Y.; Zhang, R.; Lou, Z.; Deng, J.; Wang, L. Structure-driven efficient NiFe2O4 materials for ultra-fast response electronic sensing platform. Sens. Actuators B-Chem. 2018, 255, 1436–1444. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, Y.; Tian, X.; Liang, Q.; Liu, X.; Sun, Y. p-p heterojunction composite of NiFe2O4 nanoparticles-decorated NiO nanosheets for acetone gas detection. Mater. Lett. 2020, 270, 127728. [Google Scholar] [CrossRef]
- Sun, Y.H.; Huang, Q.T.; Liu, Y.F.; Gu, Z.Q.; Bo, L.B.; Zheng, X.H. Fabrication of NiO nanosheet/NiFe2O4 nanoparticle sensor through one-step hydrothermal method for acetone detection. J. Electrochem. Soc. 2025, 172, 017517. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, T.T.; Huo, L.H.; Gao, S.; Li, B.S.; Guo, C.Y.; Yu, H.X.; Major, Z.; Zhang, X.F.; Cheng, X.L.; et al. Small size porous NiO/NiFe2O4 nanocubes derived from Ni-Fe bimetallic metal-organic frameworks for fast volatile organic compounds detection. Appl. Surf. Sci. 2023, 623, 157075. [Google Scholar] [CrossRef]
- Wang, P.; Guo, S.S.; Zhao, Y.N.; Hu, Z.X.; Tang, Y.T.; Zhou, L.C.; Li, T.K.; Li, H.Y.; Liu, H. WO3 nanoparticles supported by Nb2CTx MXene for superior acetone detection under high humidity. Sens. Actuators B-Chem. 2024, 398, 134710. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Hillie, K.T.; Tshabalala, Z.P.; Jozela, M.; Tshilongo, J.; Motaung, D.E. Enhanced propanol gas sensing performance of p-type NiO gas sensor induced by exceptionally large surface area and crystallinity. Appl. Surf. Sci. 2022, 571, 151121. [Google Scholar] [CrossRef]
- Meng, F.; Yang, J.; Wang, Y.-n.; Zhang, R.; Yuan, Z. Acetone gas sensor based on spinel-structured hollow sea urchin-like core–shell ZnO/ZnFe2O4 spheres. ACS Appl. Nano Mater. 2024, 7, 16066–16074. [Google Scholar] [CrossRef]
- Liang, X.; Fu, S.Y.; Song, X.Y.; Yao, Y.; Gao, N.; Zhang, J. Enhanced acetone gas sensing performance of SnO2/Zn2SnO4 heterojunctions incorporated with MXene: Experimental and theoretical insights. J. Alloys Compd. 2025, 1020, 179489. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.Y.; San, X.G.; Shen, Y.B.; Wang, G.S.; Zhang, L.; Meng, D. Ti3C2Tx/SnO2 P-N heterostructure construction boosts room-temperature detecting formaldehyde. Rare Met. 2024, 43, 267–279. [Google Scholar] [CrossRef]
- He, X.X.; Chai, H.F.; Zhou, Y.W.; Liu, K.W.; Yu, Z.X.; Zhang, C. Sensing properties and mechanisms of LaF3-Co3O4 nanorods for low-concentration methanol detection. Rare Met. 2024, 43, 2193–2204. [Google Scholar] [CrossRef]
- Li, L.L.; Diao, Q.; Liu, Z.K.; Zhu, G.X.; Jiao, M.L.; Liu, C.; Du, M.L.; Shi, Y.S.; Wang, Y.L.; Cheng, P.F.; et al. Preparation of Co3O4 by tunable oxygen defect engineering and its application in acetone gas sensor. Microchem. J. 2025, 212, 113481. [Google Scholar] [CrossRef]
- Kaixuan, F.; Yun, S.; Lizhe, Y.; Na, J.; Chunfeng, S.; Degang, M.; Xuebin, L.; Rui, H.; Qingling, L. Pt loaded manganese oxide nanoarray-based monolithic catalysts for catalytic oxidation of acetone. Chem. Eng. J. 2022, 432, 134397. [Google Scholar] [CrossRef]
- Zhaoxia, S.; Gongke, L.; Yufei, H. Cataluminescence sensor based on Pt/NU-901 nanocomposite for rapid capture, catalysis and detection of acetone in exhaled breath. Anal. Chim. Acta 2022, 1206, 339787. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.L.; Chen, T.X.; Lu, Q.X.; Rehman, S.U.; Zhu, L. Rationally designed mesoporous In2O3 nanofibers functionalized Pt catalysts for high-performance acetone gas sensors. Sens. Actuators B-Chem. 2019, 298, 126871. [Google Scholar] [CrossRef]
- Qiu, C.K.; Wang, L.; An, F.; Zhang, H.; Li, Q.R.; Wang, H.Z.; Li, M.J.; Guo, J.Y.; Jia, P.L.; Liu, Z.W.; et al. One-dimensional hollow porous Ru-CuO nanofibers covered with ZIF-71 for H2S gas sensing and its first-principle study. Rare Met. 2025, 44, 1170–1181. [Google Scholar] [CrossRef]
- Sanam, P.K.J.; Shah, M.D.; Pradyumnan, P.P. Enhanced thermoelectric properties in dual cation doped CuCrO2 nanocrystals mediated by magnon-carrier drag. Mater. Res. Bull. 2023, 164, 112244. [Google Scholar] [CrossRef]
- Zeb, S.; Yang, Z.; Hu, R.M.; Umair, M.; Naz, S.; Cui, Y.; Qin, C.Y.; Liu, T.Y.; Jiang, X.C. Electronic structure and oxygen vacancy tuning of Co & Ni co-doped W18O49 nanourchins for efficient TEA gas sensing. Chem. Eng. J. 2023, 465, 142815. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Yang, M.; Zhang, X.F.; Cheng, X.L.; Zhou, X.; Gao, S.; Xu, Y.M.; Huo, L.H. Three-in-one Ni doped porous SnO2 nanorods sensor: Controllable oxygen vacancies content, surface site activation and low power consumption for highly selective NO2 monitoring. Sens. Actuators B-Chem. 2023, 382, 133550. [Google Scholar] [CrossRef]


| Materials | Working Temperature (℃) | Concentration (ppm) | Response (Ra/Rg or Rg/Ra) | Response/Recovery Time (s) | Selectivity | Platinum Loading Mass Fraction (wt %) | Ref. |
|---|---|---|---|---|---|---|---|
| Pt-BiVO4 | 300 | 100 | 12.5 (Ra/Rg) | 2/61 | 2.33 | 0.476 | [59] |
| Pt-TiO2 | 260 | 200 | 42.5 (Ra/Rg) | 8/11 | 1.93 | 2.03 | [60] |
| Pt-ZnO-In2O3 | 300 | 100 | 57.1 (Ra/Rg) | 1/44 | 1.89 | —— | [61] |
| Pt-α-Fe2O3 | 220 | 100 | 27.2 (Ra/Rg) | 1/46 | 1.7 | 0.611 | [62] |
| 5 wt % Pt/NFO | 180 | 100 | 221 (Ra/Rg) | 12/13 | 5.45 | 5 | [31] |
| NiO-WO3 | 375 | 100 | 22.5 (Ra/Rg) | 6/11 | 2.5 | —— | [63] |
| GO-SnO2-NiO | 350 | 100 | 33.5 (Ra/Rg) | 4/130 | —— | —— | [64] |
| NiO-Zn2SnO4 | 300 | 100 | 49.8 (Ra/Rg) | 1/60 | 2.2 | —— | [65] |
| ZnS-NiO | 225 | 50 | 36 (Rg/Ra) | 26.31/16.7 | 1.5 | —— | [66] |
| NiFe2O4 core-shell spheres | 280 | 100 | 10.6 (Rg/Ra) | 1/7 | 3.03 | —— | [67] |
| NiO/NiFe2O4-1.5 | 200 | 100 | 19.9 (Rg/Ra) | 2.4/19.6 | 1.54 | —— | [27] |
| NiFe2O4-NiO composites | 280 | 50 | 23 (Rg/Ra) | —— | 1.28 | —— | [68] |
| NiO/NFO-A | 170 | 50 | 45 (Rg/Ra) | 55/261 | 1.91 | —— | [69] |
| NiO/NiFe2O4 nanocubes | 170 | 100 | 150.3 (Rg/Ra) | 12.8/15.6 | 1.17 | —— | [70] |
| PNFO-1.5 | 200 | 100 | 58.33 ± 1.13 (Rg/Ra) | 7/245 | 2.56 | 1.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Sun, Z.; Su, Y.; Sun, C.; Wang, P.; Yang, S.; Yang, X.; Pan, G. Platinum-Functionalized Hierarchically Structured Flower-like Nickel Ferrite Sheets for High-Performance Acetone Sensing. Chemosensors 2025, 13, 234. https://doi.org/10.3390/chemosensors13070234
Yang Z, Sun Z, Su Y, Sun C, Wang P, Yang S, Yang X, Pan G. Platinum-Functionalized Hierarchically Structured Flower-like Nickel Ferrite Sheets for High-Performance Acetone Sensing. Chemosensors. 2025; 13(7):234. https://doi.org/10.3390/chemosensors13070234
Chicago/Turabian StyleYang, Ziwen, Zhen Sun, Yuhao Su, Caixuan Sun, Peishuo Wang, Shaobin Yang, Xueli Yang, and Guofeng Pan. 2025. "Platinum-Functionalized Hierarchically Structured Flower-like Nickel Ferrite Sheets for High-Performance Acetone Sensing" Chemosensors 13, no. 7: 234. https://doi.org/10.3390/chemosensors13070234
APA StyleYang, Z., Sun, Z., Su, Y., Sun, C., Wang, P., Yang, S., Yang, X., & Pan, G. (2025). Platinum-Functionalized Hierarchically Structured Flower-like Nickel Ferrite Sheets for High-Performance Acetone Sensing. Chemosensors, 13(7), 234. https://doi.org/10.3390/chemosensors13070234







