Abstract
The electronic tongue (ET) and bioelectronic tongue (bioET) technologies have emerged as innovative and promising tools for the characterization and quality control of complex liquid matrices such as grape musts and wines. These multisensor systems, based on electrochemical detection and chemometric analysis, provide global and rapid information about taste-related attributes, antioxidant content, and other critical parameters, offering an alternative or complement to traditional analytical methods. This review explores the principles, development, and applications of ET and bioET in the wine industry, highlighting their capacity to assess grape ripeness, monitor fermentation, determine wine aging, detect adulterations, and support geographical and varietal authentication. Special attention is paid to advances in sensing materials—such as conducting polymers, metal nanoparticles, and enzymes—and the construction techniques of sensors and biosensors, which have improved ET performance. Finally, the potential of these technologies as cost-effective, portable, and on-site tools aligns with the demands of Industry 4.0 and next-generation smart agriculture and food production systems.
1. Introduction
The concept of electronic tongues (ETs or e-tongues) or taste sensors has grown rapidly during recent years due to their large potential. They are based on electrochemical sensors combined with multivariate data analysis. The development of new methods of analysis to characterize food is of vital importance for improving the current quality control systems of food products. According to the IUPAC (International Union of Pure and Applied Chemistry), an electronic tongue is a multisensor system, which consists of a number of low selective sensors and uses advanced mathematical procedures for signal processing based on the pattern recognition (PARC) and/or multivariate analysis [artificial neural networks (ANNs), principal component analysis (PCA), etc.] [1]. Therefore, ETs are holistic systems that provide global and qualitative information about the sample instead of quantitative data about specific compounds. However, if the data matrix obtained by such multisensor systems is analyzed with adequate chemometric processing tools, descriptive or predictive information of particular parameters could be extracted [2,3]. There is a most recent term in the field of electronic tongues widely named bioelectronics tongue (bioET), which includes the use of one or several biosensors implemented in the ETs [4,5].
This review examines how ETs and bioETs can be applied to study red grapes and wines in order to better predict the harvesting time of grapes and quality parameters of interest to produce high-quality wines. Grapes, the primary fruit used in wine production, are rich in essential nutrients and antioxidants. They contain a variety of vitamins, including vitamin C and K, as well as minerals like potassium and magnesium. Grapes are also known for their high content of polyphenols, such as tannins and anthocyanins, which have been linked to potential health benefits, including heart health and anti-inflammatory properties. The process of wine production begins with the cultivation and harvesting of grapes. Once the grapes are harvested, they undergo crushing and fermentation. During fermentation, yeast converts the sugars in the grapes into alcohol, producing wine. The length of fermentation, as well as factors like temperature and the presence of oxygen, significantly influence the flavor and quality of the wine. After fermentation, the wine is often aged in various types of containers such as stainless-steel tanks, wooden barrels, or concrete vats, each imparting unique organoleptic characteristics to the final product. The aging process can range from a few months to several years, and the wine may undergo additional steps such as filtration and bottling before being ready for consumption. In addition to the traditional red and white wines, sparkling wines, rosé, and dessert wines offer diverse flavors and styles, depending on the grape variety, region of cultivation, and winemaking techniques. The diversity of grapes and the winemaking process contribute to the wide array of wines available on the global market.
Routine analytical tests from the harvesting of grapes to the final bottled end-product include a number of varying chromatographic techniques, as well as more traditional ones (spectroscopy, photometry, enzymatic assays, etc.). These analytical techniques are combined to provide an in-depth study of the composition of wines, leading to analyses that are very expensive, time-consuming, and demand highly qualified personnel. It is therefore necessary to develop new technologies that allow us to obtain information concerning the spectrum of compounds, including global information about the sample instead of information about specific components [6]. ETs can become an alternative or complement to the traditional methods of wine and grape analysis that could be used, not only in the analytical laboratories, but also in situ as a quality control tool for the farmers and wine growers in the vineyards.
In addition, nowadays, we are immersed in the fourth revolution of the manufacturing industry, also called Industry 4.0 (or smart factory, cloud-based manufacturing, factory of the future, smart manufacturing, and digital manufacturing). This big manufacturing revolution will demand benefits from joining multidisciplinary competences related to new sensing technologies, big data, cloud computing, artificial intelligence, adaptive robots, smart valves, and autonomous smart control applications to bring maximum added value in future smart processes. Existing technologies exploited by measurement, control, and automation systems are mainly based on measuring physical parameters. The next big improvement will be to deploy real-time chemical information from the measurement objects to an automation database and use it in automated control decisions. Spectroscopic technologies are well-established in off-line analysis in chemical laboratories, but it is obvious that future smart factories will gain huge benefits when qualitative and quantitative material information can be generated with affordable and reliable sensors. Real-time chemical sensors will open up a new era of process control in several industries. Industrial processes of food and beverages will benefit more and more in the future from next-generation smart sensor platforms [7,8]. In this context, recent studies have demonstrated the potential of electronic tongues in detecting wine spoilage up to four weeks before human sensory panels can identify off-flavors. These devices utilize advanced sensor technologies to monitor chemical changes in wine, offering winemakers an early-warning system to prevent spoilage and maintain product quality. Such advancements exemplify how next-generation sensor platforms are poised to revolutionize quality control in the food and beverage industry [9,10]. Sensor array technologies for real-time monitoring are being used for quality control in the food and beverage industry, improving production processes [11]. For instance, they have been used for detecting changes in chemical and organoleptic profiles of wines during fermentation and aging processes [12,13].
This review stands out from previous works by offering a broad and integrated perspective on electronic tongue (ET) and bioelectronic tongue (bioET) technologies, covering their historical development, key pioneering researchers (such as Toko, Vlasov, and Winquist), and the evolution of their design and use in food analysis. Unlike earlier reviews that are either too general or limited to specific technical or analytical aspects, this article provides a comprehensive account of both technological principles and practical applications in the wine industry. In addition, it compiles and analyzes a wide range of relevant studies, highlighting successful implementations of ETs in real wine and grape analysis. Special attention is given to their role in quality control, authentication, and process monitoring, but also to the major challenges of ETs and bioETs in wine analysis.
Why Look for Other Analytical Methods in Wine Analysis?
Wine, one of the oldest alimentary products known for more than 8000 years, is an alcoholic beverage obtained through the fermentation of grapes. The origin of viticulture dates back to 6000–5000 BC in Georgia and Iran. Winemaking culture still remains unchanged: modern wine is very similar to what our ancestors knew, while modern viticulture and oenology practices still refer to those of the ancient Greeks, preserved and later evolved by the Roman Empire [14].
The wine matrix has one of the most complex compositions, with more than 800–1000 volatile compounds ranging from alcohols, esters, acids, ketones, ethers, aldehydes, terpenes, lactones, phenolic compounds, etc., that arise from the grape and/or are formed during the fermentation process, with concentrations from a few parts per billion to a few percent in weight [14,15,16,17]. The abundance of these compounds depends on many factors: grape variety and origin, viticulture practices (blending, aging, yeast used during fermentation), production treatment, storage, and oxygen exposure [17,18,19].
From the analytical point of view, wine analysis is a challenging task due to the complexity of the mixture; moreover, minor differences in the concentration of certain compounds lead to wines with completely different organoleptic characteristics. Furthermore, the synergy between groups of compounds often has a stronger influence on the organoleptic characteristics than individual compounds [17].
Over the last few years, food control has become more appreciated by consumers. They have a right to expect that the foods they consume will be safe and of high quality. The term food control includes a large number of factors such as: safety, setting standards for toxicological and microbiological hazards, implementing procedures and practices to ensure those standards; nutrition, ensuring the necessary nutrient levels and formulation in foods that contribute to a healthy diet; quality, which provides the expected sensory characteristics in foods, such as taste, aroma and/or appearance; and finally value, giving the consumers utility and economic advantages. Some of these factors, such as value, are exclusively in the domain of industry and consumers, whereas others, such as safety, are shared interests of government, industry, and consumers [20].
Although there are several methods to determine quality parameters of interest in the wine industry, they are usually long, tedious, and require very expensive laboratory equipment and qualified personnel. Moreover, the quality control that evaluates the organoleptic properties of wines, such as flavor, taste and color, is usually tested by a trained, expert human panel (wine-tasters) turning out a strong subjective element in the results that are influenced by their skills, mood and/or physical state [14]. Moreover, the number of tests that panelists can perform is limited to a few assessments per day, due to the saturation of tongue receptors. As time is a critical factor in the food industry, it is necessary to develop new, simple, and fast analytic tools able to assess antioxidant activity and organoleptic properties, as well as the authentication of wines and grapes, and geographic origin verification. These factors are becoming very important due to wine regulations, which include rules concerning the marketing, production, designations of origin and geographical indications, among others, becoming more restrictive than in the past with the final objective of protecting consumers in order to supply beverages and food that are safe, of good quality and exempt from fraud.
On the other hand, analytical tools for determining grape and wine characteristics are critical for oenologists. The standard analytical methods of alcoholic beverages, including wine, are based on traditional chemical analysis and on wet chemistry procedures or instrumental methods, involving such separation techniques as high-performance liquid chromatography (HPLC) or gas chromatography (GC) combined with mass spectrometry (MS), and such optical techniques as Fourier transform infrared spectroscopy (FTIR) [17,21,22,23].
A variety of new analytical tools are increasingly used to profile the volatile, non-volatile, and elemental composition of grapes and wines in order to analyze aroma, taste, color, mouthfeel, and even for authentication. Methods range from classical chemical analysis through to the use of modern analytical instrument techniques. For example, some improvements in standard analytical tools that have been implemented are: gas chromatography combined with tandem mass spectrometry (GC-MS/MS), which offers significant advantages for trace quantification of aroma-active volatiles and contaminant compounds [24,25]; the ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC/Q-TOF-MS) technique is a new approach in the chromatographic separations used for profiling non-volatiles and for varietal, geographic and vintage authentication purposes [26,27]; inductively coupled plasma mass spectrometry (ICP-MS) has been used to analyze metals that affect the chemical stability and oxidative reactions in wines [28]; nuclear magnetic resonance spectroscopy (NMR) has become popular for characterizing and profiling primary and secondary metabolites [29,30]; isotope ratio mass spectrometry (IRMS) is applied to detect the authentication of wines [31]; while, in connection with methods using IRMS, stable isotope ratio analysis (SIRA), based on the measurement of the ratios of the stable isotopes of carbon (13C/12C), hydrogen (2H/1H), and oxygen (18O/16O) within molecules and between different molecules, is one of the most powerful analytical tools for the authentication of wine [32,33]; and finally, gas chromatography combined with olfactometry (GC-O) for the characterization of aroma qualities of complex mixtures [34].
Table 1 collects the analytical methods, including both classical and modern, traditionally used in the analytical chemistry of wine [24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Although these techniques are sophisticated and provide accurate results, they only give information on individual wine components, but they cannot give a global assessment of the flavor or quality of wines that are produced by the synergistic interaction between several chemical compounds present in the complex chemical wine matrix [15,17]. Moreover, the equipment used is costly, large, requires calibration procedures, sample preparation, and the process is time-consuming and needs skilled and qualified personnel.

Table 1.
Overview of classical chemical analysis and modern analytical instrument techniques usually applied to test wines.
In order to reduce the cost and time-consumption of the previously described analytical procedures, electronic tongues (e-tongues) have emerged as a new analytical tool to assess a global, rapid, and easy response with information concerning parameters related to the quality and organoleptic properties of grapes and wines, such as the phenolic content. E-tongues based on electrochemical sensors (potentiometric, amperometric, voltammetric, or impedimetric) have been developed and used in food quality control [6,38,39,40]. In this sense, electrochemical techniques can lead to advantages in analytical chemistry due to their higher sensitivity and relatively low cost in comparison with the chromatography and spectroscopic methods, and they can be used as an alternative and/or complementary tool to the standard methods of analysis [41,42].
In addition, compared to conventional tasting panels, electronic tongues have the clear advantage that they can be applied in continuous flow for quality control in industrial processes. Whereas an expert taster cannot evaluate samples on a continuous basis. Table 2 shows the main advantages of electronic tongues over the use of tasting panels, traditional chemical analysis (gas or liquid chromatography, spectroscopy techniques, etc.), and modern analysis with high-generation equipment (GC-MS/MS, UHPLC/Q-TOF-MS, ICP-MS, SIRA).

Table 2.
Advantages of electronic tongues over other methodologies.
The development of new analytical tools includes the manufacture of portable devices conceived for use by wine farmers in situ in the vineyards. In this way, wine farmers could test their grapes with a simple, rapid experiment in the vineyard in order to decide the optimal point of maturity for harvesting grapes with no need to test samples in the oenological laboratory.
Electronic tongue technology has already been successfully applied to analyze such beverages as wines, beers, and strong alcoholic beverages (spirits and liqueurs) as well as to evaluate the quality of non-alcoholic beverages [43,44,45,46].
In Figure 1, the trend in the number of publications related to electronic tongues (ETs) and bioelectronic tongues (bioETs) over the past two decades is shown. Over the past several years, there has been a steady increase in the number of publications related to electronic tongues and bioelectronic tongues, particularly since 2011. This upward trend reflects a growing scientific interest in these technologies. However, the number of publications specifically focused on the application of ETs and bioETs in the wine industry remains relatively limited, although it has shown a consistent presence since 2008, with a notable peak in 2016. This graph suggests that while the potential of these technologies in the wine sector is being explored, there is still significant room for further research and development.
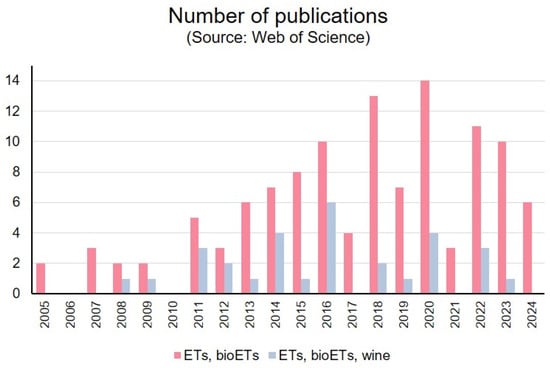
Figure 1.
Number of publications on ET and BioET in the wine industry published in the past few years (source: database Web of Science). Topics: Electronic tongue, bioelectronic tongue, and wine.
2. Electronic Tongues Technology
The main taste evaluation method in the food industry currently consists of a sensory analysis in which experienced evaluators, called sensory panelists, test the samples for evaluation [47,48,49,50,51,52]. However, in wine tastings, these analyses have some drawbacks, such as low objectivity and reproducibility, saturation of tongue receptors, as well as the high stress imposed on the panelists. Moreover, cross-mouth contamination between samples can compromise the validity of the sensory results [6,14,53,54].
A new sensory technology has been developed to evaluate the taste of food and beverages in an objective way. It is called the electronic tongue, and it consists of a multisensor system of low selective sensors and uses advanced mathematical procedures for signal processing based on pattern recognition and/or multivariate analysis [1]. The concept of the electronic tongue was first developed by Toko, Vlasov, and Winquist [55,56,57], although the first works concerning these systems were published mainly by Toko and Hayashi using the term multichannel taste sensors [58,59,60].
Electronic tongues are systems inspired to detect taste in a similar manner to the human gustatory sensation [61]. The human tongue is equipped with different receptors (taste buds) able to distinguish thousands of substances. The information on taste substances provided by the receptors is transduced into an electric signal and sensory nerves are responsible for transmitting it to the brain, and then, our biological computer, trained with knowledge acquired earlier, is able to identify what we eat and also perceive information about the quantity of individual compounds (such as the amount of salt or sucrose). The performance of the taste system is attributed to the capability of taste buds to respond to different stimuli, called cross-selectivity, and to the ability to analyze the signal from all the tongue receptors at once. These two qualities (cross-selectivity and multivariate analysis) are the key concepts involving electronic tongue systems. In electronic tongues, sensors play the role of taste receptors, and the brain is substituted by a computer equipped with appropriate data-processing techniques (Figure 2) [6].
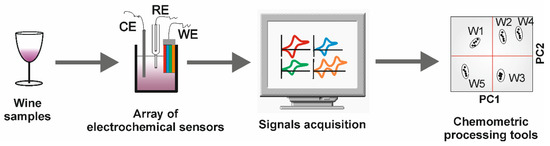
Figure 2.
Schematic presentation of the electronic tongue system. (In the WE, each color corresponds with an electrochemical sensor, which gives its corresponding signal, different from the others).
As stated before, the IUPAC defines an electronic tongue as a multisensor system, which consists of a number of low selective sensors and uses advanced mathematical procedures for signal processing based on the pattern recognition (PARC) and/or multivariate analysis artificial neural networks (ANNs), principal component analysis (PCA), etc. [1]. In addition, many research groups working in this topic have summarized the definition of an electronic tongue as an analytical instrument comprising an array of non-specific, poorly selective chemical sensors with partial specificity (cross sensitivity) to different compounds in a solution, coupled to an appropriate chemometric tool for data processing [62,63,64].
Although the concept of chemical sensors is generally used to detect a chemical substance at high sensitivity, human taste receptors do not necessarily recognize individual chemical substances. Each of the receptors for the five basic tastes simultaneously receives multiple chemical substances, showing a semi-selective response. It is practically impossible to measure the taste of foods containing hundreds of taste substances by chemical analysis methods, such as gas or liquid chromatography, although these techniques can measure the concentration of chemical substances. Therefore, in electronic tongues, sensors do not give the amounts of specific molecules that contribute to a certain taste, but rather the taste quality and intensity, in other words, a global evaluation of the analyzed matrix [1,6]. Moreover, a taste sensor should be able to measure such interactions between taste substances as the synergistic effect and the suppression effect [38]. The discrimination of each chemical substance is not an important task here, but recognition of the taste itself is, and its quantitative expression is necessary [56,61,62].
Arrays of sensors in electronic tongues combine non-specific chemical sensors with partial sensitivity that provides responses with cross-sensitivity. The difference in the electrical response of different sensors serves as a fingerprint for the analyzed sample. When combining these systems with pattern recognition software, we use the fingerprints of different samples to discriminate or classify them.
In the 1990s, while research was being carried out on the biological and molecular processes involved in taste receptors, sensitive technology was also being developed for objective evaluation in the discrimination and/or quantification of tastes, prior to the discoveries of taste receptors. Thus, in 1989, Toko and co-workers applied for a patent for their potentiometric taste sensor. They developed a taste sensor equipped in a multichannel electrode system using an immobilized lipid membrane with a polymer as transducer [60]. This taste sensor is considered to be the first electronic tongue with global selectivity and has been applied to the analysis of numerous beverages such as beer, sake, coffee, tea, or milk, among others [62].
After this first electronic tongue, and up to the present time, numerous papers have been published on this technology, mainly applied to the analysis of food and beverages, as it is considered a novel technology that can contribute greatly to quality purposes. Thus, in the last few years, the design and application of these new systems have become very popular. One of the most important research groups in this field, which can also be considered a pioneer in the design of electronic tongues, is Winquist and co-workers. In 1997, Winquist and Lundström developed an electronic tongue based on voltammetric techniques for the analysis of fruit juices, beverages, and milk. They were able to detect the aging process of milk and orange juice with the developed system [57,65]. Following this work, in 2000, they combined potentiometry, voltammetry, and conductivity measurements to produce a hybrid electronic tongue for the analysis of fermented milk [66]. Selective ion electrodes were used to measure pH, carbon dioxide, and chlorine ion concentration. The electronic tongue consisted of six working electrodes of different metals. In 2002, Winquist and collaborators developed a measurement system based on the flow injection analysis technique applied to a voltammetric electronic tongue for the analysis of different standard solutions and for the classification of different apple juices [67].
Another research group dedicated to the design of electronic tongues since the 1990s is led by Prof. Vlasov, who, together with the group of Prof. Di Natale and Paolesse, has made important advances in the development of potentiometric electronic tongues. In 1997, this group applied solid-state selective ion potentiometric sensors based on chalcogenide glass in an electronic tongue to detect heavy metal ions in solutions [68], in the analysis of beverages (tea, beer, coffee, juices, soft drinks, etc.) [69], and polluted waters [70]. Later, between 2003 and 2007, they published some works in which these electrodes were applied to the analysis of mineral waters, wines, and alcoholic beverages [71,72].
One of the first works based on a voltammetric electronic tongue based on chemically modified electrodes was developed in 2003–2004 in the Laboratory of Inorganic Chemistry and Physical Chemistry of the University of Valladolid, directed by Prof. Rodríguez-Méndez. This electronic tongue was based on sensors prepared with graphite powder mixed with phthalocyanine, and it was applied to the analysis of wine [73,74]. There are currently several international research groups of experts in electronic tongues that have made important advances in the design and application of these systems in the field of food and beverages [44,46,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90].
In the case of the wine industry, it is important to monitor changes occurring during grape ripening, wine fermentation, and aging to ensure the quality of the wines, to assess the origin and authentication, and to avoid falsifications or adulterations. Traditional techniques for analyzing food and beverages, as well as human tasting panels, have some drawbacks and need new analytical tools. In this sense, electronic tongues are reliable and reproducible analytical tools, which have demonstrated their utility for the rapid and inexpensive assessment of different chemical matrices, including wines.
3. Principles of Detection: Electrochemical Methods
The heart of an electronic tongue is the array of sensors that captures physical or chemical signals from its surroundings and converts them into electrical signals. The sensors used in electronic tongues are mainly optical, electrochemical, and gravimetric. In food analysis applications, the most commonly used are the electrochemical sensors (potentiometric, amperometric, voltammetric, and impedimetric), due to their simple instrumentation, high sensitivity, fast response, and ease of operation [91,92,93].
In potentiometric sensors, the voltage is measured at null current. A working electrode covered with a membrane is introduced into the sample to be analyzed. The potential is created at the membrane/solution interface, which is needed to retain the balance of the electrochemical process and depends on the nature of the electrode material and the composition of the solution. The advantages of potentiometric electronic tongues are the well-known principle of operation, low cost, ease of commercial production, and possibility of obtaining selective sensors. Their disadvantages are the influence of the temperature on the measurements and the adsorption of solution components on the electrode surfaces that can modify the potentials [38]. These sensors can be prepared from different materials, membranes, and techniques [93,94].
In voltammetric/amperometric sensors, the measurements are based on the electric current established between the working electrode and the counter electrode in an electrochemical cell, whereas the potential is measured between the working and the reference electrode. In voltammetric sensors, a polarization voltage is applied while the current is measured. When a potential change occurs, the current flows through the working electrode, thus oxidizing or reducing the compound under analysis. The response current is the result of the electrochemical reaction that occurs at the electrode/electrolyte interface layer. Voltamograms show peaks associated with the oxidation and reduction of electroactive species from the solution, and the intensity is a function of the analyte concentration. Moreover, voltammograms can show peaks associated with the electroactive material deposited on the electrode surface. In amperometric sensors, voltammetry is applied as a preliminary step to study the oxidation potentials of the analyte, and once established, amperometric measurements are carried out to measure the electrical current between the electrodes as a function of a constant potential applied to the working electrode. The current intensity signal from the oxidation or reduction of the substance analyzed is proportional to its concentration. These sensors are sensitive, very versatile, and different materials can be used in the electrodes for sensing. Moreover, using voltammetric sensors, different techniques can be applied (cyclic voltammetry, linear sweep voltammetry, differential pulse voltammetry, or square-wave voltammetry) [93,95,96,97]. The main disadvantage of such sensors is the lack of selectivity.
The impedimetric sensors, which are considered part of the group of electrochemical methods, are based on impedance spectroscopy measurements. The main advantage over the previous sensors is that there is no need for a reference electrode. In this method, the impedance is measured at one constant frequency or for a frequency spectrum. This system can be described by an equivalent electrical circuit, in which the electrostatic double-layer formed at the electrode/electrolyte interface governs the response at low frequencies, the solution conductance and ultrathin films coating the electrodes rule the total impedance at intermediate frequencies, and the geometric capacitance is most relevant at high frequencies. The advantage of these sensors is their high sensitivity [38,93]. They could be employed in an electronic tongue; however, in practice, the works reporting impedimetric electronic tongues are quite rare compared with potentiometric, amperometric, or voltammetric systems.
Table 3 shows some examples of electrochemical electronic tongues based on potentiometric, amperometric, voltammetric, and impedimetric sensors applied to food analysis. Hybrid systems have also been developed, mainly combining voltammetric and potentiometric sensors.

Table 3.
Examples of electrochemical electronic tongues applied to food analysis.
4. Distinct Materials for the Sensing Units
Sensing materials used for electrochemical sensors can be classified as: (1) materials for the electrode and supporting substrate, (2) materials for improving electroanalytical performances, (3) materials for the immobilization of biological recognition elements, and (4) biological elements, the last two being applicable to electrochemical biosensors. Sensing units of traditional electronic tongues are mainly lipid membranes, ion-selective electrodes, or noble metals functioning as working electrodes, with the voltage difference measured between the sensing unit and a reference electrode [38]. The choice of materials for the sensing units is crucial to obtain high performance. Even though e-tongues do not require specific interactions with the analyte, the sensing units need to respond electrically to small changes in the liquid under analysis. Moreover, depending on the method of detection, materials with electrical conductivity and/or electroactivity might be required. Materials used for the electrode and supporting substrate are usually conductive materials exhibiting low currents in an electrolyte solution, free of any electroactive species, over a relatively wide potential window. Although these sensing materials play different roles in different electrochemical sensing systems, depending on their properties, their basic functions are mainly grouped as: (1) enhancement of electron transfer, (2) catalysis of electrochemical reactions, (3) immobilization of biomolecules, (4) labeling biomolecules, and (5) acting as reactant.
Lipid membranes were one of the first classes of materials used in potentiometric sensors for e-tongues to mimic the materials of the human tongue [56]. In addition, chalcogenide glasses were largely used in electrochemical measurements due to the ease of electrode preparation and cross-sensitivity [70]. However, due to the extensive use of electrochemical methods, research began to explore electroactive materials. Among the most frequently used materials for the electrode and supporting substrate, the following may be mentioned: phthalocyanines, porphyrins, metal nanoparticles, metal oxide nanoparticles, carbon-based materials, and conducting polymers.
In recent years, conducting polymers (CPs), which consist of organic π-conjugated systems, have been employed in electrochemical sensors due to their light weight, workability, resistance to corrosion, low cost, and excellent electrical, mechanical, optical, and conducting properties [127,128]. CPs are generally semi-conductive in nature and have low conductivity, but can be made more conductive by doping. This means that the oxidation and reduction of a π-conjugated system generate p- and n-doped CPs. The doping process can be controlled by chemical and electrochemical methods. Therefore, to overcome their low conductivity, composites of CPs have been extensively studied and applied in electrochemical applications [127]. Conducting polymers such as polypyrrole, polyaniline, PEDOT -poly(3,4-ethylenedioxythiophene)-, or polythiophene have been used as sensing materials to construct electrochemical sensors [129,130,131,132,133,134].
Other sensing materials for electrochemical sensors are metallophthalocyanines (MPCs) and metalloporphyrins (MPs), which are N4-macrocyclic metal complexes. They contain N4-ligands that co-ordinate to the metal ions. In their nature, the N4-ligands of phthalocyanine and porphyrin are 18-π conjugated ring systems with a highly conjugated structure. Metal ions are from the transition metals, group I–V metals, lanthanides, and actinides. These MPc and MP complexes have a very rich electrochemistry owing to their ability to accept and donate electrons, either from central metal ions or 18-π-conjugated ring systems [135,136]. Phthalocyanines and porphyrins have been extensively used as sensing materials in electrochemical sensors due to their remarkable electrochemical and electrocatalytic properties [137,138,139,140,141,142,143,144].
Important advances have been made recently in the field of sensors thanks to the introduction of new platforms for sensor design, such as nanotechnological materials and nanostructured architectures (i.e., nanoparticles, carbon nanotubes and nanofibres, graphenes, nanostructured surfaces, etc.), which have improved their sensitivity. Many kinds of nanoparticles, including metal nanoparticles, oxide nanoparticles, semiconductor nanoparticles, and even composite nanoparticles, have been widely used in electrochemical sensors and biosensors. These nanoparticles exhibit unique optical, electrical, thermal, and catalytic properties. Nanoparticles with different sizes and compositions are now available for electroanalysis applications. Metal nanoparticles have shown excellent conductivity and catalytic properties, which make them suitable for acting as “electronic wires” to enhance the electron transfer between redox centers in proteins and electrode surfaces and as catalysts to increase electrochemical reactions. Oxide nanoparticles are often used to immobilize biomolecules due to their biocompatibility, while semiconductor nanoparticles are often used as labels or tracers for electrochemical analysis [145,146,147,148,149]. The use of nanoparticles applied to electrochemical sensors has been widely reported [145,146,147,148,149,150,151,152,153].
The combination of more than one sensing material to make composite materials can show excellent sensing and catalytic properties. The advantage of the synergistic interaction between different sensing materials produces a higher performance of electrochemical sensors. This effect has been observed in the interaction between gold nanoparticles and nickel phthalocyanine [154]. A synergetic effect between silver-gold nanoparticles and zinc phthalocyanine has also been demonstrated in the electrocatalytic reduction of O2 on vitreous carbon electrodes [155]. Gold nanoparticles-ZnO composite is one of the most widely discussed electrocatalytic materials with high catalytic activity, optical sensitivity, universal biocompatibility, and high chemical stability [156]. Pt nanoparticles and PANI aero-gel have demonstrated a synergetic effect that results in the immobilization of a high density of GOx for an efficient oxidation of glucose [157].
Biochemical sensors are currently being successfully introduced into electronic tongue technology (bioelectronic tongues) by adding enzymes as a biorecognition element to the electrode surfaces. The biorecognition elements are classified into biological (enzymes, whole cells, cell organelles, tissue) and artificial (biomimetic) receptors (aptamers, ribosymes, and molecularly imprinted polymers (MIPs)) [42]. Biosensors based on enzymes combine the advantages of classical electrochemical sensors, which provide high sensitivity and cross-selectivity, with the typical specificity of biosensors [158,159]. In these biosensors, the selectivity for the target analyte is mainly determined by the biorecognition element, whereas the sensitivity of the biosensor is greatly influenced by the transducer [42]. Therefore, the electron transfer between the active sites of the enzyme and the electrode surface can be improved through an electronic mediator, such as the above-mentioned sensing materials, acting as an electrical wire. Specific enzymes for the detection of phenols (e.g., tyrosinase, laccase, or peroxidase) or sugars (e.g., glucose oxidase or fructose dehydrogenase), among others, have been incorporated into bioelectronic tongues [42,85,145,146,148,158,159,160].
5. Sensor and Biosensor Construction
Electrochemical sensors can be chemically modified with sensing materials by means of several techniques to improve their electroanalytical performance. E-tongue technology covers different types of sensors and biosensors, from classical sensors prepared by means of simple methods (carbon paste electrodes) [73,74] or simple techniques (casting, electrodeposition, etc.) [161,162,163,164,165] to sophisticated and costly nanostructured sensors, including techniques of preparation such as self-assembled monolayer (SAM), Layer by Layer (LbL), or Langmuir–Blodgett (LB) [166,167,168,169,170,171,172].
Therefore, the major challenge in the field of electrochemical sensors, consisting in the improvement of their electroanalytical performance, as already mentioned, was addressed by using nanotechnology, which includes not only the use of sensing nanomaterials, such as graphene oxide (GO), reduced graphene oxide (rGO), and gold nanoparticles, which improve conductivity, surface area, and biocompatibility, thereby enhancing sensitivity and selectivity, but also the preparation of nanostructured architectures and the use of miniaturized electrodes, including microelectrodes and nanoelectrodes, which offer advantages like enhanced mass transport and reduced double-layer capacitance, leading to faster response times and improved sensitivity. Sensors based on thin films at the nanoscale show a high surface-to-volume ratio, small dimensions, and enable the detection of low-concentration analytes with high precision. The high surface ratio facilitates the interaction between the sensing material and the analyte, which leads to improvements in the sensitivity, and the small dimensions enable fast adsorption and desorption kinetics for the analyte in the sensing material, and therefore, provide a rapid response time [173].
5.1. Classical Methods
Carbon paste-based methods: sensors are prepared by mixing a carbon-based material (graphite, carbon nanotubes, graphene, carbon nanoparticles) with the sensing material and a mineral oil. The carbon matrix acts as a support to prevent agglomeration and provides a large surface area, high mechanical strength, fast electron transfer rate, excellent thermal and electrical conductivities, which in turn enhance the electrocatalytic activity. These sensors are cheap, easy to prepare, and have the great advantage that they can be easily recovered by cleaning the surface with a filter paper [73,74].
Coating-based methods: drop-casting, dip-coating, or spin-coating are the most commonly used coating methods for modifying electrodes with nanomaterials. These methods are easy and low-cost in sensor manufacture and do not require complicated instruments. However, only soluble or solution-processible nanomaterials can be deposited onto an electrode surface [174].
Direct deposition-based methods: these methods are diverse and include electrochemical, electrospinning, electrospray, sputtering, and vapor deposition. Electrochemical deposition is one of the most used methods to deposit thin films of polymers or polymer composites onto conducting electrode surfaces. This technique can be performed at room temperature and is carried out using three electrodes dipped into the solution at a potential (or current, depending on the method used) that is applied to polymerize the monomer and/or reduce metal ions in the simultaneous preparation of polymer composites with metal nanoparticles [174].
Printing-based methods: these methods include the well-known screen-printed method as well as newly developed methods, such as inkjet printing, nozzle-jet printing, or the laser-scribing process. Printing-based methods have been used to deposit materials onto rigid and flexible substrates to make devices on a large scale at low cost. One advantage of these methods is that they allow nanomaterials to be deposited directly onto predesigned patterns [174].
5.2. Methods Based on Nanostructured Architectures
Self-assembled monolayer (SAM): this technique is used to form monomolecular films by the spontaneous adsorption of amphiphilic adsorbates in an orderly manner onto a solid support. It has been widely used to coat electrode surfaces. The initial driving force for the assembly is the chemical affinity between the adsorbates and the substrate. In the initial stage of SAMs, mainly alkyl compounds, such as alkylated thiols and silanols, were used to construct well-organized SAM structures. However, there are various functional groups that can be used to generate SAMs [175].
Langmuir–Blodgett (LB): these films are formed by the deposition of a Langmuir film onto a solid substrate. Langmuir films are composed of an organized monolayer of amphiphilic molecules at a liquid/gas interface. These molecules, with their hydrophobic tail and hydrophilic heads, are spontaneously oriented at a liquid/gas interface. Unlike the SAM technique, the LB method can provide multi-layered film structures with defined thickness and sequence, together with well-ordered molecular arrangement and orientation [175].
Layer by Layer (LbL): these films are formed by depositing onto a substrate alternating layers of oppositely charged materials. This technique has been given much attention as a versatile method for nanostructured films. Most of the LbL assemblies are based on electrostatic interactions between the layered materials. However, LbL assemblies can be driven by other interactions, such as hydrogen bonding, metal interactions, charge transfer complexation, or bio-specific recognition, among others. A wide range of materials can be applied in the assembling process, such as polymers, biomaterials, supramolecular assemblies, and inorganic substances. This method only requires the use of beakers and tweezers in the procedure [175].
5.3. Methods Used for Biosensor Construction
Biomaterials can be incorporated into sensors to enhance specificity. In enzymatic biosensors, the immobilization of enzymes to construct biosensors depends on the materials and procedure used and can include: (1) entrapment behind a permeable membrane as a thin film covering the electrochemical detector; (2) entrapment within a conducting polymeric matrix or sol-gel; (3) entrapment within self-assembled monolayers or within LbL layers; (4) immobilization onto an LB structure; (5) covalent bonding on membranes or surfaces activated by means of bifunctional groups or spacers (glutaraldehyde, carbodiimide, silanization, etc.); (6) bulk modification of the electrode material (graphite epoxy resin or modified carbon paste, clays, zeolites, nanomaterials, organic polymers, complex compounds, etc.) [85,140,151,160,175,176,177,178,179].
Electrochemical enzymatic biosensors have evolved over three main generations. First-generation biosensors detect changes in molecules such as O₂, H⁺, or H₂O₂, which are directly related to the enzyme activity. In the second generation, electron mediators are added to the sensing layer to facilitate electron transfer between the biochemical reaction and the electrode, increasing efficiency. The third generation takes a step further by enabling direct electron transfer (DET) between the enzyme and the electrode, eliminating the need for mediators and simplifying the signal transduction mechanism [180].
6. Methods of Data Analysis
The correct utilization of e-tongues requires an appropriate data analysis. The main task of these systems is to classify the samples under study [6,38,93]. As the number of samples may be very large and many measurements are needed to distinguish between very similar samples—whose variability may also be considerable for complex liquids such as wines, juices, etc.—an enormous and complex amount of data is generated that must be processed using chemometric or pattern recognition methods. Four stages can be distinguished during data analysis: signal pre-processing, dimensionality reduction, prediction, and validation [6]. Data pre-processing is carried out mainly to scale the data, discard outliers, and extract representative parameters. Subsequently, a dimensionality reduction of variables is needed in many cases; for example, when using cyclic voltammetry, since the information obtained is large and complex. Then, one solution is to simplify the high dimensionality, maintaining the meaningful information from the data and suppressing redundant and non-significant information without loss of information by using a feature extraction stage, such as Legendre polynomials, Fourier transform, kernel method, wavelet transformation, or genetic algorithms. Depending on the type of application, different methods for dimensionality reduction can be applied. The most used process in the literature for e-tongues is principal component analysis (PCA), a non-supervised method used to discriminate between samples with different characteristics. PCA plots can be obtained in 2 or 3 dimensions (2D or 3D), depending on the need to distinguish the samples. This tool reduces the number of variables to new latent variables (principal components) in a reduced variable space to facilitate identification or classification. In addition, this new space simplifies the interpretation of the variability contained in the raw information. Therefore, PCA is a very powerful non-supervised linear pattern recognition method that reduces the dimensionality of multivariate data and helps to visualize the different categories of multivariate profiles with high similarities and differences between sample clusters.
When the number of classes of samples is very large, for instance, if one wishes to distinguish between a set of dozens of wines, the number of points placed leads to overcrowding the 2D or 3D PCA plot. Hence, other methods to treat the data are required [38]. In this context, methods involving artificial neural networks (ANNs), parallel factor analysis (PARAFAC), soft independent modelling of class analogy (SIMCA), linear discriminant analysis (LDA), or support vector machines (SVM) are used.
The low dimensional feature may also be used for prediction and/or regression purposes, where the sensors respond as independent variables and a second set of variables (e.g., chemical parameters of the samples) as dependent variables are used to establish regression models that will be able to predict and validate some parameters of the analyzed samples. These models are usually established using partial least squares (PLS), principal component regression (PCR), or support vector machine regression (SVMR), among others.
7. Summary of the Applications
Electronic tongues have several applications in various industrial areas, mainly in the pharmaceutical and food industries. In the pharmaceutical industry, it has been widely used to quantify the taste-making efficiency of medicines, as well as to analyze the stability of medicines regarding taste [181,182]. In addition, e-tongues have also been applied to the field of medicine for non-invasive diagnostics (urine, sweat, saliva, etc.) or clinical analysis [183,184], and in the field of the environment to analyze water contamination [185,186].
In the food industry, this new technology has been used in quality control during production, in food storage stages, in the control of aging processes, in the analysis of tastes, to evaluate authentication, to identify brand and/or geographical origin, or to detect adulterations (see Table 3).
Given the advantages and numerous applications of electronic tongue technology, many efforts have been made to make miniaturized and commercial prototypes of electronic tongue devices at a low cost. In this field, the main issues to overcome are miniaturization and the integration with existing silicon technology for microelectronics [38]. The first commercial electronic tongues, SA402B and TS-5000Z Taste Sensing Systems, based on 7 potentiometric electrodes with lipid-polymeric membranes, were developed by Toko and co-workers (Intelligent Sensor Technology Inc., Tokyo, Japan) [56,62]. These devices have been used to determine and quantify the intensity of taste in food products and pharmaceuticals [187,188,189,190,191].
Another commercial e-tongue is Astree II (Alpha MOS, Toulouse, France), which is based on seven ion-selective field effect transistors (ISFETs) and has been applied to quality control, food recognition, taste assessment, process monitoring, and the pharmaceutical industry [191,192,193,194,195]. Other existing commercial systems, although their applications are rather scarce compared with those already mentioned, are: the Multiarray Chemical Sensor (McScience Inc., Seoul, Republic of Korea) built of PVC and polyurethane membranes selective to ions (H+, Na+, Ca2+, NH4+, NO3−, Cl−) [196] and Sensor System (St. Petersburg, Russia) comprising seven potentiometric ion-selective sensors [197].
7.1. Electronic Tongues in the Wine Industry
The wine production process comprises a series of steps that need to be carefully managed, and for which continuous checking and measurements are required. Electronic tongues have been applied to the study of grapes, musts, and wines in different stages of the winemaking process.
The chemical composition of wines has a direct influence on their organoleptic properties. Each wine has a different chemical composition that depends on the variety and state of ripeness of the grapes, the extraction of different components in the must and the reactions that occur during the winemaking process, the post-fermentation treatment, and the aging of wine. The ripeness and quality of the grapes used to elaborate wines are usually established by their external appearance or the taste of the grape juices. The analysis of sugar and phenolic content is also a common practice to establish the quality [15,63].
Electronic tongues have been applied to discriminate between grapes according to their variety and vintage [85]. After crushing, during maceration, the contact between the must and the skins increases the concentration of phenols. Moreover, by applying different pressing techniques, the extraction of phenols can be increased. E-tongues have been used to study the increase in phenol concentration after using Flash Release and micro-oxygenation pressing techniques [198].
The fermentation of fresh grape juice or must transforms sugars into alcohols, producing wine. In white wines, only one fermentation is needed, which is the alcoholic fermentation producing ethanol, whilst red wines need two fermentation processes, the alcoholic fermentation followed by the malolactic fermentation, where malic acid is transformed into lactic acid. Here, the control by means of chemical sensors is quite difficult because fermentation is a turbulent process that produces changes in temperature that affect the performance of the sensors. For this reason, only a few papers have described the use of e-tongues to monitor wine fermentation. Using infrared spectroscopy, combined with an e-nose and e-tongue, the kinetics of the fermentation process of musts has been analyzed, and acceptable correlations with sugar consumption and alcohol production have been found [77].
After the fermentation of the grape must, the final product, the wine, is obtained. It is then aged in oak barrels for 1–2 years before bottling. This stage has a great influence on the organoleptic characteristics that wine acquires, influenced by the retention and release of volatile compounds from the lees during ageing. The oxygen that diffuses through the small pores of the barrels, the origin of the oak wood, and its degree of toasting also influence the final taste of the wine. Subsequently, the wine continues to be aged in bottles in a reducing environment that further improves its organoleptic properties. Electronic tongues have been applied to discriminate red wines according to their ageing [199] and the origin of oak barrels and toasting level [98,200].
In addition, as the ageing stage in barrels requires a lot of time and high costs, innovative techniques are applied to accelerate the ageing. One of these techniques consists of adding pieces of wood of different sizes to stainless steel tanks with built-in micro-oxygenation. An electronic tongue has been applied to discriminate between wines aged by traditional techniques and wines aged with alternative practices [100].
After ageing, the wines are bottled. Traditionally, the bottles are sealed with stoppers made of natural cork, ideal for sealing the liquid while allowing oxygenation from the outside through small pores. Traditional natural cork stoppers are associated with high-quality wines. Nowadays, the wine industry uses synthetic stoppers, made of polymers, with a degree of porosity. The effect of oxygen transfer in synthetic closures has been studied using an electronic tongue [99,198].
The organoleptic properties of final wines produced with different varieties of grapes, vintage, appellation, etc., have been evaluated with e-tongues. The grapes used to make wine will determine both the taste and the final organoleptic characteristics of the product. There are many grape varieties, and each winery uses different oenological techniques to produce different styles of wine with different flavors and characteristics. An electronic tongue has been used to discriminate wines with different Spanish appellations (Rioja, Rueda, Ribera de Duero) [73] and, in addition, this e-tongue was also used to determine differences in these wines from vintage to vintage [97]. A voltammetric e-tongue based on screen-printed electrodes has been implemented to evaluate red wines elaborated with different grape varieties from different regions and with different aging. Here, the results were compared with FTIR analysis to determine the phenolic content using these two multiparametric methods based on different working principles (electrochemical signals and vibrational spectroscopy) [201]. In addition, an impedimetric electronic tongue has been used to discriminate red wines with similar characteristics (same region, vintage, and ageing method) [202]. Bioelectronic tongues have also been used to analyze cava wines [203] or to discriminate grape juices prepared with different varieties of grape [85]. Moreover, a bioelectronic tongue based on combinations of enzymes (tyrosinase and glucose oxidase) and polypyrrole (Ppy) or polypyrrole/gold nanoparticles (Ppy/AuNP) composites was built up and applied to the analysis and discrimination of musts and wines [204]. Weather conditions or improper storage, among other variables, can spoil the final wine. E-tongues have been used to monitor the spoilage of wines by monitoring their acidity levels [205].
Finally, e-tongues have been used to evaluate possible adulterations or frauds in wines [100,206]. Some adulteration practices consist of dilution with water, blending with wine of lower quality or made with grapes from a different region, the use of prohibited ageing techniques, and the addition of ethanol or other substances (tartaric acid, tannic acid, SO2, acetic acid, or sucrose).
7.2. Major Challenges of ETs and bioETs in Wine Analysis
As stated before, the major challenge in the field of electrochemical sensors is to improve the electrochemical performance of the sensors. This issue is being overcome using nanotechnology. However, there are other challenges that should be considered in the field of electronic tongues. For example, the use of CPE can be advantageous because of its ease of preparation, low cost, renewability, and versatility in chemical modification, but there remain some shortcomings or limitations, including poor reproducibility, limited mechanical stability, surface fouling, and restricted long-term durability. Recent advances in material science and electrode engineering have provided effective strategies to overcome many of these issues. One of the primary concerns with CPEs is batch-to-batch variability, due to manual preparation and inhomogeneous mixing of graphite and binder (typically paraffin oil). However, this issue has been substantially addressed through the use of automated mixing systems and well-defined carbon allotropes such as graphene, carbon nanotubes (CNTs), and ordered mesoporous carbons. It has been demonstrated that incorporating MWCNTs (Multi-Walled Carbon Nanotubes) into CPEs improves reproducibility and surface homogeneity, thereby enhancing the analytical electrode performance [207]. Regarding the mechanical and chemical stability (especially in aggressive media), it has been improved by introducing polymeric binders, composite matrices, or nanomaterials. The use of ionic liquids as binders in CPEs helps to enhance the electrode stability and reduce surface fouling, durability, and also the stability and sensitivity at higher temperatures [208,209]. Furthermore, the integration of conductive polymers such as polyaniline or polypyrrole into the carbon paste matrix offers mechanical robustness and enhanced electrochemical response [210,211]. Additionally, to combat surface fouling, especially in complex biological or environmental matrices, self-cleaning or renewable surface strategies have been developed. The use of electrochemical pretreatment and in-situ surface renewal via gentle polishing allows for quick regeneration of the active surface. Moreover, functionalization with anti-fouling agents like Nafion or polyethylene glycol derivatives can significantly reduce surface passivation. Another frequently noted limitation is the short lifespan and limited stability over time. This is mitigated by storing the electrodes under controlled conditions and employing pre-packaged, standardized paste compositions, which have been shown to retain activity for extended periods [212].
Also, the integration into modern sensing platforms (such as screen-printed or miniaturized electrode arrays) has helped CPEs evolve into reliable, portable systems. Advances in 3D printing and microfluidics have also allowed for more precise and reproducible fabrication methods, opening new avenues for the use of CPEs in point-of-care diagnostics and environmental sensing. As a result, CPEs remain a viable and increasingly reliable choice for a wide range of electrochemical applications [213].
On the other hand, the development of coating-based electrodes, especially for electrochemical sensing, often faces significant challenges when transitioning from lab-scale to large-scale production. While drop-casting techniques are widely used at the laboratory level due to their simplicity and cost-effectiveness, they suffer from issues related to reproducibility and precision, making scaling up difficult. The manual nature of the drop-casting process introduces variability in film thickness, surface coverage, and electrode homogeneity, which ultimately affects the consistency and reliability of the sensors [214]. To assess the possibility of large-scale production, several approaches can be considered, such as the automation of coating processes with techniques such as paper-based methods, 3D printed techniques, and microfluidic electrochemical methods, which offer higher reproducibility and precision compared to manual drop-casting [215]. These methods allow for better control over film thickness, uniformity, and electrode-to-electrode consistency, which are critical for scaling production. These processes can also be adapted for mass production and integrated into automated manufacturing lines, making them feasible for large-scale fabrication. Moreover, it is essential to achieve quality control and standardization in large-scale production, implementing strict protocols and processes to meet the required specifications.
Another challenge to overcome is the biofouling and chemical fouling of electrodes, resulting in higher background currents, low accuracy, and low sensitivity and reproducibility [216]. Since grapes and wines contain large amounts of phenolic compounds, the passivation of the surface electrode can occur due to the polymerization of phenolic compounds on the surface during electrochemical measurements. Several strategies have been explored to mitigate these effects. The electrode surface modification with antifouling materials such as Nafion, polyethylene glycol (PEG), or ionic polymers can create barriers that resist non-specific adsorption and reduce passivation [216]. Moreover, the integration of nanomaterials or nanostructured materials like carbon nanotubes (CNTs), graphene, or metal nanoparticles can enhance electron transfer, increase surface area, and reduce fouling through more efficient catalytic and mass transport properties [217]. The use of renewable or disposable electrodes, such as screen-printed electrodes (SPEs), allows for easy replacement or regeneration, minimizing the long-term effects of fouling without requiring complex cleaning steps. Electrochemical pretreatments or pulsed potential cleaning are also implemented, where a periodic application of cleaning potentials can remove fouling layers and refresh the electrode surface in situ during measurements [218]. In biosensors, using selective membranes or immobilizing enzymes that are less susceptible to fouling by phenolics can also help maintain long-term functionality [180,219].
Bioelectronic tongues (bioETs), which integrate biological elements such as enzymes, cells, tissues, or receptors with electronic transducers, show great promise in complex sample analysis. However, their application in wine quality assessment remains limited due to several critical challenges focused on both the complexity of the wine matrix, the instability of biological components, the reproducibility, shelf-life, and their commercialization. On one hand, wine is a chemically complex beverage, rich in polyphenols, organic acids, sugars, alcohols, and volatile compounds. This complexity can interfere with the biorecognition elements, leading to signal suppression, biofouling, or enzymatic inhibition. Moreover, biological substances such as enzymes are highly sensitive to environmental factors (pH, temperature, and oxidative conditions prevalent in wine samples), which can result in diminished sensor performance over time. The reproducibility of the bioETs is also an issue of importance due to variations in the biological components and their interactions with the wine matrix can lead to inconsistent results, affecting the reproducibility and shelf-life of the biosensing systems. Finally, the scalability of bioETs from laboratory prototypes to commercially viable products requires addressing issues related to manufacturing consistency, cost-effectiveness, and user-friendliness. To overcome these challenges, several approaches have been explored [78,180,220]. These include advanced immobilization techniques, employing methods such as covalent bonding, entrapment in polymeric matrices (e.g., sol-gel, alginate), and cross-linking agents to enhance the stability of the immobilized biocomponents. These techniques can help in maintaining the functional integrity of the biological elements over extended periods. The employment of nanomaterials like AuNPs can also improve electron transfer rates and provide a more conductive environment for enzyme activity, thereby enhancing sensor sensitivity and longevity. Another strategy is to improve durability of bioETs using more stable biological components, such as genetically engineered enzymes or microbial cells with higher resistance to environmental stressors and/or applying protective coatings and membranes such as Nafion, which can prevent the biorecognition elements from interfering substances in wine, reducing fouling and extending sensor life. Finally, the storage of bioETs under controlled conditions (e.g., low temperatures, inert atmospheres) is crucial to preserve the activity of the biological components, thereby prolonging the shelf-life of the devices.
Finally, the application of ET and bioET in food analysis is often challenged by the lack of standardized calibration procedures. This issue arises because food matrices are highly complex and variable in composition, which makes it difficult to develop universal calibration standards that apply across different sample types. In electronic tongues, which rely on cross-sensitive sensor arrays and pattern recognition, the absence of well-defined calibration standards can hinder quantitative analysis and limit reproducibility across different studies or instruments. Since ET systems are trained on empirical data, they often require extensive samples, specific training, and recalibration, which restricts their broader applicability. In biosensing systems, which integrate biological recognition elements such as enzymes, antibodies, or aptamers, the challenge is compounded. These biosensors can be highly selective, but their performance is affected by matrix interferences and may vary with storage, sample type, or environmental conditions. Without standardized calibration protocols, it becomes difficult to validate sensor performance or compare results between different biosensor platforms. To overcome these limitations, there is a growing need for the development of synthetic or representative calibration standards for key food matrices; the use of internal standards or matrix-matched calibration; cross-laboratory validation protocols, and the integration of machine learning models to compensate for matrix variability in ET/bioET [196,221].
8. Conclusions
Electronic tongue (ET) and bioelectronic tongue (bioET) technologies have shown considerable potential as rapid, reproducible, and cost-effective analytical tools for the assessment of grape and wine quality. Compared to traditional analytical techniques and sensory panels, these systems offer significant advantages, including reduced analysis time, lower cost, ease of use, and the possibility of on-site implementation, making them highly attractive for applications in viticulture and enology. Their ability to discriminate between grape varieties, geographical origins, vintages, and aging methods, as well as to monitor fermentation and detect adulterations, positions them as valuable tools in both quality control and authentication processes.
However, the widespread implementation of ET and bioET technologies still faces several key challenges. One of the main limitations lies in the reproducibility and long-term stability of the sensor systems, as well as the surface fouling, mechanical degradation, and variability in preparation. Recent advances in materials science, such as the incorporation of carbon nanotubes, ionic liquids, and conductive polymers, have improved sensor performance, but further standardization in fabrication methods is necessary to ensure batch-to-batch consistency and robustness under real operating conditions.
BioETs, while offering enhanced selectivity due to the incorporation of biological recognition elements (e.g., enzymes), also present additional complications. The stability of these bioelements is often compromised by the physicochemical complexity of wine matrices, which include phenolic compounds, ethanol, and fluctuating pH levels that may interfere with enzymatic activity. Strategies such as covalent immobilization, encapsulation within protective matrices, and the use of genetically engineered enzymes are being explored to mitigate these effects. Furthermore, the integration of nanomaterials like gold nanoparticles, graphene, or metal oxide nanostructures has demonstrated the potential to improve electron transfer rates, reduce biofouling, and enhance sensor sensitivity.
Looking ahead, future perspectives for ET and bioET technologies include the development of miniaturized, portable, and fully automated sensing platforms, suitable for real-time, in-field applications. The integration of these devices into Industry 4.0 frameworks—incorporating wireless communication, cloud computing, and machine learning for data processing—will be crucial to their scalability and industrial adoption. Moreover, the establishment of universal calibration standards and inter-laboratory validation protocols will be essential to improve reproducibility and facilitate regulatory acceptance. Continued interdisciplinary collaboration—linking materials science, bioengineering, analytical chemistry, and data science—will be key to overcoming current limitations and enabling the transition of these technologies from laboratory prototypes to widely adopted tools in smart agriculture and food production systems.
Author Contributions
C.G.-H.: Writing—original draft, Writing—review & editing. C.G.-C.: Writing—review & editing, Funding acquisition. M.L.R.-M.: Writing—review & editing, Supervision, Project administration, Funding acquisition. F.M.-P.: Writing—review & editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
We appreciate the financial support of MINECO-FEDER Plan Nacional (PID2021-122365OB-100). Junta de Castilla y Leon-FEDER VA202P20. BIOECOUVA-CLU-2019-04, and Interreg España–Portugal Programme (POCTEP), grant 0039_MINORSENS_2_E.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Kirsanov, D.; Mednova, O.; Vietoris, V.; Kilmartin, P.A.; Legin, A. Towards reliable estimation of an “electronic tongue” predictive ability from PLS regression models in wine analysis. Talanta 2012, 90, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, P.; Casolino, M.C.; Forina, M. Chemometric brains for artificial tongues. Adv. Food Nutr. Res. 2010, 61, 57–117. [Google Scholar]
- Bachmann, T.T.; Schmid, R.D. A disposable multielectrode biosensor for rapid simultaneous detection of the insecticides paraoxon and carbofuran at high resolution. Anal. Chim. Acta 1999, 401, 95–103. [Google Scholar] [CrossRef]
- Tonning, E.; Sapelnikova, S.; Christensen, J.; Carlsson, C.; Winther-Nielsen, M.; Dock, E.; Solna, R.; Skladal, P.; Norgaard, L.; Ruzgas, T.; et al. Chemometric exploration of an amperometric biosensor array for fast determination of wastewater quality. Biosens. Bioelectron. 2005, 21, 608–617. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, M.L. Electronic Noses and Tongues in the Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Travassos Rosário, A.; Carmo Dias, J. How Industry 4.0 and Sensors Can Leverage Product Design: Opportunities and Challenges. Sensors 2023, 23, 1165. [Google Scholar] [CrossRef]
- LePree, J. Smart Sensors Enable Industry 4.0. 1 May 2019. Available online: https://www.chemengonline.com/smart-sensors-enable-industry-4-0/ (accessed on 1 April 2025).
- Potter, R.I.; Warren, C.A.; Lee, J.; Ross, C.F. Comparative assessment of Riesling wine fault development by the electronic tongue and a sensory panel. J. Food Sci. 2024, 89, 3006–3018. [Google Scholar] [CrossRef]
- Paup, V.D.; Cook-Barton, T.; Diako, C.; Edwards, C.G.; Ross, C.F. Detection of red wine faults over time with flash profiling and the electronic tongue. Beverages 2021, 7, 52. [Google Scholar] [CrossRef]
- Cho, S.; Shakir Moazzem, M.D. Recent applications of potentiometric electronic tongue and electronic nose in sensory evaluation. Prev. Nutr. Food Sci. 2022, 27, 354–364. [Google Scholar] [CrossRef]
- Gabrieli, G.; Muszynski, M.; Ruch, P.W. A reconfigurable integrated electronic tongue and its use in accelerated analysis of juices and wines. arXiv 2022, arXiv:2205.15018v1. [Google Scholar]
- Kutyła-Olesiuk, A.; Wesoły, M.; Wróblewski, W. Hybrid electronic tongue as a tool for the monitoring of wine fermentation and storage process. Electroanalysis 2018, 30, 1983–1989. [Google Scholar] [CrossRef]
- Branchini, C.G.; Lvova, L.; Di Natale, C.; Paolesse, R. Wine and combined electronic nose and tongue. In Electronic Noses and Tongues in Food Science; Rodríguez Méndez, M.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 301–307. [Google Scholar]
- Rodríguez Méndez, M.L.; De Saja, J.A.; Medina-Plaza, C.; García-Hernández, C. Electronic tongues for the organoleptic characterization of wines. In Electronic Noses and Tongues in Food Science; Rodriguez Mendez, M.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 265–273. [Google Scholar]
- Vilanova, M.; Genisheva, Z.; Graña, M.; Oliveira, J.M. Determination of Odorants in Varietal Wines from International Grape Cultivars (Vitis vinífera) Grown in NW Spain. South Afr. J. Enol. Vitic. 2013, 34, 212–222. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Wirth, J.; Morel-Salmi, C.; Souquet, J.M.; Dieval, J.B.; Aagaard, O.; Vidal, S.; Fulcrand, H.; Cheynier, V. The impact of oxygen exposure before and after bottling on the polyphenolic composition of red wines. Food Chem. 2010, 123, 107–116. [Google Scholar] [CrossRef]
- Atasanova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Gardner, S. Consumers and food safety: A food industry perspective. In Food, Nutrition and Agriculture; Albert, J.L., Ed.; Food and Agriculture Organization (FAO): Rome, Italy, 1993. [Google Scholar]
- Borbalán, A.M.A.; Zorro, L.; Guillén, D.A.; García Barroso, C. Study of the polyphenol content of red and white grape varieties by liquid chromatography–mass spectrometry and its relationship to antioxidant power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- De la Cruz, A.A.; Hilbert, G.; Riviere, C.; Mengin, V.; Ollat, N.; Bordenave, L.; Decroocq, S.; Delaunay, J.C.; Delrot, S.; Merillon, J.M.; et al. Anthocyanin identification and composition of wild Vitis spp. accessions by using LC-MS and LC-NMR. Anal. Chim. Acta 2012, 732, 145–152. [Google Scholar] [CrossRef]
- Gishen, M.; Dambergs, R.G.; Coxxolino, D. Grape and wine analysis—Enhancing the power of spectroscopy with chemometrics. A review of some application in the Australian wine industry. Aust. J. Grape Wine Res. 2005, 11, 296–305. [Google Scholar] [CrossRef]
- Nóbrega, I.C.C.; Pereira, G.E.; Silva, M.; Pereira, E.V.S.; Medeiros, M.M.; Telles, D.L.; Albuquerque, E.C., Jr.; Oliveira, J.B.; Lachenmeier, D.W. Improved sample preparation for GC–MS–SIM analysis of ethyl carbamate in wine. Food Chem. 2015, 177, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Thibon, C.; Pons, A.; Mouakka, N.; Redon, P.; Méreau, R.; Darriet, P. Comparison of electron and chemical ionization modes for the quantification of thiols and oxidative compounds in white wines by gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2015, 1415, 123–133. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalan, J.; Medina-Remon, A.; Sasot, G.; Mercader-Martí, M.; Llobet, J.M.; Lamuela-Raventos, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Malacarne, M.; Larcher, R. Identification and quantification of 56 targeted phenols in wines, spirits, and vinegars by online solid-phase extraction–ultrahigh-performance liquid chromatography–quadrupole-Orbitrap mass spectrometry. J. Chromatogr. A 2015, 1423, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Kruzlicova, D.; Fiket, Ž.; Kniewald, G. Classification of Croatian wine varieties using multivariate analysis of data obtained by high resolution ICP-MS analysis. Food Res. Int. 2013, 54, 621–626. [Google Scholar] [CrossRef]
- Skogerson, K.; Runnebaum, R.; Wohlgemuth, G.; de Ropp, J.; Heymann, H.; Fiehn, O. Comparison of gas chromatography-coupled time-of-flight mass spectrometry and 1H nuclear magnetic resonance spectroscopy metabolite identification in white wines from a sensory study investigating wine body. J. Agric. Food Chem. 2009, 57, 6899–6907. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Kokkotou, K.; Zoumpoulakis, P.; Zervou, M. NMR metabolite fingerprinting in grape derived products: An overview. Food Res. Int. 2013, 54, 1184–1194. [Google Scholar] [CrossRef]
- Raco, B.; Dotsika, E.; Poutoukis, D.; Battaglini, R.; Chantzi, P. O–H–C isotope ratio determination in wine in order to be used as a fingerprint of its regional origin. Food Chem. 2015, 168, 588–594. [Google Scholar] [CrossRef]
- Versini, G.; Camin, F.; Ramponi, M.; Dellacassa, E. Stable isotope analysis in grape products: 13C-based internal standardization methods to improve the detection of some types of adulterations. Anal. Chim. Acta 2006, 563, 325–330. [Google Scholar] [CrossRef]
- Dordevic, N.; Camin, F.; Marianella, R.M.; Postma, G.J.; Buydens, L.M.C.; Wehrens, R. Detecting the addition of sugar and water to wine. Aust. J. Grape Wine Res. 2013, 19, 324–330. [Google Scholar] [CrossRef]
- Johnson, A.J.; Hirson, G.D.; Ebeler, S.E. Perceptual characterization and analysis of aroma mixtures using gas chromatography recomposition-olfactometry. PLoS ONE 2012, 7, e42693. [Google Scholar] [CrossRef]
- Di Egidio, V.; Sinelli, N.; Giovanelli, G.; Moles, A.; Casiraghi, E. NIR and MIR spectroscopy as rapid methods to monitor red wine fermentation. Eur. Food Res. Technol. 2010, 230, 947–955. [Google Scholar] [CrossRef]
- Oliva, J.; Martinez, G.; Cermeno, S.; Motas, M.; Barba, A.; Camara, M.A. Influence of matrix on the bioavailability of nine fungicides in wine grape and red wine. Eur. Food Res. Technol. 2018, 244, 1083–1090. [Google Scholar] [CrossRef]
- Prusisz, B.; Mulica, K.; Pohl, P. Ion exchange and ion exclusion chromatographic characterization of wines using conductivity detection. J. Food Drug Anal. 2008, 16, 95–103. [Google Scholar] [CrossRef]
- Riul, A., Jr.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recent advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to assess food authenticity and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef]
- Bakker, E.; Qin, Y. Electrochemical Sensors. Anal. Chem. 2006, 78, 3965–3983. [Google Scholar] [CrossRef]
- Sandulescu, R.; Tertis, M.; Cristea, C.; Bodoki, E. New Materials for the Construction of Electrochemical Biosensors in Biosensors—Micro and Nanoscale Applications; Rinken, T., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Rudnitskaya, A.; Schmidtke, L.M.; Reis, A.; Domingues, M.R.M.; Delgadillo, I.; Debus, B.; Kirsanov, D.; Legin, A. Measurements of the effects of wine maceration with oak chips using an electronic tongue. Food Chem. 2017, 229, 20–27. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Rodriguez-Mendez, M.L.; Mohtasebi, S.S.; Apetrei, C.; Lozano, J.; Ahmadi, H.; Razavi, S.H.; de Saja, J.A. Monitoring the aging of beers using a bioelectronic tongue. Food Control 2012, 25, 216–224. [Google Scholar] [CrossRef]
- Novakowski, W.; Bertotti, M.; Paixao, T.R.L.C. Use of copper and gold electrodes as sensitive elements for fabrication of an electronic tongue: Discrimination of wines and whiskies. Microchem. J. 2011, 99, 145–151. [Google Scholar] [CrossRef]
- Winquist, F.; Olsson, J.; Eriksson, M. Multicomponent analysis of drinking water by a voltammetric electronic tongue. Anal. Chim. Acta 2011, 683, 192–197. [Google Scholar] [CrossRef]
- Hood White, M.R.; Heymann, H. Assessing the sensory profiles of sparkling wine over time. Am. J. Enol. Vitic. 2015, 66, 156–163. [Google Scholar] [CrossRef]
- Lucherk, L.W.; O’Quinn, T.G.; Legako, J.F.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Consumer and trained panel evaluation of beef strip steaks of varying marbling and enhancement levels cooked to three degrees of doneness. Meat Sci. 2016, 122, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.S.; Ahn, B.H.; Kim, H.R.; Lee, S.Y. Identification of sensory attributes that drive the likeability of Korean rice wines by American panelists. J. Food Sci. 2015, 80, 161–170. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Rosenvald, S.; Laaksonen, O.; Vanag, A.; Ollikka, T.; Vene, K.; Yang, B.R. Sensory and chemical profiles of Finnish honeys of different botanical origins and consumer preferences. Food Chem. 2018, 246, 351–359. [Google Scholar] [CrossRef]
- McKay, M.; Bauer, F.F.; Panzeri, V.; Buica, A. Testing the sensitivity of potential panelists for wine taint compounds using a simplified sensory strategy. Foods 2018, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.; Etaio, I.; Guerrero, L.; Fernandez-Gil, M.P.; Perez-Elortondo, F.J. Does consumer liking fit the sensory quality assessed by trained panelists in traditional food products? A study on PDO Idiazabal cheese. J. Sens. Stud. 2018, 33, e12318. [Google Scholar] [CrossRef]
- Lvova, L.; Yaroshenko, I.; Kirsanov, D.; Di Natale, C.; Paolesse, R.; Legin, A. Electronic tongue for brand uniformity control: A case study of Apulian red wines recognition and defects evaluation. Sensors 2018, 18, 2584. [Google Scholar] [CrossRef]
- Taladrid, D.; Lorente, L.; Bartolome, B.; Moreno-Arribas, M.V.; Laguna, L. An integrative salivary approach regarding palate cleansers in wine tasting. J. Texture Stud. 2019, 50, 75–82. [Google Scholar] [CrossRef]
- Di Natale, C.; Davide, F.; D’Amico, A.; Legin, A.; Rudinitskaya, A.; Selezenev, B.L.; Vlasov, Y. Applications of an electronic tongue to the environmental control. In Proceedings of the The 10th European Conference on Solid-State Transducers (Eurosensors X), Leuven, Belgium, 8–11 September 1996; pp. 1345–1348. [Google Scholar]
- Toko, K. Taste sensor with global selectivity. Mater. Sci. Eng. C 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Winquist, F.; Wide, P.; Lundstrom, I. An electronic tongue based on voltammetry. Anal. Chim. Acta 1997, 357, 21–31. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamanaka, M.; Toko, K.; Yamafuji, K. Multichannel taste sensor using lipid membranes. Sens. Actuators B 1990, 2, 205–213. [Google Scholar] [CrossRef]
- Toko, K.; Matsuno, T.; Yamafuji, K.; Hayashi, K.; Ikezaki, H.; Sato, K.; Toukubo, R.; Kawarai, S. Multichannel taste sensor using electric potential changes in lipid membranes. Biosens. Bioelectron. 1994, 9, 359–364. [Google Scholar] [CrossRef]
- Toko, K.; Murata, T.; Matsuno, T.; Kikkawa, Y.; Yamafuji, K. Taste map of beer by a multichannel taste sensor. Sens. Mater. 1992, 4, 145–151. [Google Scholar]
- Toko, K. A taste sensor. Meas. Sci. Technol. 1998, 9, 1919–1936. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic Tongues—A review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, M.L.; De Saja, J.A.; Gonzales-Anton, R.; Garcia-Hernandez, C.; Medina-Plaza, C.; Garcia-Cabezon, C.; Martin-Pedrosa, F. Electronic Noses and Tongues in Wine Industry. Front. Bioeng. Biotechnol. 2016, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, M. Sensor arrays and electronic tongue systems. Int. J. Electrochem. 2012, 2012, 986025. [Google Scholar] [CrossRef]
- Wide, P.; Winquist, F.; Bergsten, P.; Petriu, E.M. The Human-based multisensor fusion method for artificial nose and tongue sensor data. IEEE Trans. Instrum. Meas. 1998, 47, 1072–1077. [Google Scholar] [CrossRef]
- Winquist, F.; Holmin, S.; Krantz-Rulcker, C.; Wide, P.; Lundstrom, I. A hybrid electronic tongue. Anal. Chim. Acta 2000, 406, 147–157. [Google Scholar] [CrossRef]
- Winquist, F.; Rydberg, E.; Holmin, S.; Krantz-Rulcker, C.; Lundstrom, I. Flow injection analysis applied to a voltammetric electronic tongue. Anal. Chim. Acta 2002, 471, 159–172. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A. Cross-sensitivity evaluation of chemical sensors for electronic tongue: Determination of heavy metal ions. Sens. Actuators B 1997, 44, 532–537. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Vlasov, Y.; Di Natale, C.; Davide, F.; D’Amico, A. Tasting of beverages using an electronic tongue. Sens. Actuators B 1997, 44, 291–296. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Davide, F.; D’Amico, A.; Legin, A.; Vlasov, Y.; Rudnitskaya, A.; Selezenev, B. Multicomponent analysis on polluted waters by means of an electronic tongue. Sens. Actuators B 1997, 44, 423–428. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Lvova, L.; Vlasov, Y.; Di Natale, C.; D’Amico, A. Evaluation of Italian wine by the electronic tongue: Recognition, quantitative analysis and correlation with human sensory perception. Anal. Chim. Acta 2003, 484, 33–34. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Seleznev, B.; Vlasov, Y. Electronic tongue for quality assessment of ethanol, vodka and eau-de-vie. Anal. Chim. Acta 2005, 534, 129–135. [Google Scholar] [CrossRef]
- Parra, V.; Hernando, T.; Rodriguez-Mendez, M.L.; de Saja, J.A. Electrochemical sensor array made from bisphthalocyanine modified carbon paste electrodes for discrimination of red wines. Electrochim. Acta 2004, 49, 5177–5185. [Google Scholar] [CrossRef]
- Arrieta, A.; Rodriguez-Mendez, M.L.; De Saja, J.A. Langmuir-Blodgett film and carbon paste electrodes based on phthalocyanines as sensing units for taste. Sens. Actuators B 2003, 95, 357–365. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Voltammetric e-tongue for the quantification of total polyphenol content in olive oils. Food Res. Int. 2013, 54, 2075–2082. [Google Scholar] [CrossRef]
- Ghosh, A.; Tudu, B.; Tamuly, P.; Bhattacharyya, N.; Bandyopadhyay, R. Prediction of theaflavin and thearubigin content in black tea using a voltammetric electronic tongue. Chemometr. Intell. Lab. Syst. 2012, 116, 57–66. [Google Scholar] [CrossRef]
- Buratti, S.; Ballabio, D.; Giovanelli, G.; Zuluanga Dominguez, C.M.; Moles, A.; Benedetti, S.; Sinelli, N. Monitoring of alcoholic fermentation using near infrared and mid infrared spectroscopies combined with electronic nose and electronic tongue. Anal. Chim. Acta 2011, 697, 67–74. [Google Scholar] [CrossRef]
- Cetó, X.; Céspedes, F.; del Valle, M. BioElectronic Tongue for the quantification of total polyphenol content in wine. Talanta 2012, 99, 544–551. [Google Scholar] [CrossRef]
- Gil, L.; Barat, J.M.; Baigts, D.; Martínez-Máñez, R.; Soto, J.; Garcia-Breijo, E.; Aristoy, M.C.; Toldrá, F.; Llobet, E. Monitoring of physical–chemical and microbiological changes in fresh pork meat under cold storage by means of a potentiometric electronic tongue. Food Chem. 2011, 126, 1261–1268. [Google Scholar] [CrossRef]
- Gutierrez-Capitan, M.; Santiago, J.L.; Vila-Planas, J.; Llobera, A.; Boso, S.; Gago, P.; Martinez, M.C.; Jimenez-Jorquera, C. Classification and characterization of different white grape juices by using a hybrid electronic tongue. J. Agric. Food Chem. 2013, 61, 9325–9332. [Google Scholar] [CrossRef]
- Lvova, L.; Denis, S.; Barra, A.; Mielle, P.; Salles, C.; Vergoignan, C.; Di Natale, C.; Paolesse, R.; Temple-Boyer, P.; Feron, G. Salt release monitoring with specific sensors in “in vitro” oral and digestive environments from soft cheeses. Talanta 2012, 97, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Peres, A.M.; Dias, L.G.; Veloso, A.C.A.; Meirinho, S.G.; Morais, J.S.; Machado, A.A.S.C. An electronic tongue for gliadins semi-quantitative detection in foodstuffs. Talanta 2011, 83, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.A.; De la Fuente, R.; Caballero, I.; Rodriguez-Mendez, M.L. Beer discrimination using a portable electronic tongue based on screen-printed electrodes. J. Food Eng. 2015, 157, 57–62. [Google Scholar] [CrossRef]
- Gay, M.; Diez-Arevalo, E.; Rodriguez-Mendez, M.L.; De Saja, J.A. Electrochemical quartz crystal microbalance analysis of the oxidation reaction of phenols found in wines at lutetium bisphthalocyanine electrodes. Sens. Actuators B 2013, 185, 24–31. [Google Scholar]
- Medina-Plaza, C.; Revilla, G.; Munoz, R.; Fernandez-Escudero, J.A.; Barajas, E.; Medrano, G.; De Saja, J.A.; Rodriguez-Mendez, M.L. Electronic tongue formed by sensors and biosensors containing phthalocyanines as electron mediators. Application to the analysis of red grapes. J. Porphyr. Phthalocyanines 2014, 18, 76–86. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Sun, D.W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Tahara, Y.; Nakashi, K.; Ji, K.; Ikeda, A.; Toko, K. Development of a portable taste sensor with a lipid/polymer membrane. Sensors 2013, 13, 1076–1084. [Google Scholar] [CrossRef]
- Dias, L.G.; Peres, A.M.; Barcelos, T.P.; Morais, J.S.; Machado, A.A.S.C. Semi-quantitative and quantitative analysis of soft drinks using an electronic tongue. Sens. Actuators B 2011, 154, 111–118. [Google Scholar] [CrossRef]
- Kutyla-Olesiuk, A.; Nowacka, M.; Wesoly, M.; Ciosek, P. Evaluation of organoleptic and texture properties of dried apples by hybrid electronic tongue. Sens. Actuators B 2013, 187, 234–240. [Google Scholar] [CrossRef]
- Ceto, X.; Gutierrez, J.M.; Mimendia, A.; Cespedes, F.; del Valle, M. Voltammetric electronic tongue for the qualitative analysis of beers. Electroanalysis 2013, 25, 1635–1644. [Google Scholar] [CrossRef]
- Del Valle, M. Electronic tongues employing electrochemical sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska, M.; Wisniewska, P.; Dymerski, T.; Namiesnik, J.; Wardencki, W. Food analysis using artificial senses. J. Agric. Food Chem. 2014, 62, 1423–1448. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, P.; Wróblewski, W. Potentiometric electronic tongues for foodstuff and biosample recognition—An overview. Sensors 2011, 11, 4688–4701. [Google Scholar] [CrossRef] [PubMed]
- Scampicchio, M.; Ballabio, D.; Arecchi, A.; Cosio, S.M.; Mannino, S. Amperometric electronic tongue for food analysis. Microchim. Acta 2008, 163, 11–21. [Google Scholar] [CrossRef]
- Cavanillas, S.; Winquist, F.; Eriksson, M. A self-polishing platinum ring voltammetric sensor and its application to complex media. Anal. Chim. Acta 2015, 859, 29–36. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, M.L.; Parra, V.; Apetrei, C.; Villanueva, S.; Gay, M.; Prieto, N.; De Saja, J.A. Electronic tongue based on voltammetric electrodes modified with materials showing complementary electroactive properties. Applications. Microchim. Acta 2008, 163, 23–31. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Rodriguez-Mendez, M.L.; De Saja, J.A. Voltametric electronic tongue application to wines classification and correlation study with the chemical and sensory characterization. Quím. Nova 2010, 33, 787–793. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Schmidtke, L.M.; Delgadillo, I.; Legin, A.; Scollary, G. Study of the influence of micro-oxygenation and oak chip maceration on wine composition using an electronic tongue and chemical analysis. Anal. Chim. Acta 2009, 642, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Gay, M.; Apetrei, C.; Nevares, I.; del Alamo, M.; Zurro, J.; Prieto, N.; De Saja, J.A.; Rodríguez-Mendez, M.L. Application of an electronic tongue to study the effect of the use of pieces of wood and micro-oxygenation in the aging of red wine. Electrochim. Acta 2010, 55, 6782–6788. [Google Scholar] [CrossRef]
- Kutyła-Olesiuk, A.; Zaborowski, M.; Prokaryn, P.; Ciosek, P. Monitoring of beer fermentation based on hybrid electronic tongue. Bioelectrochemistry 2012, 87, 104–113. [Google Scholar] [CrossRef]
- Yoshida, M.; Shinohara, H.; Sugiyama, T.; Kumagai, M.; Muto, H.; Kodama, H. Taste of milk from inflamed breasts of breastfeeding mothers with mastitis evaluated using a taste sensor. Breastfeed. Med. 2014, 9, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, J.; Zhang, X. Monitoring of quality and storage time of unsealed pasteurized milk by voltammetric electronic tongue. Electrochim. Acta 2013, 88, 231–239. [Google Scholar] [CrossRef]
- Esbensen, K.; Kirsanov, D.; Legin, A.; Rudnitskaya, A.; Mortensen, J.; Pedersen, J.; Vognsen, L.; Makarychev-Mikhailov, S.; Vlasov, Y. Fermentation monitoring using multisensor systems: Feasibility study of the electronic tongue. Anal. Bioanal. Chem. 2004, 378, 391–395. [Google Scholar] [CrossRef]
- Gil, L.; Barat, J.M.; Escriche, I.; Garcia-Breijo, E.; Martínez-Mañeez, R.; Soto, J. An electronic tongue for fish freshness analysis using a thick-film array of electrodes. Microchim. Acta 2008, 163, 121–129. [Google Scholar] [CrossRef]
- Gil, L.; Barat, J.M.; Garcia-Breijo, E.; Ibañez, J.; Martínez-Mañeez, R.; Soto, J.; Llobet, E.; Brezemes, J.; Aristoy, J.C.; Toldra, F. Fish freshness analysis using metallic potentiometric electrodes. Sens. Actuators B 2008, 131, 362–370. [Google Scholar] [CrossRef]
- Rodríguez-Mendez, M.L.; Gay, M.; Apetrei, C.; De Saja, J.A. Biogenic amines and fish freshness assessment using a multisensory system based on voltammetric electrodes. Comparison between CPE and screen-printed electrodes. Electrochim. Acta 2009, 54, 7033–7041. [Google Scholar] [CrossRef]
- Winquist, F.; Krantz-Rulcker, C.; Wide, P.; Lundström, I. Monitoring of freshness of milk by an electronic tongue on the basis of voltammetry. Meas. Sci. Technol. 1998, 9, 1937–1946. [Google Scholar] [CrossRef]
- Tran, T.U.; Suzuki, K.; Okadome, H.; Homma, S.; Ohtsubo, K. Analysis of the tastes of brown rice and milled rice with different milling yields using a taste sensing system. Food Chem. 2004, 88, 557–566. [Google Scholar] [CrossRef]
- Parra, V.; Arrieta, A.A.; Fernandez-Escudero, J.A.; Rodríguez-Mendez, M.L.; De Saja, J.A. Electronic tongue based on chemically modified electrodes and voltammetry for the detection of adulterations in wines. Sens. Actuators B 2006, 118, 448–453. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Macagnano, A.; Mantini, A.; D’Amico, A.; Ubigli, M.; Legin, A.; Lvova, L.; Rudnitskaya, A.; Vlasov, Y. Application of a combined artificial olfaction and taste system to the quantification of relevant compounds in red wine. Sens. Actuators B 2000, 69, 342–347. [Google Scholar] [CrossRef]
- Riul, A.; de Sousa, H.C.; Malmegrim, R.R.; dos Santos, D.S.; Carvalho, A.C.P.L.F.; Fonseca, F.J.; Oliveira, O.N.; Mattoso, L.H.C. Wine classification by taste sensors made from ultra-thin films and using neural networks. Sens. Actuators B 2004, 98, 77–82. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Haddi, Z.; Amari, A.; Bouchikhi, B.; Mimendia, A.; Ceto, X.; del Valle, M. Hybrid electronic tongue based on multisensor data fusion for discrimination of beers. Sens. Actuators B 2013, 177, 989–996. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Polshin, E.; Kirsanov, D.; Lammertyn, J.; Nicolai, B.; Saison, D.; Delvaux, F.R.; Delvaux, F.; Legin, A. Instrumental measurement of beer taste attributes using an electronic tongue. Anal. Chim. Acta 2009, 646, 111–118. [Google Scholar] [CrossRef]
- Polshin, E.; Rudnitskaya, A.; Kirsanov, D.; Legin, A.; Saison, D.; Delvaux, F.; Delvaux, F.R.; Nicolai, B.; Lammertyn, J. Electronic tongue as a screening tool for rapid analysis of beer. Talanta 2010, 81, 88–94. [Google Scholar] [CrossRef]
- Sliwinska, M.; García-Hernández, C.; Koscinski, M.; Dymerski, T.; Wardencki, W.; Namiesnik, J.; Sliwinska-Bartkowiak, M.; Jurga, S.; García-Cabezón, C.; Rodríguez-Méndez, M.L. Discrimination of apple liqueurs (Nalewka) using a voltammetric electronic tongue, UV-Vis and Raman spectroscopy. Sensors 2016, 16, 1654. [Google Scholar] [CrossRef]
- Paixao, T.R.L.C.; Bertotti, M. Fabrication of disposable voltammetric electronic tongues by using Prussian Blue films electrodeposited onto CD-R gold surfaces and recognition of milk adulteration. Sens. Actuators B 2009, 137, 266–273. [Google Scholar] [CrossRef]
- Dias, L.A.; Peres, A.M.; Veloso, A.C.A.; Reis, F.S.; Vilas-Boas, M.; Machado, A.A.S.C. An electronic tongue taste evaluation: Identification of goat milk adulteration with bovine milk. Sens. Actuators B 2009, 136, 209–217. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Jin, W. Evaluation of varieties of set yogurts and their physical properties using a voltammetric electronic tongue based on various potential waveforms. Sens. Actuators B 2013, 177, 684–694. [Google Scholar] [CrossRef]
- Major, N.; Markovic, K.; Krpan, M.; Šaric, G.; Hruškar, M.; Vahčić, N. Rapid honey characterization and botanical classification by an electronic tongue. Talanta 2011, 85, 569–574. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Domenech, E.; Gil-Sanchez, L. A potentiometric electronic tongue for the discrimination of honey according to the botanical origin. Comparison with traditional methodologies: Physicochemical parameters and volatile profile. J. Food Eng. 2012, 109, 449–456. [Google Scholar] [CrossRef]
- Ciosek, P.; Maminska, R.; Dybko, A.; Wroblewski, W. Potentiometric electronic tongue based on integrated array of microelectrodes. Sens. Actuators B 2007, 127, 8–14. [Google Scholar] [CrossRef]
- He, W.; Hu, X.; Zhao, L.; Liao, X.; Zhang, Y.; Zhang, M.; Wu, J. Evaluation of Chinese tea by the electronic tongue: Correlation with sensory properties and classification according to geographical origin and grade level. Food Res. Int. 2009, 42, 1462–1467. [Google Scholar] [CrossRef]
- Ivarsson, P.; Holmin, S.; Hojer, N.E.; Krantz-Rulcker, C.; Winquist, F. Discrimination of tea by means of a voltammetric electronic tongue and different applied waveforms. Sens. Actuators B 2001, 76, 449–454. [Google Scholar] [CrossRef]
- Apetrei, C.; Apetrei, I.M.; Villanueva, S.; De Saja, J.A.; Gutierrez-Rosales, F.; Rodríguez-Mendez, M.L. Combination of an e-nose, an e-tongue and an e-eye for the characterisation of olive oil with different degree of bitterness. Anal. Chim. Acta 2010, 663, 91–97. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Kirsanov, D.; Legin, A.; Beullens, K.; Lammertyn, J.; Nicolai, B.; Irudayaraj, J. Analysis of apples varieties–comparison of electronic tongue with different analytical techniques. Sens. Actuators B 2006, 116, 23–28. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef]
- Wang, W.; Xu, G.; Cui, X.T.; Sheng, G.; Luo, X. Enhanced catalytic and dopamine sensing properties of electrochemically reduced conducting polymer nanocomposite doped with pure graphene oxide. Biosens. Bioelectron. 2014, 58, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, D.; Bossi, A.; Whitcombe, M.J.; Chianella, I.; Fowler, S.A.; Subramanyam, S.; Piletska, E.V.; Piletsky, S.A. Electrochemical sensor for catechol and dopamine based on a catalytic molecularly imprinted polymer-conducting polymer hybrid recognition element. Anal. Chem. 2009, 81, 3576–3584. [Google Scholar] [CrossRef]
- Gao, Y.S.; Xu, J.K.; Lu, L.M.; Wu, L.P.; Zhang, K.X.; Nie, T.; Zhu, X.F.; Wu, Y. Overoxidized polypyrrole/graphene nanocomposite with good electrochemical performance as novel electrode material for the detection of adenine and guanine. Biosens. Bioelectron. 2014, 52, 90–95. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, A.; Sun, Y.; Ru, X.; Ge, D.; Shi, W. Poly(1-(2-carboxyethyl)pyrrole)/polypyrrole composite nanowires for glucose biosensor. Electrochim. Acta 2012, 70, 278–285. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Simon, D.T.; Pham, M.C.; Noel, V.; Berggren, M. Detection of glutamate and acetylcholine with organic electrochemical transistors based on conducting polymer/platinum nanoparticle composites. Adv. Mater. 2014, 26, 5658–5664. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, G.; Armelin, E.; Aleman, C. Selective detection of dopamine combining multilayers of conducting polymers with gold nanoparticles. J. Phys. Chem. B 2014, 118, 4669–4682. [Google Scholar] [CrossRef]
- Zagal, J.; Bedioui, F.; Dodelet, J.P. N4-Macrocyclic Metal Complexes; Springer: New York, NY, USA, 2006. [Google Scholar]
- Dini, D.; Hanack, M. Physical properties of phthalocyanine-based materials. In The Porphyrin Handbook, Phthalocyanines: Properties and Materials; Kadish, K., Smith, K., Guilard, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 17. [Google Scholar]
- Sivalingam, Y.; Pudi, R.; Lvova, L.; Pomarico, G.; Basoli, F.; Catini, A.; Legin, A.; Paolesse, R.; Di Natale, C. The light modulation of the interaction of L-cysteine with porphyrins coated ZnO nanorods. Sens. Actuators B 2015, 209, 613–621. [Google Scholar] [CrossRef]
- Legin, A.; Makarychev-Mikhailov, S.; Goryacheva, O.; Kirsanov, D.; Vlasov, Y. Cross-sensitive chemical sensors based on tetraphenylporphyrin and phthalocyanine. Anal. Chim. Acta 2002, 457, 297–303. [Google Scholar] [CrossRef]
- Lvova, L.; Paolesse, R.; Di Natale, C.; D’Amico, A. Detection of alcohols in beverages: An application of porphyrin-based Electronic tongue. Sens. Actuators B 2006, 118, 439–447. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, M.L.; Medina-Plaza, C.; de Saja, J.A.; Apetrei, C.; Muñoz, R. Sensor arrays based on phthalocyanines: New developments on nanostructured and biomimetic electrochemical sensors. In Multisensor Systems for Chemical Analysis: Materials and Sensors; Lvova, L., Kirsanov, D., Di Natale, C., Legin, A., Eds.; Pan Stanford Publishing: Singapore, 2012; pp. 70–109. [Google Scholar]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Kalimuthu, P.; Sivanesan, A.; John, S.A. Fabrication of optochemical and electrochemical sensors using thin films of porphyrin and phthalocyanine derivatives. J. Chem. Sci. 2012, 124, 1315–1325. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, M.L.; Gay, M.; de Saja, J.A. New insights into sensors based on radical bisphthalocyanines. J. Porphyr. Phthalocyanines 2009, 13, 1159–1167. [Google Scholar] [CrossRef]
- Hou, K.Y.; Huang, L.; Qi, Y.B.; Huang, C.X.; Pan, H.B.; Du, M.A. Bisphenol A sensor based on novel self-assembly of zinc phthalocyanine tetrasulfonic acid-functionalized graphene nanocomposites. Mater. Sci. Eng. C 2015, 49, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 258. [Google Scholar] [CrossRef]
- Campbell, F.W.; Compton, R.G. The use of nanoparticles in electroanalysis: An updated review. Anal. Bioanal. Chem. 2010, 396, 241–259. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Wang, F.; Hu, S. Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim. Acta 2009, 165, 1–22. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I.; Wang, J. Electroanalytical and bioelectroanalytical systems based on metal and semiconductor nanoparticles. Electroanalysis 2004, 16, 19–44. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; García-Cabezón, C.; García-Hernández, C.; Bramorski, C.; Blanco-Val, Y.; Martín-Pedrosa, F.; Kawai, T.; de Saja, J.A.; Rodríguez-Méndez, M.L. Analysis of organic acids and phenols of interest in the wine industry using Langmuir–Blodgett films based on functionalized nanoparticles. Anal. Chim. Acta 2015, 853, 572–578. [Google Scholar] [CrossRef]
- Alizadeh, T.; Mirzagholipur, S. A nafion-free non-enzymatic amperometric glucose sensor based on copper oxide nanoparticles—Graphene nanocomposite. Sens. Actuators B 2014, 198, 438–447. [Google Scholar] [CrossRef]
- Fouladgar, M.; Karimi-Maleh, H.; Gupta, V. Highly sensitive voltammetric sensor based on NiO nanoparticle room temperature ionic liquid modified carbon paste electrode for levodopa analysis. J. Mol. Liq. 2015, 208, 78–83. [Google Scholar] [CrossRef]
- Alencar, W.S.; Crespilho, F.N.; Martins, M.V.A.; Zucolotto, V.; Oliveira, O.N.; Silva, W.C. Synergistic interaction between gold nanoparticles and nickel phthalocyanine in layer-by-layer (LbL) films: Evidence of constitutional dynamic chemistry (CDC). Phys. Chem. Chem. Phys. 2009, 11, 5086–5091. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Ganesan, V. Zinc phthalocyanine and silver/gold nanoparticles incorporated MCM-41 type materials as electrode modifiers. Langmuir 2009, 25, 13264–13272. [Google Scholar] [CrossRef]
- Hou, C.; Liu, H.; Zhang, D.; Yang, C.; Zhang, M. Synthesis of ZnO nanorods- Au nanoparticles hybrids via in-situ plasma sputtering-assisted method for simultaneous electrochemical sensing of ascorbic acid and uric acid. J. Alloys Compd. 2016, 666, 178–184. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Zhang, G.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef]
- Zeravik, J.; Hlavacek, A.; Lacina, K.; Skládal, P. State of the art in the field of electronic and bioelectronic tongues—Towards the analysis of wines. Electroanalysis 2009, 21, 2509–2520. [Google Scholar] [CrossRef]
- Toko, K. Biochemical Sensors: Mimicking Gustatory and Olfactory Senses; Jenny Standford Publishing: New York, NY, USA, 2013. [Google Scholar]
- Medina-Plaza, C.; de Saja, J.A.; Rodríguez-Méndez, M.L. Bioelectronic tongue based on lipidic nanostructured layers containing phenol oxidases and lutetium bisphthalocyanine for the analysis of grapes. Biosens. Bioelectron. 2014, 57, 276–286. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Yang, L.L.; Yan, S.L.; Wang, M.M.; Cheng, D.; Chen, Q.; Dong, Y.L.; Liu, P.; Cai, W.Q.; et al. The Cu-MOF-199/single-walled carbon nanotubes modified electrode for simultaneous determination of hydroquinone and catechol with extended linear ranges and lower detection limits. Anal. Chim. Acta 2015, 899, 57–65. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. The biocomposite screen-printed biosensor based on immobilization of tyrosinase onto the carboxyl functionalised carbon nanotube for assaying tyramine in fish products. J. Food Eng. 2015, 149, 1–8. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Amperometric biosensor based on polypyrrole and tyrosinase for the detection of tyramine in food samples. Sens. Actuators B 2013, 178, 40–46. [Google Scholar] [CrossRef]
- Apetrei, C.; Rodríguez-Méndez, M.L.; De Saja, J.A. Amperometric tyrosinase based biosensor using an electropolymerized phosphate-doped polypyrrole film as an immobilization support. Application for detection of phenolic compounds. Electrochim. Acta 2011, 56, 8919–8925. [Google Scholar] [CrossRef]
- Wang, K.; Dai, L.N.; Liu, Q.; Li, H.N.; Ju, C.; Wu, J.; Li, H.M. Electrodeposition of unsubstituted iron phthalocyanine nano-structure film in a functionalized ionic liquid and its electrocatalytic and electroanalysis applications. Analyst 2011, 136, 4344–4349. [Google Scholar] [CrossRef] [PubMed]
- Ponce, I.; Silva, J.F.; Oñate, R.; Rezende, M.C.; Paez, M.A.; Pavez, J.; Zagal, J.H. Enhanced catalytic activity of Fe phthalocyanines linked to Au(111) via conjugated self-assembled monolayers of aromatic thiols for O2 reduction. Electrochem. Commun. 2011, 13, 1182–1186. [Google Scholar] [CrossRef]
- Han, J.B.; Xu, X.Y.; Rao, X.Y.; Wei, M.; Evans, D.G.; Duan, X.J. Layer-by-layer assembly of layered double hydroxide/cobalt phthalocyanine ultrathin film and its application for sensors. J. Mater. Chem. 2011, 21, 2126–2130. [Google Scholar] [CrossRef]
- Aoki, P.H.B.; Volpati, D.; Riul, A., Jr.; Caetano, W.; Constantino, C.J.L. Layer-by-Layer technique as a new approach to produce nanostructured films containing phospholipids as transducers in sensing applications. Langmuir 2009, 25, 2331–2338. [Google Scholar] [CrossRef]
- Volpati, D.; Alessio, P.; Zanfolim, A.A.; Storti, F.C.; Job, A.E.; Ferreira, M.; Riul, A.; Oliveira, O.N.; Constantino, C.J.L. Exploiting distinct molecular architectures of ultrathin films made with iron phthalocyanine for sensing. J. Phys. Chem. B 2008, 112, 15275–15282. [Google Scholar] [CrossRef]
- Santos, A.C.; Zucolotto, V.; Constantino, C.J.L.; Cunha, H.N.; dos Santos, J.R., Jr.; Eiras, C. Electroactive LBL films of metallic-phthalocyanines and poly (o-methoxyaniline) for sensing. J. Solid State Electrochem. 2007, 11, 1505–1510. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Furini, L.N.; Constantino, C.J.L.; de Saja, J.A.; Rodríguez-Méndez, M.L. Synergistic electrocatalytic effect of nanostructured mixed films formed by functionalised gold nanoparticles and bisphthalocyanines. Anal. Chim. Acta 2014, 851, 95–102. [Google Scholar] [CrossRef]
- Furini, L.N.; Feitosa, E.; Alessio, P.; Shimabukuro, M.H.; Riul, A., Jr.; Constantino, C.J.L. Tuning the nanostructure of DODAB/nickel tetrasulfonated phthalocyanine bilayers in LbL films. Mat. Sci. Eng. C-Mater. 2013, 33, 2937–2946. [Google Scholar] [CrossRef]
- Ferreira, M.; Zucolotto, V.; Oliveira, O.N., Jr.; Wohnrath, K. Encyclopedia of Nanoscience and Nanotechnology 4. H.S; Nalwa, H.S., Ed.; American Scientific Publishers: Los Angeles, CA, USA, 2003; pp. 441–460. [Google Scholar]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of nanomaterials: A crucial step in biosensor fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef]
- Ariga, K.; Nakanishi, T.; Michinobu, T. Immobilization of biomaterials to nano-assembled films (Self-Assembled Monolayers, Langmuir-Blodgett Films, and Layer-by-Layer Assemblies) and their related functions. J. Nanosci. Nanotechnol. 2006, 6, 2278–2301. [Google Scholar] [CrossRef]
- Apetrei, C.; Alessio, P.; Constantino, C.J.L.; De Saja, J.A.; Rodriguez-Mendez, M.L.; Pavinatto, F.; Fernandes, E.G.R.; Zucolotto, V.; Oliveira, O.N. Biomimetic biosensor based on lipidic layers containing tyrosinase and lutetium bisphthalocyanine for the detection of antioxidants. Biosens. Bioelectron. 2011, 26, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Aoki, P.H.B.; Alessio, P.; Volpati, D.; Paulovich, F.V.; Riul, A., Jr.; Oliveira, O.N., Jr.; Constantino, C.J.L. On the distinct molecular architectures of dipping- and spray-LbL films containing lipid vesicles. Mater. Sci. Eng. C 2014, 41, 363–371. [Google Scholar] [CrossRef]
- Alessio, P.; Martin, C.S.; de Saja, J.A.; Rodriguez-Mendez, M.L. Mimetic biosensors composed by layer-by-layer films of phospholipid, phthalocyanine and silver nanoparticles to polyphenol detection. Sens. Actuators B 2016, 233, 654–666. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Fernandes, E.G.R.; Alessio, P.; Constantino, C.J.L.; de Saja, J.A.; Zucolotto, V.; Apetrei, C.; Oliveira, O.N., Jr.; Rodriguez-Mendez, M.L. Optimized architecture for Tyrosinase-containing Langmuir–Blodgett films to detect pyrogallol. J. Mater. Chem. 2011, 21, 4995–5003. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, M.L. Nanostructured thin films as electrochemical sensors and biosensors for milk analysis. Sens. Actuators Rep. 2023, 6, 100179. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Performance qualification of an electronic tongue based on ICH guideline Q2. J. Pharm. Biomed. Anal. 2010, 51, 497–506. [Google Scholar] [CrossRef]
- Pein, M.; Kirsanov, D.; Ciosek, P.; del Valle, M.; Yaroshenko, I.; Wesoły, M.; Zabadaj, M.; Gonzalez-Calabuig, A.; Wróblewski, W.; Legin, A. Independent comparison study of six different electronic tongues applied for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2015, 114, 321–329. [Google Scholar] [CrossRef]
- Lvova, L.; Martinelli, E.; Dini, F.; Bergamini, A.; Paolesse, R.; Di Natale, C.; D’Amico, A. Clinical analysis of human urine by means of potentiometric electronic tongue. Talanta 2009, 77, 1097–1104. [Google Scholar] [CrossRef]
- Yaroshenko, I.; Kirsanov, D.; Kartsova, L.; Sidorova, A.; Borisova, I.; Legin, A. Determination of urine ionic composition with potentiometric multisensor system. Talanta 2015, 131, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.E.; Grassi, V.; Scagion, V.P.; Mattoso, L.H.C.; Glenn, G.M.; Medeiros, E.S. Sensor array for water analysis based on interdigitated electrodes modified with fiber films of poly(lactic acid)/multiwalled carbon nanotubes. IEEE Sens. J. 2013, 13, 759–766. [Google Scholar] [CrossRef]
- Zadorozhnaya, O.; Kirsanov, D.; Buzhinsky, I.; Tsarev, F.; Abramova, N.; Bratov, A.; Muñoz, F.J.; Ribó, J.; Bori, J.; Riva, M.C.; et al. Water pollution monitoring by an artificial sensory system performing in terms of Vibrio fischeri bacteria. Sens. Actuators B 2015, 207, 1069–1075. [Google Scholar] [CrossRef]
- Fujita, A.; Isogai, A.; Endo, M.; Utsunomiya, H.; Nakano, S.; Iwata, H. Effects of sulfur dioxide on formation of fishy off-odor and undesirable taste in wine consumed with seafood. J. Agric. Food Chem. 2010, 58, 4414–4420. [Google Scholar] [CrossRef] [PubMed]
- Ujihara, T.; Hayashi, N.; Ikezaki, H. Objective evaluation of astringent and umami taste intensities of Matcha using a taste sensor system. Food Sci. Technol. Res. 2013, 19, 1099–1105. [Google Scholar] [CrossRef]
- Akitomi, H.; Tahara, Y.; Yasuura, M.; Kobayashi, Y.; Ikezaki, H.; Toko, K. Quantification of tastes of amino acids using taste sensors. Sens. Actuators B 2013, 179, 276–281. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formulation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Zhang, X. Discrimination of preserved licorice apricot using electronic tongue. Math. Comput. Model. 2013, 58, 737–745. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Baldwin, E.A.; Plotto, A.; Rosskopf, E.; Hong, J.C.; Bai, J. Electronic tongue discrimination of four tomato cultivars harvested at six maturities and exposed to blanching and refrigeration treatments. Postharvest Biol. Technol. 2018, 136, 42–49. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kwak, H.S.; Kim, M.J.; Kim, Y.; Kim, K.O.; Kim, S.S. Comparison of a descriptive analysis and instrumental measurements (electronic nose and electronic tongue) for the sensory profiling of Korean fermented soybean paste (doenjang). J. Sens. Stud. 2017, 32, 12282. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, P.; Chen, W.J.; Chen, H.M. Monitoring the quality change of fresh Coconut milk using an electronic tongue. J. Food Process. Preserv. 2017, 41, e13110. [Google Scholar]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing—Electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef]
- Zakaria, A.; Shakaff, A.Y.M.; Masnan, M.J.; Ahmad, M.N.; Adom, A.H.; Jaafar, M.N.; Ghani, S.A.; Abdullah, A.H.; Aziz, A.H.A.; Kamarudin, L.M.; et al. A biomimetic sensor for the classification of honeys of different floral origin and the detection of adulteration. Sensors 2011, 11, 7799–7822. [Google Scholar] [CrossRef] [PubMed]
- Prieto, N.; Gay, M.; Vidal, S.; Aagaards, O.; de Saja, J.A.; Rodriguez-Mendez, M.L. Analysis of the influence of the type of closure in the organoleptic characteristics of a red wine by using an electronic panel. Food Chem. 2011, 129, 589–594. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Rocha, S.M.; Legin, A.; Pereira, V.; Marques, J.C. Evaluation of the feasibility of the electronic tongue as a rapid analytical tool for wine age prediction and quantification of the organic acids and phenolic compounds. The case-study of Madeira wine. Anal. Chim. Acta 2010, 662, 82–89. [Google Scholar] [CrossRef]
- Parra, V.; Arrieta, A.; Fernández-Escudero, J.A.; Iñiguez, M.; de Saja, J.A.; Rodríguez-Méndez, M.L. Monitoring of the ageing of red wines in oak barrels by means of a hybrid electronic tongue. Anal. Chim. Acta 2006, 563, 229–237. [Google Scholar] [CrossRef]
- Garcia-Hernandez, C.; Salvo-Comino, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Analysis of red wines using an electronic tongue and infrared spectroscopy. Correlations with phenolic content and color parameters. LWT-Food Sci. Technol. 2020, 118, 108785. [Google Scholar] [CrossRef]
- Garcia-Hernandez, C.; Salvo Comino, C.; Martín-Pedrosa, F.; Rodríguez-Méndez, M.L.; Garcia-Cabezon, C. Impedimetric electronic tongue based on nanocomposites for the analysis of red wines. Improving the variable selection method. Sens. Actuators B Chem. 2018, 277, 365–372. [Google Scholar] [CrossRef]
- Cetó, X.; Capdevilla, J.; Minguez, S.; del Valle, M. Voltammetric bioelectronic tongue for the analysis of phenolic compounds in rosé cava wines. Food Res. Int. 2014, 55, 455–461. [Google Scholar] [CrossRef]
- Garcia-Hernandez, C.; Garcia-Cabezon, C.; Martin-Pedrosa, F.; Rodríguez-Mendez, M.L. Analysis of musts and wines by means of a bio-electronic tongue based on tyrosinase and glucose oxidase using polypyrrole/gold nanoparticles as the electron mediator. Food Chem. 2019, 289, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sanchez, L.; Soto, J.; Martinez-Mañez, R.; Garcia-Breijo, E.; Ibañez, J.; Llobet, E. A novel humid electronic nose combined with an electronic tongue for assessing deterioration of wine. Sens. Actuators A 2011, 171, 152–158. [Google Scholar] [CrossRef]
- Verrelli, G.; Francioso, L.; Paolesse, R.; Siciliano, P.; Di Natale, C.; D’Amico, A.; Logrieco, A. Development of silicon-based potentiometric sensors: Towards a miniaturized electronic tongue. Sens. Actuators B 2007, 123, 191–197. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Richtera, L. Preparation and characterization of carbon paste electrode bulk-modified with multiwalled carbon nanotubes and its application in a sensitive assay of antihyperlipidemic Simvastatin in biological samples. Molecules 2019, 24, 2215. [Google Scholar] [CrossRef]
- Švancara, I.; Kalcher, K. Carbon paste electrodes. In Advances in Electrochemical Sciences and Engineering; Alkire, R.C., Bartlett, P.N., Lipkowski, J., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2015; pp. 379–423. [Google Scholar]
- Sarakatsanou, C.; Karastogianni, S.; Girousi, S. Promising electrode surfaces, modified with nanoparticles, in the sensitive and selective electroanalytical determination of antibiotics: A review. Appl. Sci. 2023, 13, 5391. [Google Scholar] [CrossRef]
- Gautam, V.; Singh, K.P.; Yadav, V.L. Polyaniline/MWCNTs/starch modified carbon paste electrode for non-enzymatic detection of cholesterol: Application to real sample (cow milk). Anal. Bioanal. Chem. 2018, 410, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Rahmadhani, S.; Setiyanto, H.; Zulfikar, M.A. Fabrication of carbon paste electrode modified with phenol imprinted polyaniline as a sensor for phenol analysis by potentiometric. Mater. Sci. Forum 2018, 936, 71–76. [Google Scholar] [CrossRef]
- Horta, D.G.; Bevilaqua, D.; Acciari, H.A.; Garcia, O., Jr.; Benedetti, A.V. Optimization of the use of carbon paste electrodes (CPE) for electrochemical study of the chalcopyrite. Quim. Nova 2009, 32, 1734–1738. [Google Scholar] [CrossRef]
- Materon, E.M.; Wong, A.; Gomes, L.M.; Ibáñez-Redín, G.; Joshi, N.; Oliveira, O.N., Jr.; Faria, R.C. Combining 3D printing and screen-printing in miniaturized, disposable sensors with carbon paste electrodes. J. Mater. Chem. C 2021, 9, 5633–5642. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, J.; Qiao, J.; Ge, F.; Yang, Y.; Zhang, Q. Advancements in Electrochemical Sensing Technology for Heavy Metal Ions Detection. Food Chem. X 2025, 25, 102204. [Google Scholar]
- Mohan, J.M.; Amreen, K.; Javed, A.; Dubey, S.K.; Goel, S. Emerging trends in miniaturized and microfluidic electrochemical sensing platforms. Curr. Opin. Electrochem. 2022, 33, 100930. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Zhu, B.; Su, B. An Overview of Antifouling Strategies for Electrochemical Analysis. Electroanalysis 2022, 34, 966–975. [Google Scholar] [CrossRef]
- Lin, P.H.; Li, B.R. Antifouling strategies in advanced electrochemical sensors and biosensors. Analyst 2020, 145, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Chea, V.; Paolucci-Jeanjean, D.; Belleville, M.P. Optimization and charac-terization of an enzymatic membrane for the degradation of phenolic compounds. Catal. Today 2012, 193, 49–56. [Google Scholar] [CrossRef]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.M.; Porumb, R.; Epure, P. Progress in electrochemical (bio)sensors for monitoring wine production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef]
- Kapoor, A.; Sundaramurthy, A. (Eds.) Sensor Technologies for Food Quality and Safety; Detection Science Series; The Royal Society of Chemistry (RSC): London, UK, 2025; Volume 29. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).