Detection of Oral Beta-Lactam Antibiotics Using a Taste Sensor with Surface-Modified Lipid/Polymer Membranes
Abstract
1. Introduction
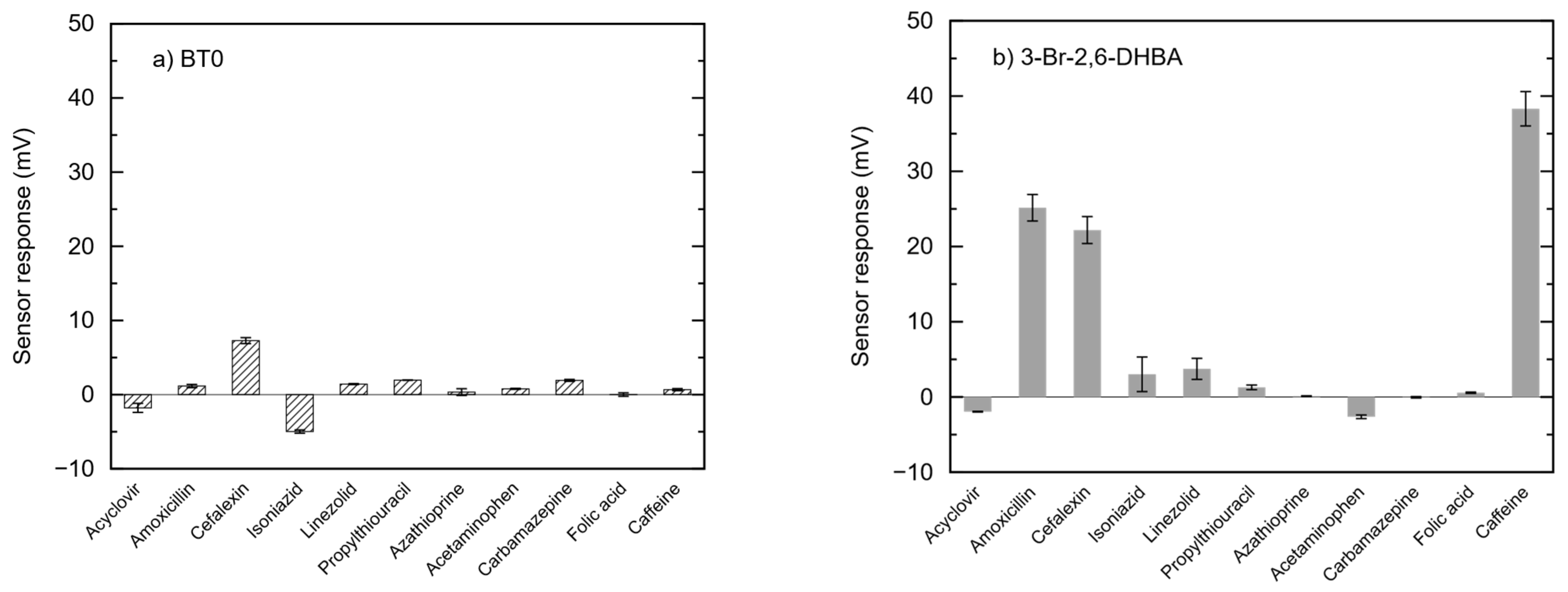
- The most important criterion was that, in the aforementioned study [24], the sensor output on the BT0 membrane for each 0.1 mM drug solution prepared using reference solution (30 mM KCl, 0.3 mM tartaric acid) was less than 1.0 mV, with no detectable response level.
- Other criteria are as follows: (1) The drug is an active pharmaceutical ingredient (API) widely used in clinical practice in Japan and worldwide, including for pediatric patients, and is administered orally. (2) The drug has the potential to cause palatability issues, including bitterness, when taken orally for repeated dosing. (3) The drug exhibits structural diversity, covering a wide range of chemical structures.
2. Materials and Methods
2.1. Reagents
- 30 mM solutions: caffeine, cefalexin, isoniazid, acetaminophen (paracetamol)
- 10 mM suspensions: acyclovir, amoxicillin, linezolid, propylthiouracil, azathioprine, carbamazepine, folic acid, cefaclor monohydrate, and cefdinir (resulting in slightly turbid suspensions)
2.2. Components of the Modified Membrane and Solvent
2.3. Sensor Preparation: Lipid/Polymer Membrane and Surface Modification
2.4. Measurement Procedure of the Taste Sensor
- (1)
- The sensor electrodes were first immersed in a reference solution containing 30 mM KCl and 0.3 mM tartaric acid, and the electric potential of this solution (Vr) was measured for 30 s. The reference solution simulates human saliva and has minimal inherent taste [47].
- (2)
- Subsequently, the electric potential of the sample solution (Vs) was measured for 30 s while the electrodes were immersed in the sample.
- (3)
- The relative response value was calculated as the difference between Vs and Vr.
- (4)
- Finally, the membrane surface was cleaned with an aqueous cleaning solution composed of 10 mM KOH, 100 mM KCl, and 30% ethanol (v/v).
3. Results
3.1. Sensor Outputs of 11 Drugs Measured Using BT0 Membranes and 3-Br-2,6-DHBA-Modified Membranes
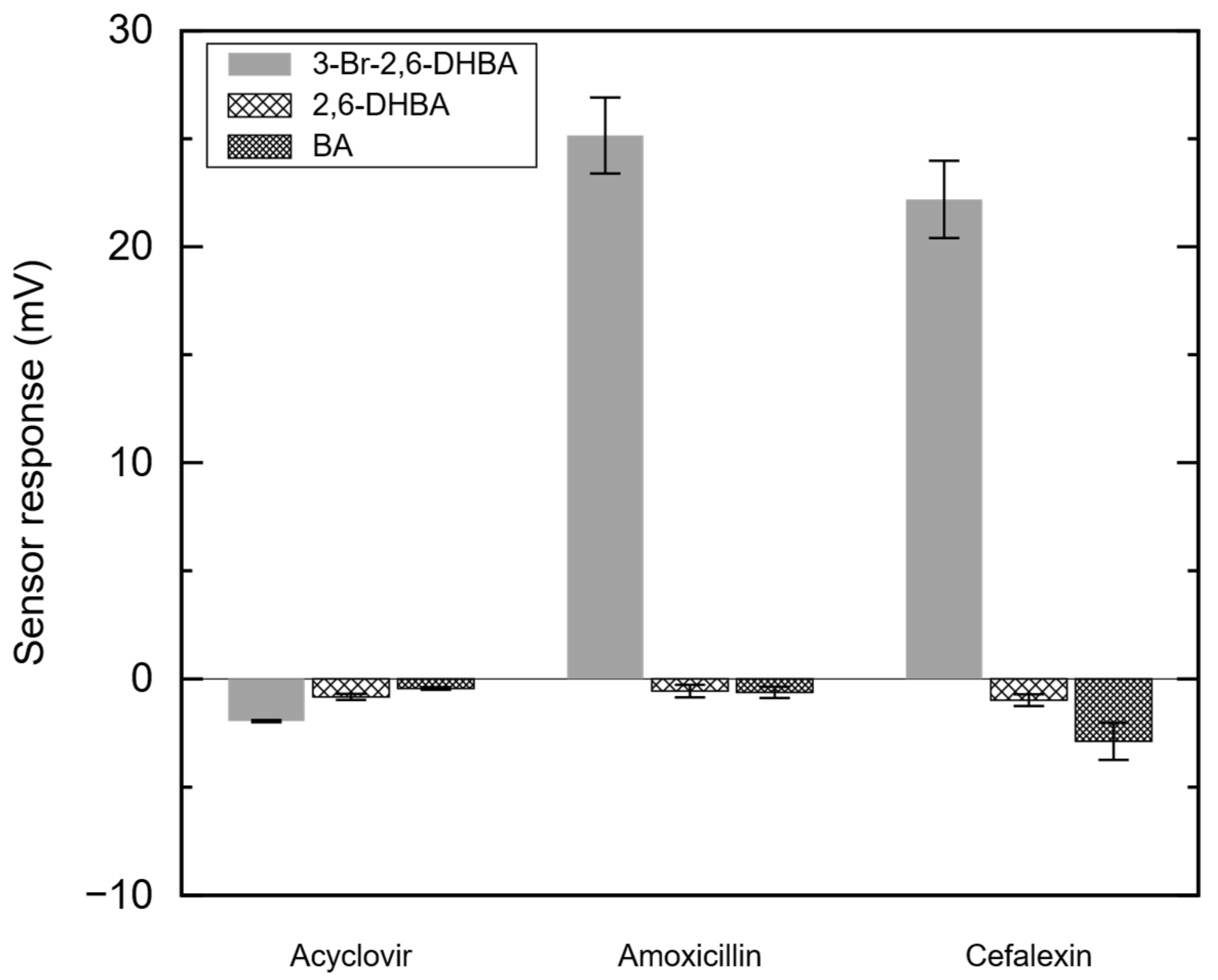
3.2. Sensor Output of Amoxicillin, Cefalexin, and Acyclovir with 3-Br-2,6-DHBA-, 2,6-DHBA, or BA-Modified Membranes
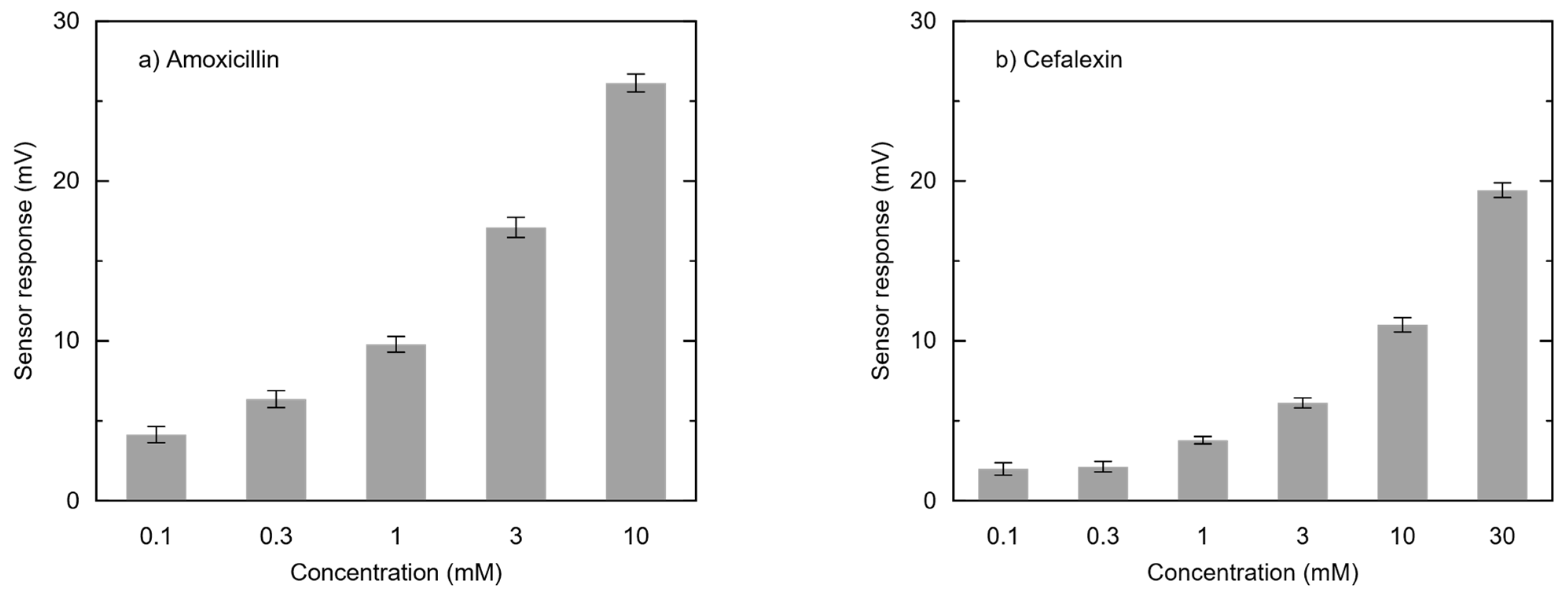
3.3. Concentration Dependence of Amoxicillin and Cefalexin on Sensor Output in Modified Membranes
3.4. Extended Response Analysis of 3-Br-2,6-DHBA-Modified Membranes for Two Oral Cephalosporins
4. Discussion
- (1)
- The molecule contains two spatially separated C=O groups.
- (2)
- Between these two C=O groups, there is a moiety capable of stacking (via π–π interactions or hydrophobic interactions) with aromatic carboxylic acids containing hydroxyl groups.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toko, K. Taste sensor with global selectivity. Mater. Sci. Eng. C 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste Sensor: Electronic Tongue with Lipid Membranes. Anal. Sci. 2020, 36, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Toko, K.; Tahara, Y.; Habara, M.; Kobayashi, Y.; Ikezaki, H. Taste Sensor: Electronic Tongue with Global Selectivity. In Essentials of Machine Olfaction and Taste; Nakamoto, T., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 87–174. ISBN 9781118768495. [Google Scholar]
- Toko, K. Research and Development of Taste Sensors as a Novel Analytical Tool. Proc. Jpn. Acad. Ser. B 2023, 99, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sasabuchi, Y.; Okada, A.; Yasunaga, H. Do Orally Disintegrating Tablets Facilitate Medical Adherence and Clinical Outcomes in Patients with Post-stroke. Dysphagia 2025, 40, 381–387. [Google Scholar] [CrossRef]
- Suryawanshi, C.P.; Wagh, R.D. Expansion and Valuation of Piroxicam Mouth Dissolving Medications. Indo Am. J. Pharm. Sci. 2024, 11, 58. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; El-Messiry, M.M.; El-Kamel, A.H. Effects of Functional Biomaterials on the Attributes of Orally Disintegrating Tablets Loaded with Furosemide Nanoparticles: In Vitro and In Vivo Evaluations. J. Funct. Biomater. 2024, 15, 161. [Google Scholar] [CrossRef]
- Ferlak, J.; Guzenda, W.; Osmałek, T. Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives. Pharmaceutics 2023, 15, 361. [Google Scholar] [CrossRef]
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V.; Rosenholm, J.M. Orodispersible Films: Conception to Quality by Design. Adv. Drug Deliv. Rev. 2021, 178, 113983. [Google Scholar] [CrossRef]
- Ikezaki, H.; Naito, Y.; Kobayashi, Y.; Toukubo, R.; Taniguchi, A.; Toko, K. Development of an Electronic Tongue. Tech. Rep. IEICE OME 2000, 100, 19–24. (In Japanese) [Google Scholar]
- Takagi, S.; Toko, K.; Wada, K.; Yamada, H.; Toyoshima, K. Detection of Suppression of Bitterness by Sweet Substance Using a Multichannel Taste Sensor. J. Pharm. Sci. 1998, 87, 552–555. [Google Scholar] [CrossRef]
- Uchida, T.; Miyanaga, Y.; Tanaka, H.; Wada, K.; Kurosaki, S.; Ohki, T.; Yoshida, M.; Matsuyama, K. Quantitative Evaluation of the Bitterness of Commercial Medicines Using a Taste Sensor. Chem. Pharm. Bull. 2000, 48, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Usui, R.; Ikezaki, H.; Tahara, K.; Takeuchi, H. An Advanced Technique Using an Electronic Taste-Sensing System to Evaluate the Bitterness of Orally Disintegrating Films and the Evaluation of Model Films. Int. J. Pharm. 2017, 530, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Morimoto, S.; Nishikawa, H.; Haraguchi, T.; Kojima, H.; Tsujino, H.; Arisawa, M.; Yamashita, T.; Nishikawa, J.; Yoshida, M.; et al. Bitterness-Suppressing Effect of Umami Dipeptides and Their Constituent Amino Acids on Diphenhydramine: Evaluation by Gustatory Sensation and Taste Sensor Testing. Chem. Pharm. Bull. 2020, 68, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced Taste Sensors Based on Artificial Lipids with Global Selectivity to Basic Taste Qualities and High Correlation to Sensory Scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef]
- Wu, X.; Onitake, H.; Huang, Z.; Shiino, T.; Tahara, Y.; Yatabe, R.; Ikezaki, H.; Toko, K. Improved Durability and Sensitivity of Bitterness-Sensing Membrane for Medicines. Sensors 2017, 17, 2541. [Google Scholar] [CrossRef]
- Haraguchi, T.; Uchida, T.; Yoshida, M.; Kojima, H.; Habara, M.; Ikezaki, H. The Utility of the Artificial Taste Sensor in Evaluating the Bitterness of Drugs: Correlation with Responses of Human TASTE2 Receptors (hTAS2Rs). Chem. Pharm. Bull. 2018, 66, 71–77. [Google Scholar] [CrossRef]
- The Japanese Pharmacopoeia, 18th ed.; The Minister of Health, Labour and Welfare: Tokyo, Japan, 2021; pp. 406–407.
- Yoshimatsu, J.; Toko, K.; Tahara, Y.; Ishida, M.; Habara, M.; Ikezaki, H.; Kojima, H.; Ikegami, S.; Yoshida, M.; Uchida, T. Development of Taste Sensor to Detect Non-Charged Bitter Substances. Sensors 2020, 20, 3455–3467. [Google Scholar] [CrossRef]
- Baguley, D.; Lim, E.; Bevan, A.; Pallet, A.; Faust, S.N. Prescribing for Children—Taste and Palatability Affect Adherence to Antibiotics: A Review. Arch. Dis. Child. 2012, 97, 293–297. [Google Scholar] [CrossRef]
- Aljebab, F.; Alanazi, M.; Choonara, I.; Conroy, S. Observational study on the palatability and tolerability of oral prednisolone and oral dexamethasone in children in Saudi Arabia and the UK. Arch. Dis. Child. 2018, 103, 83–88. [Google Scholar] [CrossRef]
- Pauli, E.; Ma, Z.; Sha, Y.; Zhang, X.; Brackett, J.; Towa, L.; Upadhyay, B.; Satcher, R. Development of an Immediate-Release Prototype Tablet Formulation of Hydroxychloroquine Sulfate with an Interwoven Taste-Masking System. J. Pharm. Sci. 2023, 112, 830–836. [Google Scholar] [CrossRef]
- Cirri, M.; Mura, P.A.; Maestrelli, F.; Benedetti, S.; Buratti, S. Pediatric Orally Disintegrating Tablets (ODTs) with Enhanced Palatability Based on Propranolol HCl Coground with Hydroxypropyl-β-Cyclodextrin. Pharmaceutics 2024, 16, 1351. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Kurihara, T.; Yoshida, M.; Haraguchi, T.; Nishikawa, H.; Ikegami, S.; Okuno, T.; Yamashita, T.; Nishikawa, J.; Tsujino, H.; et al. A New Bitterness Evaluation Index Obtained Using the Taste Sensor for 48 Active Pharmaceutical Ingredients of Pediatric Medicines. Chem. Pharm. Bull. 2021, 69, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Gumpper, R.H.; Liu, Y.; Kocak, D.D.; Xiong, Y.; Cao, C.; Deng, Z.; Krumm, B.E.; Jain, M.K.; Zhang, S.; et al. Bitter Taste Receptor Activation by Cholesterol and an Intracellular Tastant. Nature 2024, 628, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Behrens, M. The Tuman Bitter Taste Receptors: A Review. Chemosensors 2019, 7, 35. [Google Scholar]
- Wiener, A.; Shudler, M.; Levit, A.; Niv, M.Y. BitterDB: A Database of Bitter Compounds. Nucleic Acids Res. 2012, 40, D413–D419. [Google Scholar] [CrossRef]
- Kuhn, D.; Neve, R.L. BitterDB: Taste Ligands and Receptors Database in 2019. Nucleic Acids Res. 2019, 47, D1179–D1185. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, F.; Kimura, S.; Onodera, T.; Uchida, T.; Toko, K. Taste Sensor for Detecting Non-charged Bitter Substances: Xanthine derivatives of pharmaceutical applications. Microchem. J. 2024, 200, 110248. [Google Scholar] [CrossRef]
- Maruishi Pharm Co., Ltd. Package Insert for Caffeine Powder. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/730119_2115004X1159_1_04 (accessed on 5 April 2025).
- GlaxoSmithKline Co., Ltd. Package Insert for 40% Acyclovir Granules. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/340278_6250002D1024_1_16 (accessed on 5 April 2025).
- LTL Co., Ltd. Package Insert for Amoxicillin 10% Fine Granules. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/171911_6131001C1210_2_07 (accessed on 5 April 2025).
- Towa pharm. Co., Ltd. Package Insert for Cefalexin Complex Granules. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/480235_6132002E2170_1_05 (accessed on 5 April 2025).
- Alflesa Co., Ltd. Package Insert for Isoniazide Powder. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/530258_6222001F3037_3_03 (accessed on 5 April 2025).
- Chouseidou Co., Ltd. Package Insert for Acetaminophen Powder. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/450064_1141001X1118_1_09 (accessed on 5 April 2025).
- Pfizer Co., Ltd. Package Insert for Isoniazide Powder. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/672212_6249002F1024_5_07 (accessed on 5 April 2025).
- Nipuro ES Pharm Co., Ltd. Package Insert for Propylthiouracil Tablet. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/530100_2432002F1054_3_01 (accessed on 5 April 2025).
- Sando Pham. Co., Ltd. Package Insert for Azathioprine Tablet. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/112268_3999005F1059_X_09 (accessed on 5 April 2025).
- Kyouwayakuhin Co., Ltd. Package Insert for Carbamazepine Tablet. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/672173_1139002C1066_2_03 (accessed on 5 April 2025).
- Fuji Pharmaceutical Co., Ltd. Package Insert for Folic Acid Tablet. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/670109_3135001F1025_2_03 (accessed on 5 April 2025).
- Sawai Pharmaceutical Co., Ltd. Package Insert for Cefaclor Fine Granule. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/300119_6132005C1266_1_02 (accessed on 5 April 2025).
- Towa pharm. Co., Ltd. Package Insert for Cefdinir 10% Fine Granule. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/480235_6132013C1163_1_06 (accessed on 5 April 2025).
- Ishida, M.; Ide, H.; Arima, K.; Zhao, Z.; Matsui, T.; Toko, K. Identification of the Principle of Taste Sensors to Detect Non-Charged Bitter Substances by 1H-NMR Measurement. Sensors 2022, 22, 2592. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, F.; Kimura, S.; Onodera, T.; Uchida, T.; Toko, K. Assessment of Bitterness in Non-Charged Pharmaceuticals with a Taste Sensor: A Study on Substances with Xanthine Scaffold and Allopurinol. Molecules 2024, 29, 2452. [Google Scholar] [CrossRef]
- Buhrer, T.; Gehrig, P.; Simon, W. Neutral-carrier-based Ion-selective Microelectrodes Design and Application. Anal. Sci. 1988, 4, 547–557. [Google Scholar] [CrossRef]
- Morf, W.E. The Principles of Ion-Selective Electrodes and of Membrane Transport, 1st ed.; Elsevier Science: Budapest, Hungary, 1981; Volume 1, ISBN 9780444597557. [Google Scholar]
- Ito, M.; Wada, K.; Yoshida, M.; Hazekawa, M.; Abe, K.; Chen, R.; Habara, M.; Ikezaki, H.; Uchida, T. Advances in Pharmaceutical Sensors. Sens. Mater. 2013, 25, 17–30. [Google Scholar]
- Eizai Co., Ltd. Package Insert for Azactam Injection. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/170033_6122400D1028_1_17 (accessed on 5 April 2025).
- Ohnuma, T.; Hayashi, Y.; Yamashita, K.; Marquess, J.; Lefor, A.K.; Sanui, M. A Nationwide Survey of Intravenous Antimicrobial Use in Intensive Care Units in Japan. Int. J. Antimicrob. Agents 2018, 51, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Egi, M.; Ogura, H.; Yatabe, T.; Atagi, K.; Inoue, S.; Kakihana, Y.; Kushimoto, S.; Saito, O.; Shiraishi, A.; Suzuki, K.; et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). Acute Med. Surg. 2021, 8, e659. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.; Patrick, M.; Fowler, W. Advances in Organic Synthesis. Tetrahedron Lett. 2014, 55, 2078–2081. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Peng, Z.; Li, A.; Liu, J. Analytical Techniques in Chemistry. Anal. Chem. 2017, 89, 2065–2072. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Shen, Y.; Zhang, J.; Zhu, J.-J. Electrogenerated Chemiluminescence of Au Nanoclusters for the Detection of Dopamine. Anal. Chem. 2011, 83, 661–665. [Google Scholar] [CrossRef]
- Turner, J.; Muraoka, A.; Bedenbaugh, M.; Childress, B.; Pernot, L.; Wiencek, M.; Peterson, Y.K. The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition. Front. Microbiol. 2022, 13, 807955. [Google Scholar] [CrossRef]
- Del Bene, J.E. Lone Pair-π Interactions. Chem. Rev. 2001, 101, 2425–2456. [Google Scholar]
- Fun, H.-K.; Hemamalini, M.; Lobo, P.L.L.; Prasad, D.J.; Poojary, B. (5E)-5-(2,4-Dichlorobenzylidene)-2-(piperidin-1-yl)-1,3-thiazol-4(5H)-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67 Pt 11, o2884. [Google Scholar] [CrossRef]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Florez, I.D.; Mathew, J.L.; Shang, L.; Zhang, G.; Tian, X.; Fu, Z.; Liu, E.; Luo, Z.; et al. Short-course vs long-course antibiotic therapy for children with nonsevere community-acquired pneumonia: A systematic review and meta-analysis. JAMA Pediatr. 2022, 176, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Pepino, M.Y.; Duke, F.F.; Reed, D.R. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Reed, D.R.; Roberts, K.M.; Mathew, P.S.; Mansfield, C.J. Age-related differences in bitter taste and efficacy of bitter blockers. PLoS ONE 2014, 9, e103107. [Google Scholar] [CrossRef]
- van den Brink, M.; IJpma, I.; Fiocco, M.; Tissing, W.J.E.; Havermans, R.C. Taste function in children: Normative values and associated factors. Pediatr. Res. 2022, 92, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Ranmal, S.R.; Nhouchi, Z.; Keeley, A.; Adler, L.; Lavarde, M.; Pensé-Lhéritier, A.-M.; Tuleu, C. Taste Assessment for Paediatric Drug Development: A Comparison of Bitterness Taste Aversion in Children versus Naïve and Expert Young Adult Assessors. Int. J. Pharm. 2023, 647, 123494. [Google Scholar] [CrossRef]
- Servant, G.; Kenakin, T.; Zhang, L.; Williams, M.; Servant, N. The Function and Allosteric Control of the Human Sweet Taste Receptor. Adv. Pharmacol. 2020, 88, 59–82. [Google Scholar] [CrossRef]
- Berto, L.; Dumazer, A.; Malhaire, F.; Cannone, G.; Kutti Ragunath, V.; Goudet, C.; Lebon, G. Recent Advances in the Structural Biology of the Class C G Protein-Coupled Receptors: The Metabotropic Glutamate Receptor 5. Biol. Aujourd’hui 2021, 215, 85–94. [Google Scholar] [CrossRef]
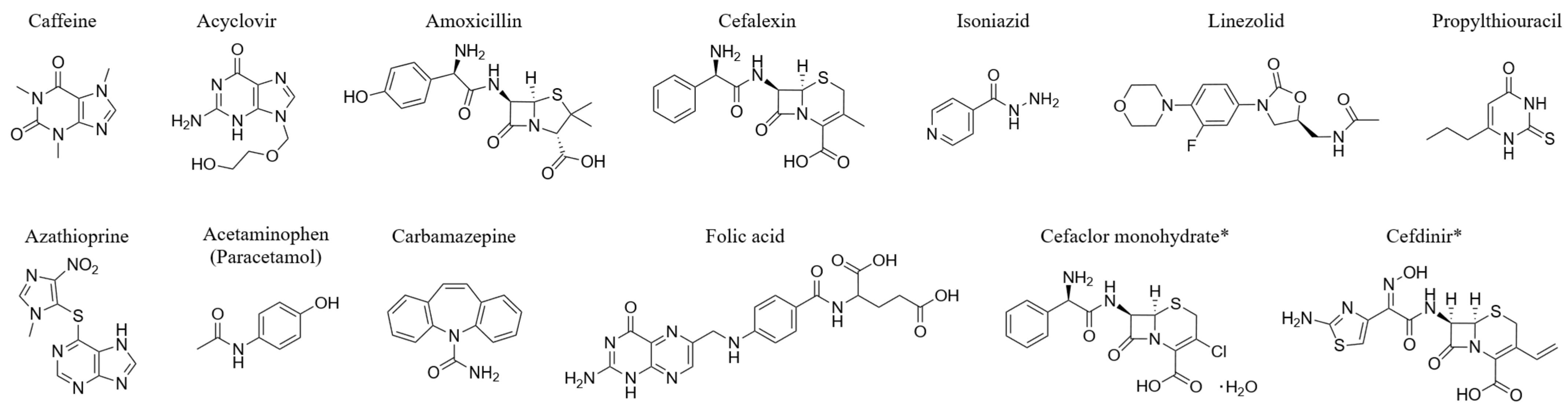


| Drug Name | Medical Effects/Indications | Formulation | Reference No. ** |
|---|---|---|---|
| Caffeine | CNS stimulant, analgesic; prevents drowsiness, relieves headaches | Powder | 30 |
| Acyclovir | Antiviral for herpes simplex, shingles | 40% granules jelly | 31 |
| Amoxicillin | Broad-spectrum penicillin for infections, including H. pylori | Capsules, fine granules | 32 |
| Cefalexin | Oral cephalosporin antibiotic for infections | Fine granules | 33 |
| Isoniazid | First-line oral antituberculosis drug | Tablets | 34 |
| Acetaminophen (Paracetamol) | Antipyretic, analgesic for pain relief | Powder | 35 |
| Linezolid | Synthetic antibacterial for MRSA, sepsis | Tablets | 36 |
| Propylthiouracil | Antithyroid agent | Tablets | 37 |
| Azathioprine | Immunosuppressant for organ transplant rejection prevention | Tablets | 38 |
| Carbamazepine | Antiepileptic, antimanic for seizures, psychiatric conditions | Tablets, 50% fine granules | 39 |
| Folic Acid | Treats/prevents folic acid deficiency, supplementation | Tablets, granules | 40 |
| Cefaclor Monohydrate * | Oral cephalosporin for infections (Staph, Strep, E. coli) | 10% fine granules | 41 |
| Cefdinir * | Oral cephalosporin for infections (Staph, Strep) | 10% granules | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, T.; Jiang, Z.; Zhao, Z.; Kimura, S.; Onodera, T.; Toko, K. Detection of Oral Beta-Lactam Antibiotics Using a Taste Sensor with Surface-Modified Lipid/Polymer Membranes. Chemosensors 2025, 13, 186. https://doi.org/10.3390/chemosensors13050186
Uchida T, Jiang Z, Zhao Z, Kimura S, Onodera T, Toko K. Detection of Oral Beta-Lactam Antibiotics Using a Taste Sensor with Surface-Modified Lipid/Polymer Membranes. Chemosensors. 2025; 13(5):186. https://doi.org/10.3390/chemosensors13050186
Chicago/Turabian StyleUchida, Takahiro, Ziyi Jiang, Zeyu Zhao, Shunsuke Kimura, Takeshi Onodera, and Kiyoshi Toko. 2025. "Detection of Oral Beta-Lactam Antibiotics Using a Taste Sensor with Surface-Modified Lipid/Polymer Membranes" Chemosensors 13, no. 5: 186. https://doi.org/10.3390/chemosensors13050186
APA StyleUchida, T., Jiang, Z., Zhao, Z., Kimura, S., Onodera, T., & Toko, K. (2025). Detection of Oral Beta-Lactam Antibiotics Using a Taste Sensor with Surface-Modified Lipid/Polymer Membranes. Chemosensors, 13(5), 186. https://doi.org/10.3390/chemosensors13050186






