Abstract
The fabrication of miniaturized and durable pH electrodes is a key requirement for developing advanced analytical devices for both industrial and biomedical applications. Glass electrodes are not an option in these cases. Electrodes based on metal oxides have been the most studied for pH sensing in these and other applications. Stainless steel pH electrodes have been an option for many years, both for measurement using steel as a sensitive material and using it as a substrate for the deposition of other metal oxides; in the latter case, the sensitive ability of stainless steel seems to play a crucial role. In addition, recent use as a substrate for materials such as polymers, carbon nanotubes, and metallic nanoparticles should be considered. This paper presents a review of this type of pH electrode, covering aspects related to the sensing mechanism, the treatment of stainless steel, potentiometric performances, applications, and the prospects of these sensors for use in modern analytical instruments. Sensing with the oxide passive layer and the artificial layer by oxidation treatments is analyzed. The use of metal oxides and other materials as the sensitive layer on stainless steel, their application in wearable devices, microneedle sensors, and combination with field-effect transistors for high-temperature pH sensing are covered as the most current and promising applications.
1. Introduction
pH is a fundamental parameter measured in any modern chemical laboratory. For this purpose, the most widely used is the glass membrane electrode, which is based on the potential difference between the internal and external sides of a glass membrane selective to H+ ions [1]. The difference in H+ activity between the solutions of both sides causes a potential difference that is quantitatively evaluated through the Nernst equation. Since the H+ ion activity in the inner solution is constant and known, the potential difference at the boundary between the inside and outside of the membrane depends on the pH of the sample. This makes it possible, after calibration, to know the H+ activity and thus the pH when measuring a potential difference from a reference electrode. The measuring cell scheme of a glass membrane pH electrode is [2,3]:
The external reference electrode (first from left to right) is currently already integrated in the glass tube of the “combined” pH electrode.
Glass membrane electrodes for pH are mainly composed of a silicate matrix containing other atoms such as sodium, lithium, calcium, barium, aluminum, and boron, depending on the requirements for the working pH range as well as the manufacturer. In the glass membrane, the oxygen atoms of silicates are negatively charged, and this charge is compensated by Na+ or Li+ ions [3,4]. These ions have a certain mobility when the membrane is hydrated, which allows an ion-exchange process with the H+ ions of the sample, establishing a potential difference dependent on the H+ activity in the external solution, which ultimately governs pH measurement with this type of electrode [3,5].
In a potentiometric cell, several potentials are developed. There are potentials associated with the reference electrodes (Eref1 and Eref2). There is also the potential associated with the junction of the solutions, known as the liquid junction potential (Ej), which affects the accuracy of the measurements because it is not strictly constant. In addition, the asymmetry potential is associated with the glass membrane due to morphological differences between the outer and inner surfaces [3]. For a pH electrode, the Nernst equation describes the potential as a function of the H+ activity, according to Equation (1) [3,5]. A constant, K, groups all constant potentials, such as the standard half-cell potential, the reference potentials, and the liquid junction potential, although the latter, as mentioned above, is not entirely constant.
where ΔE is the potential difference (V), K is a constant (V), R is the ideal gas constant (8.314 J/mol K), T is the temperature (K), F is the Faraday constant (96,485.33 C/mol), and is the activity of the H+ ions.
The effect of interfering ions, such as sodium, can be considered by introducing the product of the potentiometric selectivity constants of the interfering ions by their activity in the logarithmic term [5].
Glass membrane pH electrodes have certain disadvantages, although they are the most used in laboratories. First, they generally exhibit an alkaline error and an acid error due to the intrinsic response mechanism of the glass. The pH at which the alkaline error becomes significant depends on the type of glass used in the membrane, but in general, it is an effect that exists for any glass electrode, resulting in a pH reading lower than the actual value of the solution. On the other hand, the acid error is mainly present in the cheaper glass electrodes and consists of a pH measurement higher than the actual value of the solution. This error is associated with saturation of the active sites in the membrane, which causes loss of response at pH values below 0.5 [3]. Another limitation of glass membrane electrodes is the fragility of the material, which prevents their use in certain conditions, such as highly agitated systems with a high content of solids; this can lead to electrode breakage. On the other hand, glass electrodes are not suitable for the miniaturized systems that can be used in industry or in vivo analysis, for decentralized analysis in confined environments, for biomedical, and other special applications.
This has led to the development of other pH sensors with increased mechanical strength and structural flexibility. Among the best known are metal/metal oxide (MMO) and metal oxide (MO) electrodes, which consist of a metal surface coated with an oxide layer. Both types are very similar, but they differ in their pH response mechanism [6]. In both cases, the oxide layer allows the recording of a potentiometric response to pH when the electrode is immersed in the solution. Oxides of iridium, zinc, platinum, tungsten, and other metals have been used for pH measurement [7,8,9,10]. Another material that has been widely used is stainless steel, which is believed to respond to the activity of H+ ions in a solution via the same mechanism. Stainless steel has been used for pH measurement for many years [11], and although other materials such as IrO2 or RuO2 have been investigated, stainless steel remains an inexpensive alternative for pH-selective electrode construction. In addition to being a sensitive material, it also provides a substrate for coating with metal oxides, polymers, and nanomaterials [12,13].
This paper summarizes, for the first time, aspects related to the application of stainless steel as a material for pH sensors. In the context of the current development of miniaturized and decentralized analytical technologies, with a clear example in wearable devices, stainless steel can be a low-cost and high-performance material for this type of application. The review discusses aspects related to the response mechanism, surface treatments, potentiometric performances, and future perspectives of stainless steel pH electrodes.
2. Mechanism Behind the pH Response of Stainless Steel Electrodes
It is important to emphasize that the possible mechanisms of potentiometric response to pH of a stainless steel electrode are based on a surface layer of metal oxides, so these are fulfilled for the oxides of the metals that compose the stainless steel, as well as for other oxides that may be deposited on the steel as a substrate.
To discuss the mechanism underlying the potentiometric response of stainless steel to pH, it is first necessary to address the chemical characteristics of this metallurgical material. Stainless steel is an alloy containing iron and carbon, but it contains at least 10.5% chromium, which gives it a high resistance to corrosion [14]. There are several types of stainless steel: austenitic, martensitic, ferritic, austenitic–ferritic, and precipitation hardening. These microstructures are defined by the chemical composition and metallurgical conditions of formation, which directly influence their properties, such as hardness, ductility, magnetic properties, etc., and at the same time, they define their applications. It is common to use the series of the American Iron and Steel Institute (AISI). The series 200 and 300 include austenitic steels, which are the most used. The series 400 includes ferritic and martensitic steels [15]. All these chemical and microstructural characteristics are essential for explaining the potentiometric behavior of a stainless steel pH electrode.
Stainless steels are protected from corrosion by a passive surface oxide layer, but in order to be used as pH electrodes, they are typically exposed to a synthetic surface oxidation process that mainly produces iron and chromium oxides (including mixed oxides), though depending on the type of stainless steel, other oxides may also form [16,17]. This suggests that the response mechanism to H+ ions in solution is the same as that proposed for metal oxide electrodes. Nomura and Ujihira [18], some of the pioneers of stainless steel pH electrodes, state that any type of oxide-coated metal can function as a pH sensor if the oxide film is properly prepared. Furthermore, they propose that the response of metal oxide electrodes occurs according to the ion-exchange mechanism at the active –OH sites formed on the metal oxide surface when the electrode is introduced into solution. This mechanism has been assumed by other authors [19].
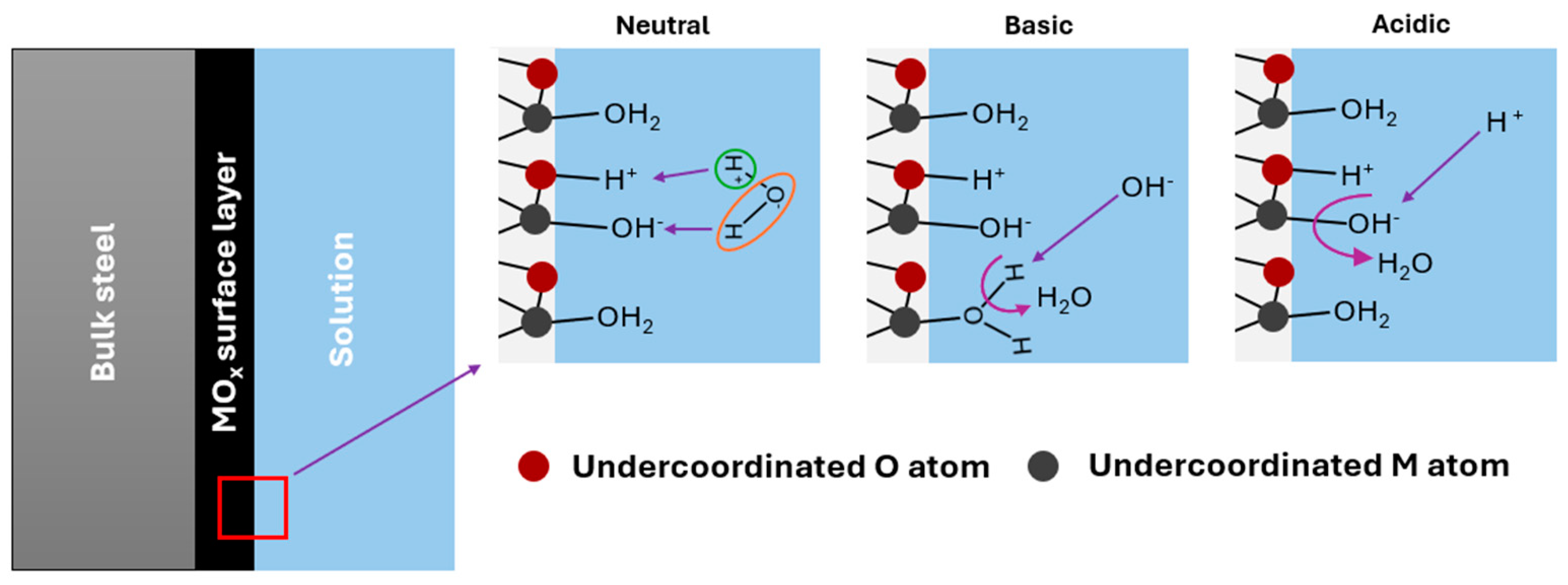
When a metal oxide film is immersed in an aqueous medium, several processes occur that depend on the surface and solution chemistry. The structure of an electric double layer (EDL) and the charge generation at the MO–electrolyte interface are not yet fully understood. However, attention must be paid to the development of acid–base surface groups in order to support what occurs with H+ ion sensing. A recent theoretical study by Zhang et al. [20] provided information on the behavior of metal oxides in neutral, basic, and acidic media, although it focused specifically on TiO2. According to these authors, water can adsorb both dissociatively and non-dissociatively on the oxide surface, and the former leads to the formation of surface OH− and H+ groups adsorbed on M and O atoms, respectively, which are undercoordinated at the interface (Figure 1). Both the adsorbed water molecules and the OH− and H+ are active sites, which is consistent with site-binding theory. In a basic medium, an OH− ion can bind to an H+ ion adsorbed to an oxygen atom of the oxide (mechanism I) to form a water molecule in the liquid or dissociate an adsorbed water molecule to form a surface OH− and a free water molecule (mechanism II). According to Zhan et al. [20], mechanism II is more probable, which does not mean that both cannot occur. This leads to a negative surface charge in alkaline media, as has been observed experimentally [21,22]. On the other hand, if the medium is acidic, the H+ ions can bind to adsorbed OH− ions and form water (mechanism III) or they can bind to the surface of the oxide via oxygen atoms (mechanism IV). Mechanism III is the most expected, but mechanism IV can also occur. In this way, a positive surface charge is created, in agreement with what has been observed experimentally for metal oxides [21,22]. These surface changes result in a potential variation that allows potentiometric pH measurement using a stainless steel electrode coated with a passive layer of metal oxides. It is important to note that all these phenomena occur in the context of an EDL. A Gouy–Chapman–Stern EDL consists of a Stern layer containing the layer of specifically adsorbed species and the counterion layer, followed by the diffuse layer [20].
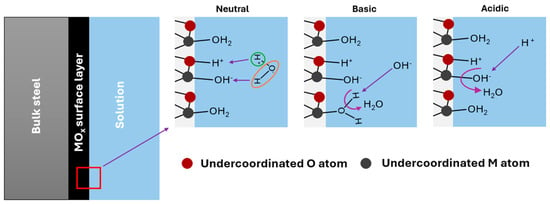
Figure 1.
Most probable mechanisms for the development of surface charge on a metal oxide layer immersed in a solution.
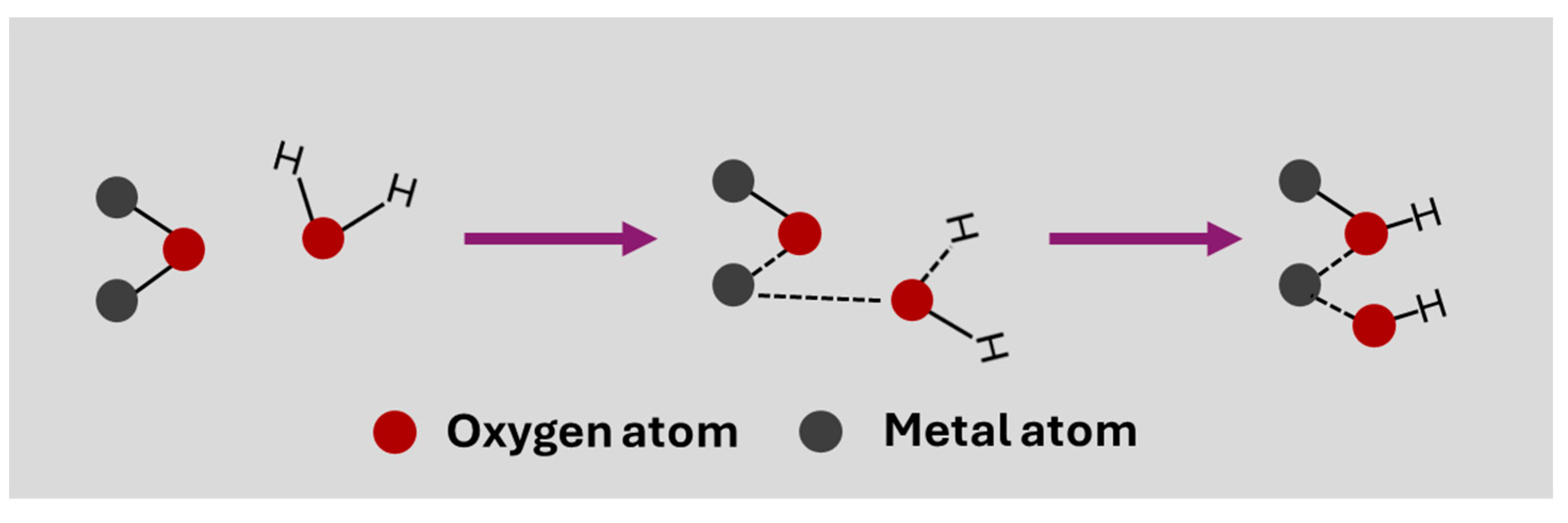
This is an updated understanding of the mechanism of pH measurement using this type of electrode. It is important to keep in mind that there is a simple theory based on the formation of a MOH layer when the electrode is immersed in water. In this case, the dissociative adsorption of a water molecule causes the formation of two surface –OH groups, due to the adsorption of the hydrogen atom on oxygen and the OH− on the metal atom of the surface oxide (Figure 2) [23]. These –OH groups are responsible for the surface ion exchange that causes potential variation in pH measurement, forming O− and –OH2+ groups depending on whether they give or accept H+.
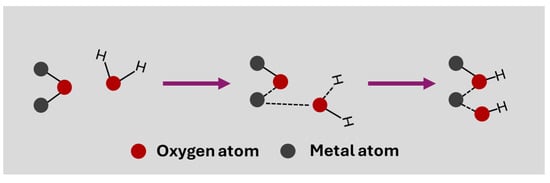
Figure 2.
A simple mechanism for the dissociative adsorption of a water molecule on a metal oxide to obtain surface –OH groups.
The ion-exchange mechanism discussed above is similar to that which occurs in a glass membrane, naturally adapted to the chemical characteristics of the MO–electrolyte interface. However, in addition to the ion-exchange mechanism, Fog and Buck [24] proposed four other mechanisms that should be considered, detailed as follows:
1. The redox equilibrium between two solid phases, such as a lower and a higher valence of an oxide, or an oxide and a pure metal. This is the operating principle of the well-known antimony pH electrode (Equation (2)).
The potential difference between the solid phase (an MO electrode in this case) and the liquid phase (solution, aq) is described according to Equation (1).
2. Solid solution or intercalation reaction, in which an H+ ion from the solution enters the metal oxide structure (Equation (3)). The potential difference governing the potentiometric response is calculated according to Equation (4).
where L and S are the liquid and solid phases, respectively. The other variables are the same as described in Equation (1).
3. The reaction between the H+ ions of the solution and the surface oxygen of the metal oxide (Equation (5)). This mechanism is also known as oxygen intercalation. The potential difference is calculated using Equation (6).
4. A steady-state corrosion of the electrode material. If there is kinetic control, there will be no Nernstian response.
Although these mechanisms were proposed in the 1980s, the exact mechanism of the potentiometric response of an electrode with a metal oxide interface remains unclear to this day. This is because it is difficult to generalize the same mechanism for all oxides, since each material has different chemical and crystalline characteristics that directly influence its behavior at the MO–electrolyte interface. Among these, the ion-exchange mechanism, two-solid-phase equilibrium, and intercalation reaction seem to be the most widely accepted. In the case of the response of the oxide layer of stainless steel, this may be even more difficult to explain by a single mechanism because the surface composition of the oxide layer in this material is complex due to the presence of oxides of different metals and even mixed oxides.
3. Surface Treatments of Stainless Steel for pH Response
In general, stainless steel is subjected to oxidative treatment in order to increase the surface oxide film, which allows a potentiometric response to H+ ions. This treatment can be performed in an aqueous medium with strong oxidizing agents, or atmospherically at high temperature.
Strong oxidants are used for wet oxidation, and a mixture of 2.5 M CrO3 and 5.0 M H2SO4 at 70 °C is common. A time between 10 and 30 min is sufficient to cause the formation of a layer that produces a potentiometric response [18]. Another alternative is to use a mixture of 2.5 M K2Cr2O7 and 5.0 M H2SO4, at 70 °C, with stirring, for up to 3 h [25]. Note that in both cases, a highly oxidizing chromic mixture is prepared.
Atmospheric oxidation is carried out in a furnace at temperatures above 400 °C, usually between 600 and 700 °C for the best results. The treatment time can usually vary from 1 h to 24 h [11,18]. After heating, the stainless steel undergoes a color change that depends on the intensity of the treatment in terms of temperature and time [18]. A yellow to violet-brown color can occur when going from a less to a more intensive treatment. These colors reflect different surface chemical characteristics that affect potentiometric measurement. Temperature is a critical factor in this case, and the potentiometric response varies depending on the type of steel used. The most common is the AISI 300 series, with 304, 303, and 316 as the most used.
On the other hand, stainless steel has been used as a substrate for the deposition of metal oxides that are generally foreign to the elemental composition of this material. Some oxides, such as iridium, ruthenium, and tungsten have shown Nernstian, stable, and reproducible pH response [7]. Methods such as electrodeposition, sol-gel dip-coating, and sputtering have been used [6,26,27,28]. Electrodeposition consists of insoluble oxide forming on the surface of stainless steel while it is used as an electrode in an electrolytic cell. Cyclic voltammetry using a solution of the metal has been used for this purpose [26,27]. On the other hand, sol-gel dip-coating combines the sol-gel method with dip-coating to form homogeneous films on substrates. The sol is prepared from the chemical precursors of the oxide, then the substrate is immersed, extracted, and covered with a film; thereafter, a surface gel is formed because of the evaporation of the solvent. The film is stabilized by a subsequent drying process and, usually, a high-temperature treatment [29]. Sputtering is a physical deposition method in which plasma is formed from an inert gas; the ionized atoms in the plasma are accelerated and, upon impact with the target material, release atoms that are deposited on the substrate to form a coating. In the case of metal oxides, oxygen gas is injected into the system [30,31]. Coatings with other materials such as polymers and nanomaterials are commonly achieved by the dip-coating and electrodeposition techniques [13,32].
4. Potentiometric Performance of Stainless Steel for pH Electrodes and Their Applications
4.1. pH Electrodes Based on Stainless Steel as a Sensitive Material
One of the most important contributions to the use of stainless steel as a pH-sensitive material was published by Nomura and Ujihira [18]. They used the austenitic steels SUS304 and SUS316 (Japanese standards similar to AISI 304 and AISI 316), which were exposed to an oxidative treatment to produce a sensitive oxide film. Both wet oxidation with a 2.5 M CrO3/5.0 M H2SO4 mixture at 70 °C and oxidation by heating in an oven at 400–700 °C were tested, whereas some electrodes were prepared by combining both techniques. The pH electrode was connected to the gate of a field-effect transistor system (FET) and a Ag/AgCl electrode was used as a reference.
Among the main findings of this work was that the SUS304 electrode only showed a Nernstian response from pH 1 to 13 with heat treatment at 700 °C, while at lower temperatures, there was a loss of response below pH 4 due to surface defects caused by insufficient coverage and changes in the oxide layer due to iron dissolution. Temperatures above 800 °C were not effective for treatment. It was observed that upon increasing the treatment time and temperature, the film thickness increases. The best film thickness was between 30 and 70 nm. The only significant interfering effect was found for Cl− ions, especially at 0.5 M, where the linear response was lost below pH 4, possibly due to the dissolution of the metal oxides in the HCl medium. Unlike SUS304, SUS316 did not produce a stable pH response when heat-treated. However, the treatment with the oxidizing mixture produced the same response as SUS304. Nevertheless, in this case, there was no effect of Cl− ions, demonstrating the better suitability of this material for pH sensing. The indifference to Cl− ions was attributed to the presence of molybdenum in SUS316, resulting in less charge in the active sites where chloride is incorporated. This suggests that the adsorption of chloride ions generates destabilization, blocking, or competition at the interface, which affects the potentiometric response. This work laid some of the groundwork for the use of stainless steel as a pH-sensitive material, showing some of its main limitations and the differences in response depending on the surface treatment. These were factors that subsequently played an important role in the abandonment of the native oxide layer of stainless steel for pH sensing.
Zampronio et al. [25] developed a potentiometric flow cell using AISI 316 stainless steel pH electrodes with oxidative pretreatment. This cell was used for the determination of acid mixtures by flow injection analysis (FIA). They constructed electrodes with two different geometries, a flat electrode and another with tubular geometry. In the work, a mixture of 2.5 M K2Cr2O7 and 5.0 M H2SO4 at 70 °C was used for oxidation for different periods ranging from 10 min to 3 h. The pH range evaluated was from 2 to 12 in buffer medium. Three FIA cell designs were tested; in one of them, the 80 μL potentiometric cell contained the reference electrode (Ag/AgCl) in direct contact with the liquid flow. Another model contained a 3 M KCl solution between the reference electrode and the flow system. The third model cell was constructed for the tubular electrode in which the stainless steel plate was perforated to create an internal cell volume of 15 μL, and the solution was passed through this hole. According to Zampronio et al. [25], the electrodes showed yellow, red, green, blue, and violet colors depending on the time, temperature, and agitation during the treatment. As mentioned above, the intensity of the treatment changes the coating color due to a different composition in terms of the amount and type of surface oxide on the stainless steel.
Calibration of the oxidized electrode for 1 h yielded a slope of −52 mV/pH, which decreased (in absolute value) to −43.7 mV/pH when recalibrated after 5 days of uninterrupted use and remained close in subsequent calibrations. The calibration of 10 different electrodes had an average slope of −45 mV/pH. In general, the response of these electrodes was sub-Nernstian. The response time of the electrode in FIA cells must be fast; in this case, the electrode responded in 5 s, a sufficient time for this kind of application. This demonstrates the potential of these electrodes for applications where traditional membrane glass electrodes have limitations, ranging from adaptation to the analytical system to performance parameters such as response time.
The electrode was stable for one month, but the authors observed that the presence of chloride ions and the use of solutions with pH < 3 reduced its durability to one week. This is one of the main limitations of stainless steel electrodes with native oxides as a sensitive material, as observed in the case of the results reported by Nomura and Ujihira [18].
The cell with the reference electrode separated by a KCl solution showed high noise (50 mV amplitude) in the potentiometric response, which the authors attributed to the pulsations of the peristaltic pump or the interference from the laboratory circuit. The noise was reduced to 6 mV by using a grounded stainless steel tube. The cell with direct contact between the sample and the Ag/AgCl reference electrode showed less noise (2 mV) without the need for the grounded stainless steel tube. However, this cell had some disadvantages related to air bubbles around the electrodes. The response of the tubular electrode was not good, due to a loss of sensitivity. The authors used the FIA system with the stainless steel electrode to titrate mixtures of succinic acid and oxalic acid (both with close pKa) with NaOH. A multivariate calibration model was built, and the data were inverted to use time as an independent variable to extract more information from the variation of the potentiometric titration process.
The work of Zampronio et al. [25] is an example of the potential that stainless steel pH electrodes could have for specific applications within analytical systems. However, it also highlights some important drawbacks compared to other sensitive materials. The fact that the sensor loses stability in very acidic conditions or in the presence of chloride ions from one month to one week limits the application of the electrode only for routine titrations where HCl is not used. However, these characteristics may be specific to each stainless steel electrode, so the fact that this was the case in this instance does not mean that it would be the same for another electrode, even if it is made of the same type of stainless steel. This is indicated by the fact that, for example, the slope of the calibration curve is not the same in all papers reporting results using the same type of stainless steel, such as the results of Zampronio et al. [25] and those of Nomura and Ujihira [18]. This is because potentiometric sensing is an interfacial phenomenon, and the surface properties of the stainless steel determine the potentiometric and analytical performance parameters. Properties such as surface roughness, surface defects, or local surface composition influence the characteristics of the oxide layer formed after oxidative treatment. However, the fact that several authors have reported the effect of chloride ions and loss of response in highly acidic media is an important reason to evaluate it when developing any stainless steel electrode.
Hashimoto et al. [11] investigated the effect of the heat treatment on the sensitivity of the stainless steel pH electrodes. SUS304 stainless steel electrodes were treated at 500 to 700 °C for 24 to 96 h in an oven under atmospheric air. The electrode potential was cyclically measured in three buffer solutions with pH values of 7, 4, and 9. The relative sensitivity of the sensor (%) was calculated using Equation (7), which is a way of measuring the change in the potential differences Ea and Eb (versus a Ag/AgCl reference electrode in this case) between pHa and pHb against the theoretical slope of −2.303 RT/F = −0.05916 V/pH, when T = 298.15 K.
Among the main findings of this work is that increasing the oxidation temperature up to 600 °C resulted in an increase in the martensite crystalline phase, although austenite remained dominant. Martensite decreased again when the treatment was carried out at 700 °C. The pH sensitivity of the material showed the same trend as the martensite composition, so it was concluded that there is a dependence between them. In addition, this work discussed the influence of the stainless steel underlayer on electrodes in which this material is coated with a metal oxide. Currently, stainless steel is used more as a substrate for other oxides than as a sensitive material. Several authors have found that the potentiometric response of these oxides is better on stainless steel than on other metal substrates. According to Hashimoto et al. [11], citing an earlier paper [28], the response of CuO/Al and Al was unstable and over-Nernstian, while that of CuO/SUS304 and SUS304 was stable and Nernstian. This shows that the electrode’s response is strongly dependent on the underlying layer of the substrate and not only on the surface oxide. For this reason, the sensitivity of stainless steel to H+ ions cannot only be seen as an aspect relevant to the application of this material as an electrode; it is also an active property in the application as a substrate.
As part of research into the use of stainless steel as a pH-sensitive material, our research group demonstrated that the response of AISI 304 can be Nernstian and reproducible without the need for artificial oxidative treatment, i.e., the response of the passive oxide layer of stainless steel can be enough, so a previous investigation of the material without artificial oxidation is necessary. In addition, the non-artificially oxidized electrode was tested in an acid–base titration. A slight underestimation of the pH was observed at pH 9.5 and above. However, this behavior did not affect the result of the potentiometric titration when the data were processed by first- and second-derivative methods. The results were comparable to those obtained with a glass electrode and with titration with a colored indicator [33].
Although the chemical properties of stainless steel allow it to be used as a pH-sensitive material, as seen above, currently, this application has been displaced because coating with oxides, polymers, nanomaterials, and others, allows a more versatile handling of the interface, which improves the potentiometric response to H+ ions in terms of sensitivity, selectivity, and stability. This makes stainless steel a preferred material as a substrate rather than as an electrode. However, there are still many aspects to be investigated in depth that could revive interest in the use of stainless steel as a sensitive material, such as the mechanism underlying chloride ion interference or loss of response in highly acidic media. All of this is important, considering that a pH electrode may be much less expensive when using stainless steel as the sensing material than when using advanced materials such as metal nanoparticles or carbon nanotubes as part of the modifier coating. This makes this material very viable for industrial applications, as will be detailed below.
4.2. pH Electrodes Based on Stainless Steel as a Substrate
The use of stainless steel as a substrate for other sensitive materials seems to be a trend for this alloy in potentiometric pH sensing. In this context, Hashimoto et al. [28] fabricated 3d-block metal oxide-coated SUS304 electrodes for pH sensing via the sol-gel dip-coating method. According to these authors, RuO2 and IrO2 materials are too expensive, while some attempts to reduce the cost—through the use of binary systems like IrOx-TiO2, RuO2-SnO2, and RuO2-Ta2O5—have produced sub- or over-Nernstian responses. In contrast, metal oxides from the 3d-block of the periodic table are a cheaper option. Therefore, they tested MOx/SUS-type systems, where M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn.
The electrode modification consisted of preparing the coating system from the metal source, where the SUS304 electrode was immersed and pulled up at a rate of 0.5 mm/s. The film was preheated at 500 °C for 10 min. This was repeated three times, and finally, the film was treated at 500 °C for 24 h. In this work, a Ag/AgCl reference electrode was used. The SUS304 electrode showed 90.9% relative sensitivity with an initial pH response time of 1 s, while SUS304 treated at 500 °C showed 94.1% relative sensitivity with the same initial pH response time. Some oxides showed lower relative sensitivity than the substrate, such as NiO/SUS, with a sensitivity of 87.7%. The best relative sensitivity was obtained for the Co3O4/SUS electrode with 99.8% and 1 s initial pH response time. The ZnO/SUS, CuO/SUS, Cr2O3/SUS, and Mn2O3/SUS all showed relative sensitivity greater than 97%, the last two being greater than 98%. The glass pH electrode showed a relative sensitivity of 99.2%, but its initial pH response time was 14 s. Most of the electrodes showed good pH repeatability. The reaction that the authors associated with the potentiometric response mechanism of these oxides to H+ ions is shown in Equation (8), where MOx is a higher-valence metal oxide and MOx−δ(OH)δ is a partially hydrolyzed lower-valence oxide. The sensitivity of the 3d-block metal oxides to pH depends on how likely this reaction is.
It is important to note the significant difference in response time compared to the glass pH electrode. This advantage of metal oxide electrodes is mainly due to their sensing mechanism. Firstly, in these electrodes, the process takes place directly at the electrode–solution interface, whereas in the glass electrode, ionic diffusion into the selective membrane is required. In the mechanism presented in Equation (8), it can be observed that, in these electrodes, the potentiometric response arises from a surface redox reaction, which results in a faster response compared to the ion-exchange mechanism governing glass membrane electrodes. In addition, metal oxide electrodes exhibit a lower electrical resistance. All these factors facilitate faster detection and transduction than when using a glass membrane electrode for pH measurement.
On the other hand, the physical properties of stainless steel can also be an important advantage for its selection as a substrate for pH electrodes. In this context, Hashimoto et al. [34] presented Fe2O3-TeO2-based glass enamel/stainless steel electrodes for pH sensors. These authors chose SUS304 stainless steel for enameling because of its ease of handling compared to carbon steel and emphasized the importance of the coefficient of thermal expansion for this type of fabrication. The work of Hashimoto et al. [11,28,34] is essential to understand the path of stainless steel in the construction of pH sensors and the reasons that this material is among the favorites for this application. The use of stainless steel as a substrate is not only beneficial in terms of cost; its chemical and physical properties also allow the construction of pH sensors with higher performance than those constructed with other materials, both metallic and non-metallic. This makes stainless steel suitable for the manufacture of advanced pH-sensitive devices for industrial applications and biomedical technologies, to name just a few.
Sadig et al. [35] fabricated pH sensors using SUS304 stainless steel and Ti wires as substrates. The technique used was sol-gel spray-coating, in which an aerosol of the coating solution was formed using a nozzle and pressure and sprayed onto the moving substrate. The spray-coating system was designed to make the technique more economical and environmentally friendly. After coating, the substrate was dried at 90 °C and then calcined at 400 °C for 2 h. This method allowed the development of an IrO2-RuO2-TiO2 film sensitive to H+ ions. The authors suggest, according to the general reaction presented in the paper, that the redox equilibrium between two solid phases is a possible mechanism among those proposed by Fog and Buck [24], with partial reduction of Ir/Ru(IV) to Ir/Ru(III) and the formation of a couple of higher- and lower-valence metal oxides. More details on the reactions can be found in the work of Sadig et al. [35]. The authors propose that TiO2 is involved in sensing, although no reaction for this oxide has been described. Perhaps it is important to keep in mind that there are several possible sensing mechanisms and that, in reality, this situation may be too complex. The slope of the calibration curve of potential (versus calomel reference electrode) as a function of pH for the SUS304-based electrode was −59.0 mV/pH, which is very close to the calibration slope of the Ti-based sensor (−59.1 mV/pH). Both values were very close to the Nernstian theoretical value of −59.16 mV/pH.
The slope of the calibration curve and standard potential remained virtually unchanged over 120 days, demonstrating the long-term stability of the sensors. In addition, a drift rate of 3 mV/h and low hysteresis were observed for both film-modified substrates. The response time for both pH sensors was between 4 and 8 s, and the response was reproducible. Furthermore, the substrates were very stable at temperatures between 10 and 60 °C, and the slope of the electrodes remained close to the Nernstian value. There was no significant effect of K+, Na+, Li+, and Mg2+ cations. The electrodes were used to measure pH in real samples of milk, yogurt, lemon juice, rainwater, distilled water, and tap water, with very similar values compared to the commercial glass electrode. As an example of how each stainless steel electrode can respond differently, regardless of whether it is the same material, the slope of the calibration curve of SUS304 was −31.75 mV/pH, well below the value obtained in other previously discussed papers. In the field of stainless steel-based pH electrodes, the work of Sadig et al. [35] is very interesting since it allows a comparison of two substrates with very similar potentiometric performances, but with very different prices. Stainless steel-based electrodes are much less expensive than titanium-based electrodes. Therefore, it is possible to remark upon the competitiveness of this material against others.
Also comparing stainless steel and titanium substrates, Fiore et al. [36] presented a functionalized orthopedic implant as an electrochemical pH-sensing tool for intelligent diagnosis of hardware infections. The work focused on the problem of orthopedic implant infections, which can be life-threatening for patients. The monitoring of bacterial proliferation in these implants is possible through monitoring the pH, since the occurrence of infection involves a decrease in pH from physiological to acidic values. Screws made of stainless steel, titanium, and titanium alloy were tested as substrates for IrO2 electrodeposition. The stainless steel screw showed a sensitivity of −0.092 ± 0.004 V/pH (R2 = 0.975); for the titanium screw, it was −0.061 ± 0.002 V/pH (R2 = 0.992); and the titanium alloy screw showed a sensitivity of −0.058 ± 0.004 V/pH (R2 = 0.957). These results indicate that the stainless steel substrate caused an over-Nernstian response, making Ti-based substrates a better choice. From these results, a sensor was developed using a Ti implant modified with electrodeposited coating. The results of this work contrast with those of Sadig et al. [35], and several reasons may justify this. First, the sensitive layer and the coating method were not the same, which caused the surface characteristics, both chemical and physical, to differ. Second, if stainless steel is different, it can lead to different results. This is a clear example of the importance of investigating not only the sensitive coating but also the type of substrate.
An interesting aspect of this work is that the performance of three reference electrodes in potentiometric pH measurements was studied. A screen-printed Ag/AgCl pseudo-reference electrode, a bulk Ag/AgCl reference electrode (the traditional electrode with a glass tube containing KCl solution), and a silver wire reference electrode were compared in terms of sensitivity, reproducibility, and correlation coefficient. The results showed similar performance for all three electrodes, allowing the Ag wire to be selected for the implantable sensor due to its size and flexibility. The reference electrode is essential in an electrochemical measurement, and this type of study provides insight into the performance of electrodes when their design differs from the traditional type used in the laboratory. In particular, for applications such as those discussed in this article, the reference electrode must be miniaturized, which often leads to the modification of the chemical system that allows a constant and known reference potential, ultimately resulting in a pseudo-reference electrode.
On the other hand, the use of analytical technologies in wearable devices is now a reality. Electrochemical analytical methods are the most appropriate instrumental methods for these applications. In this context, sensors play a fundamental role in these devices, as do electronic circuits suitable for miniaturized and high-accuracy systems. The sensing of pH is of particular importance in the field of sports medicine. Athletes are exposed to conditions that can cause physiological changes in a very short time, which can lead to health problems. Sweat pH is an important indicator of these changes, so monitoring this parameter in athletes and other people is relevant to medical professionals. In this context, Zamora et al. [37] presented the development of textile potentiometric sensors for pH measurement. These authors tested different conductive fabrics (Argenmesh, Ripstop silver, and stainless steel mesh) as substrates for a sensitive layer of iridium oxide that was electrodeposited. An Ag/AgCl/KCl (3 M) reference electrode was used in this work. According to the authors, the Argenmesh fabric is made of nylon threads, 55% of which are coated with Ag; the Ripstop fabric is also made of nylon threads, all of which are coated with Ag; and the stainless steel mesh fabric is made of 100% surgical stainless steel threads. They also point out that the wearability and comfort of these fabrics are similar to those of traditional fabrics used in the textile industry, which will facilitate their integration into athletic or medical garments without discomfort.
The morphological and surface composition study showed a higher amount of electrodeposited metal oxide (i.e., IrO2) on the stainless steel mesh, and, unlike the other substrates, it did not undergo surface changes detrimental to the electrodeposition process. The others, however, suffered a loss of Ag coating, exposing non-conductive polymer fibers, which resulted in lower conductivity. The best potentiometric response was obtained for the stainless steel mesh electrode, whose calibration slope was sub-Nernstian (−47.57 mV/pH) but higher than that of −25.25 mV/pH for Argenmesh and −17.15 mV/pH for Ripstop. The difference in sensitivity was related to the amount of IrO2 electrodeposited in each of the fabrics. These results were obtained in a configuration in which the fabric was folded to form a double layer, but a new configuration was tested in which the steel fabric was stretched to provide a better contact surface between the wires. In this case, a decrease in slope (−32.11 mV/pH) was observed, which was related to the fact that the previous configuration provided a larger surface area for IrO2 electrodeposition. On the other hand, in response to the temperature change from 35 to 40 °C, the stretched fabric configuration proved to be more robust.
The pH of a sweat-like saline solution (pH 7.0) was measured using the stretched fabric configuration. The pH calculated from the measured potential difference was 7.011, an error of only 0.15%, demonstrating the accuracy of the measurement. The measurement was then carried out on real human skin, which gave a pH of 6.2, compared with 6.5 using a commercial strip test, giving a relative error of 4%. The sensor gave a response in a few seconds, whereas other reports took up to 30 min. It must be emphasized that the reference electrode used for these measurements, i.e., Ag/AgCl/KCl (3 M), was used within a small square flat device intended for this type of application. This further demonstrates the feasibility of the prototype for technological development and eventual real-world use.
The results of this work demonstrate the competitiveness and superiority of the sensor developed using a stainless steel textile substrate. The authors see this sensor as a viable device for wearable applications with wireless communication. From our point of view, the existing data supporting stainless steel as the material of choice for the fabrication of pH electrodes are strengthened by this work. In this case, the best results were attributed solely to the amount of IrO2 deposited on the fiber, but as previously demonstrated, the sensitivity of stainless steel may also play a role in the results.
As seen previously, in vivo pH monitoring is impractical with conventional analytical technologies. However, the use of advanced microsensors allows this type of analysis to be performed with results that are competitive with traditional methods in terms of analytical performance. García-Guzmán et al. [38] used stainless steel microneedles as a substrate for in vivo transdermal potentiometric pH sensing. The indicator electrode was based on a three-layer structure of carbon ink, functionalized multi-walled carbon nanotubes as an ion-to-electron transducer, and a hydrogen-selective membrane. The reference electrode was a layer of Ag/AgCl covered by a polyvinyl butyral membrane in one of the microneedles. The sensing system allowed responses close to Nernstian value, with repeatability and reproducibility. In the same vein, Liu et al. [32] presented a microneedle electrode array for multiparameter biochemical sensing in gouty arthritis. Gouty arthritis is one of the most common forms of inflammatory arthritis caused by the accumulation of uric acid in the joints. It is a health problem that affects many people and often becomes a cause of temporary disability due to the inflammatory process. This makes it necessary to monitor the patient’s clinical parameters in order to control the chronic disease. The monitoring system was developed in a plug-in design for a portable device controlled by a mobile application, allowing real-time, in situ, and dynamic monitoring of biomarkers. AISI 201 stainless steel microneedles were used. The parameters monitored by this device were pH, uric acid, and reactive oxygen species. First, the microelectrodes were pickled and electroplated with Au. For pH monitoring, the Au-coated microelectrode was modified with carbon nanotubes and polyaniline. In this work, the reference electrode was also integrated into the microneedle device by coating one of the gold electroplated microneedles with a Ag/AgCl paste and then with a layer of polyvinyl butyral, as in the work of García-Guzmán et al. [38]. The slope of the calibration curve was −62.8 mV/pH, close to the Nernstian value. This system showed good response to pH in the presence of the other analytes, indicating interference-free detection. In addition, the response was reproducible and stable. In vivo application demonstrated potential for real-world scenarios.
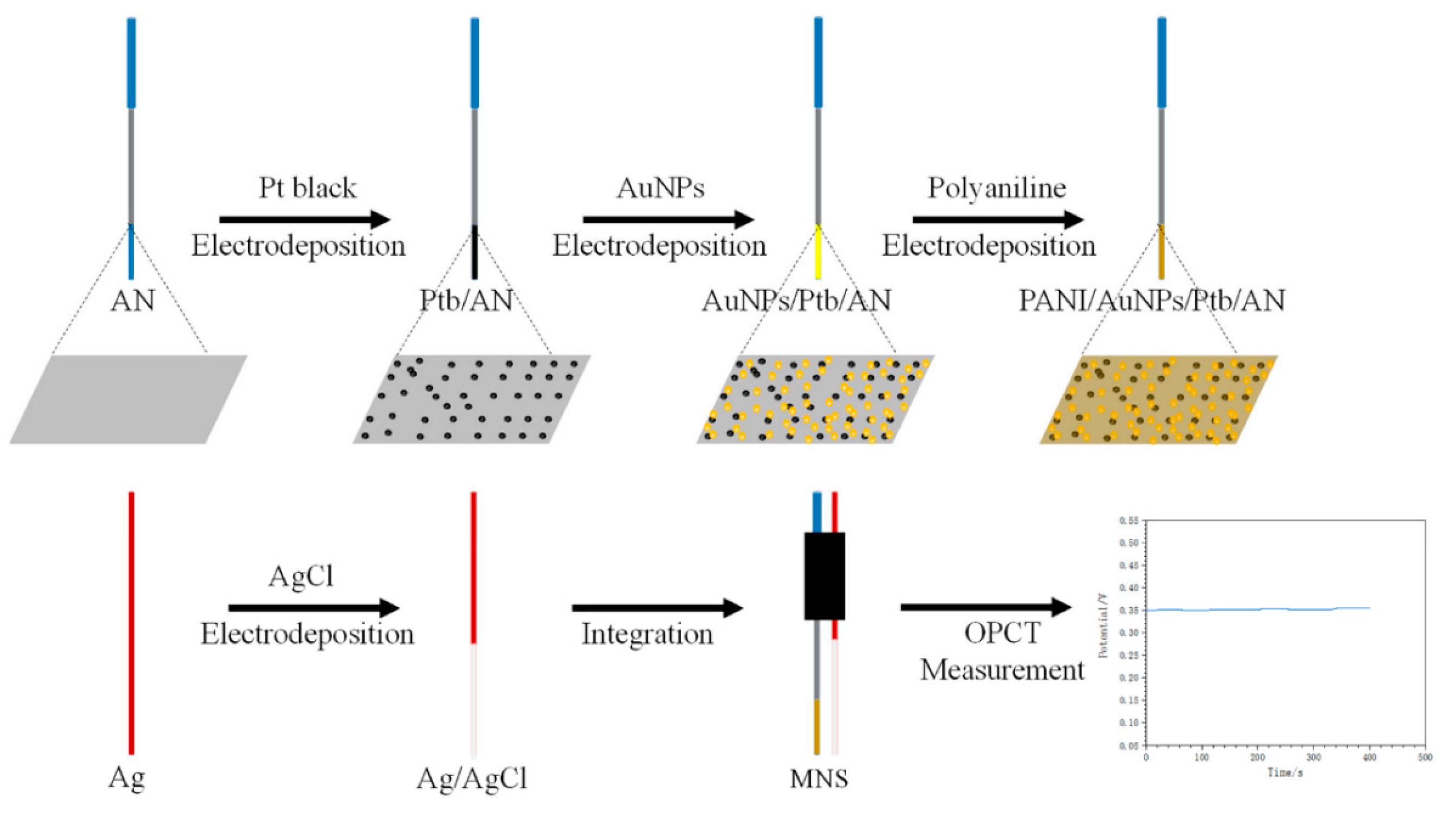
On the other hand, Ming et al. [13] presented an implantable microneedle sensor for pH monitoring (MNS). A stainless steel acupuncture needle (AN) was used as the sensitive substrate to construct the sensor. A layer of platinum black and gold nanoparticles was prepared by electrodeposition and subsequently modified with polyaniline to increase the pH sensitivity. An Ag/AgCl reference electrode was prepared and integrated for the sensing system (Figure 3).
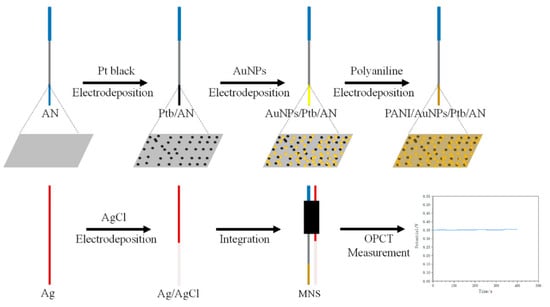
Figure 3.
Schematic of the preparation of the implantable microneedle sensor for pH monitoring (MNS). AN: acupuncture needle, Ptb: platinum black, AuNPs: gold nanoparticles, PANI: polyaniline, OPCT: open-circuit voltage–time. Image taken from Ming et al. [13] under CC-BY 4.0 license [39].
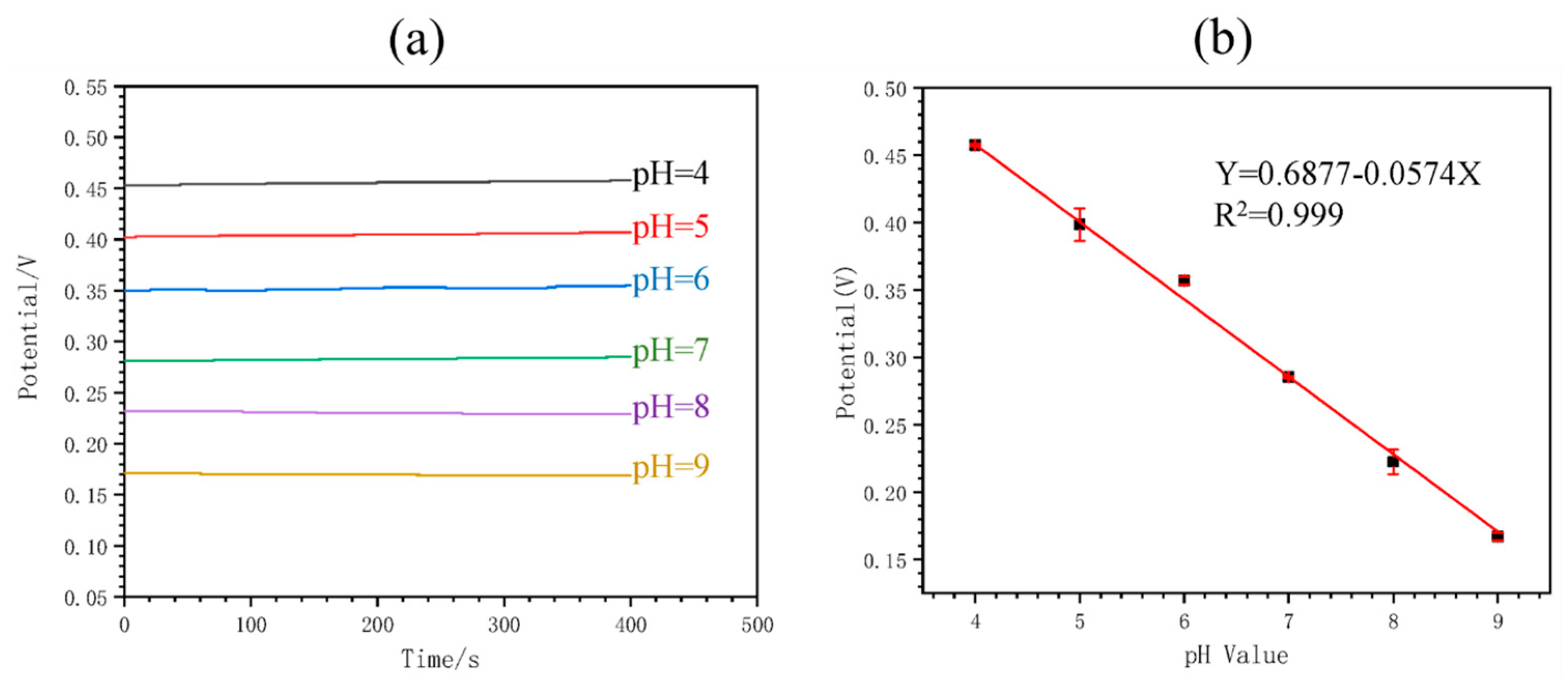
Figure 4 shows the calibration of the MNS. It can be noted that the OPCT decreases with the increasing pH of the solution (Figure 4a), yielding a calibration curve with a near-Nernstian slope value of −57.4 mV/pH (Figure 4b). This sensor demonstrated the ability to monitor pH in real time by analyzing buffer solutions and blood serum. In both cases, there was a minimal change in the potential with time for each pH value. The estimated pH response time was 420 s. Continuous in vivo pH monitoring was performed in rats by implanting the MNS in the main abdominal vein, demonstrating the functionality of the device for this application. The sensor response was selective to H+ ions in the presence of potential interferents Na+, K+, and Mg2+. In addition, the response was repeatable. After 7 days of storage of the sensor in serum, a decrease in the difference between the potential for pH 6 and pH 8 of 15.99% was caused, which the authors did not consider significant. Therefore, the sensor was found to be stable for continuous pH monitoring.
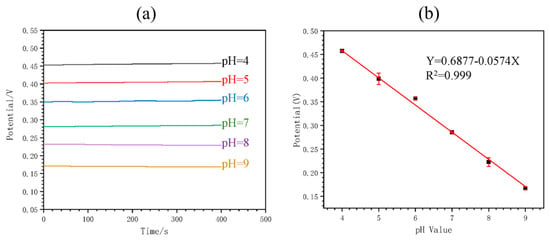
Figure 4.
Calibration of the implantable microneedle sensor for pH monitoring: (a) potential versus time for different pH values, and (b) calibration curve of potential versus pH. Image taken from Ming et al. [13] under the CC-BY 4.0 license [39].
The authors pointed out the following main limitations; firstly, the trauma during implantation because the sensor is composed of a needle electrode and a reference electrode. Second, the sensor needs to be connected to an electrochemical workstation. However, the contribution in terms of operability with respect to conventional methods is remarkable. In a clinical laboratory, blood pH measurement requires sample extraction, preservation, and preparation. All this is avoided by this potentiometric sensor. It should also be noted that these limitations can be overcome by using a sensor that integrates the indicator and reference electrodes in a single needle. On the other hand, working on the electronic system allows portability by using a smaller potentiometer and a wireless communication system. The authors mentioned this as an avenue for future work.
Note that the sensors presented by García-Guzmán et al. [38], Liu et al. [32], and Ming et al. [13] are not based on metal oxides. Instead, they integrate other types of materials such as nanoparticles, carbonaceous materials, polymers, and others, demonstrating the potential of stainless steel for applications in the context of electrochemical sensing based on advanced materials. There have been other works using materials such as polypyrrole with hydroquinone monosulfonate and oxalate co-doping, achieving a response of −54.67 mV/pH, close to the Nernstian value, for a pH ranging from 2 to 12 [40]. All these materials improve the potentiometric response in different ways, in some cases making it more selective and in others improving the conductivity or increasing the surface area, depending on the sensitive mechanism of the active layer.
4.3. pH Electrodes Based on Stainless Steel for Industrial Applications
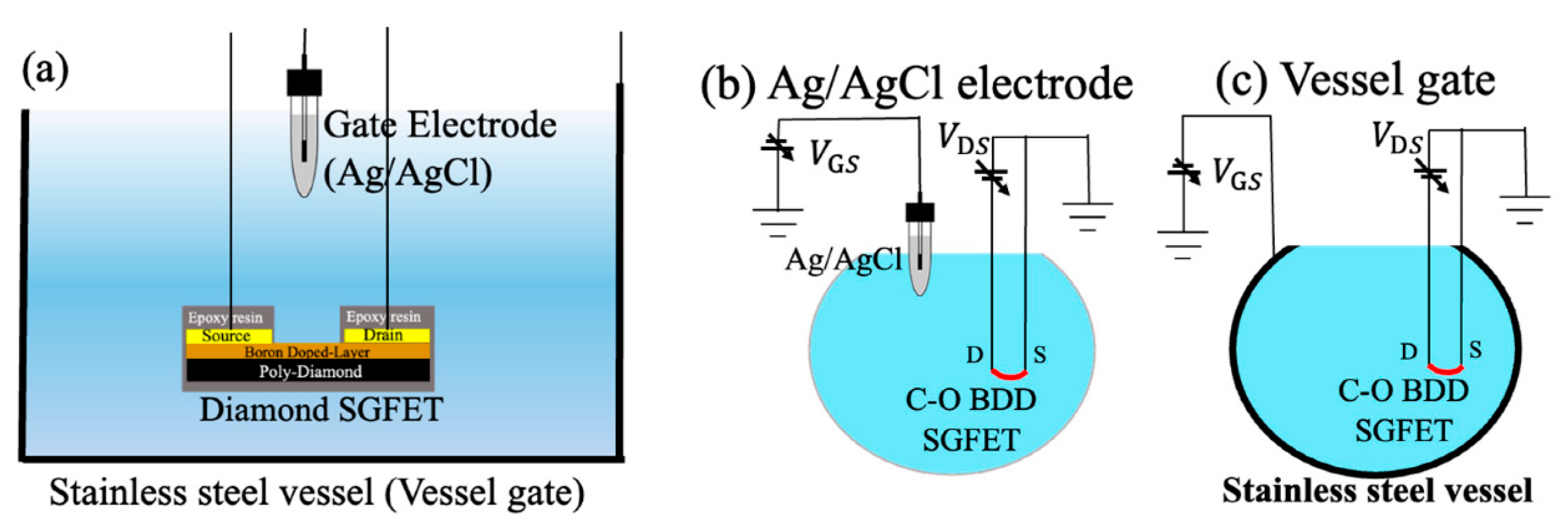
The industrial approach to stainless steel as a pH-sensitive material has not been neglected. For example, measuring pH at elevated temperatures can be complicated using traditional sensors, and in this context, Kawaguchi et al. [41] investigated pH measurement at elevated temperatures using a vessel gate and an oxygen-terminated boron-doped diamond solution gated FET (C-O BDD SGFET). Solution-gated field-effect transistors (SGFETs) are well known in the field of electrochemical sensing. These FETs operate in a solution, and the drain current is controlled by the potential induced by the electrical double layer on a gate electrode. According to the authors, diamond SGFETs are good candidates for pH-sensing applications because the hole concentration of boron-doped diamond SGFETs varies with different ion concentrations in the solution. These semiconductor sensors have a smaller size and higher mechanical resistance than glass electrodes, making them an alternative in cases where the traditional electrode cannot be used. However, the authors point out that these sensor systems use a glass gate electrode, which makes their use in the food industry, where high temperatures are required, unfeasible. They used a stainless steel (SUS304) vessel called a “vessel gate” as the gate electrode instead of a glass electrode. Stainless steel was chosen because of its proven sensitivity to H+ ions and its widespread use in the food industry due to its low cost and corrosion resistance. Figure 5a shows the cross-sectional view of the C-O BDD SGFET and the Ag/AgCl electrode in contact with a solution inside the vessel gate. Figure 5b shows the measurement schematic of the design with the Ag/AgCl electrode as a gate, while Figure 5c shows it with the vessel gate. When the Ag/AgCl electrode is used as a gate, the gate voltage is applied between the tip of the electrode and the FET channel, which is the sensing surface. When the vessel gate is used, the entire stainless steel surface becomes the sensing surface.
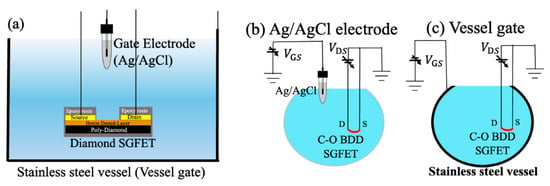
Figure 5.
(a) Cross-sectional view of the C-O BDD SGFETs and the Ag/AgCl electrode in contact with a solution inside the vessel gate, (b) measurement schematic of the design with the Ag/AgCl electrode as a gate, and (c) measurement schematic of the design with the vessel gate. The blue colors represent a solution. Image taken from Kawaguchi et al. [41] under the CC-BY 4.0 license [39].
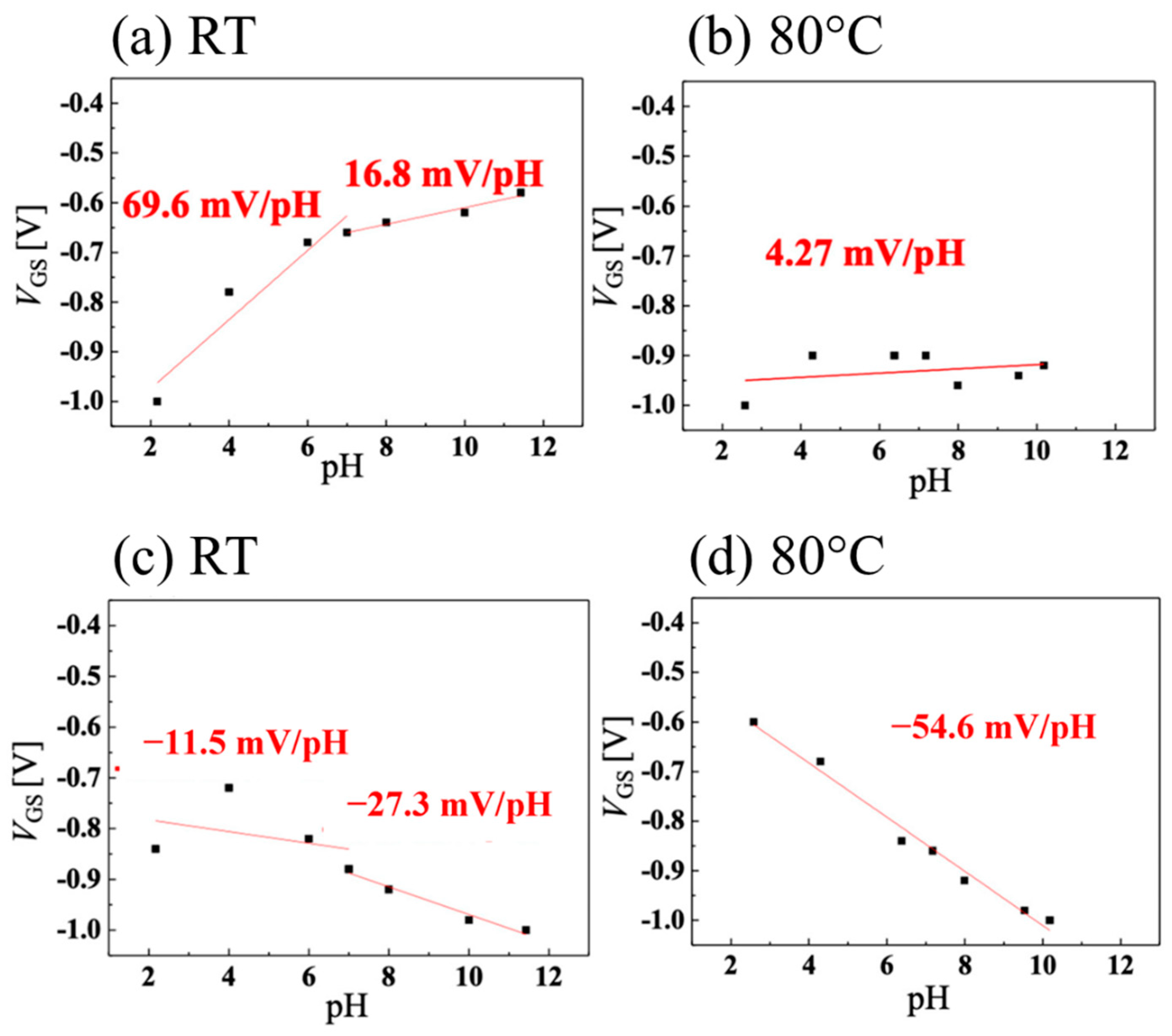
The sensitivities of the pH measurements for the system using the Ag/AgCl electrode as a gate are shown in Figure 6a for room temperature and in Figure 6b for 80 °C. The slope is high in the acidic medium but decreases in alkaline pH at room temperature. At 80 °C, however, the sensitivity drops sharply to 4.27 mV/pH over the entire pH range. The authors attribute this loss of sensitivity at high temperatures to an increase in the amount of activated boron, which reduces the effect of changes in the drain current caused by ions adsorbed on the surface due to changes in pH, ultimately resulting in a reduction in sensitivity. On the other hand, the sensitivity of the system using the vessel gate is shown in Figure 6c for room temperature and in Figure 6d for 80 °C. It is observed that at room temperature, the system becomes insensitive at acidic pH, with a slope of −11.5 mV/pH, increasing (in absolute value) to −27.3 mV/pH in alkaline medium. At room temperature, the sensitivity of the system is worse than when the Ag/AgCl electrode is used. The authors attributed the difference in the signs of the slope between the two systems to the fact that, in a sensing circuit, the direction of the surface dipole in the FET channel is the opposite of that of the vessel surface. Consequently, the effects of the ions on the two EDL capacitors of the FET channel and the vessel surface were opposite. At 80 °C, the system with the pH-sensitive vessel gate showed a slope of −54.6 mV/pH. At this temperature (353.15 K), the theoretical Nernst slope was −70.1 mV/pH, indicating that a system with 77.9% sensitivity was achieved.
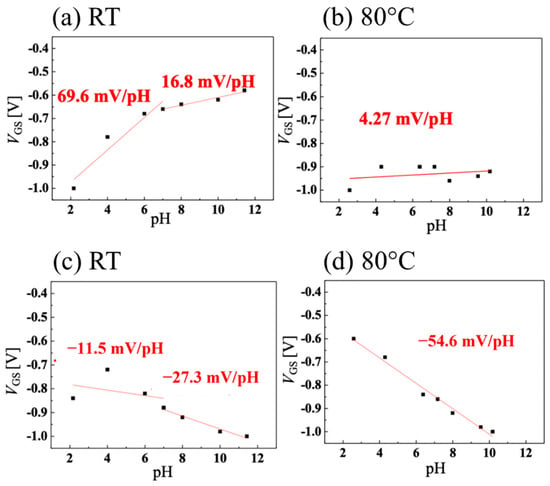
Figure 6.
pH sensitivity when (a) using a Ag/AgCl electrode at room temperature (RT), (b) using a Ag/AgCl electrode at 80 °C, (c) using the vessel gate at room temperature, and (d) using the vessel gate at 80 °C. Images taken from Kawaguchi et al. [41] under the CC-BY license [39] and combined into a single figure.
In a similar work, Chang et al. [42] presented an ion-sensitive stainless steel vessel for an all-solid-state pH-sensing system incorporating pH-insensitive hydrogen-terminated diamond SGFETs. In this case, the sensitivity with the Ag/AgCl electrode as the gate was 0.60 mV/pH, while when the stainless steel vessel was used as the gate, the sensitivity was −54.18 mV/pH, which is close to the Nernstian value.
The works of Kawaguchi et al. [41] and Chang et al. [42] are very interesting because they combine a system based on field-effect transistors with the pH sensitivity of stainless steel. This makes it possible to take advantage of this material for a specific application wherein traditional materials in potentiometric systems cannot be used. This work is undoubtedly a clear example of the evolution of scientific knowledge in order to combine the best of each study to make new systems with technological value. Although already mentioned by Chang et al. [42], it is necessary to emphasize the need for a study of the response to the presence of other ions, so that we might then know the selectivity of the pH-sensing systems developed by these authors, especially considering the discussed effect of chloride ions.
5. Conclusions and Prospects of the Use of Stainless Steel in pH Electrodes
Stainless steel is a material with great potential for the development of pH sensors. Among the types of stainless steel, austenitic AISI 304 is the most widely used. Surface oxidation of stainless steel by heat treatment between 600 and 700 °C or by wet oxidation with chromic mixture is recommended to obtain a sufficiently sensitive oxide film. However, if the material is not subjected to surface oxidation, i.e., using the natural passive layer of stainless steel, a response close to Nernstian is possible. Therefore, it is advisable to test the response and stability of the electrode without any artificial oxidation before proceeding with this process.
Artificially oxidized stainless steel can show a significant effect of chloride ions in the solution and lose sensitivity to extremely acidic pH values, so these are aspects that must be considered when studying a pH sensor made of this material to define its limitations and scope.
Stainless steel is a good substrate material for metal oxides for pH sensing. The fact that this material is sensitive to hydronium ions enhances the response of the outermost layer of metal oxides, so its use is recommended not only for its conductivity and low-cost but also for its active role in sensing. In addition, it can also be used as a substrate for polymeric, carbonaceous, nanometric, and other materials that enable or enhance pH sensing.
For some years, stainless steel was a forgotten material for pH sensing, and it is now a promising material for the development of wearable pH sensors for medicine and sports science. In addition, its combination with sensors based on field effect transistors allows the development of systems sensitive to H+ at high temperatures, such as those used in industry.
In general, stainless steel is a promising sensitive and substrate material for applications wherein the glass electrode cannot be used due to its mechanical fragility or loss of response with an increase in temperature. In addition, it allows use in miniaturized, decentralized, wireless, and low-cost systems that can be used in many applications.
Author Contributions
Conceptualization, J.E.V.-C., J.H., M.A.A.-P., I.A.R.-D., G.L.T., R.C., J.J.P.L.-V., L.A.G.-F., M.S.-P. and L.H.; methodology, J.E.V.-C., I.A.R.-D. and G.L.T.; investigation, J.E.V.-C., J.H., J.J.P.L.-V., L.A.G.-F. and L.H.; resources, I.A.R.-D., G.L.T., R.C., M.A.A.-P. and M.S.-P.; writing—original draft preparation, J.E.V.-C., J.H., J.J.P.L.-V., L.A.G.-F. and L.H.; writing—review and editing, M.A.A.-P., I.A.R.-D., G.L.T., R.C. and M.S.-P.; visualization, J.E.V.-C., J.H., L.A.G.-F. and J.J.P.L.-V.; supervision, I.A.R.-D., M.A.A.-P. and G.L.T.; project administration, I.A.R.-D., R.C. and G.L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Acknowledgments
Javier E. Vilasó-Cadre and Lázaro A. González-Fernández thank the SECIHTI for the Ph.D. scholarship at the Universidad Autónoma de San Luis Potosí. Iván A. Reyes-Domínguez thanks SECIHTI for the professorship at the Institute of Metallurgy, Universidad Autónoma de San Luis Potosí. Juan Hidalgo-Viteri expresses gratitude for the Ph.D. fellowship offered by the Tempus Foundation under the Stipendium Hungaricum Scholarship Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krull, U.J.; Thompson, M. Analytical Chemistry. In Encyclopedia of Physical Science and Technology; Meyers, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 543–579. [Google Scholar] [CrossRef]
- Cummings, W.G.; Torrance, K.; Verhappen, I. Chemical Analysis: Electrochemical Techniques. In Instrumentation Reference Book; Boyes, W., Ed.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 363–399. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis, 7th ed.; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Zhao, X.; Chen, Y.; Xu, C.; Wei, G.; Yuan, A. Calibration-free pH long-time measurement method based on electrode potential drift dynamic compensation-applying self-adaptive dynamic optimization exponential smoothing method. Meas. Sci. Technol. 2022, 34, 025103. [Google Scholar] [CrossRef]
- Bard, A.; Faulkner, L.; White, H. Electrochemical Methods: Fundamentals and Applications, 3rd ed.; John Wiley & Sons Ltd.: New York, NY, USA, 2022. [Google Scholar]
- Głáb, S.; Hulanicki, A.; Edwall, G.; Ingman, I. Metal-Metal Oxide and Metal Oxide Electrodes as pH Sensors. Crit. Rev. Anal. Chem. 1989, 21, 29–47. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Young, S.-J.; Tang, W.-L. Wireless Zinc Oxide Based pH Sensor System. J. Electrochem. Soc. 2019, 166, B3047–B3050. [Google Scholar] [CrossRef]
- Uppuluri, K.; Lazouskaya, M.; Szwagierczak, D.; Zaraska, K.; Tamm, M. Fabrication, potentiometric characterization, and application of screen-printed RuO2 pH electrodes for water quality testing. Sensors 2021, 21, 5399. [Google Scholar] [CrossRef]
- Zea, M.; Moya, A.; Fritsch, M.; Ramon, E.; Villa, R.; Gabriel, G. Enhanced Performance Stability of Iridium Oxide-Based pH Sensors Fabricated on Rough Inkjet-Printed Platinum. ACS Appl. Mater. Interfaces 2019, 11, 15160–15169. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kitabayashi, H.; Ito, K.; Nasu, H.; Ishihara, A.; Nishio, Y. Effect of heat-treatment on the pH sensitivity of stainless-steel electrodes as pH sensors. Heliyon 2019, 5, e01239. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Moo, J.G.S.; Pumera, M. Helical 3D-Printed Metal Electrodes as Custom-Shaped 3D Platform for Electrochemical Devices. Adv. Funct. Mater. 2016, 26, 698–703. [Google Scholar] [CrossRef]
- Ming, T.; Lan, T.; Yu, M.; Wang, H.; Deng, J.; Kong, D.; Yang, S.; Shen, Z. Platinum Black/Gold Nanoparticles/Polyaniline Modified Electrochemical Microneedle Sensors for Continuous In Vivo Monitoring of pH Value. Polymers 2023, 15, 2796. [Google Scholar] [CrossRef]
- Miranda-Pérez, A.F.; Rodríguez-Vargas, B.R.; Calliari, I.; Pezzato, L. Corrosion Resistance of GMAW Duplex Stainless Steels Welds. Materials 2023, 16, 1847. [Google Scholar] [CrossRef]
- Cobb, H. The naming and numbering of stainless steels. Adv. Mater. Process. 2008, 5, 173–178. [Google Scholar]
- Habib, K.A.; Damra, M.S.; Saura, J.J.; Cervera, I.; Bellés, J. Breakdown and Evolution of the Protective Oxide Scales of AISI 304 and AISI 316 Stainless Steels under High-Temperature Oxidation. Int. J. Corros. 2011, 2011, 824676. [Google Scholar] [CrossRef]
- de Carvalho, C.E.R.; da Costa, G.M.; Cota, A.B.; Rossi, E.H. High temperature oxidation behavior of AISI 304 and AISI 430 stainless steels. Mater. Res. 2006, 9, 393–397. [Google Scholar] [CrossRef]
- Nomura, K.; Ujihira, Y. Response of Oxide Films on Stainless Steel as a pH Sensor. Anal. Chem. 1988, 60, 2564–2567. [Google Scholar] [CrossRef]
- Kurzweil, P. Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook. Sensors 2009, 9, 4955–4985. [Google Scholar] [CrossRef]
- Zhang, C.; Andrade, M.F.C.; Goldsmith, Z.K.; Raman, A.S.; Li, Y.; Piaggi, P.M.; Wu, X.; Car, R.; Selloni, A. Molecular-scale insights into the electrical double layer at oxide-electrolyte interfaces. Nat. Commun. 2024, 15, 10270. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Albetran, H.M.; Abdelrahman, B.H.; Al-Yaseri, A.; Yekeen, N.; Low, I.M. Wettability of Nanostructured Transition-Metal Oxide (Al2O3, CeO2, and AlCeO3) Powder Surfaces. Materials 2022, 15, 5485. [Google Scholar] [CrossRef]
- Veloso, C.H.; Filippov, L.O.; Filippova, I.V.; Ouvrard, S.; Araujo, A.C. Adsorption of polymers onto iron oxides: Equilibrium isotherms. J. Mater. Res. Technol. 2020, 9, 779–788. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Vorobéva, N.A.; Rumyantseva, M.N.; Gaśkov, A.M. Active sites on the surface of nanocrystalline semiconductor oxides ZnO and SnO2 and gas sensitivity. Russ. Chem. Bull. 2018, 66, 1728–1764. [Google Scholar] [CrossRef]
- Fog, A.; Buck, R.P. Electronic semiconducting oxides as pH sensors. Sens. Actuators 1984, 5, 137–146. [Google Scholar] [CrossRef]
- Zampronio, C.G.; Rohwedder, J.J.R.; Poppi, R.J. Development of a potentiometric flow cell with a stainless steel electrode for pH measurements. Determination of acid mixtures using flow injection analysis. Talanta 2000, 51, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.C.M.; Madrid, R.E.; Felice, C.J. A pH sensor based on a stainless steel electrode electrodeposited with iridium oxide. IEEE Trans. Educ. 2009, 52, 133–136. [Google Scholar] [CrossRef]
- Shahrestani, S.; Ismail, M.C.; Kakooei, S.; Beheshti, M.; Zabihiazadboni, M.; Zavareh, M.A. Iridium Oxide pH Sensor Based on Stainless Steel Wire for pH Mapping on Metal Surface. IOP Conf. Ser. Mater. Sci. Eng. 2018, 328, 012014. [Google Scholar] [CrossRef]
- Hashimoto, T.; Miwa, M.; Nasu, H.; Ishihara, A.; Nishio, Y. pH Sensors Using 3d-Block Metal Oxide-Coated Stainless Steel Electrodes. Electrochim. Acta 2016, 220, 699–704. [Google Scholar] [CrossRef]
- Puetz, J.; Aegerter, M.A. Dip Coating Technique. In Sol-Gel Technologies for Glass Producers and Users; Aegerter, M., Mennig, M., Eds.; Springer: New York, NY, USA, 2004; pp. 37–48. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chou, J.C. Preparation and characteristics of ruthenium dioxide for pH array sensors with real-time measurement system. Sens. Actuators B Chem. 2008, 128, 603–612. [Google Scholar] [CrossRef]
- Xi, Y.; Guo, Z.; Wang, L.; Xu, Q.; Ruan, T.; Liu, J. Fabrication and Characterization of Iridium Oxide pH Microelectrodes Based on Sputter Deposition Method. Sensors 2021, 21, 4996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, X.; Liu, Z.; Zheng, S.; Yao, C.; Zhang, T.; Huang, S.; Zhang, J.; Wang, J.; Farah, S.; et al. Plug-In Design of the Microneedle Electrode Array for Multi-Parameter Biochemical Sensing in Gouty Arthritis. ACS Sens. 2025, 10, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Vilasó-Cadre, J.E.; Benítez-Fernández, D.; López-Álvarez, I.A.; Ftovar-Vázquez, Y.; Arada-Pérez, M.A.; Reyes-Domínguez, I.A. Acid-base potentiometric titration using a stainless steel electrode without oxidative treatment. Turk. J. Chem. 2023, 47, 801–813. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kuno, T.; Ito, D.; Ishihara, A.; Nishio, Y. pH response and mechanical properties of Fe2O3–TeO2-based glass/stainless steel enamel electrodes for pH sensors. Heliyon 2023, 9, e12966. [Google Scholar] [CrossRef]
- Sadig, H.R.; Cheng, L.; Xiang, T. Using sol-gel supported by novel economic and environment-friendly spray-coating in the fabrication of nanostructure tri-system metal oxide-based pH sensor applications. J. Electroanal. Chem. 2018, 827, 93–102. [Google Scholar] [CrossRef]
- Fiore, L.; Mazzaracchio, V.; Gosti, C.; Duranti, L.; Vitiello, R.; Maccauro, G.; Arduini, F. Functionalized orthopaedic implant as pH electrochemical sensing tool for smart diagnosis of hardware infection. Analyst 2024, 149, 3085–3096. [Google Scholar] [CrossRef]
- Zamora, M.L.; Dominguez, J.M.; Trujillo, R.M.; Goy, C.B.; Sánchez, M.A.; Madrid, R.E. Potentiometric textile-based pH sensor. Sens. Actuators B Chem. 2018, 260, 601–608. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; Pérez-Ràfols, C.; Cuartero, M.; Crespo, G.A. Toward in Vivo Transdermal pH Sensing with a Validated Microneedle Membrane Electrode. ACS Sens. 2021, 6, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Creative Commons. Available online: https://creativecommons.org/licenses/by/4.0/ (accessed on 18 April 2025).
- Prissanaroon-Ouajai, W.; Pigram, P.J.; Jones, R.; Sirivat, A. A sensitive and highly stable polypyrrole-based pH sensor with hydroquinone monosulfonate and oxalate co-doping. Sens. Actuators B Chem. 2009, 138, 504–511. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Nomoto, R.; Sato, H.; Takarada, T.; Chang, Y.H.; Kawarada, H. pH Measurement at Elevated Temperature with Vessel Gate and Oxygen-Terminated Diamond Solution Gate Field Effect Transistors. Sensors 2022, 22, 1807. [Google Scholar] [CrossRef]
- Chang, Y.H.; Iyama, Y.; Kawaguchi, S.; Takarada, T.; Sato, H.; Nomoto, R.; Tadenuma, K.; Falina, S.; Syamsul, M.; Shintani, Y.; et al. Ion-Sensitive Stainless Steel Vessel for All-Solid- State pH Sensing System Incorporating pH-Insensitive Diamond Solution Gate Field-Effect Transistors. IEEE Sens. J. 2023, 23, 9110–9119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).