A Review on Xanthine Oxidase-Based Electrochemical Biosensors: Food Safety and Quality Control Applications
Abstract
1. Introduction
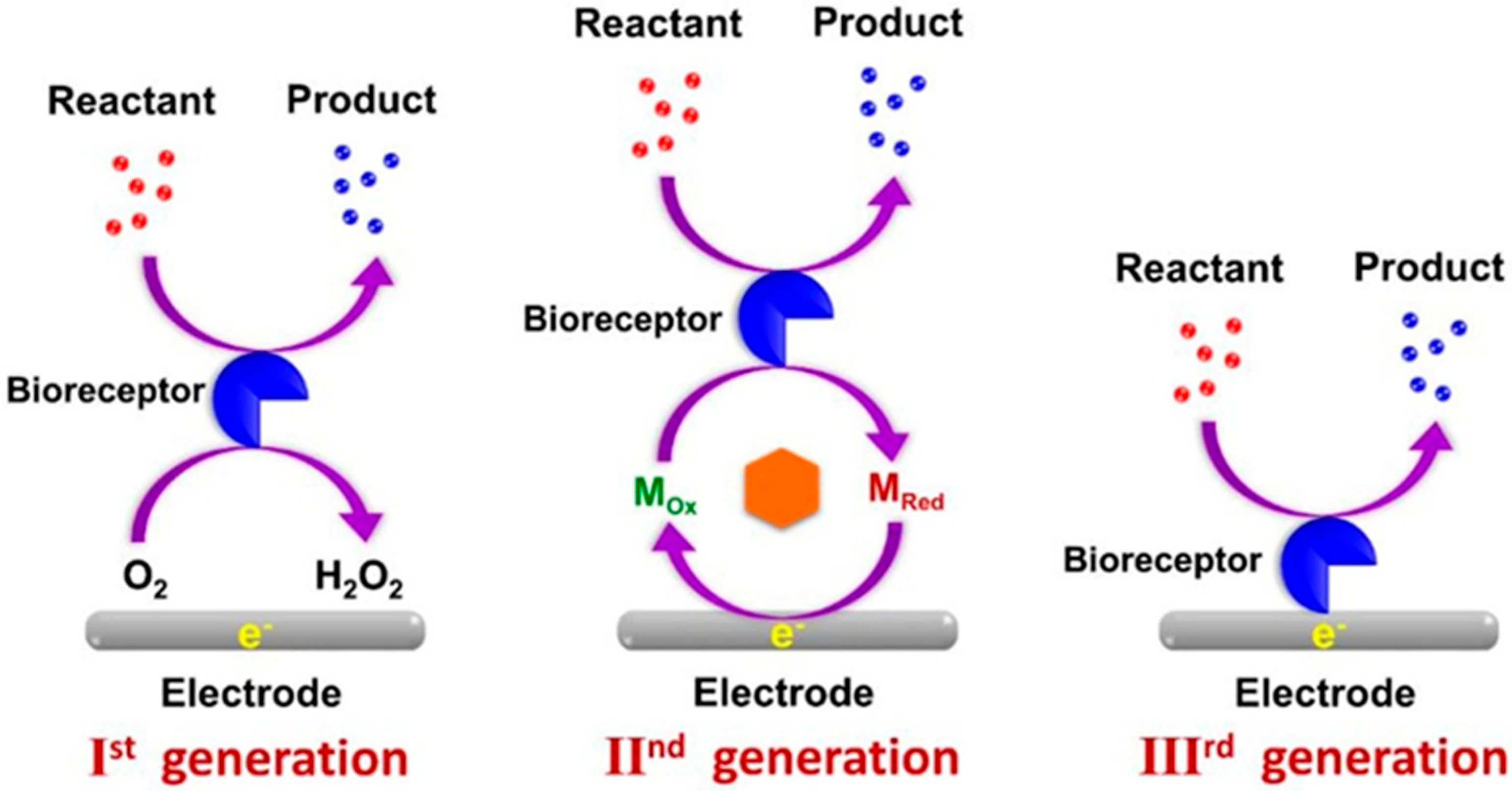
2. Electrochemical Biosensors—General Concepts
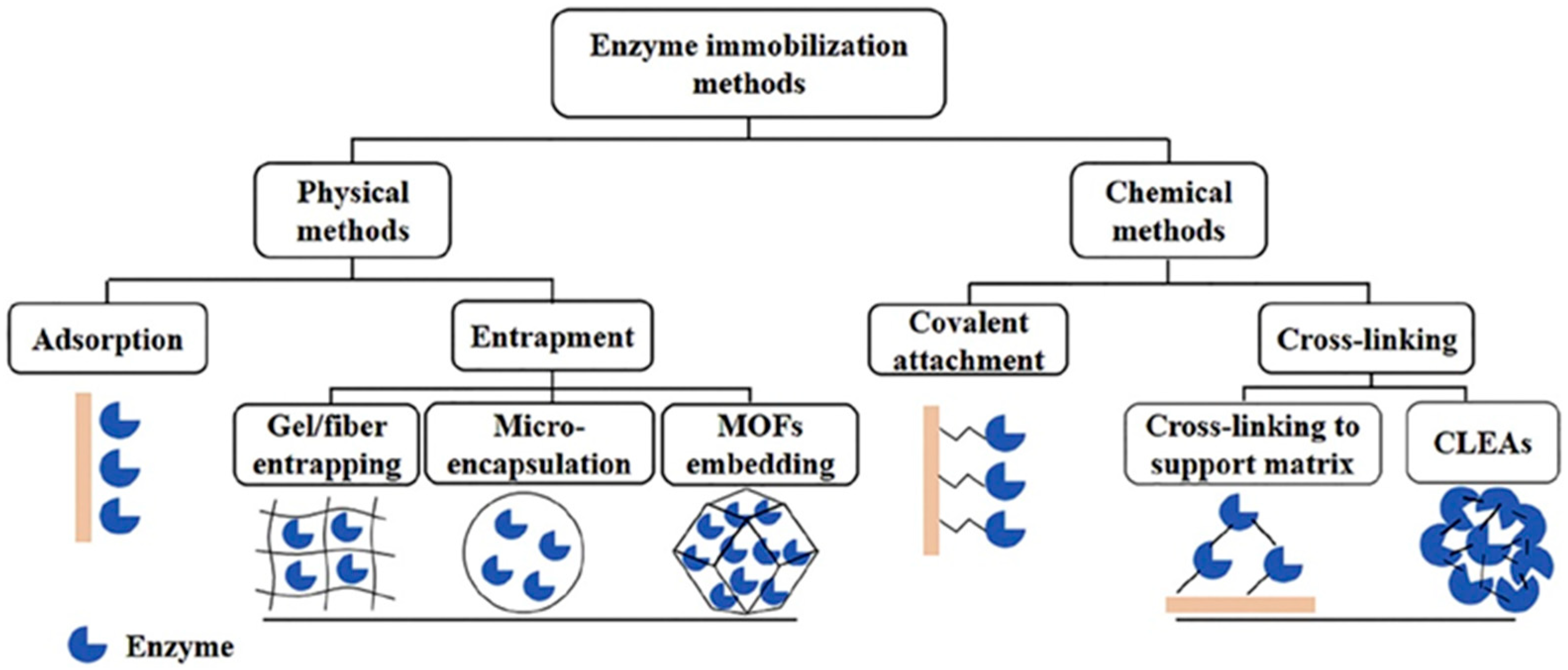
- Physical adsorption
- Physical entrapment or encapsulation
- Chemical immobilization
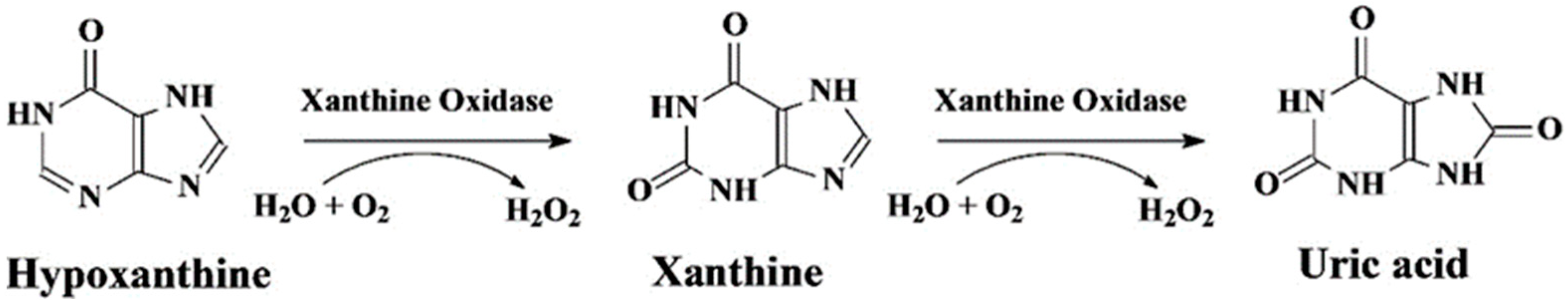
3. Xanthine Oxidase-Based Electrochemical Biosensors
- (i)
- the consumed O2;
- (ii)
- the produced uric acid;
- (iii)
- the produced H2O2. Generally, direct detection of H2O2 requires anodic potentials (around 0.6 V vs. Ag/AgCl) or cathodic potentials below 0.0 V vs. Ag/AgCl.
- (iv)
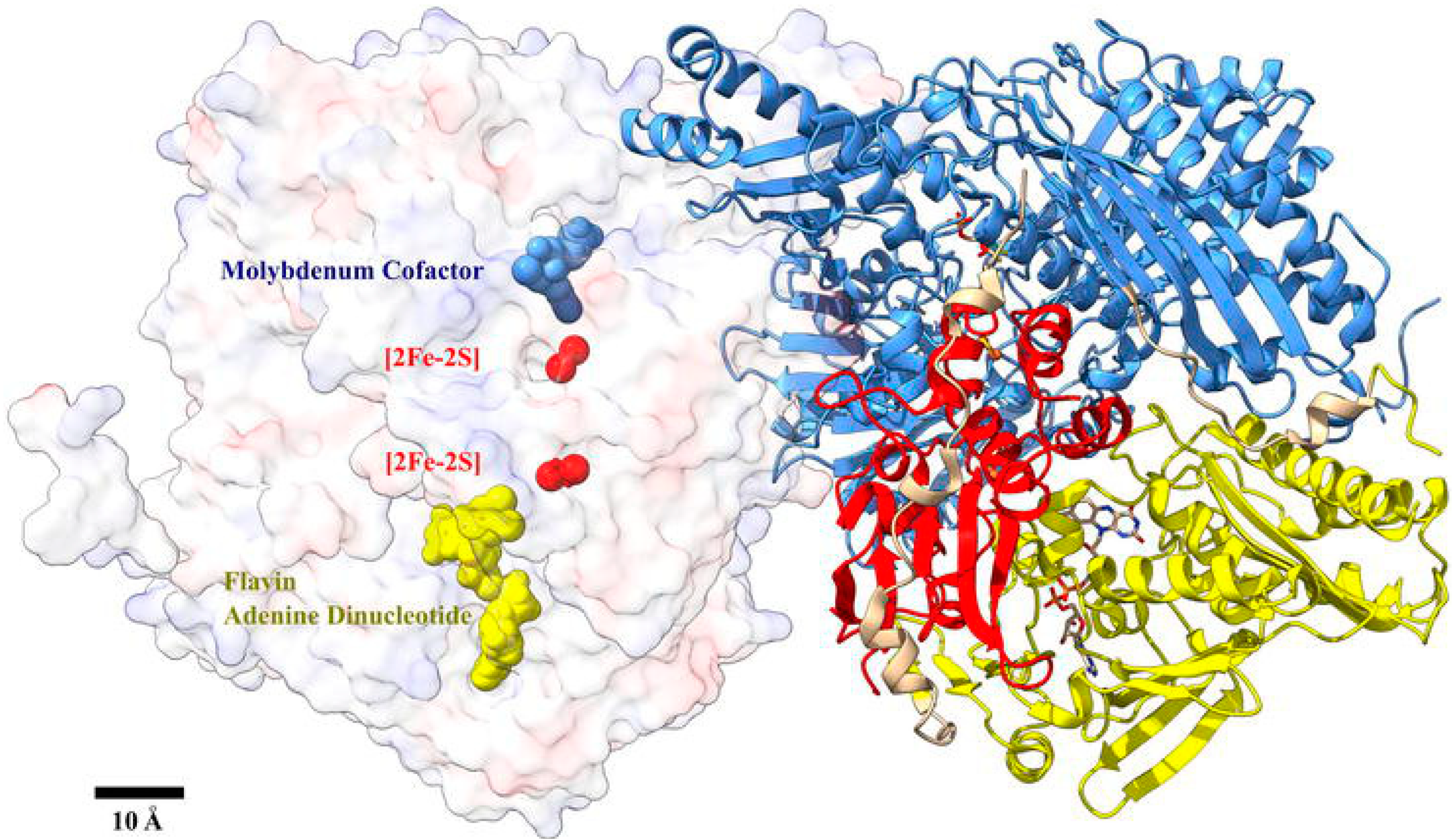
- the current generated as a result of DET between the redox-active sites of XOx and the electrode surface. Here, it should be noted that XOx is characterized by a pronounced spatial shielding effect of the active center due to its location at a significant depth (over 2 nm) in a hydrophobic cavity of the molecule, which hinders DET. Although various innovative strategies have been applied to realize DET, most often the turnover rate of electrons is considerably lower than the electron exchange rate between the redox-active site of the XOx macromolecule and O2 (the native electron acceptor).
3.1. First-Generation XOx Biosensors
3.1.1. Biosensors Based on the Electrooxidation of H2O2 and/or Uric Acid
Nanoparticle-Based Biosensors
Biosensors Based on Polymers
Biosensors Based on Metal-Organic Frameworks (MOFs)
3.1.2. Biosensors Based on the Electroreduction of H2O2
3.2. Second-Generation XOx Biosensors
3.3. Third-Generation XOx Biosensors
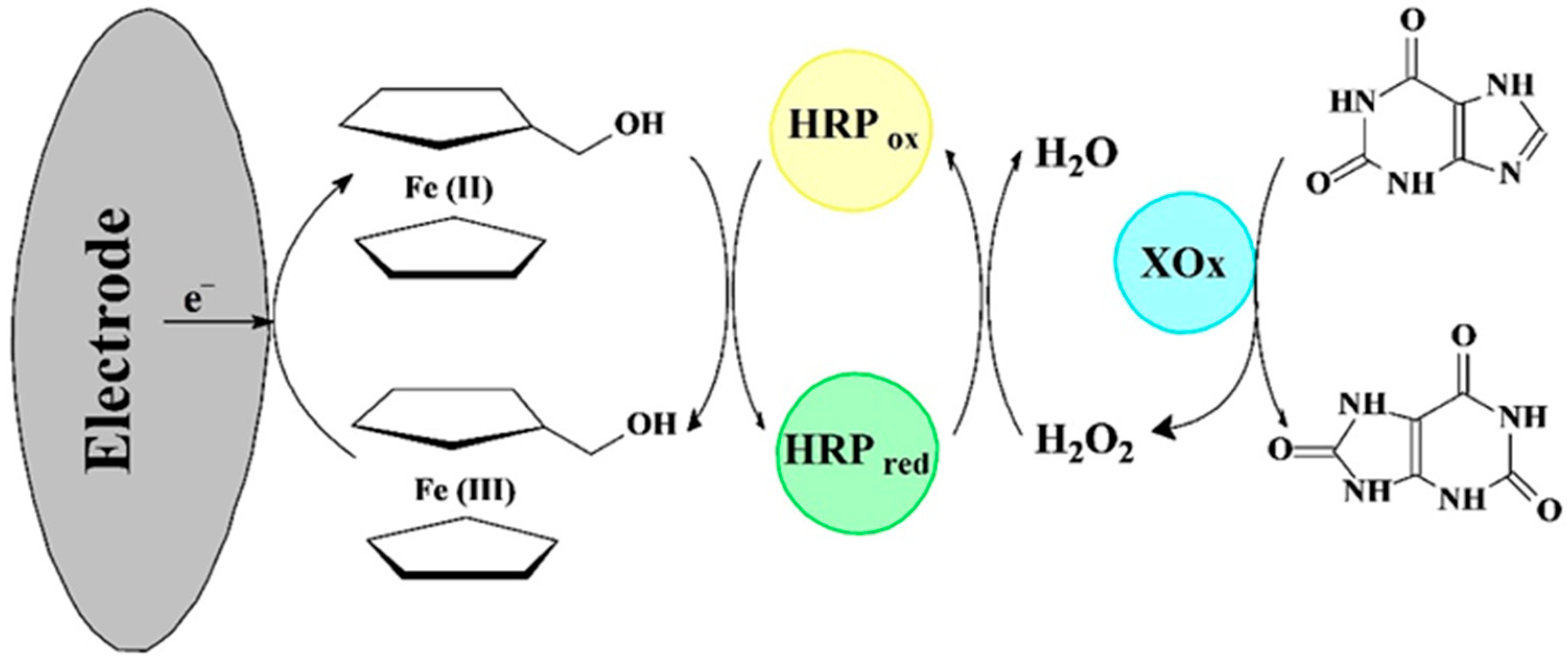
3.4. Bi-Enzyme Biosensors
4. Concluding Remarks
- -
- Extending the shelf life and stability of the biorecognition component. Reliable XOx immobilization should be the main focus in the innovative design of optimized biosensing platforms. Storage stability and operational stability can be improved by introducing novel nanomaterial-assisted enzyme immobilization techniques. At the same time, employing mild conditions, high quantities of enzyme molecules can be immobilized uniformly. For example, the biocompatibility and large surface area of gold nanowires provide high enzyme loading efficiency and a compatible microenvironment for XOx immobilization.
- -
- Improving low-level detection efficiency. Electrochemical enzyme biosensors have become more versatile, robust, and flexible with the induction of novel classes of nanocomposites. The analytical features in terms of repeatability and reproducibility may be adjusted, including functionalization or doping of the host matrices of biosensors, allowing fine control over biosensor performance. For example, chemical doping of heteroatoms (N, S, B, etc.) within carbon nanomaterials such as graphene and CNTs could substantially enhance their electrocatalytic properties. Utilization of recent advancements in quantum dots and dendrimers has also opened up new prospects for the development of efficient and high-performance XOx-based biosensors. There is increasing interest in nanocomposites with regular nanostructures in enzymatic biosensor interface design. Furthermore, the results obtained have shown that bimetallic nanocrystals with core-shell structures greatly affect the analytical performance of electrochemical biosensors. Introducing a third metal in the bimetallic structure may be a promising strategy for enhancing the catalytic activity and sensing performance of bimetallic nanocrystals.
- -
- Using synergies in material science, bioelectronics, and nanofabrication technologies, these devices should be miniaturized into smart hand-held analyzers. Electrochemical XOx-based biosensors need transducers assembled within a carefully designed sensing interface that can be fabricated into a portable unit. Thus, the electrochemical biosensor can be miniaturized into a compact device connected to a smartphone for powering, processing, data analysis, and visualization. In the foreseeable future, we expect artificial intelligence (AI) algorithms to be introduced to power bioelectroanalytical methods for Hx/X assay.
Funding
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| BQ | 1,4-Benzoquinone |
| CHIT | Chitosan |
| CLEAs | Cross-linked enzyme aggregates |
| CMC | Carboxymethylcellulose |
| CPE | Carbon paste electrode |
| DET | Direct electron transfer |
| FAD | Flavin adenine dinucleotide |
| Fc | Ferrocene |
| GCE | Glassy carbon electrode |
| GCPE | Glassy carbon paste electrode |
| GMA | Glycidyl methacrylate |
| GR | Graphene |
| HMTES | Hydroxymethyltriethoxysilane |
| HPLC | High-performance liquid chromatography |
| HRP | Horseradish peroxidase |
| Hx | Hypoxanthine |
| KMapp | Apparent Michaelis–Menten constant |
| LbL | Layer-by-layer |
| L-Cys | L-cysteine |
| LOD | Limit of detection |
| MeOHFc | Ferrocenemethanol |
| MNP | Magnetic nanoparticles |
| MOFs | Metal-organic frameworks |
| MV | Methyl viologen |
| MWCNTs | Multi-walled carbon nanotubes |
| NPs | Nanoparticles |
| OMIEC | Organic mixed ionic-electronic conductor |
| PAMAM | Polyamidoamine |
| PANI | Polyaniline |
| PBS | Phosphate buffer solution |
| PGE | Pencil graphite electrode |
| PPD | Poly(o-phenylenediamine) |
| PPy | Polypyrrole |
| PU | Polyurethane |
| PVS | Polyvinyl sulphonate |
| REGO | Reduced expanded graphene oxide |
| rGO | Reduced graphene oxide |
| SA | Sodium alginate |
| SCE | Saturated calomel electrode |
| SG | Silica sol–gel |
| SWCNH | Single-walled carbon nanohorn |
| SWCNTs | Single-walled carbon nanotubes |
| SWy | Sodium montmorillonite |
| TCNQ | 7,7′,8,8′-Tetracyanoquinodimethane |
| X | Xanthine |
| XOx | Xanthine oxidase |
| VFc | Vinylferrocene |
References
- Wijayanti, S.D.; Tsvik, L.; Haltrich, D. Recent Advances in Electrochemical Enzyme-Based Biosensors for Food and Beverage Analysis. Foods 2023, 12, 3355. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, V.K.; Srivastava, S.; Singh, R. A systematic review on recent trends and perspectives of biosensors in food industries. J. Food Saf. 2023, 43, e13071. [Google Scholar] [CrossRef]
- Wang, K.; Lin, X.; Zhang, M.; Li, Y.; Luo, C.; Wu, J. Review of Electrochemical Biosensors for Food Safety Detection. Biosensors 2022, 12, 959. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Lv, C.; Liu, X.; Yang, M.; Guo, M.; Fu, Q. Electrochemical biosensors represent promising detection tools in medical field. Adv. Sens. Energy Mater. 2023, 2, 100081. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, J.; Ko, S.H. Electrochemical biosensors for point-of-care testing. Bio-Des. Manuf. 2024, 7, 548–565. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- Dolmacı, N.; Çete, S.; Arslan, F.; Yaşar, A. An amperometric biosensor for fish freshness detection from xanthine oxidase immobilized in polypyrrole-polyvinylsulphonate film. Artif. Cells Blood Substit. Biotechnol. 2012, 40, 275–279. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Benvidi, A.; Abbasi, S.; Rezaeinasab, M. Enzyme-based ultrasensitive electrochemical biosensor using poly(l-aspartic acid)/MWCNT bio-nanocomposite for xanthine detection: A meat freshness marker. Microchem. J. 2019, 149, 104000. [Google Scholar] [CrossRef]
- Jeyasanta, I.; Giftson, K.H.; Patterson, J. Quality Indicator Hypoxanthine Compared with Other Volatile Amine Indicators of Sea Foods Stored in Refrigerator. Asian J. Anim. Vet. Adv. 2018, 13, 144–154. Available online: https://scialert.net/abstract/?doi=ajava.2018.144.154 (accessed on 10 February 2025). [CrossRef]
- Gunasekaran, S.; Sankari, G.; Ponnusamy, S. Vibrational spectral investigation on xanthine and its derivatives—Theophylline, caffeine and theobromine. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 117–127. [Google Scholar] [CrossRef]
- Song, D.; Chen, Q.; Zhai, C.; Tao, H.; Zhang, L.; Jia, T.; Lu, Z.; Sun, W.; Yuan, P.; Zhu, B. Label-Free ZnIn2S4/UiO-66-NH2 Modified Glassy Carbon Electrode for Electrochemically Assessing Fish Freshness by Monitoring Xanthine and Hypoxanthine. Chemosensors 2022, 10, 158. [Google Scholar] [CrossRef]
- Kasare, P.K.; Wang, S.-F. Hydrothermally Synthesized Cerium Phosphate with Functionalized Carbon Nanofiber Nanocomposite for Enhanced Electrochemical Detection of Hypoxanthine. Chemosensors 2024, 12, 84. [Google Scholar] [CrossRef]
- Rognum, T.O.; Holmen, S.; Musse, M.A.; Dahlberg, P.S.; Stray-Pedersen, A.; Saugstad, O.D.; Opdal, S.H. Estimation of time since death by vitreous humor hypoxanthine, potassium, and ambient temperature. Forensic Sci. Int. 2016, 262, 160–165. [Google Scholar] [CrossRef]
- Liao, L.; Xing, Y.; Xiong, X.; Gan, L.; Hu, L.; Zhao, F.; Tong, Y.; Deng, S. An electrochemical biosensor for hypoxanthine detection in vitreous humor: A potential tool for estimating the post-mortem interval in forensic cases. Microchem. J. 2020, 155, 104760. [Google Scholar] [CrossRef]
- Dzyadevych, S.V.; Arkhypova, V.N.; Soldatkin, A.P.; El’skaya, A.V.; Martelet, C.; Jaffrezic-Renault, N. Amperometric enzyme biosensors: Past, present and future. IRBM 2008, 29, 171–180. [Google Scholar] [CrossRef]
- Fernández, H.; Zon, M.A.; Maccio, S.A.; Alaníz, R.D.; Di Tocco, A.; Carrillo Palomino, R.A.; Cabas Rodríguez, J.A.; Granero, A.M.; Arévalo, F.J.; Robledo, S.N.; et al. Multivariate Optimization of Electrochemical Biosensors for the Determination of Compounds Related to Food Safety—A Review. Biosensors 2023, 13, 694. [Google Scholar] [CrossRef]
- Hosseinikebria, S.; Khazaei, M.; Dervisevic, M.; Judicpa, M.A.; Tian, J.; Razal, J.M.; Voelcker, N.H.; Nilghaz, A. Electrochemical biosensors: The beacon for food safety and quality. Food Chem. 2025, 475, 143284. [Google Scholar] [CrossRef]
- Ghaani, M.; Azimzadeh, M.; Büyüktaş, D.; Carullo, D.; Farris, S. Electrochemical Sensors in the Food Sector: A Review. J. Agric. Food Chem. 2024, 72, 24170–24190. [Google Scholar] [CrossRef]
- Dube, A.; Malode, S.J.; Alodhayb, A.N.; Mondal, K.; Shetti, N.P. Conducting polymer-based electrochemical sensors: Progress, challenges, and future perspectives. Talanta Open 2025, 11, 100395. [Google Scholar] [CrossRef]
- Dodevska, T.; Hadzhiev, D.; Shterev, I. A Review on Electrochemical Microsensors for Ascorbic Acid Detection: Clinical, Pharmaceutical, and Food Safety Applications. Micromachines 2023, 14, 41. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Ruzgas, T.; Larpant, N.; Shafaat, A.; Sotres, J. Wireless, Battery-Less Biosensors Based on Direct Electron Transfer Reactions. ChemElectroChem 2019, 6, 5167. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Seychell, B.C.; Vella, M.; Hunter, G.J.; Hunter, T. The Good and the Bad: The Bifunctional Enzyme Xanthine Oxidoreductase in the Production of Reactive Oxygen Species. In Reactive Oxygen Species-Advances and Developments; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Dervisevic, M.; Dervisevic, E.; Şenel, M. Recent progress in nanomaterial-based electrochemical and optical sensors for hypoxanthine and xanthine. A review. Microchim. Acta 2019, 186, 749. [Google Scholar] [CrossRef]
- Felicia, W.X.L.; Rovina, K.; ‘Aqilah, N.M.N.; Vonnie, J.M.; Yin, K.W.; Huda, N. Assessing Meat Freshness via Nanotechnology Biosensors: Is the World Prepared for Lightning-Fast Pace Methods? Biosensors 2023, 13, 217. [Google Scholar] [CrossRef]
- Wang, B.; Liu, K.; Wei, G.; He, A.; Kong, W.; Zhang, X. A Review of Advanced Sensor Technologies for Aquatic Products Freshness Assessment in Cold Chain Logistics. Biosensors 2024, 14, 468. [Google Scholar] [CrossRef]
- Ahlawat, J.; Sharma, M.; Pundir, C.S. Advances in xanthine biosensors and sensors: A review. Enzym. Microb. Technol. 2024, 174, 110377. [Google Scholar] [CrossRef]
- Garg, D.; Singh, M.; Verma, N.; Monika. Review on recent advances in fabrication of enzymatic and chemical sensors for hypoxanthine. Food Chem. 2022, 375, 131839. [Google Scholar] [CrossRef]
- Sakthivel, K.; Balasubramanian, S.; Chang-Chien, G.-P.; Wang, S.-F.; Ahammad; Billey, W.; Platero, J.; Soundappan, T.; Sekhar, P. Editors’ Choice—Review—Advances in Electrochemical Sensors: Improving Food Safety, Quality, and Traceability. ECS Sens. Plus 2024, 3, 020605. [Google Scholar] [CrossRef]
- Majer-Baranyi, K.; Székács, A.; Adányi, N. Application of Electrochemical Biosensors for Determination of Food Spoilage. Biosensors 2023, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Ando, K.; Karube, I.; Matsuoka, H.; Suzuki, S. Determination of Hypoxanthine in Fish Meat with an Enzyme Sensor. J. Food Sci. 1983, 48, 496–500. [Google Scholar] [CrossRef]
- Boluda, A.; Casado, C.M.; Alonso, B.; García Armada, M.P. Efficient Oxidase Biosensors Based on Bioelectrocatalytic Surfaces of Electrodeposited Ferrocenyl Polycyclosiloxanes—Pt Nanoparticles. Chemosensors 2021, 9, 81. [Google Scholar] [CrossRef]
- Sriramulu, G.; Verma, R.; Singh, K.R.B.; Singh, P.; Chakra, C.S.; Mallick, S.; Singh, R.P.; Sadhana, K.; Singh, Y. Self-assembled copper oxide nanoflakes for highly sensitive electrochemical xanthine detection in fish-freshness biosensors. J. Mol. Struct. 2024, 1304, 137640. [Google Scholar] [CrossRef]
- Thandavan, K.; Gandhi, S.; Sethuraman, S.; Rayappan, J.B.B.; Krishnan, U.M. Development of electrochemical biosensor with nano-interface for xanthine sensing—A novel approach for fish freshness estimation. Food Chem. 2013, 139, 963–969. [Google Scholar] [CrossRef]
- Jain, U.; Narang, J.; Chauhan, N. Enhanced electrochemical performance of xanthine biosensor by core—Shell magnetic nanoparticles and carbon nanotube interface. Adv. Mater. Lett. 2016, 7, 472–479. [Google Scholar] [CrossRef]
- Shan, D.; Wang, Y.; Xue, H.; Cosnier, S. Sensitive and selective xanthine amperometric sensors based on calcium carbonate nanoparticles. Sens. Actuators B Chem. 2009, 136, 510–515. [Google Scholar] [CrossRef]
- Çubukçu, M.; Timur, S.; Anik, Ü. Examination of performance of glassy carbon paste electrode modified with gold nanoparticle and xanthine oxidase for xanthine and hypoxanthine detection. Talanta 2007, 74, 434–439. [Google Scholar] [CrossRef]
- Tripathi, A.; Elias, A.L.; Jemere, A.B.; Harris, K.D. Amperometric Determination of Xanthine Using Nanostructured NiO Electrodes Loaded with Xanthine Oxidase. ACS Food Sci. Technol. 2022, 2, 1307–1317. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singhal, C.; Pundir, C.S. Evaluation of Freshness of Fishes Using MWCNT/TiO2 Nanobiocomposites Based Biosensor. Food Anal. Methods 2017, 10, 522–528. [Google Scholar] [CrossRef]
- Jain, U.; Narang, J.; Rani, K.; Burna, B.; Sunny, S.; Chauhan, N. Synthesis of cadmium oxide and carbon nanotube based nanocomposites and their use as a sensing interface for xanthine detection. RSC Adv. 2015, 5, 29675–29683. [Google Scholar] [CrossRef]
- Devi, R.; Yadav, S.; Naranga, J.; Pundir, C. Electrochemical biosensor based on gold coated iron nanoparticles/chitosan composite bound xanthine oxidase for detection of xanthine in fish meat. J. Food Eng. 2013, 115, 207–214. [Google Scholar] [CrossRef]
- Agüí, L.; Manso, H.; Yáñez-Sedeño, P.; Pingarrón, J.M. Amperometric biosensor for hypoxanthine based on immobilized xanthine oxidase on nanocrystal gold–carbon paste electrodes. Sens. Actuators B Chem. 2006, 113, 272–280. [Google Scholar] [CrossRef]
- Saadaoui, M.; Sánchez, A.; Díez, P.; Raouafi, N.; Pingarrón, J.M.; Villalonga, R. Amperometric xanthine biosensors using glassy carbon electrodes modified with electrografted porous silica nanomaterials loaded with xanthine oxidase. Microchim. Acta 2016, 183, 2023–2030. [Google Scholar] [CrossRef]
- Pundir, C.S.; Devi, R.; Narang, J.; Singh, S.; Nehra, J.; Chaudhry, S. Fabrication of an amperometric xanthine biosensor based on polyvinylchloride membrane. J. Food Biochem. 2011, 36, 21–27. [Google Scholar] [CrossRef]
- Devi, R.; Yadav, S.; Pundir, C.S. Electrochemical detection of xanthine in fish meat by xanthine oxidase immobilized on carboxylated multiwalled carbon nanotubes/polyaniline composite film. Biochem. Eng. J. 2011, 58–59, 148–153. [Google Scholar] [CrossRef]
- Devi, R.; Thakur, M.; Pundir, C.S. Construction and application of an amperometric xanthine biosensor based on zinc oxide nanoparticles–polypyrrole composite film. Biosens. Bioelectron. 2011, 26, 3420–3426. [Google Scholar] [CrossRef]
- Devi, R.; Yadav, S.; Pundir, C.S. Au-colloids–polypyrrole nanocomposite film based xanthine biosensor. Colloids Surf. A Physicochem. Eng. Asp. 2012, 394, 38–45. [Google Scholar] [CrossRef]
- Devi, R.; Batra, B.; Lata, S.; Yadav, S.; Pundir, C.S. A method for determination of xanthine in meat by amperometric biosensor based on silver nanoparticles/cysteine modified Au electrode. Process Biochem. 2013, 48, 242–249. [Google Scholar] [CrossRef]
- Sahyar, B.Y.; Kaplan, M.; Ozsoz, M.; Celik, E.; Otles, S. Electrochemical xanthine detection by enzymatic method based on Ag doped ZnO nanoparticles by using polypyrrole. Bioelectrochemistry 2019, 130, 107327. [Google Scholar] [CrossRef] [PubMed]
- Thakur, D.; Pandey, C.M.; Kumar, D. Highly Sensitive Enzymatic Biosensor Based on Polyaniline-Wrapped Titanium Dioxide Nanohybrid for Fish Freshness Detection. Appl. Biochem. Biotechnol. 2022, 194, 3765–3778. [Google Scholar] [CrossRef]
- Devi, R.; Yadav, S.; Pundir, C.S. Amperometric determination of xanthine in fish meat by zinc oxide nanoparticle/chitosan/multiwalled carbon nanotube/polyaniline composite film bound xanthine oxidase. Analyst 2012, 137, 754–759. [Google Scholar] [CrossRef]
- Das, J.; Mishra, H.N. Electrochemical biosensor for monitoring fish spoilage based on nanocellulose as enzyme immobilization matrix. Food Meas. 2023, 17, 3827–3844. [Google Scholar] [CrossRef]
- Borisova, B.; Sánchez, A.; Jiménez-Falcao, S.; Martín, M.; Salazar, P.; Parrado, C.; Pingarrón, J.M.; Villalonga, R. Reduced graphene oxide-carboxymethylcellulose layered with platinum nanoparticles/PAMAM dendrimer/magnetic nanoparticles hybrids. Application to the preparation of enzyme electrochemical biosensors. Sens. Actuators B Chem. 2016, 232, 84–90. [Google Scholar] [CrossRef]
- Siciliano, G.; Alsadig, A.; Chiriacò, M.S.; Turco, A.; Foscarini, A.; Ferrara, F.; Gigli, G.; Primiceri, E. Beyond traditional biosensors: Recent advances in gold nanoparticles modified electrodes for biosensing applications. Talanta 2024, 268 Pt 1, 125280. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Dervisevic, E.; Çevik, E.; Şenel, M. Novel electrochemical xanthine biosensor based on chitosan–polypyrrole–gold nanoparticles hybrid bio-nanocomposite platform. J. Food Drug Anal. 2017, 25, 510–519. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, J.; Zhang, J.; Ding, L.; Ju, H. Amperometric detection of hypoxanthine and xanthine by enzymatic amplification using a gold nanoparticles–carbon nanohorn hybrid as the carrier. Analyst 2012, 137, 3126. [Google Scholar] [CrossRef]
- Labban, N.; Wayu, M.B.; Steele, C.M.; Munoz, T.S.; Pollock, J.A.; Case, W.S.; Leopold, M.C. First Generation Amperometric Biosensing of Galactose with Xerogel-Carbon Nanotube Layer-By-Layer Assemblies. Nanomaterials 2019, 9, 42. [Google Scholar] [CrossRef]
- Hughes, L.B.; Labban, N.; Conway, G.E.; Pollock, J.A.; Leopold, M.C. Adaptable Xerogel-Layered Amperometric Biosensor Platforms on Wire Electrodes for Clinically Relevant Measurements. Sensors 2019, 19, 2584. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.M.; Wemple, A.H.; Leopold, M.C. Nanomaterial-Doped Xerogels for Biosensing Measurements of Xanthine in Clinical and Industrial Applications. Gels 2023, 9, 437. [Google Scholar] [CrossRef]
- Salvigni, L.; Mariani, F.; Gualandi, I.; Decataldo, F.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Selective detection of liposoluble vitamins using an organic electrochemical transistor. Sens. Actuators B Chem. 2023, 393, 134313. [Google Scholar] [CrossRef]
- Gentile, F.; Vurro, F.; Janni, M.; Manfredi, R.; Cellini, F.; Petrozza, A.; Zappettini, A.; Coppedè, N. A Biomimetic, Biocompatible OECT Sensor for the Real-Time Measurement of Concentration and Saturation of Ions in Plant Sap. Adv. Electron. Mater. 2022, 8, 2200092. [Google Scholar] [CrossRef]
- Kim, H.; Won, Y.; Song, H.W.; Kwon, Y.; Jun, M.; Oh, J.H. Organic Mixed Ionic–Electronic Conductors for Bioelectronic Sensors: Materials and Operation Mechanisms. Adv. Sci. 2024, 11, 2306191. [Google Scholar] [CrossRef]
- Lin, Y.; Kroon, R.; Zeglio, E.; Herland, A. P-type accumulation mode organic electrochemical transistor biosensor for xanthine detection in fish. Biosens. Bioelectron. 2025, 269, 116928. [Google Scholar] [CrossRef]
- Kidanemariam, A.; Cho, S. Recent Advances in the Application of Metal–Organic Frameworks and Coordination Polymers in Electrochemical Biosensors. Chemosensors 2024, 12, 135. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, B.; Shen, C.; Lai, O.-M.; Tan, C.-P.; Cheong, L.-Z. Electrochemical Biosensing of Chilled Seafood Freshness by Xanthine Oxidase Immobilized on Copper-Based Metal–Organic Framework Nanofiber Film. Food Anal. Methods 2019, 12, 1715–1724. [Google Scholar] [CrossRef]
- Joon, A.; Ahlawat, J.; Aggarwal, V.; Jaiwal, R.; Pundir, C.S. An improved amperometric determination of xanthine with xanthine oxidase nanoparticles for testing of fish meat freshness. Sens. Bio-Sens. Res. 2021, 33, 100437. [Google Scholar] [CrossRef]
- Dalkıran, B.; Erden, P.; Kılıç, E. Construction of an Electrochemical Xanthine Biosensor Based on Graphene/Cobalt Oxide Nanoparticles/Chitosan Composite for Fish Freshness Detection. J. Turk. Chem. Soc. Sect. A Chem. 2017, 4, 23–44. [Google Scholar] [CrossRef][Green Version]
- Öztürk, F.; Erden, P.; Kacar, C.; Kilic, E. Amperometric biosensor for xanthine determination based on Fe3O4 nanoparticles. Acta Chim. Slov. 2014, 61, 19–26. Available online: https://acta-arhiv.chem-soc.si/61/61-1-19.pdf (accessed on 10 February 2025).
- Dodevska, T.; Horozova, E.; Dimcheva, N. Electrochemical characteristics and structural specifics of carbonaceous electrodes, modified with micro- and nanodeposits of platinum metals. Bulg. Chem. Commun. 2013, 45, 171–178. Available online: http://bcc.bas.bg/BCC_Volumes/Volume_45_Special_A_2013/BCC-45-SE-A-171-178.pdf (accessed on 10 February 2025).
- Horozova, E.; Dodevska, T.; Dimcheva, N. Modified graphite electrodes as catalysts for electroreduction of hydrogen peroxide. Bulg. Chem. Commun. 2008, 40, 233–239. Available online: http://www.bcc.bas.bg/BCC_Volumes/Volume_40_Number_3_2008/Volume_40_Number_3_2008_PDF/2817-RR.pdf (accessed on 10 February 2025).
- Dodevska, T.; Horozova, E.; Dimcheva, N. Electrocatalytic reduction of hydrogen peroxide on modified graphite electrodes: Application to the development of glucose biosensors. Anal. Bioanal. Chem. 2006, 386, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Dimcheva, N.; Horozova, E.; Jordanova, Z. An amperometric xanthine oxidase enzyme electrode based on hydrogen peroxide electroreduction. Z. Naturforschung C 2002, 57, 883–889. [Google Scholar] [CrossRef]
- Horozova, E.; Dodevska, T.; Dimcheva, N. Modified with microquantities of platinum metals graphites: Application to the development of xanthine oxidase enzyme electrode. J. Univ. Chem. Technol. Metall. 2008, 43, 59–64. Available online: https://journal.uctm.edu/node/j2008-1/8_Horozova_Dodevska-59-64.pdf (accessed on 10 February 2025).
- Horozova, E.; Dodevska, T.; Dimcheva, N. Modified graphites: Application to the development of enzyme-based amperometric biosensors. Bioelectrochemistry 2009, 74, 260–264. [Google Scholar] [CrossRef]
- Dodevska, T.; Horozova, E.; Dimcheva, N. Design of an amperometric xanthine biosensor based on a graphite transducer patterned with noble metal microparticles. Cent. Eur. J. Chem. 2010, 8, 19–27. [Google Scholar] [CrossRef]
- Albelda, J.A.V.; Uzunoglu, A.; Santos, G.N.C.; Stanciu, L.A. Graphene-titanium dioxide nanocomposite based hypoxanthine sensor for assessment of meat freshness. Biosens. Bioelectron. 2017, 89, 518–524. [Google Scholar] [CrossRef]
- Kilinc, E.; Erdem, A.; Gokgunnec, L.; Dalbasti, T.; Karaoglan, M.; Ozsoz, M. Buttermilk based cobalt phthalocyanine dispersed ferricyanide mediated amperometric biosensor for the determination of xanthine. Electroanalysis 1998, 10, 273–275. [Google Scholar] [CrossRef]
- Arslan, F.; Yaşar, A.; Kılıç, E. An Amperometric Biosensor for Xanthine Determination Prepared from Xanthine Oxidase Immobilized in Polypyrrole Film. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Erden, P.E.; Pekyardımcı, Ş.; Kılıç, E. Amperometric enzyme electrodes for xanthine determination with different mediators. Acta Chim. Slov. 2012, 59, 824–832. Available online: https://acta-arhiv.chem-soc.si/59/59-4-824.pdf (accessed on 10 February 2025). [PubMed]
- Dervisevic, M.; Custiuc, E.; Çevik, E.; Durmus, Z.; Şenel, M.; Durmus, A. Electrochemical biosensor based on REGO/Fe3O4 bionanocomposite interface for xanthine detection in fish sample. Food Control 2015, 57, 402–410. [Google Scholar] [CrossRef]
- Can, E.; Selvi, C.K.; Canel, E.; Erden, P.E.; Kılıç, E. Electrochemical xanthine biosensor based on carbon nanofiber and ferrocene carboxylic acid for the assessment of fish freshness. Ionics 2024, 30, 4215–4226. [Google Scholar] [CrossRef]
- Bollella, P. Enzyme-based amperometric biosensors: 60 years later … Quo Vadis? Anal. Chim. Acta 2022, 1234, 340517. [Google Scholar] [CrossRef]
- Takahashi, S.; Anzai, J.-I. Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors. Materials 2013, 6, 5742–5762. [Google Scholar] [CrossRef]
- Teng, Y.; Chen, C.; Zhou, C.; Zhao, H.; Lan, M. Disposable amperometric biosensors based on xanthine oxidase immobilized in the Prussian blue modified screen-printed three-electrode system. Sci. China Chem. 2010, 53, 2581–2586. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, L.; Tao, W.; Yao, S. Amperometric Study of Au-Colloid Function on Xanthine Biosensor Based on Xanthine Oxidase Immobilized in Polypyrrole Layer. Electroanalysis 2004, 16, 1271–1278. [Google Scholar] [CrossRef]
- Dalkıran, B.; Erden, P.E.; Kılıç, E. Amperometric biosensors based on carboxylated multiwalled carbon nanotubes-metal oxide nanoparticles-7,7,8,8-tetracyanoquinodimethane composite for the determination of xanthine. Talanta 2017, 167, 286–295. [Google Scholar] [CrossRef]
- Dalkıran, B.; Kaçar, C.; Erden, P.E.; Kılıç, E. Electrochemical xanthine biosensor based on zinc oxide nanoparticles–multiwalled carbon nanotubes–1,4-benzoquinone composite. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 317–332. [Google Scholar] [CrossRef]
- Dervisevic, M.; Custiuc, E.; Çevik, E.; Şenel, M. Construction of novel xanthine biosensor by using polymeric mediator/MWCNT nanocomposite layer for fish freshness detection. Food Chem. 2015, 181, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, C.; Luo, J.; Luo, J.; Cui, D. Biosensor for detection of hypoxanthine based on xanthine oxidase immobilized on chemically modified carbon paste electrode. Anal. Chim. Acta 2000, 412, 55–61. [Google Scholar] [CrossRef]
- Dodevska, T.; Horozova, E.; Dimcheva, N. Electrochemical behavior of ascorbate oxidase immobilized on graphite electrode modified with Au-nanoparticles. Mater. Sci. Eng. B 2013, 178, 1497–1502. [Google Scholar] [CrossRef]
- Schachinger, F.; Chang, H.; Scheiblbrandner, S.; Ludwig, R. Amperometric Biosensors Based on Direct Electron Transfer Enzymes. Molecules 2021, 26, 4525. [Google Scholar] [CrossRef]
- Horozova, E.; Dimcheva, N.; Jordanova, Z. Enzymatic and electrochemical reactions of xanthine oxidase immobilized on carbon materials. Z. Naturforschung C 1997, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.C.; Ghica, M.E.; Brett, C.M.A. Design of a new hypoxanthine biosensor: Xanthine oxidase modified carbon film and multi-walled carbon nanotube/carbon film electrodes. Anal. Bioanal. Chem. 2013, 405, 3813–3822. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, Z. Direct Electrochemistry of Xanthine Oxidase at a Gold Electrode Modified with Single-Wall Carbon Nanotubes. Anal. Sci. 2004, 20, 635–638. [Google Scholar] [CrossRef]
- Gao, Y.; Shen, C.; Di, J.; Tu, Y. Fabrication of amperometric xanthine biosensors based on direct chemistry of xanthine oxidase. Mater. Sci. Eng. C 2009, 29, 2213–2216. [Google Scholar] [CrossRef]
- Shan, D.; Wang, Y.-N.; Xue, H.-G.; Cosnier, S.; Ding, S.-N. Xanthine oxidase/laponite nanoparticles immobilized on glassy carbon electrode: Direct electron transfer and multielectrocatalysis. Biosens. Bioelectron. 2009, 24, 3556–3561. [Google Scholar] [CrossRef]
- Sharma, P.; Thakur, D.; Kumar, D. Novel Enzymatic Biosensor Utilizing a MoS2/MoO3 Nanohybrid for the Electrochemical Detection of Xanthine in Fish Meat. ACS Omega 2023, 8, 31962–31971. [Google Scholar] [CrossRef]
- Sen, S.; Sarkar, P. A novel third-generation xanthine biosensor with enzyme modified glassy carbon electrode using electrodeposited MWCNT and nanogold polymer composite film. RSC Adv. 2015, 5, 95911–95925. [Google Scholar] [CrossRef]
- Dalkiran, B.; Kaçar, C.; Erden, P.E.; Kiliç, E. Amperometric xanthine biosensors based on chitosan-Co3O4-multiwall carbon nanotube modified glassy carbon electrode. Sens. Actuators B Chem. 2014, 200, 83–91. [Google Scholar] [CrossRef]
- Görgülü, M.; Çete, S.; Arslan, H.; Yaşar, A. Preparing a new biosensor for hypoxanthine determination by immobilization of xanthine oxidase and uricase in polypyrrole-polyvinyl sulphonate film. Artif. Cells Nanomed. Biotechnol. 2013, 41, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Erol, E.; Yildirim, E.; Cete, S. Construction of biosensor for hypoxanthine determination by immobilization of xanthine oxidase and uricase in polypyrrole-paratoluenesulfonate film. J. Solid State Electrochem. 2020, 24, 1695–1707. [Google Scholar] [CrossRef]
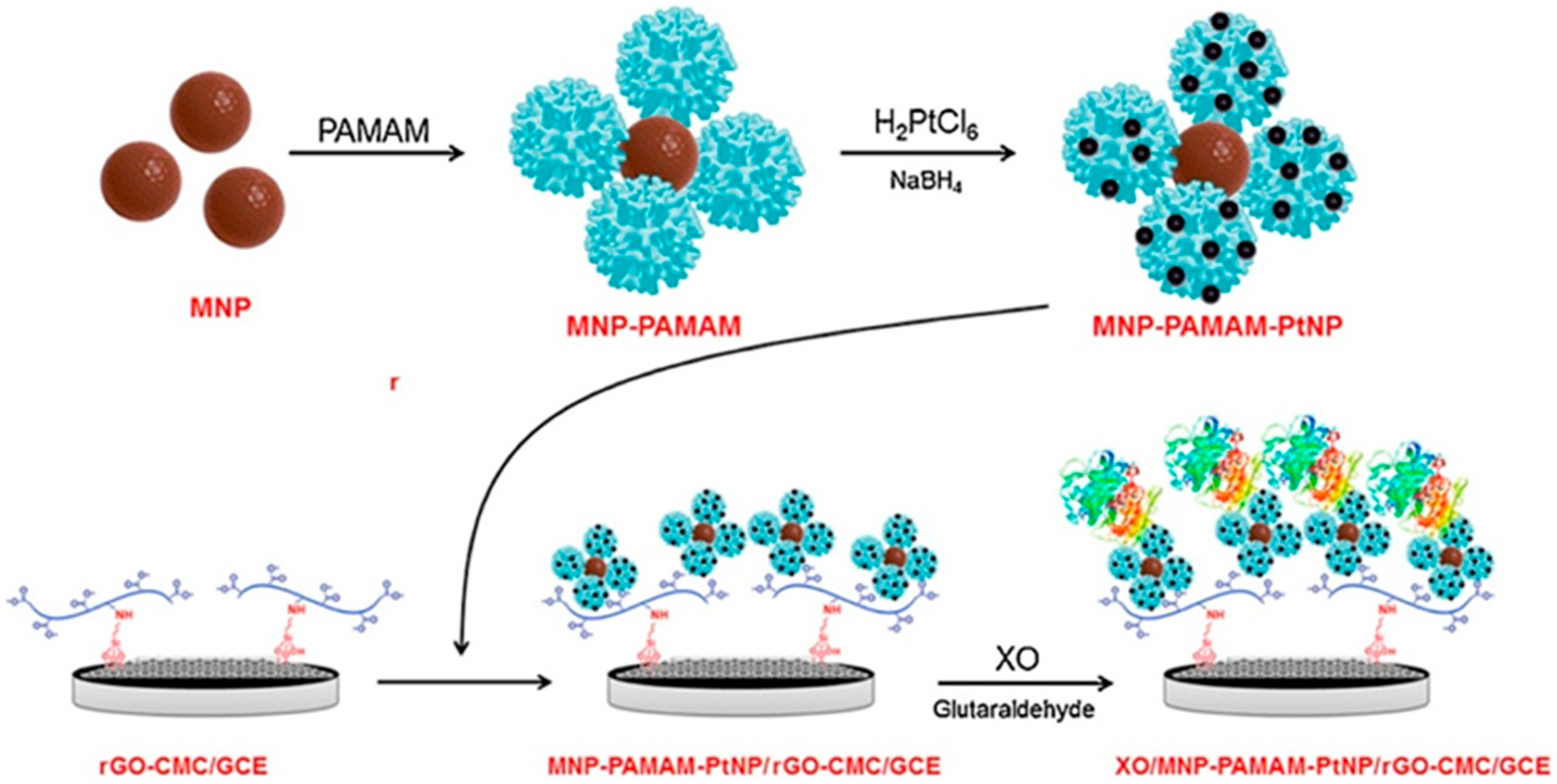

| Electrode | Analyte | Technique (Potential) | Linear Range (LOD) | Stability | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| XOx-PVS-PPy/Pt 3 | X | Amp. | 0.1–1000 µM | 49% | fish | [10] |
| (0.3 V **) | (0.1 µM) | (30 days) | ||||
| XOx/PtNPs/FPP 4 | X | Amp. | 10–1400 µM | 70% (24 days) | fish | [36] |
| (−0.1 V **) | (45 nM) | |||||
| XOx/PtNPs/FPP 1 | Amp. | 30–800 µM | ||||
| (0.4 V **) | (30 nM) | |||||
| XOx/Nano Fe3O4/Au 1 | X | Amp. | 0.4–2.4 nM | 80% (11 days) | fish | [38] |
| (0.5 V *) | (2.5 pM) | |||||
| XOx/Fe3O4-NPs/c-MWCNT/FTO | X | Amp. | 0.05–150 µM | 50% (120 days) | fish | [39] |
| (0.2 V *) | (0.05 µM) | |||||
| XOx/c-MWCNT/PANI/Pt 1 | X | Amp. | 0.6–58 µM | 50% (100 days) | fish | [49] |
| (0.4 V *) | (0.6 µM) | |||||
| XOx/CHIT/Fe-NPs@Au/PGE 1 | X | Amp. | 0.1–300 µM | 75% (100 days) | fish | [45] |
| (0.5 V *) | (0.1 µM) | |||||
| GA–BSA–XOx–AuNPs–CPE 2 | Hx | Amp. | 0.5–10 µM | 15 days | sardines, chicken | [46] |
| (0.0 V *) | (0.22 µM) | |||||
| GA–BSA–XOx–AuNPs–CPE 1 | Amp. | 0.5–10 µM | ||||
| (0.6 V *) | (0.1 µM) | |||||
| XOx/ZnO-NPs–PPy/Pt 1 | X | Amp. | 0.8–40 µM | 60% (100 days) | fish | [50] |
| (0.38 V *) | (0.8 µM) | |||||
| XOx/AgNPs/L-Cys/Au 1 | X | Amp. | 2–16 µM | 80% (60 days) | chicken, beef, pork | [52] |
| (0.5 V *) | (0.15 µM) | |||||
| XOx/nano Ag-ZnO/PPy/PGE 1 | X | Amp. | 0.06-0.6 µM | 78% (20 days) | fish | [53] |
| (0.7 V *) | (0.07 µM) | |||||
| XOx/ZnO-NP/CHIT/c-MWCNT/PANI/Pt 1 | X | CV | 1–100 µM | 70% (30 days) | fish | [55] |
| (0.5 V *) | (0.1 µM) | |||||
| XOx/PtNPs-PAMAM-MNP/GO-CMC/GCE 1 | X | Amp. | 50 nM–12 µM | 73% (28 days) | fish | [57] |
| (0.6 V *) | (13 nM) | |||||
| CHIT–PPy/Au–XOx/GCE 1 | X | Amp. | 1–200 µM | 85% (18 days) | fish, chicken, beef | [59] |
| (0.7 V *) | (0.25 µM) | |||||
| Pt/HMTES (XOx) + C6-MPCs/PU(75:25) 1 | X | Amp. | up to 600 µM | – | fish | [63] |
| (0.4 V *) | (5.2 µM) | |||||
| Amp. | up to 600 µM | |||||
| (0.65 V *) | (3.1 µM) | |||||
| XOx@Cu-MOF/SA/GCE 1 | X | DPV | 0.01–10 µM | 80% (20 days) | squid, large yellow croaker | [69] |
| (0.579 V **) | (6.4 nM) | |||||
| Hx | DPV | 0.01–10 µM | ||||
| (0.749 V **) | (2.3 nM) | |||||
| XOxNPs/Au 1 | X | Amp. | 0.01–1 µM | 50% (60 days) | fish | [70] |
| (0.25 V *) | (0.01 µM) | |||||
| Nafion/XOx/Co3O4/CHIT/GR 1 | X | Amp. | 0.5–80 µM | 83% (60 days) | fish | 71 |
| (0.7 V *) | (0.2 µM) | |||||
| Nafion/XOx/TiO2-G/GCE 1 | Hx | Amp. | 20–512 µM | 77% (10 days) | pork | [80] |
| (0.8 V *) | (9.5 µM) |
| Electrode | Mediator | Analyte | Technique (Potential) | Linear Range (LOD) | Stability | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| XOx/c-MWCNTs/Fe3O4/TCNQ/CHIT/GCE | TCNQ | X | Amp. (0.3 V *) | 1.9–230 µM (0.2 µM) | 70% (30 days) | coffee | [90] |
| XOx/BQ-MWCNTs-ZnO-CHIT/GCE | BQ | X | Amp. (0.25 V *) | 0.9–110 µM (0.21 µM) | 95% (25 days) | chicken, beef | [91] |
| Poly(GMA-co-VFc)/REGO-Fe3O4/XOx/PGE | VFc | X | Amp. (0.35 V *) | 2–36 µM (0.17 µM) | 70% (25 days) | fish | [92] |
| XOx/SWy-2-MV/CPE | MV | Hx | CV (−0.72 V **) | 1–400 µM (0.8 µM) | 60% (5 weeks) | fish | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodevska, T. A Review on Xanthine Oxidase-Based Electrochemical Biosensors: Food Safety and Quality Control Applications. Chemosensors 2025, 13, 159. https://doi.org/10.3390/chemosensors13050159
Dodevska T. A Review on Xanthine Oxidase-Based Electrochemical Biosensors: Food Safety and Quality Control Applications. Chemosensors. 2025; 13(5):159. https://doi.org/10.3390/chemosensors13050159
Chicago/Turabian StyleDodevska, Totka. 2025. "A Review on Xanthine Oxidase-Based Electrochemical Biosensors: Food Safety and Quality Control Applications" Chemosensors 13, no. 5: 159. https://doi.org/10.3390/chemosensors13050159
APA StyleDodevska, T. (2025). A Review on Xanthine Oxidase-Based Electrochemical Biosensors: Food Safety and Quality Control Applications. Chemosensors, 13(5), 159. https://doi.org/10.3390/chemosensors13050159






