Recent Advances in Nanomaterials for Enhanced Colorimetric Detection of Viruses and Bacteria
Abstract
1. Introduction
2. Colorimetric Detection Based on Aggregation
3. Plasmonic Properties for Enhanced Sensitivity
3.1. Antibody Functionalization
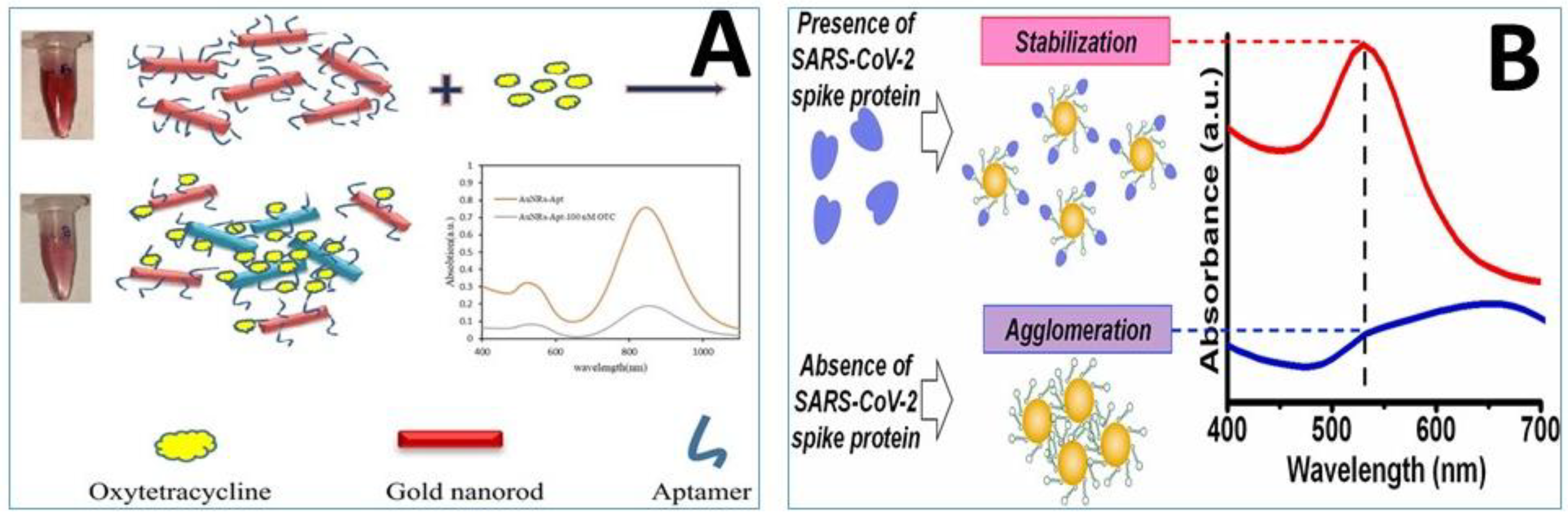
3.2. Aptamer-Functionalized
3.3. Other News Approaches
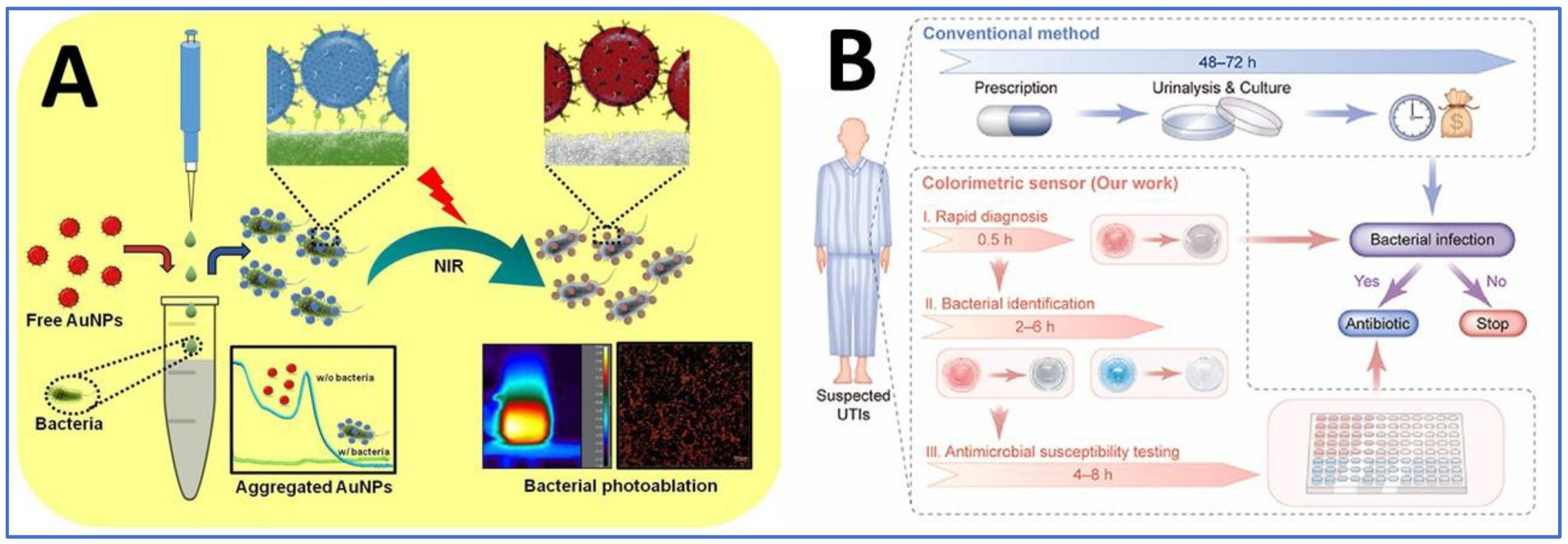
4. Point-of-Care Testing Based on Colorimetric Sensors
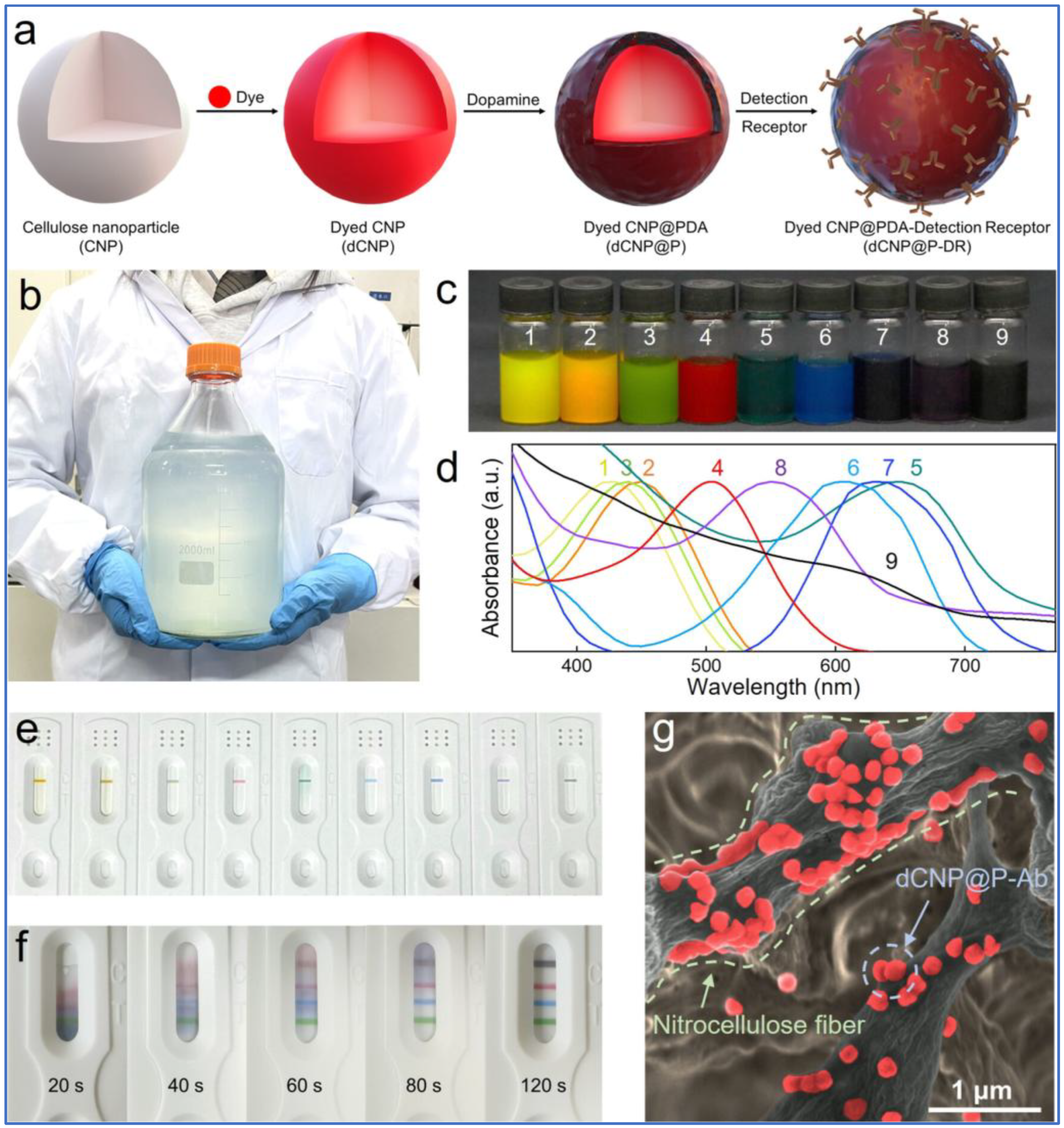
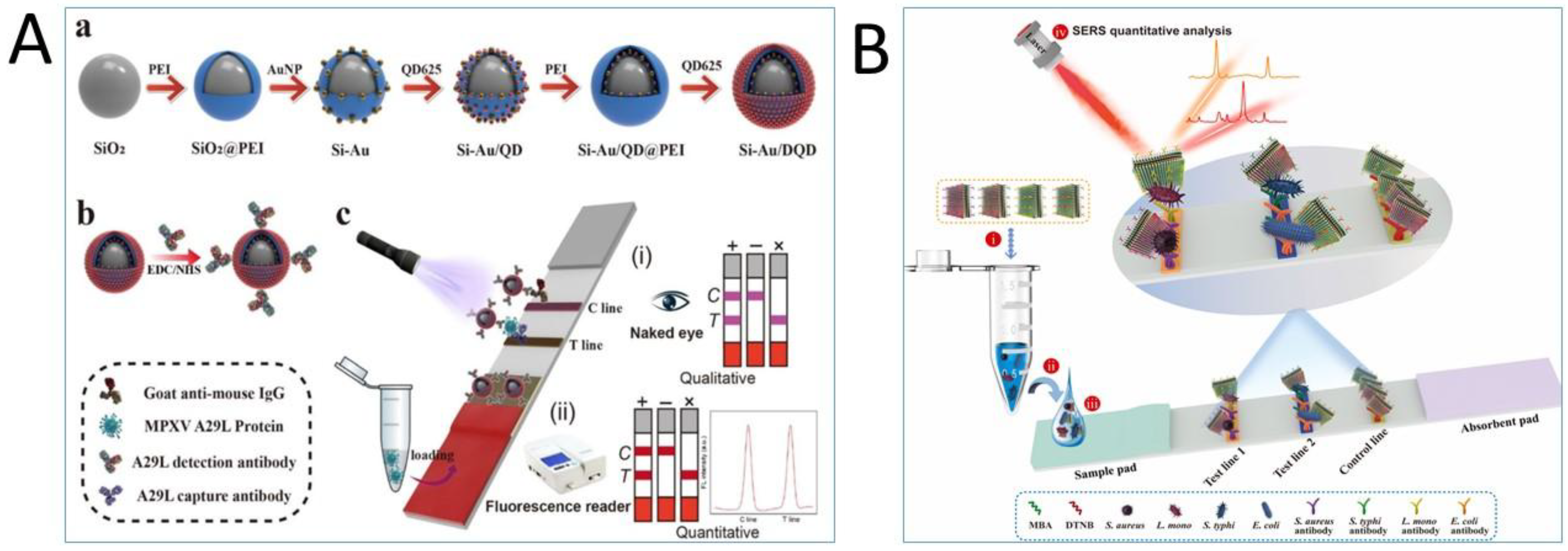
5. Dual and Multiplexed Detection Platforms
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 15 January 2025).
- Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial resistance and its drivers—A review. Antibiotics 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Song, H.A.; Pierson, S.L.; Shen-Gunther, J.; Xia, Q. Emerging infectious diseases are virulent viruses—Are we prepared? An overview. Microorganisms 2023, 11, 2618. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Mishra, P.; Arora, N.K. Linkages between environmental issues and zoonotic diseases: With reference to COVID-19 pandemic. Environ. Sustain. 2021, 4, 455–467. [Google Scholar] [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms-From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Wu, J.; Coin, L.; O’Brien, J.; Hai, F.; Jiang, G. Molecular Methods for Pathogenic Bacteria Detection and Recent Advances in Wastewater Analysis. Water 2021, 13, 3551. [Google Scholar] [CrossRef]
- Tabatabaei, M.S.; Ahmed, M. Enzyme-linked immunosorbent assay (ELISA). In Cancer Cell Biology; Christian, S.L., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2508. [Google Scholar]
- Zhao, Q.; Lu, D.; Zhang, G.; Zhang, D.; Shi, X. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta 2021, 223, 121722. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.V.; Jeevanantham, S.; Kamalesh, R.; Sneha, S.; Yaashikaa, P.R. Methods of Detection of Food-Borne Pathogens: A Review. Environ. Chem. Lett. 2021, 19, 189–207. [Google Scholar] [CrossRef]
- Otoo, J.A.; Schlappi, T.S. REASSURED multiplex diagnostics: A critical review and forecast. Biosensors 2022, 12, 124. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, B.; Wei, G. Two-dimensional material-based colorimetric biosensors: A review. Biosensors 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Cui, Y.; Cai, Z.; Li, L. Applications of smartphone-based colorimetric biosensors. Biosens. Bioelectron. 2022, 11, 100173. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, N.Y. A Foldable Thermoplastic Microdevice Integrating Isothermal Amplification and Schiff-Reaction-Based Colorimetric Assay for the Detection of Infectious Pathogens. Chemosensors 2024, 12, 75. [Google Scholar] [CrossRef]
- Geleta, G.S. A colorimetric aptasensor based on gold nanoparticles for detection of microbial toxins: An alternative approach to conventional methods. Anal. Bioanal. Chem. 2022, 414, 7103–7122. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Hu, G.; Zhang, E.; Yu, H.-H. Colorimetric Sensors for Chemical and Biological Sensing Applications. Sensors 2023, 23, 2749. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Huang Y, Guo X, Wu Y, Chen X, Feng L, Xie N, Shen G Nanotechnology’s frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Sig. Transduct. Target. Ther. 2024, 9, 34. [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2023, 13, 40. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Sun, Y.; Chen, B.; Hu, F.; Guo, C.; Yang, T. Recent Advances in Colorimetric Sensors Based on Gold Nanoparticles for Pathogen Detection. Biosensors 2023, 13, 29. [Google Scholar] [CrossRef]
- Kim, D.-M.; Yoo, S.-M. Colorimetric Systems for the Detection of Bacterial Contamination: Strategy and Applications. Biosensors 2022, 12, 532. [Google Scholar] [CrossRef]
- Abdolhosseini, M.; Zandsalimi, F.; Moghaddam, F.S.; Tavoosidana, G. A review on colorimetric assays for DNA virus detection. J. Virol. Methods 2022, 301, 114461. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Avan, A.A. Nanotechnology-based Colorimetric Approaches for Pathogenic Virus Sensing: A Review. Curr. Med. Chem. 2022, 29, 2691–2718. [Google Scholar] [CrossRef] [PubMed]
- Pirzada, M.; Altintas, Z. Nanomaterials for virus sensing and tracking. Chem. Soc. Rev. 2022, 51, 5805–5841. [Google Scholar] [CrossRef]
- Ozin, G.A. Nanochemistry: Synthesis in Diminishing Dimensions. Adv. Mater. 1992, 4, 612–649. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on Nanomaterials: Synthesis and Applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Verma, M.S.; Rogowski, J.L.; Jones, L.; Gu, F.X. Colorimetric Biosensing of Pathogens Using Gold Nanoparticles. Biotechnol. Adv. 2015, 33, 666–680. [Google Scholar] [CrossRef]
- Babakhani, P. The Impact of Nanoparticle Aggregation on Their Size Exclusion During Transport in Porous Media: One- and Three-Dimensional Modelling Investigations. Sci. Rep. 2019, 9, 14071. [Google Scholar] [CrossRef]
- Jin, Z.; Yim, W.; Retout, M.; Housel, E.; Zhong, W.; Zhou, J.; Strano, M.S.; Jokerst, J.V. Colorimetric Sensing for Translational Applications: From Colorants to Mechanisms. Chem. Soc. Rev. 2024, 53, 7681–7741. [Google Scholar] [CrossRef]
- Liu, G.; Lu, M.; Huang, X.; Li, T.; Xu, D. Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening. Sensors 2018, 18, 4166. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Li, X.; Wang, X.; Liu, J.-Z.; She, W.-Z.; Liu, J.; Ling, J.; Li, R.S.; Cao, Q. Colorimetric Detection and Killing of Bacteria by Enzyme-Instructed Self-Aggregation of Peptide-Modified Gold Nanoparticles. Chemosensors 2023, 11, 484. [Google Scholar] [CrossRef]
- Baetsen-Young, A.M.; Vasher, M.; Matta, L.L.; Colgan, P.; Alocilja, E.C.; Day, B. Direct Colorimetric Detection of Unamplified Pathogen DNA by Dextrin-Capped Gold Nanoparticles. Biosens. Bioelectron. 2018, 101, 29–36. [Google Scholar] [CrossRef]
- Basso, C.R.; Tozato, C.C.; Araujo, J.C.; Pedrosa, V.A. A fast and highly sensitive method for the detection of canine distemper virus by the naked eye. Anal. Methods 2015, 7, 2264–2267. [Google Scholar] [CrossRef]
- Verdoodt, N.; Basso, C.R.; Rossi, B.F.; Pedrosa, V.A. Development of a rapid and sensitive immunosensor for the detection of bacteria. Food Chem. 2017, 221, 1792–1796. [Google Scholar] [CrossRef]
- Basso, C.R.; Tozato, C.C.; Crulhas, B.P.; Castro, G.R.; Junior, J.P.A.; Pedrosa, V.A. An easy way to detect dengue virus using nanoparticle–antibody conjugates. Virology 2018, 513, 85–90. [Google Scholar] [CrossRef]
- Basso, C.R.; Crulhas, B.P.; Magro, M.; Vianello, F.; Pedrosa, V.A. A new immunoassay of hybrid nanomaterial conjugated to aptamers for the detection of dengue virus. Talanta 2019, 197, 482–490. [Google Scholar] [CrossRef]
- Basso, C.R.; Cruz, T.F.; Silva, B.L.; Pedrosa, V.A.; Araújo Junior, J.P. A Methodology for Porcine Circovirus 2 (PCV-2) Quantification Based on Gold Nanoparticles. Materials 2020, 13, 1087. [Google Scholar] [CrossRef]
- Basso, C.R.; Cruz, T.F.; Vieira, L.B.; Pedrosa, V.A.; Possebon, F.S.; Araújo Junior, J.P. Development of a Gold Nanoparticle-Based ELISA for Detection of PCV2. Pathogens 2024, 13, 108. [Google Scholar] [CrossRef]
- Ngernpimai, S.; Srijampa, S.; Thongmee, P.; Teerasong, S.; Puangmali, T.; Maleewong, W.; Chompoosor, A.; Tippayawat, P. Insight into the Covalently Oriented Immobilization of Antibodies on Gold Nanoparticle Probes to Improve Sensitivity in the Colorimetric Detection of Listeria monocytogenes. Bioconjug. Chem. 2022, 33, 2103–2112. [Google Scholar] [CrossRef]
- Sung, Y.J.; Suk, H.J.; Sung, H.Y.; Li, T.; Poo, H.; Kim, M.G. Novel Antibody/Gold Nanoparticle/Magnetic Nanoparticle Nanocomposites for Immunomagnetic Separation and Rapid Colorimetric Detection of Staphylococcus aureus in Milk. Biosens. Bioelectron. 2013, 43, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Mortezaei, M.; Dadmehr, M.; Korouzhdehi, B.; Hakimi, M.; Ramshini, H. Colorimetric and Label-Free Detection of Gelatinase Positive Bacteria and Gelatinase Activity Based on Aggregation and Dissolution of Gold Nanoparticles. J. Microbiol. Methods 2021, 191, 106349. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Kim, M.I. Nanomaterial-Mediated Paper-Based Biosensors for Colorimetric Pathogen Detection. TrAC Trends Anal. Chem. 2020, 132, 116038. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.; Tran, V.T.; Lee, D.K.; Ahmed, S.R.; Hong, J.C.; Lee, J.; Park, E.Y.; Lee, J. Magnetic Nanozyme-Linked Immunosorbent Assay for Ultrasensitive Influenza A Virus Detection. ACS Appl. Mater. Interfaces 2018, 10, 12534–12543. [Google Scholar] [CrossRef]
- Meléndez-Villanueva, M.A.; Morán-Santibañez, K.; Martínez-Sanmiguel, J.J.; Rangel-López, R.; Garza-Navarro, M.A.; Rodríguez-Padilla, C.; Zarate-Triviño, D.G.; Trejo-Ávila, L.M. Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses 2019, 11, 1111. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Corredor, J.C.; Nagy, É.; Neethirajan, S. Amplified visual immunosensor integrated with nanozyme for ultrasensitive detection of avian influenza virus. Nanotheranostics 2017, 1, 338–345. [Google Scholar] [CrossRef]
- Savas, S.; Altintas, Z. Graphene Quantum Dots as Nanozymes for Electrochemical Sensing of Yersinia enterocolitica in Milk and Human Serum. Materials 2019, 12, 2181. [Google Scholar] [CrossRef]
- Liu, D.; Ju, C.; Han, C.; Shi, R.; Chen, X.; Duan, D.; Yan, J.; Yan, X. Nanozyme Chemiluminescence Paper Test for Rapid and Sensitive Detection of SARS-CoV-2 Antigen. Biosens. Bioelectron. 2020, 173, 112817. [Google Scholar] [CrossRef]
- Alam, N.; Ravikumar, C.H.; Sreeramareddygari, M.; Somasundrum, M.; Surareungchai, W. Label-free ultra-sensitive colorimetric detection of hepatitis E virus based on oxidase-like activity of MnO2 nanosheets. Anal Bioanal Chem. 2023, 415, 703. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, M.; Liu, Y.; Jin, M.; Zhao, H. Selection and Characterization of DNA Aptamers for Constructing a Colorimetric Biosensor for Detection of PBP2a. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117735. [Google Scholar] [CrossRef]
- Ferrari, E. Gold Nanoparticle-Based Plasmonic Biosensors. Biosensors 2023, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Su, R.; Li, X.; Chen, W.; Chen, Q.; Yang, T.; Wang, J.; Liu, H.; Fang, Q.; et al. Structure of RNA Polymerase Complex and Genome within a dsRNA Virus Provides Insights into the Mechanisms of Transcription and Assembly. Proc. Natl. Acad. Sci. USA 2018, 115, 7344. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.A.; Narovec, C.M.; Whelan, R.J. Effects of Cationic Proteins on Gold Nanoparticle/Aptamer Assays. ACS Omega 2017, 2, 8222. [Google Scholar] [CrossRef] [PubMed]
- Storhoff, J.J.; Lucas, A.D.; Garimella, V.; Bao, Y.P.; Müller, U.R. Homogeneous Detection of Unamplified Genomic DNA Sequences Based on Colorimetric Scatter of Gold Nanoparticle Probes. Nat. Biotechnol. 2004, 22, 883. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Wang, S.; Liu, Y. A Smartphone-Integrated Paper Sensing System for Fluorescent and Colorimetric Dual-Channel Detection of Foodborne Pathogenic Bacteria. Anal. Bioanal. Chem. 2020, 412, 611. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwon, C.; Noh, H. Paper-Based Diagnostic System Facilitating Escherichia coli Assessments by Duplex Coloration. ACS Sens. 2019, 4, 2435. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Tu, Z.; Wei, H.; Rong, Z. PDA-mediated colorimetric-fluorescence co-enhanced immunochromatography assay for the simultaneous and rapid detection of SARS-CoV-2 and HAdV. Chem. Eng. J. 2024, 481, 148756. [Google Scholar] [CrossRef]
- Lee, A.S.; Kim, S.M.; Kim, K.R.; Park, C.; Lee, D.-G.; Heo, H.R.; Cha, H.J.; Kim, C.S. A colorimetric lateral flow immunoassay based on oriented antibody immobilization for sensitive detection of SARS-CoV-2. Sens. Actuators B 2023, 379, 133245. [Google Scholar] [CrossRef]
- Raji, M.A.; Aloraij, Y.; Alhamlan, F.; Suaifan, G.; Weber, K.; Cialla-May, D.; Popp, J.; Zourob, M. Development of rapid colorimetric assay for the detection of Influenza A and B viruses. Talanta 2021, 221, 121468. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Salena, B.J.; Li, Y. Aptamer and DNAzyme based colorimetric biosensors for pathogen detection. Angew. Chem. Int. Ed. Engl. 2025, 64, e202418725. [Google Scholar] [CrossRef]
- Schmitz, F.R.W.; Cesca, K.; Valério, A.; de Oliveira, D.; Hotza, D. Colorimetric Detection of Pseudomonas aeruginosa by Aptamer-Functionalized Gold Nanoparticles. Appl. Microbiol. Biotechnol. 2023, 107, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, J.; Pan, X.; Liu, B.; Wang, L. Aptamer-functionalized polydiacetylene biosensor for the detection of three foodborne pathogens. Anal. Sci. 2024, 40, 199. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Tang, L.; Chen, F.; Chen, C.; Cheng, Y.; Cai, C. Self-service multimodal detection of subtype influenza A virus H5N1 based on a molecularly imprinted virus sensor. Chem. Eng. J. 2024, 483, 148946. [Google Scholar] [CrossRef]
- Serra, T.; Nieddu, S.; Cavalera, S.; Pérez-Juste, J.; Pastoriza-Santos, I.; Di Nardo, F.; Testa, V.; Baggiani, C.; Anfossi, L. Plasmonic nanosized molecularly imprinted polymer (nanoMIP) as innovative optical lateral flow immunoassay probes. Sens. Actuators B 2025, 428, 137249. [Google Scholar] [CrossRef]
- Stephens, N.; Di Silvio, L.; Dunsford, I.; Ellis, M.; Glencross, A.; Sexton, A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. Technol. 2018, 78, 155–166. [Google Scholar] [CrossRef]
- Tran, T.A.H.; Vu, V.T.; Huang, C.-J. Development of functional biointerface using mixed zwitterionic silatranes. Langmuir 2024, 40, 24516. [Google Scholar] [CrossRef]
- Chen, W.; Peng, X.; Wei, Y.; Dong, S.; Zhang, J.; Zhao, Y.; Sun, F. Nanozyme-catalyzed and zwitterion-modified swabs for the detection of Listeria monocytogenes in complex matrices. Talanta 2024, 280, 126777. [Google Scholar] [CrossRef]
- Zhang, P.; Hou, H.; Xu, S.; Wen, Y.; Zhang, Y.; Xing, F. Localized surface plasmon resonance sensing based on monometallic gold nanoparticles: From material preparation to detection of bioanalytes. Anal. Methods. 2025, 16, 6072. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. LSPR and SPR-based fiber-optic cholesterol sensor using immobilization of cholesterol oxidase over silver nanoparticles coated graphene oxide nanosheets. IEEE Sens. J. 2018, 18, 1039. [Google Scholar] [CrossRef]
- Semwal, V.; Jensen, O.R.; Bang, O.; Janting, J. Investigation of performance parameters of spherical gold nanoparticles in localized surface plasmon resonance biosensing. Micromachines. 2023, 14, 1717. [Google Scholar] [CrossRef]
- Sanromán-Iglesias, M.; Garrido, V.; Gil-Ramírez, Y.; Aizpurua, J.; Grzelczak, M.; Grilló, M.-J. Plasmon-Assisted Fast Colorimetric Detection of Bacterial Nucleases in Food Samples. Sensors Actuators B Chem. 2021, 349, 130780. [Google Scholar] [CrossRef]
- Hassan, H.; Sharma, P.; Hasan, M.R.; Singh, S.; Thakur, D.; Narang, J. Gold nanomaterials – The golden approach from synthesis to applications. Mater. Sci. Energy Technol. 2022, 5, 375. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano. 2020, 7, 2195. [Google Scholar] [CrossRef]
- Huang, R.; Cai, X.; Du, J.; Lian, J.; Hui, P.; Gu, M.; Li, F.; Wang, J.; Chen, W. Bioinspired plasmonic nanosensor for on-site antimicrobial susceptibility testing in urine samples. ACS Nano. 2022, 16, 19229. [Google Scholar] [CrossRef]
- Yaghubi, F.; Zeinoddini, M.; Saeedinia, A.R.; Azizi, A.; Samimi Nemati, A. Design of Localized Surface Plasmon Resonance (LSPR) biosensor for immunodiagnostic of E. coli O157:H7 using gold nanoparticles conjugated to the chicken antibody. Plasmonics 2020, 15, 1481. [Google Scholar] [CrossRef]
- Pereira, R.H.A.; Keijok, W.J.; Prado, A.R.; de Oliveira, J.P.; Guimarães, M.C.C. Rapid and sensitive detection of ochratoxin A using antibody-conjugated gold nanoparticles based on localized surface plasmon resonance. Toxicon 2021, 199, 139. [Google Scholar] [CrossRef]
- Tanabe, S.; Itagaki, S.; Matsui, K.; Nishii, S.; Yamamoto, Y.; Sadanaga, Y.; Shiigi, H. Simultaneous Optical Detection of Multiple Bacterial Species Using Nanometer-Scaled Metal–Organic Hybrids. Anal. Chem. 2022, 94, 10984. [Google Scholar] [CrossRef]
- Wenck, C.; Leopoldt, D.; Habib, M.; Hegermann, J.; Stiesch, M.; Doll-Nikutta, K.; Heisterkamp, A.; Torres-Mapa, M.L. Colorimetric detection of oral bacteria using functionalized gold nanoparticles as a plasmonic biosensor array. Nanoscale Adv. 2024, 6, 1447. [Google Scholar] [CrossRef]
- Marin, M.; Rizzotto, F.; Léguillier, V.; Péchoux, C.; Borezee-Durant, E.; Vidic, J. Naked-eye detection of Staphylococcus aureus in powdered milk and infant formula using gold nanoparticles. J. Microbiol. Methods 2022, 201, 106578. [Google Scholar] [CrossRef]
- Seele, P.P.; Dyan, B.; Skepu, A.; Maserumule, C.; Sibuyi, N.R.S. Development of gold-nanoparticle-based lateral flow immunoassays for rapid detection of TB ESAT-6 and CFP-10. Biosensors 2023, 13, 354. [Google Scholar] [CrossRef]
- Kaushal, S.; Pinnaka, A.K.; Soni, S.; Singhal, N.K. Antibody-assisted graphene oxide coated gold nanoparticles for rapid bacterial detection and near-infrared photothermal therapy. Sens. Actuators B 2020, 320, 129141. [Google Scholar]
- Zhao, M.; Cao, F.; Chen, J.; Hong, J.; Deng, D.; Wang, Q.; Sun, Y.; Li, Q.; Xin, H.; Wang, X. Rapid, direct, visualized and antibody-free bacterial detection with extra species identification and susceptibility evaluation capabilities. Biosens. Bioelectron. 2023, 221, 114902. [Google Scholar] [CrossRef]
- Lew, T.T.S.; Aung, K.M.M.; Ow, S.Y.; Amrun, S.N.; Sutarlie, L.; Ng, L.F.P.; Su, X. Epitope-functionalized gold nanoparticles for rapid and selective detection of SARS-CoV-2 IgG antibodies. ACS Nano 2021, 15, 12286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, P.; Wang, H.; Wang, X.; Hu, Z.; Wang, F.; Li, Z. Dual protein corona-mediated target recognition system for visual detection and single-molecule counting of nucleic acids. ACS Nano 2025, 19, 6929–6941. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, D.L.; Bessa, I.A.A.; Souza, A.B.C.; Monteiro, L.M.; Borges, R.M.; Allonso, D.; Ligiero, C.B.P.; Ronconi, C.M. Zika Virus NS1 Protein Detection Using Gold Nanoparticle-Assisted Dynamic Light Scattering. Chem. Asian J. 2024, 19, e202400826. [Google Scholar] [CrossRef]
- Yang, Y.; Murray, J.; Haverstick, J.; Tripp, R.A.; Zhao, Y. Silver Nanotriangle Array-Based LSPR Sensor for Rapid Coronavirus Detection. Sens. Actuators B 2022, 359, 131604. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Y.; Zhao, W.; Guo, R.; Qian, S.; Xu, Y.; Li, Y.; Liu, Y.; Liu, H. A multifunctional biosensor for selective identification, sensitive detection and efficient photothermal sterilization of Salmonella typhimurium and Staphylococcus aureus. Anal. Chim. Acta 2025, 1338, 343589. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Y.; Li, J.; Wu, J. A trigger-based aggregation of aptamer-functionalized gold nanoparticles for colorimetry: An example on detection of Escherichia coli O157:H7. Sens. Actuators B 2021, 320, 129865. [Google Scholar] [CrossRef]
- Zhan, L.; Li, C.M.; Fu, Z.F.; Zou, H.Y.; Huang, C.Z. Dual-aptamer-based enzyme linked plasmonic assay for pathogenic bacteria detection. Colloids Surf. B Biointerfaces 2022, 214, 112471. [Google Scholar] [CrossRef]
- Deb, R.; Kumari, S.; Prusty, S.; Singh, S.P.; Sahoo, P.R.; Mohapatra, S. Leveraging localized surface plasmon resonance (LSPR) properties of gold nanoparticles for a point-of-care aptasensor enabling rapid screening of Klebsiella pneumoniae in urinary tract infections. ACS Appl. Bio Mater. 2023, 6, 3117. [Google Scholar]
- Fathollahi Arani, S.; Zeinoddini, M.; Saeedinia, A.R.; Danesh, N.M.; Robatjazi, S.M. LSPR-based colorimetric aptasensor design for rapid and simple detection of Vibrio cholerae O1. Appl. Biochem. Microbiol. 2024, 60, 967–975. [Google Scholar] [CrossRef]
- Mehrabi, F.; Dadmehr, M.; Ranjbar, B.; Hosseini, M. Aptamer-based colorimetric detection of oxytetracycline by a LSPR-based assay of gold nanorods in honey sample. Chem. Select 2024, 9, e202305069. [Google Scholar] [CrossRef]
- Aithal, S.; Mishriki, S.; Gupta, R.; Sahu, R.P.; Botos, G.; Tanvir, S.; Hanson, R.W.; Puri, I.K. SARS-CoV-2 detection with aptamer-functionalized gold nanoparticles. Talanta 2022, 236, 122841. [Google Scholar] [CrossRef]
- Yao, S.; Li, J.; Pang, B.; Wang, X.; Shi, Y.; Song, X.; Xu, K.; Wang, J.; Zhao, C. Colorimetric immunoassay for rapid detection of Staphylococcus aureus based on etching-enhanced peroxidase-like catalytic activity of gold nanoparticles. Mikrochim. Acta 2020, 187, 504. [Google Scholar] [CrossRef]
- Xie, M.; Chen, T.; Cai, Z.; Lei, B.; Dong, C. A digital microfluidic platform coupled with colorimetric loop-mediated isothermal amplification for on-site visual diagnosis of multiple diseases. Lab Chip 2023, 12, 2778. [Google Scholar] [CrossRef]
- Zhao, C.; Jian, X.; Gao, Z.; Song, Y.-Y. Plasmon-mediated peroxidase-like activity on an asymmetric nanotube architecture for rapid visual detection of bacteria. Anal. Chem. 2022, 94, 14038. [Google Scholar] [CrossRef]
- Du, J.Q.; Luo, W.C.; Zhang, J.T.; Li, Q.Y.; Bao, L.N.; Jiang, M.; Yu, X.; Xu, L. Machine learning-assisted fluorescence/fluorescence colorimetric sensor array for discriminating amyloid fibrils. Sens. Actuators B 2024, 320, 136173. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Chen, S.; Su, X.; Xu, D.; Zhai, Y.; Liu, Y.; Hu, G.; Guo, C.; Yang, H.B.; et al. Machine learning-assisted colorimetric sensor arrays for intelligent and rapid diagnosis of urinary tract infection. ACS Sens. 2024, 9, 1945. [Google Scholar] [CrossRef]
- Xue, L.; Qi, W.; Li, Y.; Duan, H.; Wang, L.; Wang, S.; Liu, Y.; Liao, M.; Lin, J. Microfluidic colorimetric biosensors based on MnO2 nanozymes and convergence–divergence spiral micromixers for rapid and sensitive detection of Salmonella. ACS Sens. 2021, 6, 2883. [Google Scholar] [CrossRef]
- Pioz, M.J.; Espinosa, R.L.; Laguna, M.F.; Santamaria, B.; Murillo, A.M.M.; Hueros, Á.L.; Quintero, S.; Tramarin, L.; Valle, L.G.; Herreros, P.; et al. A review of Optical Point-of-Care devices to Estimate the Technology Transfer of These Cutting-Edge Technologies. Biosensors 2022, 12, 1091. [Google Scholar] [CrossRef]
- Kim, M.J.; Haizan, I.; Ahn, M.J.; Park, D.-H.; Choi, J.-H. Recent Advances in Lateral Flow Assays for Viral Protein Detection with Nanomaterial-Based Optical Sensors. Biosensors 2024, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Hasan, M.R.; Jain, A.; Pilloton, R.; Narang, J. LFA: The Mysterious Paper-Based Biosensor: A Futuristic Overview. Chemosensors 2023, 11, 255. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593. [Google Scholar] [CrossRef]
- Chowdhury, P.; Lawrance, R.; Lu, Z.-Y.; Lin, H.-C.; Chan, Y.-H. Recent progress in dual/multi-modal detection modes for improving sensitivity and specificity of lateral flow immunoassays applied for point-of-care diagnostics. TrAC Trends Anal. Chem. 2024, 177, 117798. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Choi, T.S.; Kim, K.T. Fluorescein-switching-based lateral flow assay for the detection of microRNAs. Org. Biomol. Chem. 2024, 22, 8182–8188. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, L.; Liu, X.; Chang, Y.; Xia, R.; Zhang, J.; Kong, Y.; Gong, Y.; Li, T.; Wang, G.; et al. Colored Cellulose Nanoparticles with High Stability and Easily Modified Surface for Accurate and Sensitive Multiplex Lateral Flow Assay. ACS Nano 2025, 19, 4704. [Google Scholar] [CrossRef]
- Yu, Y.C.; Wang, Z.; Ji, X.; Williamson, E.J.; Cordoba, H.M.; Ulloa-Gomez, A.M.; Deering, A.J.; Chiu, G.T.C.; Allebach, J.P.; Stanciu, L.A. Application of a dual-modality colorimetric analysis method to inkjet printing lateral flow detection of Salmonella typhimurium. Microchim. Acta 2024, 191, 559. [Google Scholar] [CrossRef]
- Jang, M.; Kim, S.; Song, J.; Kim, S. Rapid and simple detection of influenza virus via isothermal amplification lateral flow assay. Anal. Bioanal. Chem. 2022, 414, 4685. [Google Scholar] [CrossRef]
- He, Z.; Duan, X.; Zhao, Z.; Chen, Y.; Fu, C.; Zhang, F.; Wang, J.; Feng, J.; Lin, N.; Chen, H. Rapid On-Site Diagnosis of PEDV and PoRV Co-Infection by Gold Magnetic Nanoparticles-Based SERS Immunochromatography. Talanta 2025, 285, 127428. [Google Scholar] [CrossRef]
- Trakoolwilaiwan, T.; Takeuchi, Y.; Leung, T.S.; Sebek, M.; Storozhuk, L.; Nguyen, L.; Tung, L.D.; Thanh, N.T.K. Development of a thermochromic lateral flow assay to improve sensitivity for dengue virus serotype 2 NS1 detection. Nanoscale 2023, 15, 12915. [Google Scholar] [CrossRef]
- Ahn, G.; Lee, S.; Lee, S.H.; Baek, Y.H.; Song, M.-S.; Kim, Y.-H.; Ahn, J.-Y. Zika Virus Lateral Flow Assays Using Reverse Transcription-Loop-Mediated Isothermal Amplification. RSC Adv. 2021, 11, 17800. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.; Yoon, D.S.; Park, J.S.; Woo, H.; Lee, D.; Cho, S.-Y.; Park, C.; Yoo, Y.K.; Lee, K.-B.; et al. Sample-to-answer platform for the clinical evaluation of COVID-19 using a deep learning-assisted smartphone-based assay. Nat. Commun. 2023, 14, 2361. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, B.; Cennamo, M.; Minopoli, A.; Campanile, R.; Bolletti Censi, S.; Terracciano, D.; Portella, G.; Velotta, R. Colorimetric Test for Fast Detection of SARS-CoV-2 in Nasal and Throat Swabs. ACS Sens. 2020, 5, 3043. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, X.; Wei, H.; Tu, Z.; Rong, Z.; Wang, C.; Wang, S. Fluorescence-enhanced dual signal lateral flow immunoassay for flexible and ultrasensitive detection of monkeypox virus. J. Nanobiotechnol. 2023, 21, 450. [Google Scholar] [CrossRef]
- Zhang, T.; Deng, R.; Wang, Y.; Wu, C.; Zhang, K.; Wang, C.; Gong, N.; Ledesma-Amaro, R.; Teng, X.; Yang, C.; et al. A paper-based assay for the colorimetric detection of SARS-CoV-2 variants at single-nucleotide resolution. Nat. Biomed. Eng. 2022, 6, 957. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhang, J.; Zhang, C.; Wang, S.; Li, N. Staining-enhanced peroxidase-mimicking gold nanoparticles in nanozyme-linked immunosorbent assay for Klebsiella pneumoniae detection. ACS Omega 2023, 8, 12345–12352. [Google Scholar]
- Wen, C.-Y.; Zhao, L.-J.; Wang, Y.; Wang, K.; Li, H.-W.; Li, X.; Zi, M.; Zeng, J.-B. Colorimetric and photothermal dual-mode lateral flow immunoassay based on Au-Fe3O4 multifunctional nanoparticles for detection of Salmonella typhimurium. Microchimica Acta 2023, 190, 57. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021, 20, 593. [Google Scholar] [CrossRef]
- Kusuma, S.A.F.; Harmonis, J.A.; Pratiwi, R.; Hasanah, A.N. Gold Nanoparticle-Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules. Sensors 2023, 23, 8172. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Liang, X.; Liu, J.; Zhao, T.-Y.; Li, X.; Zhang, Y.; Guo, G.; Zhang, Z.; Zeng, J. An achromatic colorimetric nanosensor for sensitive multiple pathogen detection by coupling plasmonic nanoparticles with magnetic separation. Talanta 2023, 256, 124271. [Google Scholar] [CrossRef]
- Bushra, R.; Ahmad, M.; Alam, K.; Seidi, F.; Qurtulen, S.; Shakeel, S.; Song, J.; Jin, Y.; Xiao, H. Recent Advances in Magnetic Nanoparticles: Key Applications, Environmental Insights, and Future Strategies. Sustain. Mater. Technol. 2024, 40, e00985. [Google Scholar] [CrossRef]
- Gao, S.; Wei, Z.; Zheng, X.; Zhu, J.; Wang, T.; Huang, X.; Shen, T.; Zhang, D.; Guo, Z.; Zou, X. Advancements in magnetic nanomaterial-assisted sensitive detection of foodborne bacteria: Dual-recognition strategies, functionalities, and multiplexing applications. Food Chem. 2025, 478, 143626. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Chowdhury, A.D.; Khoris, I.M.; Doong, R.-A.; Li, T.-C.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Hollow Magnetic-Fluorescent Nanoparticles for Dual-Modality Virus Detection. Biosens. Bioelectron. 2020, 170, 101013. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Chowdhury, A.D.; Khoris, I.M.; Nasrin, F.; Takemura, K.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Dual modality sensor using liposome-based signal amplification technique for ultrasensitive norovirus detection. Biosens. Bioelectron. 2020, 157, 112169. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Q.; Li, J.; Zheng, S.; Wang, S.; Gu, B. Colorimetric–Fluorescent Dual-Signal Enhancement Immunochromatographic Assay Based on Molybdenum Disulfide-Supported Quantum Dot Nanosheets for the Point-of-Care Testing of Monkeypox Virus. Chem. Eng. J. 2023, 472, 144889. [Google Scholar] [CrossRef]
- Kakkar, S.; Gupta, P.; Yadav, S.P.S.; Raj, D.; Singh, G.; Chauhan, S.; Mishra, M.K.; Martín-Ortega, E.; Chiussi, S.; Kant, K. Lateral Flow Assays: Progress and Evolution of Recent Trends in Point-of-Care Applications. Mater. Today Bio 2024, 28, 101188. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, W.; Duan, H.; Su, Y.; Li, X.; Jiang, H.; Xiong, Y.; Tang, B.Z.; Huang, X. Crescent-Shaped Janus Nanoassemblies of Gold Nanoparticles and AIEgens Enhancing Plasmonic and Fluorescent Activities for Colorimetric and Ratiometric Fluorescence Lateral Flow Immunoassay. Nano Today 2024, 58, 102452. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Li, J.; Tu, Z.; Gu, B.; Wang, S. Ultrasensitive and Multiplex Detection of Four Pathogenic Bacteria on a Bi-Channel Lateral Flow Immunoassay Strip with Three-Dimensional Membrane-Like SERS Nanostickers. Biosens. Bioelectron. 2022, 214, 114525. [Google Scholar] [CrossRef]
- Atta, S.; Zhao, Y.; Li, J.Q.; Vo-Dinh, T. Dual-Modal Colorimetric and Surface-Enhanced Raman Scattering (SERS)-Based Lateral Flow Immunoassay for Ultrasensitive Detection of SARS-CoV-2 Using a Plasmonic Gold Nanocrown. Anal. Chem. 2024, 96, 4783. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, S.; Liu, Y.; Wang, C.; Gu, B.; Zhang, L.; Wang, S. Isothermal Amplification and Hypersensitive Fluorescence Dual-Enhancement Nucleic Acid Lateral Flow Assay for Rapid Detection of Acinetobacter baumannii and Its Drug Resistance. Biosensors 2023, 1, 945. [Google Scholar] [CrossRef]
- Drobysh, M.; Liustrovaite, V.; Kanetski, Y.; Brasiunas, B.; Zvirbliene, A.; Rimkute, A.; Gudas, D.; Kucinskaite-Kodze, I.; Simanavicius, M.; Ramanavicius, S.; et al. Electrochemical Biosensing Based Comparative Study of Monoclonal Antibodies Against SARS-CoV-2 Nucleocapsid Protein. Sci. Total Environ. 2024, 908, 168154. [Google Scholar] [CrossRef] [PubMed]
- Jofre, C.F.; Regiart, M.; Fern, M.A. Electrochemical Microfluidic Immunosensor Based on TES-AuNPs@Fe3O4 and CMK-8 for IgG Anti-Toxocara canis Determination. Anal. Chim. Acta 2020, 1096, 120. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Jiang, H.; Juhas, M.; Zhang, Y. Multiplex biosensing for simultaneous detection of mutations in SARS-CoV-2. ACS Omega 2021, 6, 25846–25859. [Google Scholar] [CrossRef] [PubMed]
| Type of Pathogens | Analyte | Strategy | LOD | Ref. |
|---|---|---|---|---|
| Staphylococcus aureus | Sugar cane | Antibody/Aggregation | 105 CFU/mL | [37] |
| Dengue | Serum | Antibody/Aggregation | TCID50 107 | [38] |
| PCV-2 | Serum | Antibody/Aggregation | 105 DNA copies/mL | [40] |
| Staphylococcus aureus | Milk | Antibody/Magnetic/Aggregation | 1.5 × 105 CFU/mL | [43] |
| Influenza virus A | Blood sample | Peroxidase mimic | 1.11 pg/mL | [48] |
| Ersinia enterocolitica | Human serum | Enzyme mimics | 30 CFU/mL | [49] |
| Methicillin-resistant Staphylococcus aureus | Food sample | Aptamer/Aggregation | 20 nM | [52] |
| E.Coli | Food sample | fluorescein-labeled aptamer | 10 CFU/mL | [58] |
| Human adenovirus | Human sample | Antibody/Aggregation | 104 copies/mL | [60] |
| Influenza A and B viruses | Human sample | Antibody/Aggregation | 0.04 ng mL−1 | [62] |
| E. coli O157 S. typhimurium V. parahaemolyticus. | Food sample | Aptamer/Aggregation | 39 CFU/mL 60 CFU/mL 60 CFU/mL | [65] |
| H5N1 virus | Human sample | Aptamer/Aggregation | 11.6 fM | [66] |
| Listeria monocytogenes | Food samples | Aptamer/Aggregation | 2.83 × 105 CFU/mL | [70] |
| E. coli Klebsiella pneumoniae | Urine samples | Catalyze H2O2 | 512 × 105 CFU/mL | [77] |
| Ochratoxin A | Food samples | Antibody/Aggregation | 0.001 pg mL−1 | [79] |
| E. coli Staphylococcus aureus | Minced chicken | Antibody/Aggregation | 50 CFU/mL | [80] |
| Aggregatibacter actinomycetemcomitans Actinomyces naeslundii Porphyromonas gingivalis Streptococcus oralis | Human sample | Positively/Negatively charged gold nanoparticles | 107 CFU/mL | [81] |
| Staphylococcus aureus | Milk and infant food | Aptamer/Aggregation | 7.5 × 104 CFU/mL 8.4 × 104 CFU/mL | [82] |
| Mycobacterium | Clinic samples | Antibody/Aggregation | 0.0625 ng/mL | [83] |
| Escherichia coli Salmonella | Food samples | Antibody/Aggregation | 103 CFU/mL 102 CFU/mL | [84] |
| Escherichia coli Staphylococcus aureu | Clinic samples | Protein/Aggregation | 105 CFU/mL 108 CFU/mL | [85] |
| Zika virus | Human serum | Antibody/Aggregation | 0.96 μg mL−1 | [88] |
| Corona virus | Saliva | Enzyme/Aggregation | 625 PFU/mL | [89] |
| Klebsiella pneumoniae | Urine | Aptamer/Aggregation | 3.4 × 103 CFU/mL | [93] |
| Vibrio cholerae | Human sample | Aptamer/Aggregation | 103 CFU/mL | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, C.R.; Filho, M.V.B.; Gavioli, V.D.; Parra, J.P.R.L.L.; Castro, G.R.; Pedrosa, V.A. Recent Advances in Nanomaterials for Enhanced Colorimetric Detection of Viruses and Bacteria. Chemosensors 2025, 13, 112. https://doi.org/10.3390/chemosensors13030112
Basso CR, Filho MVB, Gavioli VD, Parra JPRLL, Castro GR, Pedrosa VA. Recent Advances in Nanomaterials for Enhanced Colorimetric Detection of Viruses and Bacteria. Chemosensors. 2025; 13(3):112. https://doi.org/10.3390/chemosensors13030112
Chicago/Turabian StyleBasso, Caroline R., Marcos V. B. Filho, Victoria D. Gavioli, Joao P. R. L. L. Parra, Gustavo R. Castro, and Valber A. Pedrosa. 2025. "Recent Advances in Nanomaterials for Enhanced Colorimetric Detection of Viruses and Bacteria" Chemosensors 13, no. 3: 112. https://doi.org/10.3390/chemosensors13030112
APA StyleBasso, C. R., Filho, M. V. B., Gavioli, V. D., Parra, J. P. R. L. L., Castro, G. R., & Pedrosa, V. A. (2025). Recent Advances in Nanomaterials for Enhanced Colorimetric Detection of Viruses and Bacteria. Chemosensors, 13(3), 112. https://doi.org/10.3390/chemosensors13030112






