Flexible Polymer-Based Electrodes for Detecting Depression-Related Theta Oscillations in the Medial Prefrontal Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
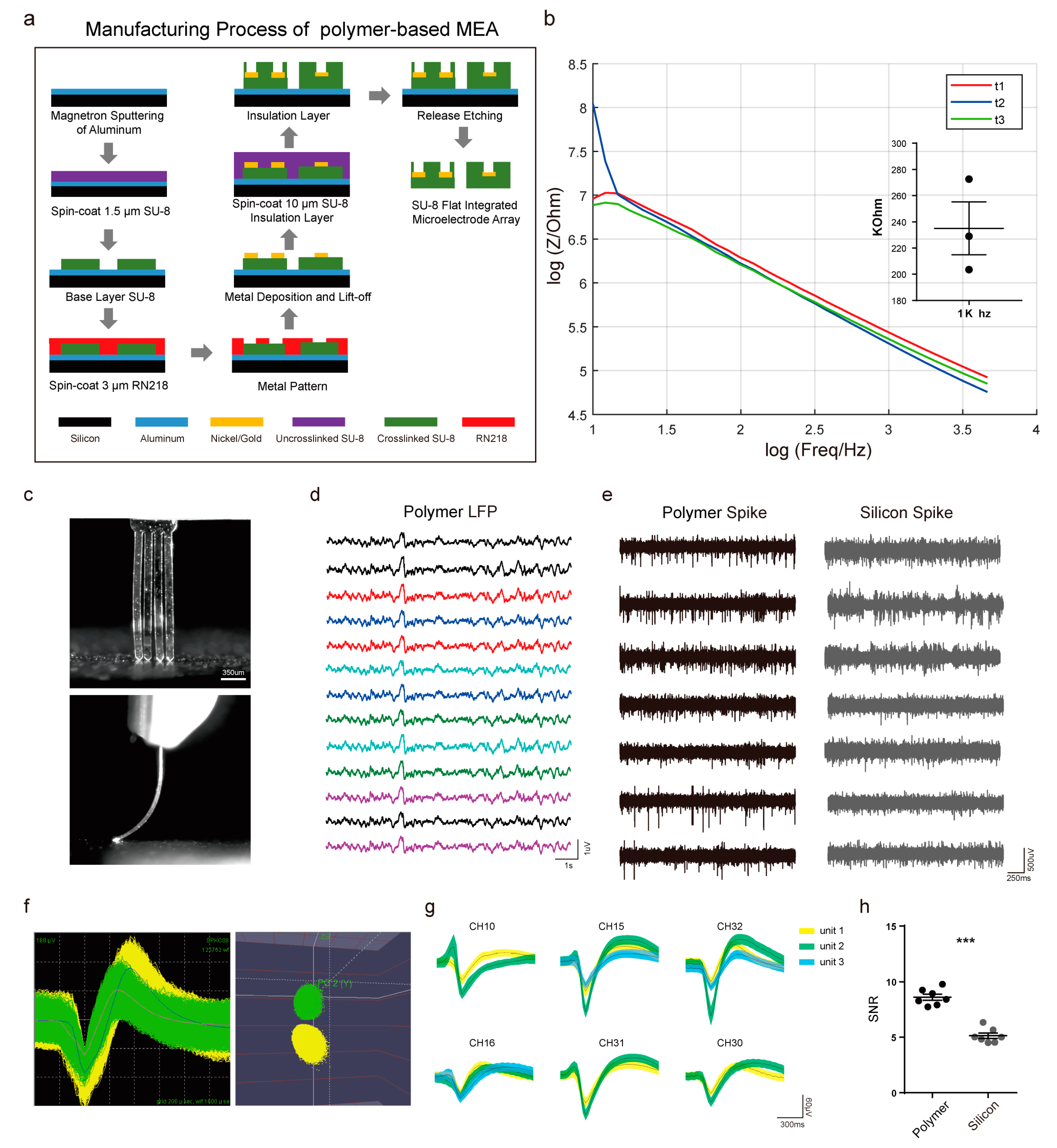
2.2. Electrode Fabrication and EIS Testing
2.3. In Vivo Electrophysiology
2.3.1. Electrode Implantation Surgery
2.3.2. Signal-to-Noise Ratio (SNR) Calculation
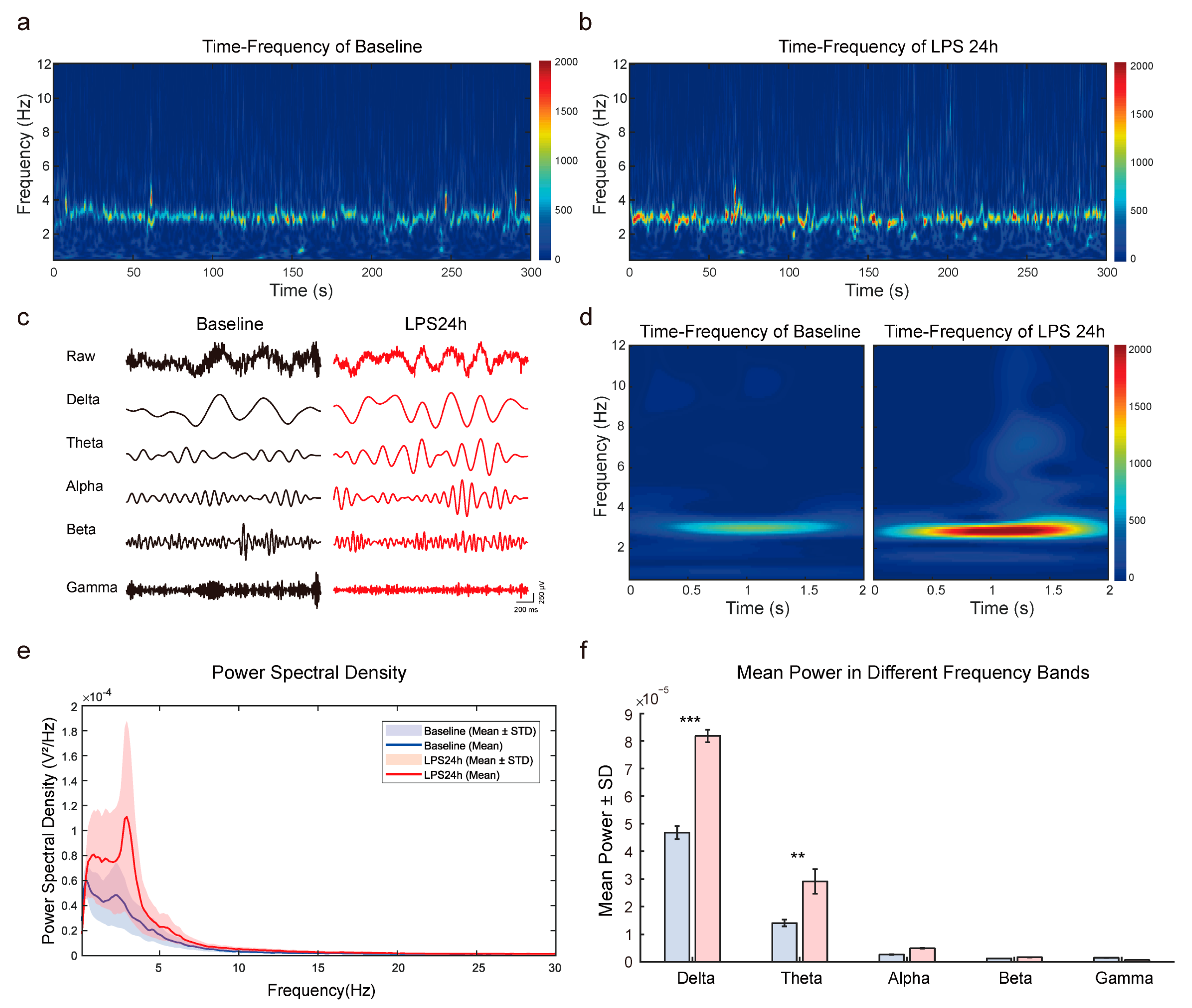
2.3.3. Power Spectral Density (PSD) Analysis
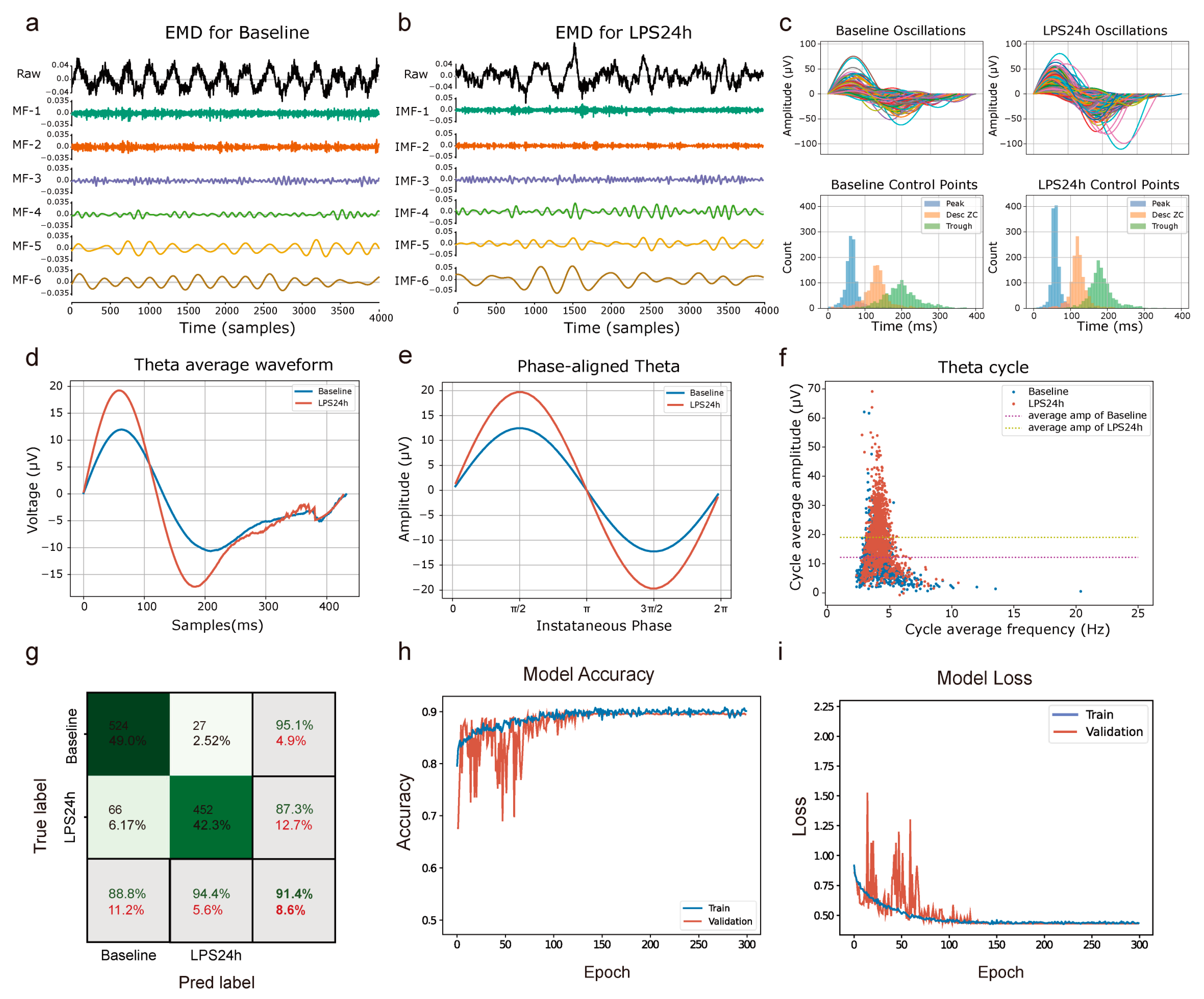
2.3.4. Time–Frequency Analysis
2.4. Behavioral Assays
2.4.1. Open Field Test (OFT)
2.4.2. Elevated Plus Maze Test (EPM)
2.4.3. Tail-Suspension Test (TST)
2.4.4. Forced Swim Test (FST)
2.4.5. Sucrose Preference Test (SPT)
2.5. Statistics and Data Visualization
3. Results
3.1. Behavioral Validation of the LPS-Induced Acute Depression Model in Mice
3.2. Fabrication and Performance Evaluation of Flexible Polymer Multichannel Electrodes
3.3. Enhanced Theta Oscillations in the mPFC of LPS-Induced Depressive Mice
3.4. Depression State Recognition Based on EMD and CNN-LSTM Machine Learning Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qiao, Y.; Wang, M.; Liang, X.; Zhang, M.; Li, C.; Cairang, J.; Wang, J.; Bi, H.; Gao, T. The influence of genetic and acquired factors on the vulnerability to develop depression: A review. Biosci. Rep. 2023, 43, BSR20222644. [Google Scholar] [CrossRef] [PubMed]
- Smart, O.L.; Tiruvadi, V.R.; Mayberg, H.S. Multimodal approaches to define network oscillations in depression. Biol. Psychiatry 2015, 77, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhang, T. Alteration of phase-phase coupling between theta and gamma rhythms in a depression-model of rats. Cogn. Neurodyn. 2013, 7, 167–172. [Google Scholar] [CrossRef]
- Hare, B.D.; Duman, R.S. Prefrontal cortex circuits in depression and anxiety: Contribution of discrete neuronal populations and target regions. Mol. Psychiatry 2020, 25, 2742–2758. [Google Scholar] [CrossRef]
- Csicsvari, J.; Hirase, H.; Czurkó, A.; Mamiya, A.; Buzsáki, G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J. Neurosci. 1999, 19, 274–287. [Google Scholar] [CrossRef]
- Kumar, S.; Black, S.J.; Hultman, R.; Szabo, S.T.; DeMaio, K.D.; Du, J.; Katz, B.M.; Feng, G.; Herbert, E.C., III; Dzirasa, K. Cortical control of affective networks. J. Neurosci. 2013, 33, 1116–1129. [Google Scholar] [CrossRef]
- Sun, Y.; Giacobbe, P.; Tang, C.W.; Barr, M.S.; Rajji, T.; Kennedy, S.H.; Fitzgerald, P.B.; Lozano, A.M.; Wong, W.; Daskalakis, Z.J. Deep Brain Stimulation Modulates Gamma Oscillations and Theta-Gamma Coupling in Treatment Resistant Depression. Brain Stimul. 2015, 8, 1033–1042. [Google Scholar] [CrossRef]
- Dantzer, R.; O’connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Normann, R.A.; Fernandez, E. Clinical applications of penetrating neural interfaces and Utah Electrode Array technologies. J. Neural Eng. 2016, 13, 061003. [Google Scholar] [CrossRef] [PubMed]
- Kipke, D.R.; Shain, W.; Buzsáki, G.; Fetz, E.; Henderson, J.M.; Hetke, J.F.; Schalk, G. Advanced Neurotechnologies for Chronic Neural Interfaces: New Horizons and Clinical Opportunities. J. Neurosci. 2008, 28, 11830–11838. [Google Scholar] [CrossRef] [PubMed]
- Zorzos, A.N.; Boyden, E.S.; Fonstad, C.G. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt. Lett. 2010, 35, 4133–4135. [Google Scholar] [CrossRef]
- Jeong, J.W.; Yeo, W.H.; Akhtar, A.; Norton, J.J.; Kwack, Y.J.; Li, S.; Jung, S.Y.; Su, Y.; Lee, W.; Xia, J.; et al. Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 2013, 25, 6839–6846. [Google Scholar] [CrossRef]
- Kim, D.H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S.; et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 2017, 3, e1601966. [Google Scholar] [CrossRef]
- Kozai TD, Y.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater 2012, 11, 1065–1073. [Google Scholar] [CrossRef]
- Quinn, A.J.; Lopes-dos-Santos, V.; Dupret, D.; Nobre, A.C.; Woolrich, M.W. EMD: Empirical Mode Decomposition and Hilbert-Huang Spectral Analyses in Python. J. Open Source Softw. 2021, 6, 2977. [Google Scholar] [CrossRef]
- Okonogi, T.; Sasaki, T. Theta-Range Oscillations in Stress-Induced Mental Disorders as an Oscillotherapeutic Target. Front. Behav. Neurosci. 2021, 15, 698753. [Google Scholar] [CrossRef]
- Ward, M.P.; Rajdev, P.; Ellison, C.; Irazoqui, P.P. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res. 2009, 1282, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Guo, L. (Ed.) Recording Electrodes. In Principles of Electrical Neural Interfacing: A Quantitative Approach to Cellular Recording and Stimulation; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 17–31. [Google Scholar]
- Neto, J.P.; Baião, P.; Lopes, G.; Frazão, J.; Nogueira, J.; Fortunato, E.; Barquinha, P.; Kampff, A.R. Does Impedance Matter When Recording Spikes With Polytrodes? Front. Neurosci. 2018, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y. Material considerations for in vitro neural interface technology. MRS Bull. 2012, 37, 566–572. [Google Scholar] [CrossRef]
- Sun, R.; Tang, M.Y.; Yang, D.; Zhang, Y.Y.; Xu, Y.H.; Qiao, Y.; Yu, B.; Cao, S.X.; Wang, H.; Huang, H.Q. C3aR in the medial prefrontal cortex modulates the susceptibility to LPS-induced depressive-like behaviors through glutamatergic neuronal excitability. Prog. Neurobiol. 2024, 236, 102614. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.-A.; Sherfey, J.; Vijayan, S. Emotional Memory Processing during REM Sleep with Implications for Post-Traumatic Stress Disorder. J. Neurosci. 2023, 43, 433–446. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Liu, S.; Su, F.; Kang, X.; Ke, Y.; Ming, D. Altered fronto-central theta-gamma coupling in major depressive disorder during auditory steady-state responses. Clin. Neurophysiol. 2023, 146, 65–76. [Google Scholar] [CrossRef]
- Porsolt, R.D. Behavioral Despair Revisited—A Citation-Classic Commentary on Depression—A New Animal-Model Sensitive to Antidepressant Treatments; Porsolt, R.D., Lepichon, M., Jalfre, M., Eds.; Institute For Scientific Information: Philadelphia, PA, USA, 1993; Volume 20, p. 9. [Google Scholar]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef]
- Mitchell, D.J.; McNaughton, N.; Flanagan, D.; Kirk, I.J. Frontal-midline theta from the perspective of hippocampal “theta”. Prog. Neurobiol. 2008, 86, 156–185. [Google Scholar] [CrossRef]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef]
- Olbrich, S.; Arns, M. EEG biomarkers in major depressive disorder: Discriminative power and prediction of treatment response. Int. Rev. Psychiatry 2013, 25, 604–618. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Redmond, S.J.; Bertoux, M.; Hodges, J.R.; Hornberger, M. A Comparison of Magnetic Resonance Imaging and Neuropsychological Examination in the Diagnostic Distinction of Alzheimer’s Disease and Behavioral Variant Frontotemporal Dementia. Front. Aging Neurosci. 2016, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Canolty, R.T.; Knight, R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010, 14, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Bland, B.H.; Oddie, S.D. Theta band oscillation and synchrony in the hippocampal formation and associated structures: The case for its role in sensorimotor integration. Behav. Brain Res. 2001, 127, 119–136. [Google Scholar] [CrossRef]
- Jacobs, J.; Hwang, G.; Curran, T.; Kahana, M.J. EEG oscillations and recognition memory: Theta correlates of memory retrieval and decision making. Neuroimage 2006, 32, 978–987. [Google Scholar] [CrossRef]
- Fell, J.; Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011, 12, 105–118. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Shang, S.; Yuan, Q.; Wang, P.; Zhuang, L. Flexible Polymer-Based Electrodes for Detecting Depression-Related Theta Oscillations in the Medial Prefrontal Cortex. Chemosensors 2024, 12, 258. https://doi.org/10.3390/chemosensors12120258
Sun R, Shang S, Yuan Q, Wang P, Zhuang L. Flexible Polymer-Based Electrodes for Detecting Depression-Related Theta Oscillations in the Medial Prefrontal Cortex. Chemosensors. 2024; 12(12):258. https://doi.org/10.3390/chemosensors12120258
Chicago/Turabian StyleSun, Rui, Shunuo Shang, Qunchen Yuan, Ping Wang, and Liujing Zhuang. 2024. "Flexible Polymer-Based Electrodes for Detecting Depression-Related Theta Oscillations in the Medial Prefrontal Cortex" Chemosensors 12, no. 12: 258. https://doi.org/10.3390/chemosensors12120258
APA StyleSun, R., Shang, S., Yuan, Q., Wang, P., & Zhuang, L. (2024). Flexible Polymer-Based Electrodes for Detecting Depression-Related Theta Oscillations in the Medial Prefrontal Cortex. Chemosensors, 12(12), 258. https://doi.org/10.3390/chemosensors12120258




