Silsesquioxanes as Promising Materials for the Development of Electrochemical (Bio)Sensors
Abstract
1. Introduction
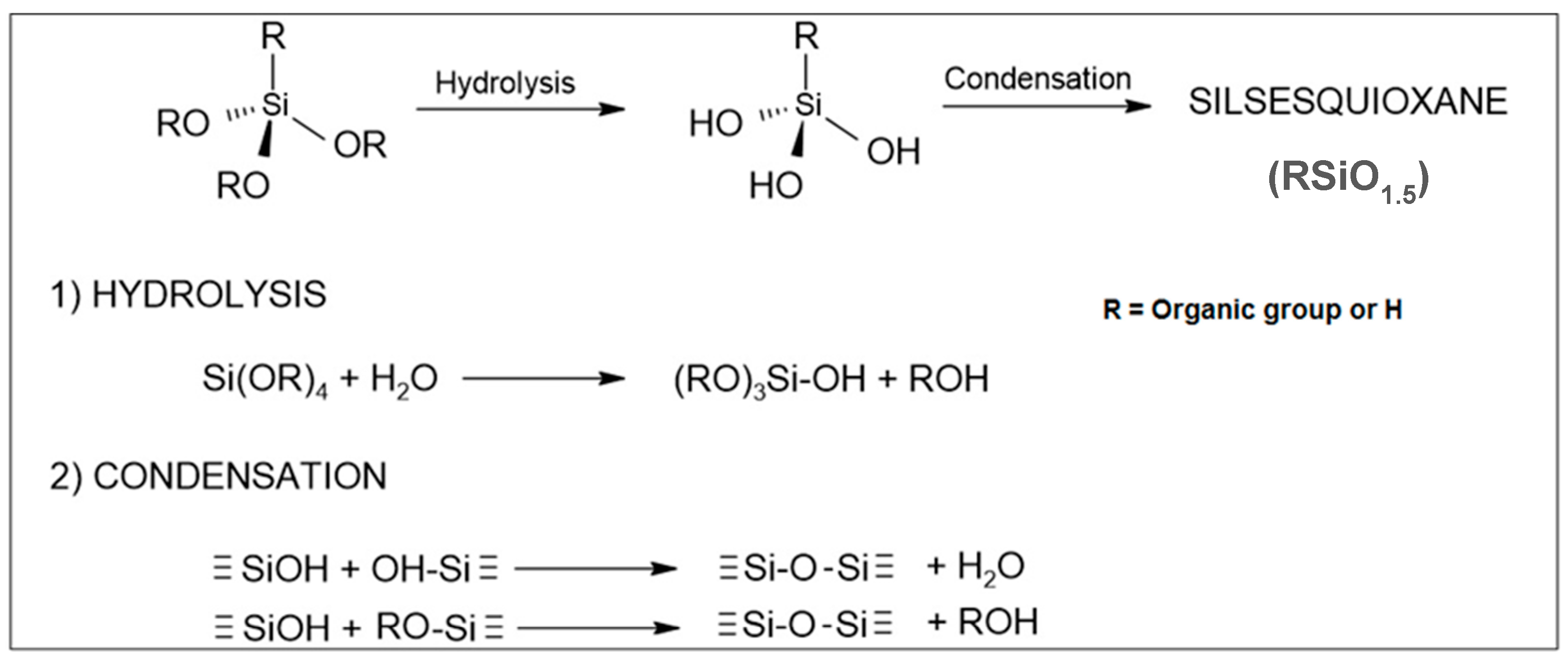
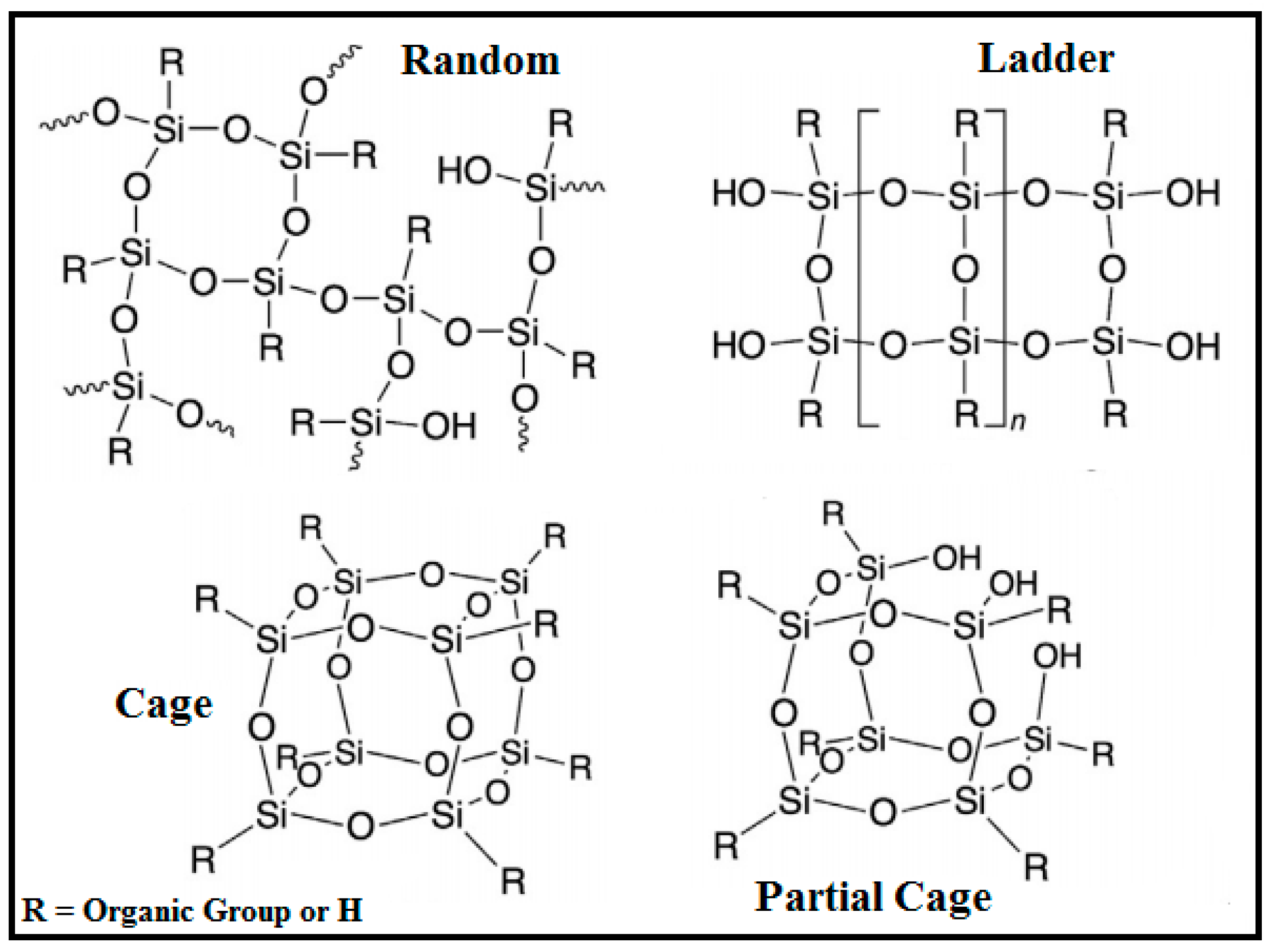
2. Synthesis of SSQs
3. SSQs and Related Nanocomposites for Applications in Electrochemical Sensing
4. Strategies for the Modification of Electrodes with SSQs and Related (Nano)Composites
5. Electrochemical Sensing Platforms Containing SSQ-Based Materials
| Electrode | Modifier | Analyte | LOD (M) | LOQ (M) | Linear Range (M) | Reference |
|---|---|---|---|---|---|---|
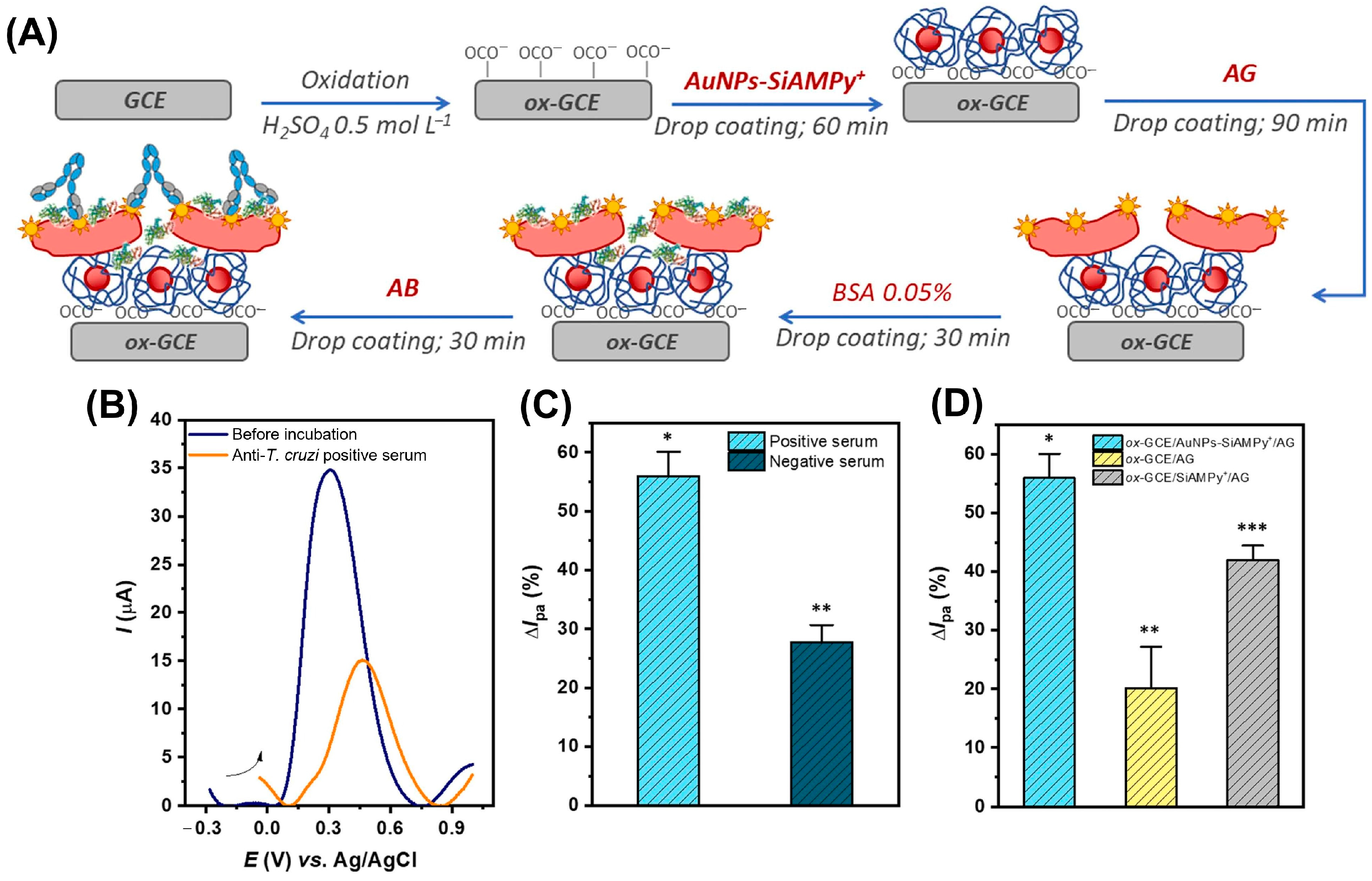
| GCE | AuNPs-SiAMPy+ | Anti-T. cruzi antibodies | – | – | – | [50] |
| GPE | ACCoN | Isoniazid | 5.53 × 10−7 | – | 6.0 × 10−7–1.0 × 10−5 | [71] |
| GCE | POSS-S-Au | Flutamide | 1.2 × 10−7 | – | 3.0 × 10−6–3.0 × 10−5 | [65] |
| CPE | APS, AEAPS and DTOAS | Ag+ | APS: 1.0 × 10−6 | – | APS: 1.6 × 10−6–1.0 × 10−2 | [18] |
| AEAPS: 1.0 × 10−8 | AEAPS: 1.0 × 10−6–1.0 × 10−2 | |||||
| DTOAS: 1.6 × 10−6 | DTOAS: 1.0 × 10−8–1.0 × 10−2 | |||||
| CPE | CuHSPD | Ascorbic acid | 1.66 × 10−6 | 5.54 × 10−6 | 5.0 × 10−6–4.0 × 10−5 | [99] |
| CNTPE | SBA-AgNPs | Sulfamethoxazol (SMZ)/paracetamol (PC) | SMZ: 1.9 × 10−8 | – | SMZ: 6.0 × 10−8–1.2 × 10−4 | [64] |
| PC: 3.1 × 10−8 | PC: 1.0 × 10−7–8.0 × 10−4 | |||||
| GCE | POSS-OH, POSS-SH and POSS-vinyl | Bisphenol A | POSS-OH 3.97 × 10−6 | – | POSS-OH: 7.49 × 10−7–7.49 × 10−6 | [105] |
| POSS-SH 1.4 × 10−7 | POSS-SH: 7.49 × 10−7–8.99 × 10−6 | |||||
| POSS-vinyl 8.0 × 10−8 | POSS-vinil 7.49 × 10−7–7.49 × 10−6 | |||||
| GCE | f-MWCNT-Ni(OH)2-Si4Pic+Cl− | Folic acid | 9.5 × 10−8 | 3.0 × 10−7 | 5.0 × 10−7–2.6 × 10−5 | [9] |
| GPE | CuHCFSSQ-H and ZnHCFSSQ-H | Ascorbic acid | 2.99 × 10−4 | – | 4.0 × 10−4–4.0 × 10−3 | [4] |
| GCE | CoTsPc–Si4DMAP+Cl− | Nifedipine (NIF)/ DehydroNIF (dHNIF) | NIF: 6.2 × 10−9 dHNIF: 4.5 × 10−9 | – | 1.5 × 10−8–1.75 × 10−6 | [7] |
| GPE | MTTiPNiH | Sulfite/sodium dipyrone (DIP) | Sulfite: 3.0 × 10−5 | – | Sulfite: 8.0 × 10−5–9.0 × 10−4 | [104] |
| DIP: 4.0 × 10−5 | Sodium dipyrone: 1.0 × 10−4–9.0 × 10−4 | |||||
| GCE | AgNPs-Si4Pic+NO3− | 4-nitrophenol | 5.0 × 10−8 | 1.5 × 10−7 | 2.9 × 10−7–3.15 × 10−5 | [57] |
| GPE | AgHSP | Ascorbic acid | 7.034 × 10−5 | – | 5.0 × 10−5–6.0 × 10−4 | [108] |
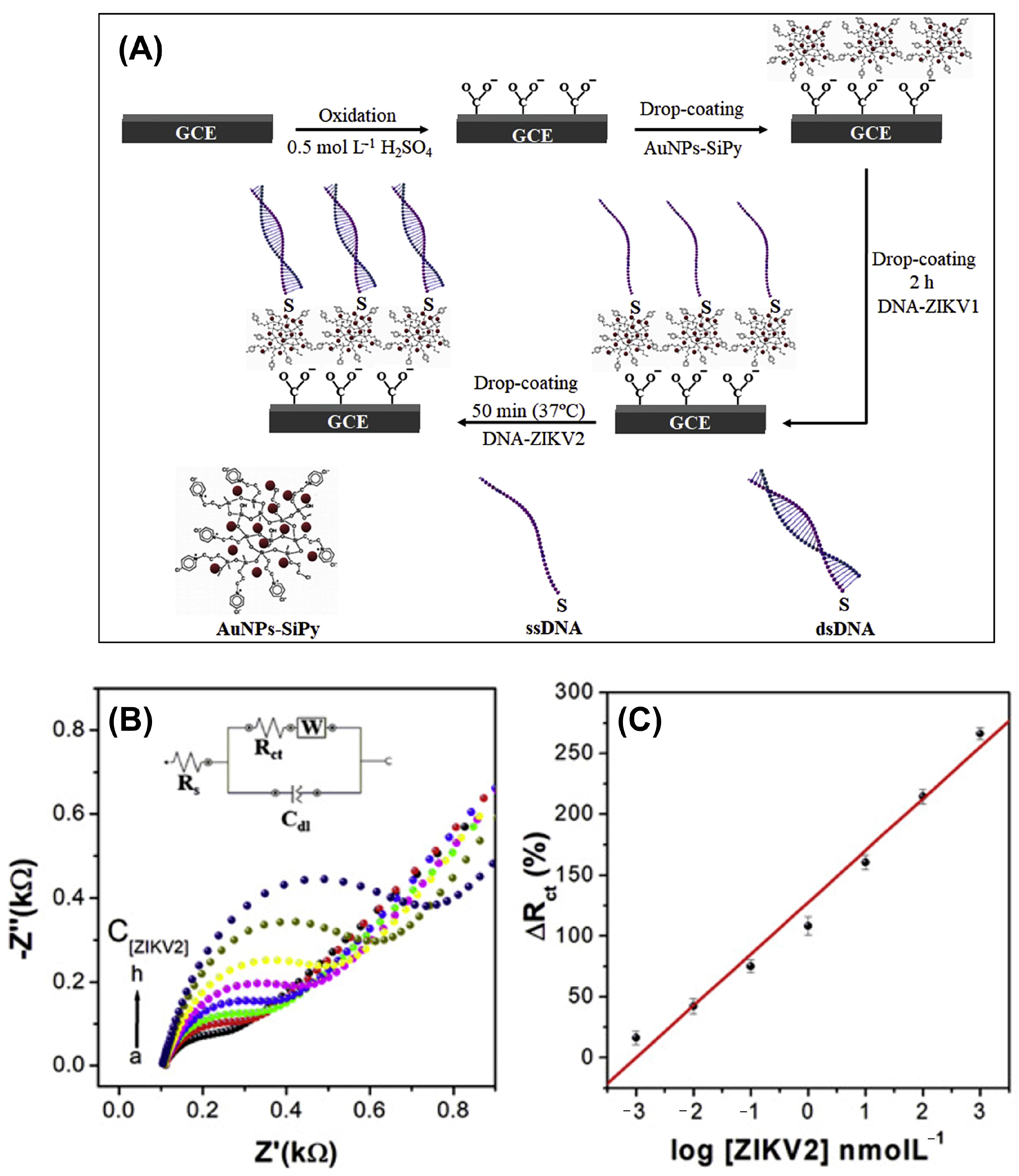
| GCE | AuNPs-SiPy | Zika virus | 8.2 × 10−13 | – | 1.0 × 10−12–1.0 × 10−6 | [8] |
| GPE | ACCuN | L-cysteine | 1.25 × 10−4 | – | 2.0 × 10−4–2.0 × 10−3 | [83] |
| CPE | Si3Pic+Cl−/NiTsPc | Sulfanilamide | 1.2 × 10−5 | 3.5 × 10−5 | 3.5 × 10−5–3.01 × 10−4 | [80] |
| GPE | POSS-SH/CuCl2/K3[Fe(CN)6] | L-dopamine | 2.08 × 10−4 | – | 2.5 × 10−5–4.0 × 10−4 | [109] |
| GPE | ZTTiPNiH | Sulfite | 5.0 × 10−5 | – | 5.0 × 10−5–8.0 × 10−4 | [106] |
| Ceramic substrate | POSS-TPPBr | Humidity | – | – | 11% to 95% (relative humidity) | [110] |
| Ceramic substrate | PMDS | Humidity | – | – | 11% to 95% (relative humidity) | [111] |
| ITO | (Si4ampy+Cl−/NiTsPc)11 | Nitrite | 2.6 × 10−5 | – | 1.13 × 10−4–8.6 × 10−4 | [48] |
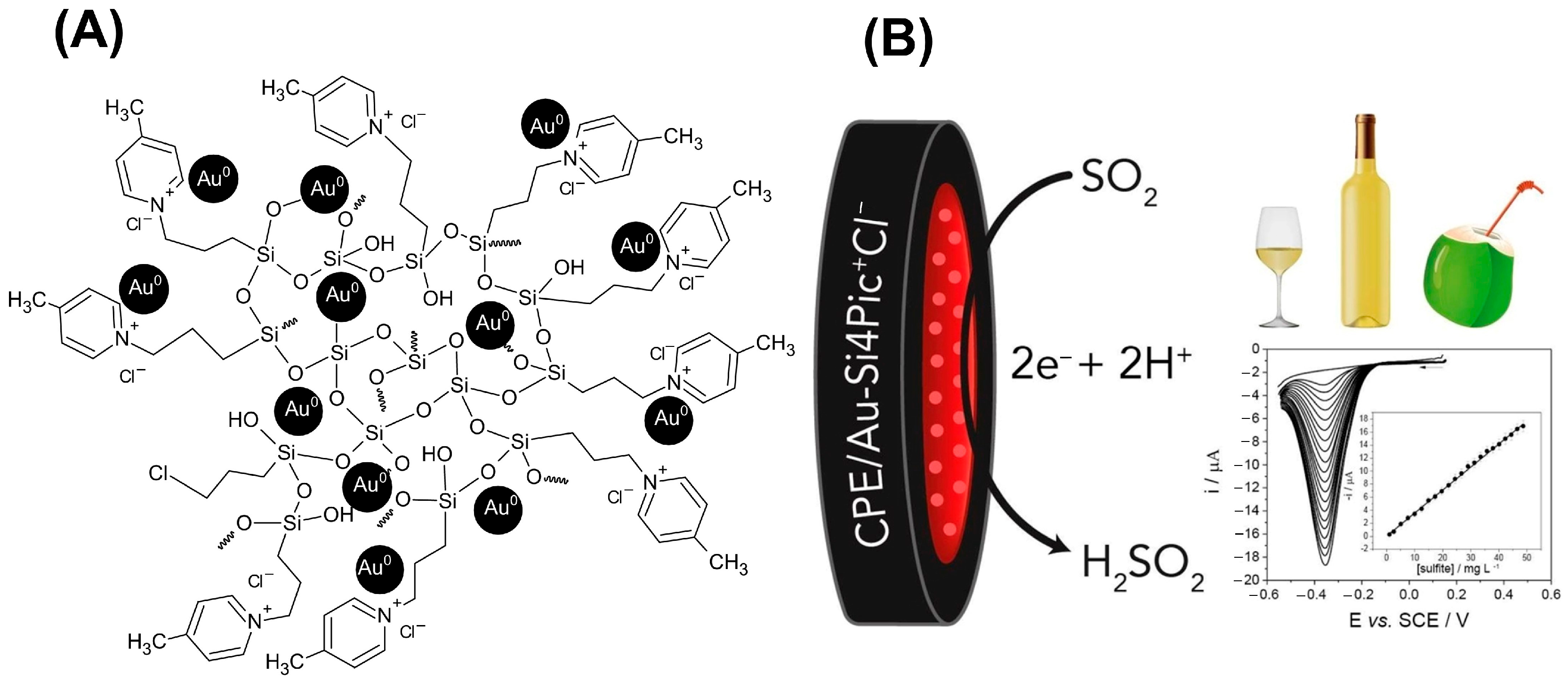
| CPE | Au-Si4Pic+Cl− | Sulfite | 0.88 mg L−1 | 2.68 mg L−1 | 2.54–48.6 mg L−1 | [10] |
| ITO | (AuNPs-SiPy+/PB)2 | H2O2 | – | – | – | [45] |
| ITO | (SiPy/NiTsPc)3 (S-SiPy/NiTsPc)3 (T-SiPy/NiTsPc)3 | Dopamine | (SiPy/NiTsPc)3: 9.672 × 10−6 | (SiPy/NiTsPc)3: 2.971 × 10−5 | 9.9 × 10−6–9.09 × 10−5 | [90] |
| (S-SiPy/NiTsPc)3: 8.845 × 10−6 | (S-SiPy/NiTsPc)3: 2.901 × 10−5 | |||||
| (T-SiPy/NiTsPc)3: 8.486 × 10−6 | (T-SiPy/NiTsPc)3: 2.828 × 10−5 | |||||
| GCE | Fe3O4 NPs-Si4Pic+Cl−/AuNPs-Si4Pic+Cl− | Bisphenol A | 7.0 × 10−9 | – | 2.0 × 10−8–1.4 × 10−6 | [59] |
| GCE | AuNps/TLA/HRP | Catechol | 8.52 × 10−7 | – | 6.0 × 10−6–4.6 × 10−5 | [11] |
| GCE | (Fe3O4-Pt)NPs | 2-, 3-, and 4-nitrophenol (2-, 3-, 4-NP) | 2-NP: 3.37 × 10−8 | 2-NP: 1.022 × 10−7 | 1.0 × 10−7–1.5 × 10−6 | [58] |
| 3-NP: 4.53 × 10−8 | 3-NP: 1.375 × 10−7 | |||||
| 4-NP: 4.82 × 10−8 | 4-NP: 1.461 × 10−7 | |||||
| GPE | GOSFeH | Diuron | 4.96 × 10−9 | – | 1.00 × 10−8–9.00 × 10−7 | [61] |
| GCE | Pd-Si4Pic+NO3− | Nimesulide | 3.90 × 10−8 | 1.30 × 10−7–6.00 × 10−5 | [16] | |
| GCE | V-POSS:MLG | 2-hydroxy benzophenone | 0.2 × 10−9 | 0.66 × 10−9 | 1.0 × 10−9–1.0 × 10−4 | [86] |
| GCE | FOD | Tryptophan | 0.01 × 10−6 | – | 0.06 × 10−6–1.0 × 10−4; 1 × 10−4–4.4 × 10−4 | [85] |
| CPE | SZnFe | 4-chlorophenol | 5.3 × 10−6 | – | 6.0 × 10−7–6.0 × 10−5 | [83] |
| CPE | SCuH | Nitrite | 2.65 × 10−5 | – | 2.0 × 10−4–2.0 × 10−3 | [82] |
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, F.; Lu, L.; Ha, C.S. Silsesquioxane-Containing Hybrid Nanomaterials: Fascinating Platforms for Advanced Applications. Macromol. Chem. Phys. 2019, 220, 1800324. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Diré, S.; Redaelli, M.; Borovin, E.; Callone, E.; di Credico, B.; Morazzoni, F.; Pegoretti, A.; Scotti, R. Unveiling the Hybrid Interface in Polymer Nanocomposites Enclosing Silsesquioxanes with Tunable Molecular Structure: Spectroscopic, Thermal and Mechanical Properties. J. Colloid Interface Sci. 2018, 512, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.S.; Fernandes, D.S.; do Carmo, D.R. A Modified Hybrid Silsesquioxane/Histidine Composite for Copper and Zinc Adsorption and It Behavior in the Electro-Oxidation of Ascorbic Acid. Mater. Sci. Eng. C 2020, 111, 110739. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Zhang, W.; Li, J. Polyhedral Oligomeric Silsesquioxane-Incorporated Gelatin Hydrogel Promotes Angiogenesis during Vascularized Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 22410–22425. [Google Scholar] [CrossRef]
- Arsalani, N.; Kazeminava, F.; Akbari, A.; Hamishehkar, H.; Jabbari, E.; Kafil, H.S. Synthesis of Polyhedral Oligomeric Silsesquioxane Nano-Crosslinked Poly(Ethylene Glycol)-Based Hybrid Hydrogels for Drug Delivery and Antibacterial Activity. Polym. Int. 2019, 68, 667–674. [Google Scholar] [CrossRef]
- Winiarski, J.P.; de Barros, M.R.; Wecker, G.S.; Nagurniak, G.R.; Parreira, R.L.T.; Affeldt, R.F.; Peralta, R.A.; Jost, C.L. A Novel Hybrid Organic-Inorganic Silsesquioxane and Cobalt(Ii) Tetrasulphophthalocyanine Material as an Efficient Electrochemical Sensor for the Simultaneous Determination of the Anti-Hypertensive Nifedipine and Its Metabolite. J. Mater. Chem. C Mater. 2020, 8, 6839–6850. [Google Scholar] [CrossRef]
- Steinmetz, M.; Lima, D.; Viana, A.G.; Fujiwara, S.T.; Pessôa, C.A.; Etto, R.M.; Wohnrath, K. A Sensitive Label-Free Impedimetric DNA Biosensor Based on Silsesquioxane-Functionalized Gold Nanoparticles for Zika Virus Detection. Biosens. Bioelectron. 2019, 141, 111351. [Google Scholar] [CrossRef]
- Winiarski, J.P.; Rampanelli, R.; Bassani, J.C.; Mezalira, D.Z.; Jost, C.L. Multi-Walled Carbon Nanotubes/Nickel Hydroxide Composite Applied as Electrochemical Sensor for Folic Acid (Vitamin B9) in Food Samples. J. Food Compos. Anal. 2020, 92, 103511. [Google Scholar] [CrossRef]
- Winiarski, J.P.; de Barros, M.R.; Magosso, H.A.; Jost, C.L. Electrochemical Reduction of Sulfite Based on Gold Nanoparticles/Silsesquioxane-Modified Electrode. Electrochim. Acta 2017, 251, 522–531. [Google Scholar] [CrossRef]
- Mossanha, R.; Erdmann, C.A.; Santos, C.S.; Wohnrath, K.; Fujiwara, S.T.; Pessoa, C.A. Construction of a Biosensor Based on SAM of Thiolactic Acid on Gold Nanoparticles Stabilized by Silsesquioxane Polyelectrolyte for Cathecol Determination. Sens. Actuators B Chem. 2017, 252, 747–756. [Google Scholar] [CrossRef]
- Zapp, E.; Da Silva, P.S.; Westphal, E.; Gallardo, H.; Spinelli, A.; Vieira, I.C. Troponin T Immunosensor Based on Liquid Crystal and Silsesquioxane-Supported Gold Nanoparticles. Bioconjug Chem. 2014, 25, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Sheng, Q.; Zheng, J. Hydrophobic Interface Controlled Electrochemical Sensing of Nitrite Based on One Step Synthesis of Polyhedral Oligomeric Silsesquioxane/Reduced Graphene Oxide Nanocomposite. Talanta 2016, 150, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Freitas, B.T.; Freitas, R.M.L.; Rodrigues, A.B.; Baroni, A.S.; dos Reis, I.C.; dos Santos Felipe, A.; Rodrigues, B.B.; Barbosa, P.F.P.; Maraldi, V.A.; dos Santos Franco, F.; et al. Application of Biohybrid Silsesquioxane Based Material Modified with Methylene Blue for Determination of Sulphite. Int. J. Electrochem. Sci. 2023, 18, 100100. [Google Scholar] [CrossRef]
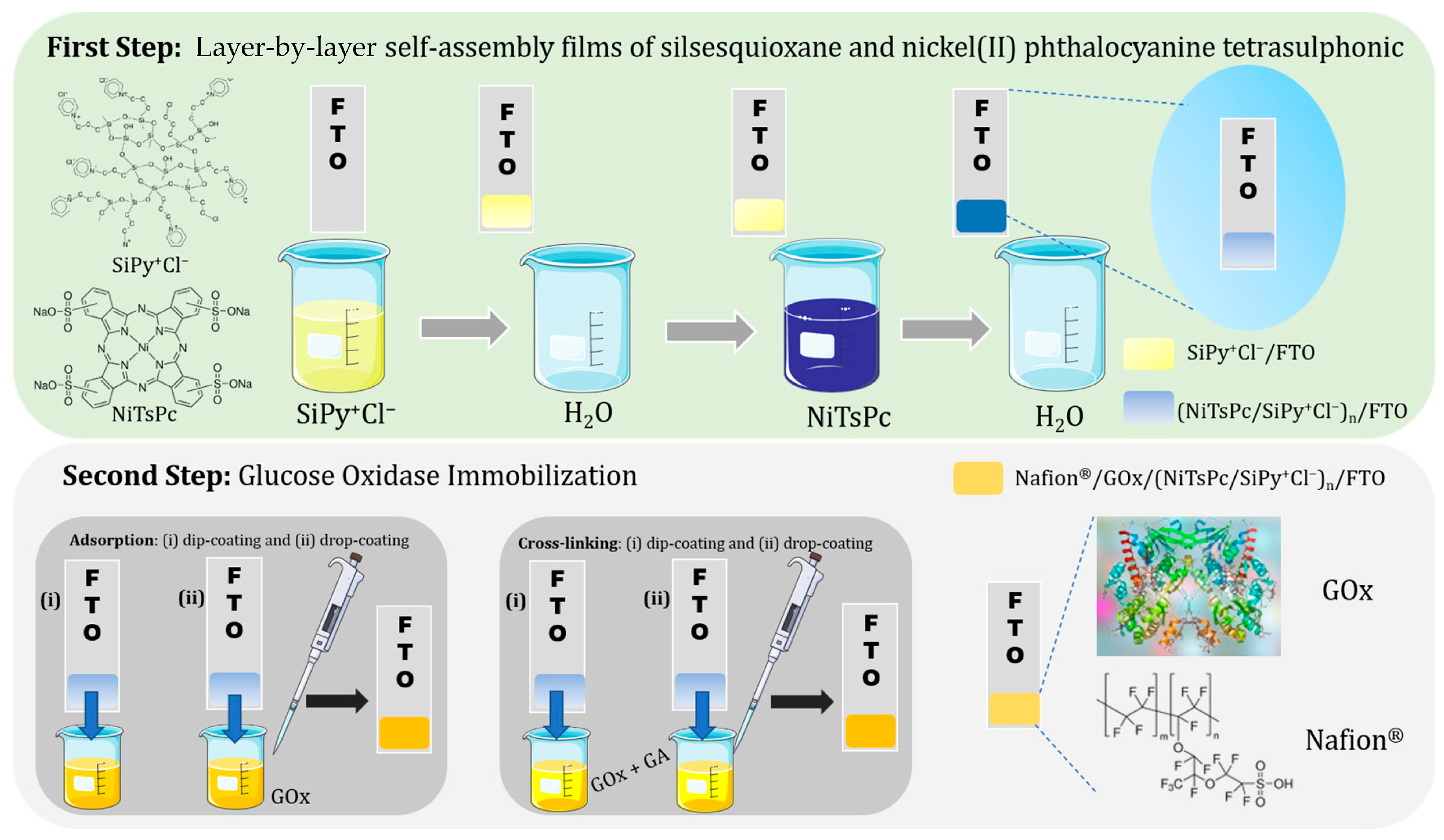
- Winiarski, J.P.; de Melo, D.J.; Santana, E.R.; Santos, C.S.; de Jesus, C.G.; Fujiwara, S.T.; Wohnrath, K.; Pessôa, C.A. Layer-by-Layer Films of Silsesquioxane and Nickel(II) Tetrasulphophthalocyanine as Glucose Oxidase Platform Immobilization: Amperometric Determination of Glucose in Kombucha Beverages. Chemosensors 2023, 11, 346. [Google Scholar] [CrossRef]
- Goularte, R.B.; Winiarski, J.P.; Latocheski, E.; Jost, C.L. Novel Analytical Sensing Strategy Using a Palladium Nanomaterial-Based Electrode for Nimesulide Electrochemical Reduction. J. Electroanal. Chem. 2022, 920, 116622. [Google Scholar] [CrossRef]
- Kaneko, Y. Ionic Silsesquioxanes: Preparation, Structure Control, Characterization, and Applications. Polymer 2018, 144, 205–224. [Google Scholar] [CrossRef]
- Evseev, M.E.; Kholmogorova, A.S.; Neudachina, L.K.; Pestov, A.V.; Puzyrev, I.S.; Osipova, V.A.; Adamova, L. V N-Substituted-3-Aminopropylsilsesquioxanes: Synthesis, Physicochemical Properties, and Application. Russ. Chem. Bull. 2021, 70, 1154–1160. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, Z.; He, C. Construction of Organic Optoelectronic Materials by Using Polyhedral Oligomeric Silsesquioxanes (POSS). In Silicon Containing Hybrid Copolymers; Wiley: Hoboken, NJ, USA, 2020; pp. 167–200. ISBN 9783527823499. [Google Scholar]
- Shi, H.; Yang, J.; You, M.; Li, Z.; He, C. Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Hybrid Soft Gels: Molecular Design, Material Advantages, and Emerging Applications. ACS Mater. Lett. 2020, 2, 296–316. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Wu, D. Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Hybrid Materials: Molecular Design, Solution Self-Assembly and Biomedical Applications. Chin. J. Chem. 2021, 39, 757–774. [Google Scholar] [CrossRef]
- Olejnik, A.; Sztorch, B.; Brząkalski, D.; Przekop, R.E. Silsesquioxanes in the Cosmetics Industry—Applications and Perspectives. Materials 2022, 15, 1126. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hayami, R.; Gunji, T. Characterization of NMR, IR, and Raman Spectra for Siloxanes and Silsesquioxanes: A Mini Review. J. Solgel Sci. Technol. 2022, 104, 36–52. [Google Scholar] [CrossRef]
- Ullah, M.S.; Yazıcı, N.; Wis, A.A.; Özkoç, G.; Kodal, M. A Review on Polyhedral Oligomeric Silsesquioxanes as a New Multipurpose Nanohybrid Additive for Poly(Lactic Acid) and Poly(Lactic Acid) Hybrid Composites. Polym. Compos. 2022, 43, 1252–1281. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.; Kwon, M.S. Recent Progress in Ladder-like Polysilsesquioxane: Synthesis and Applications. Mater. Chem. Front. 2024, 8, 2689–2726. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Tong, Y.; Umar, A.; Luo, Y.; Zhao, S. Progress of Polyhedral Oligomeric Silsesquioxanes in Proton Exchange Membrane Fuel Cells: A Review. Process Saf. Environ. Prot. 2024, 187, 1322–1337. [Google Scholar] [CrossRef]
- Brinker, J.C.; Scherer, G.W. Sol-Gel Science—The Physics and Chemistry of Sol-Gel Processing; Academic Press: New York, NY, USA, 1990; ISBN 978-0-08-057103-4. [Google Scholar]
- Walcarius, A.; Collinson, M.M. Analytical Chemistry with Silica Sol-Gels: Traditional Routes to New Materials for Chemical Analysis. Annu. Rev. Anal. Chem. 2009, 2, 121–143. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The Sol-Gel Route to Advanced Silica-Based Materials and Recent Applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef]
- Benvenutti, E.V.; Moro, C.C.; Costa, T.M.H.; Gallas, M.R. Materiais Híbridos à Base de Sílica Obtidos Pelo Método Sol-Gel. Quim. Nova 2009, 32, 1926–1933. [Google Scholar] [CrossRef]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid Inorganic-Organic Mesoporous Silicates—Nanoscopic Reactors Coming of Age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol-Gel Based Materials for Biomedical Applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Sato, Y.; Sugimoto, A.; Iwashina, T.; Hayami, R.; Yamamoto, K.; Gunji, T. Hydrolysis and Condensation Behavior of Tetraethoxysilane, Hexaethoxydisiloxane, and Octaethoxytrisiloxane. J. Solgel Sci. Technol. 2023, 108, 377–391. [Google Scholar] [CrossRef]
- Kickelbick, G. Concepts for the Incorporation of Inorganic Building Blocks into Organic Polymers on a Nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoën, X.; Durand, J.O.; Wong Chi Man, M.; Khashab, N.M. Organosilica Hybrid Nanomaterials with a High Organic Content: Syntheses and Applications of Silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, M.; Grzelak, M.; Januszewski, R.; Januszewski, R.; Marciniec, B.; Marciniec, B. Synthesis and Hydrosilylation of Vinyl-Substituted Open-Cage Silsesquioxanes with Phenylsilanes: Regioselective Synthesis of Trifunctional Silsesquioxanes. Inorg. Chem. 2020, 59, 7830–7840. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like Silsesquioxanes-Based Hybrid Materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Lorenzini, C.; Versace, D.L.; Gaillet, C.; Lorthioir, C.; Boileau, S.; Renard, E.; Langlois, V. Hybrid Networks Derived from Isosorbide by Means of Thiol-Ene Photoaddition and Sol-Gel Chemistry. Polymer 2014, 55, 4432–4440. [Google Scholar] [CrossRef]
- Fujiwara, S.T.; Gushikem, Y.; Alfaya, R.V.S. Adsorption of FeCl3, CuCl2 and ZnCl2 on Silsesquioxane 3-n-Propylpyridiniumchloride Polymer Film Adsorbed on Al2O3 Coated Silica Gel. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 135–141. [Google Scholar] [CrossRef]
- Yang, L.; Gao, J.; Qiu, Q.; Qin, J.; Peng, X.; Tang, C. Insulating Paper Cellulose with High Mechanical Performance and Low Polarizability: The Effect of Doping Concentration on Polyhedral Oligomeric Silsesquioxanes-Modified Cellulose. Adv. Eng. Mater. 2021, 23, 2100022. [Google Scholar] [CrossRef]
- Mishra, K.; Babu, L.K.; Vaidyanathan, R. Improvement of Fracture Toughness and Thermo-Mechanical Properties of Carbon Fiber/Epoxy Composites Using Polyhedral Oligomeric Silsesquioxane. J. Compos. Mater. 2020, 54, 1273–1280. [Google Scholar] [CrossRef]
- Jeleń, P.; Szumera, M.; Gawęda, M.; Długoń, E.; Sitarz, M. Thermal Evolution of Ladder-like Silsesquioxanes during Formation of Black Glasses. J. Therm. Anal. Calorim. 2017, 130, 103–111. [Google Scholar] [CrossRef]
- Huo, L.; Wu, X.; Tian, C.; Gao, J. Thermal, Mechanical, and Electrical Properties of Alkyd-Epoxy Resin Nanocomposites Modified with 3-Glycidyloxypropyl-POSS. Polym. Plast. Technol. Eng. 2018, 57, 371–379. [Google Scholar] [CrossRef]
- Divakaran, N.; Kale, M.B.; Dhamodharan, D.; Mubarak, S.; Wu, L.; Wang, J. Effect of POSS-Modified Montmorillonite on Thermal, Mechanical, and Electrical Properties of Unsaturated Polyester Nanocomposites. Polymers 2020, 12, 2031. [Google Scholar] [CrossRef] [PubMed]
- Calaça, G.N.; Erdmann, C.A.; Soares, A.L.; Pessôa, C.A.; Fujiwara, S.T.; Garcia, J.R.; Vidotti, M.; Wohnrath, K. Layer-by-Layer AuNPs-SiPy+/Prussian Blue Nanoparticles Modified Electrodes: Characterization and Electrocatalytic Effects. Electrochim. Acta 2017, 249, 104–112. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Kuciński, K.; Hreczycho, G. Highly Efficient Catalytic Route for the Synthesis of Functionalized Silsesquioxanes. Inorg. Chem. 2017, 56, 9337–9342. [Google Scholar] [CrossRef] [PubMed]
- Alfaya, R.V.S.; Alfaya, A.A.S.; Gushikem, Y. Processo de Preparação Do Polieletrólito Cloreto de 3-n-Propilpiridínio Ligado a Uma Estrutura de Silsesquioxano. BR Patent BR9803053-1, 17 August 1998. [Google Scholar]
- Ribicki, A.C.; Chemin, B.G.; Van Haandel, V.J.; Winiarski, J.P.; de Castro Rozada, T.; Pessoa, C.A.; Estrada, R.A.; Fiorin, B.C.; Fujiwara, S.T. Sol Gel Synthesis of 3-n-Propyl(4-Aminomethyl)Pyridinium Silsesquioxane Chloride and the Enhanced Electrocatalytic Activity of LbL Films. J. Solgel Sci. Technol. 2018, 87, 216–229. [Google Scholar] [CrossRef]
- Ribicki, A.C.; Sperandio, M.L.; van Haandel, V.J.; Estrada, R.A.; Fujiwara, S.T. Synthesis and Characterization of Hybrid Polymer Based on Functionalized Silica as Efficient Adsorbent for Heavy Metal Ions from Aqueous Solution. J. Braz. Chem. Soc. 2020, 31, 2049–2057. [Google Scholar] [CrossRef]
- Lima, D.; Ribicki, A.; Gonçalves, L.; Hacke, A.C.M.; Lopes, L.C.; Pereira, R.P.; Wohnrath, K.; Fujiwara, S.T.; Pessôa, C.A. Nanoconjugates Based on a Novel Organic-Inorganic Hybrid Silsesquioxane and Gold Nanoparticles as Hemocompatible Nanomaterials for Promising Biosensing Applications. Colloids Surf. B Biointerfaces 2022, 213, 112355. [Google Scholar] [CrossRef]
- Cruz-Quesada, G.; Espinal-Viguri, M.; López-Ramón, M.V.; Garrido, J.J. Hybrid Xerogels: Study of the Sol-Gel Process and Local Structure by Vibrational Spectroscopy. Polymers 2021, 13, 2082. [Google Scholar] [CrossRef]
- Cruz-Quesada, G.; Espinal-Viguri, M.; López-Ramón, M.V.; Garrido, J.J. Novel Silica Hybrid Xerogels Prepared by Co-Condensation of TEOS and ClPhTEOS: A Chemical and Morphological Study. Gels 2022, 8, 677. [Google Scholar] [CrossRef]
- Mageste Fonseca, R.L.; Sampaio, D.; Mayrink, T.F.; Alcamand, H.A.; Palhares, H.G.; Nunes, E.H.M.; Houmard, M. Influence of the Alcoholic Solvent and Gelation Temperature on the Structural and Water Vapor Adsorption Properties of Silica Adsorbents Synthesized by Sol-Gel Method without Catalyst. Microporous Mesoporous Mater. 2022, 342, 112114. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral Oligomeric Silsesquioxane-Based Hybrid Materials and Their Applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Ajdari, F.B.; Kowsari, E.; Nadri, H.R.; Maghsoodi, M.; Ehsani, A.; Mahmoudi, H.; Eshkalak, S.K.; Chinnappan, A.; Jayathilak, W.A.D.M.; Ramakrishna, S. Electrochemical Performance of Silsesquioxane-GO Loaded with Alkoxy Substituted Ammonium-Based Ionic Liquid and POAP for Supercapacitor. Electrochim. Acta 2020, 354, 136663. [Google Scholar] [CrossRef]
- de Oliveira, R.D.; Calaça, G.N.; Santos, C.S.; Fujiwara, S.T.; Pessôa, C.A. Preparation, Characterization and Electrochemistry of Layer-by-Layer Films of Silver Nanoparticles and Silsesquioxane Polymer. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 638–647. [Google Scholar] [CrossRef]
- Crocomo, P.Z.; Winiarski, J.P.; de Barros, M.R.; Latocheski, E.; Nagurniak, G.R.; Parreira, R.L.T.; Siebert, D.A.; Micke, G.A.; Magosso, H.A.; Jost, C.L. Silver Nanoparticles-Silsesquioxane Nanomaterial Applied to the Determination of 4-Nitrophenol as a Biomarker. Electroanalysis 2019, 31, 2319–2329. [Google Scholar] [CrossRef]
- Gerent, G.G.; Spinelli, A. Magnetite-Platinum Nanoparticles-Modified Glassy Carbon Electrode as Electrochemical Detector for Nitrophenol Isomers. J. Hazard. Mater. 2017, 330, 105–115. [Google Scholar] [CrossRef]
- Santana, E.R.; de Lima, C.A.; Piovesan, J.V.; Spinelli, A. An Original Ferroferric Oxide and Gold Nanoparticles-Modified Glassy Carbon Electrode for the Determination of Bisphenol A. Sens. Actuators B Chem. 2017, 240, 487–496. [Google Scholar] [CrossRef]
- Moradi, N.; Soufi, G.; Kabir, A.; Karimi, M.; Bagheri, H. Polyester Fabric-Based Nano Copper-Polyhedral Oligomeric Silsesquioxanes Sorbent for Thin Film Extraction of Non-Steroidal Anti-Inflammatory Drugs. Anal. Chim. Acta 2023, 1270, 341461. [Google Scholar] [CrossRef]
- Maraldi, V.A.; do Carmo, D.R. Prussian Blue Nanoparticles Supported on a Hybrid Platform of Graphene Oxide and Cubic Silsesquioxane Applied to Diuron Detection. Mater. Res. Bull. 2023, 167, 112426. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Erdmann, C.A.; Inaba, J.; Viana, A.G.; Pessoa, C.A.; Wohnrath, K.; Garcia, J.R. Electrochemical Immunosensor for Diagnostic of Parasitical Human Diseases. ECS Trans. 2013, 50, 393–399. [Google Scholar] [CrossRef]
- Tkachenko, O.S.; Souza, L.V.; Deon, M.; Becker, E.M.; de Menezes, E.W.; Arenas, L.T.; Benvenutti, E.V. AgNP-Decorated SBA-15 for MWCNT Paste Modified Electrode: A Sensor for Simultaneous Voltammetric Determination of Paracetamol and Sulfamethoxazole. Electroanalysis 2021, 33, 29–37. [Google Scholar] [CrossRef]
- Kannan, A.; Venkatesvaran, H.; Ananthakrishnan, D.; Mayavan, A.; Gandhi, S. An Early Detection of Prostate Cancer Drug in Water to Prevent Loss of Biodiversity. Process Saf. Environ. Prot. 2021, 151, 51–62. [Google Scholar] [CrossRef]
- dos Santos, V.; de Jesus, C.G.; dos Santos, M.; Canestraro, C.D.; Zucolotto, V.; Fujiwara, S.T.; Garcia, J.R.; Pessoa, C.A.; Wohnrath, K. Platinum Nanoparticles Incorporated in Silsesquioxane for Use in LbL Films for the Simultaneous Detection of Dopamine and Ascorbic Acid. J. Nanoparticle Res. 2012, 14, 1081. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Pei, F.; Liu, Q.; Fang, Y.; Hu, Q. Thiol-Rich Polyhedral Oligomeric Silsesquioxane-Modified Magnetic Nanoparticles for the Highly Efficient Separation and Preconcentration of Cd(Ii) and Pb(Ii) in Food and Water Prior to ICP-OES Determination. J. Anal. At. Spectrom. 2018, 33, 1974–1980. [Google Scholar] [CrossRef]
- Ge, Q.; Liu, H. Au Nanoparticles in Silsesquioxane-Based Hybrid Networks by Simultaneous Recovery and Reduction of Au(III) in Wastewater. ACS Appl. Nano Mater. 2022, 5, 9861–9870. [Google Scholar] [CrossRef]
- Da Silva, P.S.; Gasparini, B.C.; Magosso, H.A.; Spinelli, A. Gold Nanoparticles Hosted in a Water-Soluble Silsesquioxane Polymer Applied as a Catalytic Material onto an Electrochemical Sensor for Detection of Nitrophenol Isomers. J. Hazard. Mater. 2014, 273, 70–77. [Google Scholar] [CrossRef]
- dos Santos, M.; Wrobel, E.C.; dos Santos, V.; Quináia, S.P.; Fujiwara, S.T.; Garcia, J.R.; Pessôa, C.A.; Scheffer, E.W.O.; Wohnrath, K. Development of an Electrochemical Sensor Based on LbL Films of Pt Nanoparticles and Humic Acid. J. Electrochem. Soc. 2016, 163, B499–B506. [Google Scholar] [CrossRef]
- Magossi, M.S.; Magossi, M.S.; Dias Filho, N.L.; do Carmo, D.R. Isoniazid-Sensing Behavior of a Hybrid Silsesquioxane and Cobalt Pentacyanonitrosylferrate-Based Nanocomposite. Electroanalysis 2021, 33, 1886–1894. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon Nanomaterials and Their Application to Electrochemical Sensors: A Review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Çakal, D.; Ertan, S.; Cihaner, A.; Önal, A.M. Electrochemical and Optical Properties of Substituted Phthalimide Based Monomers and Electrochemical Polymerization of 3,4-Ethylenedioxythiophene-Polyhedral Oligomeric Silsesquioxane (POSS) Analogue. Dye. Pigment. 2019, 161, 411–418. [Google Scholar] [CrossRef]
- Gao, S.; Hu, X.; Zhang, L.; Mai, Y.; Pang, H.; Dai, Y.; Liao, B. Silsesquioxane-Polythiophene Hybrid Copolymer as an Efficient Modifier for Single-Walled Carbon Nanotubes. Int. J. Polym. Sci. 2020, 2020, 7659405. [Google Scholar] [CrossRef]
- Gon, M.; Tanaka, K.; Chujo, Y. Recent Progress on Designable Hybrids with Stimuli-Responsive Optical Properties Originating from Molecular Assembly Concerning Polyhedral Oligomeric Silsesquioxane. Chem. Asian J. 2022, 17, e202200144. [Google Scholar] [CrossRef] [PubMed]
- Wannasiri, C.; Chanmungkalakul, S.; Bunchuay, T.; Chuenchom, L.; Uraisin, K.; Ervithayasuporn, V.; Kiatkamjornwong, S. Cross-Linking Silsesquioxane Cages with Polyaromatics as Fluorescent Porous Polymers for Fluoride Sensing and Removal. ACS Appl. Polym. Mater. 2020, 2, 1244–1255. [Google Scholar] [CrossRef]
- Kakuta, T.; Tanaka, K.; Chujo, Y. Synthesis of Emissive Water-Soluble Network Polymers Based on Polyhedral Oligomeric Silsesquioxane and Their Application as Optical Sensors for Discriminating the Particle Size. J. Mater. Chem. C Mater. 2015, 3, 12539–12545. [Google Scholar] [CrossRef]
- Narikiyo, H.; Kakuta, T.; Matsuyama, H.; Gon, M.; Tanaka, K.; Chujo, Y. Development of the Optical Sensor for Discriminating Isomers of Fatty Acids Based on Emissive Network Polymers Composed of Polyhedral Oligomeric Silsesquioxane. Bioorganic Med. Chem. 2017, 25, 3431–3436. [Google Scholar] [CrossRef]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Hanprasit, S.; Masik, M.; Prigyai, N.; Kiatkamjornwong, S. Silsesquioxane Cages as Fluoride Sensors. Chem. Commun. 2017, 53, 12108–12111. [Google Scholar] [CrossRef]
- Vanoni, C.R.; Winiarski, J.P.; Nagurniak, G.R.; Magosso, H.A.; Jost, C.L. A Novel Electrochemical Sensor Based on Silsesquioxane/Nickel (II) Phthalocyanine for the Determination of Sulfanilamide in Clinical and Drug Samples. Electroanalysis 2019, 31, 867–875. [Google Scholar] [CrossRef]
- Magossi, M.S.; Fernandes, D.S.; do Carmo, D.R. Synthesis of a Novel Hybrid Nanocomposite Based on Copper Pentacyanonitrosylferrate and Octa(Aminopropyl)Silsesquioxane and Its Behavior on L-Cysteine Electrooxidation. Solid. State Sci. 2019, 95, 105931. [Google Scholar] [CrossRef]
- da Silva Carvalho, V.A.; Peixoto, M.S.; dos Santos Felipe, A.; Sales, A.S.B.; Masteralo, V.R.; Dias Filho, N.L.; do Carmo, D.R. A Metallo- Silsesquioxane Synthesized from Cu (II) via a Cyanide Fe (III) Bridge: Synthesis and Application as an Electrochemical Sensor. ChemistrySelect 2024, 9, e202401702. [Google Scholar] [CrossRef]
- Franco, F.D.S.; Peixoto, M.S.; Baroni, A.S.; Felipe, A.D.S.; Barbosa, P.F.P.; Maraldi, V.A.; Freitas, R.M.L.; Da Silva, S.F.V.; Alves, V.P.; Moraes, A.A.C.; et al. Voltammetric Behaviour of an Electroactive of Zn2+ -Octa(Aminopropyl)Silsesquioxane Nanocoposite: An Application to 4-Chlorophenol Detection in Tap Water. Int. J. Electrochem. Sci. 2022, 17, 220512. [Google Scholar] [CrossRef]
- Alsaiari, M.; Saleem, A.; Alsaiari, R.; Muhammad, N.; Latif, U.; Tariq, M.; Almohana, A.; Rahim, A. SiO2/Al2O3/C Grafted 3-n Propylpyridinium Silsesquioxane Chloride-Based Non-Enzymatic Electrochemical Sensor for Determination of Carcinogenic Nitrite in Food Products. Food Chem. 2022, 369, 130970. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Herrero, M.; Alonso, B.; Casado, C.M.; Armada, M.P.G. Three-Dimensional Electrocatalytic Surface Based on an Octasilsesquioxane Dendrimer for Sensing Applications. J. Electroanal. Chem. 2019, 839, 16–24. [Google Scholar] [CrossRef]
- Kannan, A.; Mayavan, A.; Gandhi, S. Polyhedral Oligomeric Silsesquioxane-Based Hydrophobic Hybrid Composite for the Electrochemical Detection of a Benzophenone Derivative. Microchem. J. 2024, 203, 110798. [Google Scholar] [CrossRef]
- Santos, C.S.; Ferreira, R.T.; Calixto, C.M.F.; Rufino, J.L.; Garcia, J.R.; Fujiwara, S.T.; Wohnrath, K.; Pessoa, C.A. The Influence of Organization of LbL Films Containing a Silsesquioxane Polymer on the Electrochemical Response of Dopamine. J. Appl. Electrochem. 2014, 44, 1047–1058. [Google Scholar] [CrossRef]
- Estevam, R.B.; Ferreira, R.T.; Bischof, A.B.H.; dos Santos, F.S.; Santos, C.S.; Fujiwara, S.T.; Wohnrath, K.; Lazaro, S.R.; Garcia, J.R.; Pessoa, C.A. Preparation and Characterization of LbL Films Based on Graphene Oxide Nanoparticles Interacting with 3-n-Propylpyridinium Silsesquioxane Chloride. Surf. Coat. Technol. 2015, 275, 2–8. [Google Scholar] [CrossRef]
- dos Santos, V.; dos Santos, M.; de Jesus, C.G.; Fujiwara, S.T.; Garcia, J.R.; Pessôa, C.A.; Wohnrath, K. The Role of a Layer-by-Layer Film Containing Pt Nanoparticle on the Performance of a Glucose Enzymatic Biosensor. Int. J. Electrochem. Sci. 2013, 8, 10601–10620. [Google Scholar] [CrossRef]
- Intema, R.C.; Wrobel, E.C.; Fujiwara, S.T.; Garcia, J.R.; Pessôa, C.A.; Wohnrath, K. The Effect of Surfactants in the Silsesquioxane Solution for LbL Films Assembly. J. Mater. Sci. 2017, 52, 7647–7663. [Google Scholar] [CrossRef]
- Zhao, S.; Caruso, F.; Dahne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The Future of Layer-by-Layer Assembly: A Tribute to ACS Nano Associate Editor Helmuth Mohwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef]
- De Jesus, C.G.; Lima, D.; Dos Santos, V.; Wohnrath, K.; Pessôa, C.A. Glucose Biosensor Based on the Highly Efficient Immobilization of Glucose Oxidase on Layer-by-Layer Films of Silsesquioxane Polyelectrolyte. Sens. Actuators B Chem. 2013, 186, 44–51. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Yamamoto, S.; Miyashita, T.; Mitsuishi, M. PH-Responsive Ultrathin Nanoporous SiO2Films for Selective Ion Permeation. Langmuir 2021, 37, 5627–5634. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Yamamoto, S.; Miyashita, T.; Mitsuishi, M. Controlled Ion Permeability of Ultrathin Nanoporous SiO2 Films from Silsesquioxane-Containing Polymer Nanosheets. ACS Appl. Nano Mater. 2020, 3, 7454–7461. [Google Scholar] [CrossRef]
- Ariga, K.; Lvov, Y.; Decher, G. There Is Still Plenty of Room for Layer-by-Layer Assembly for Constructing Nanoarchitectonics-Based Materials and Devices. Phys. Chem. Chem. Phys. 2022, 24, 4097–4115. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.; Weng, G.M.; Röhr, J.A.; Wang, H.; Taylor, A.D. Layer-by-Layer Assembly of Two-Dimensional Materials: Meticulous Control on the Nanoscale. Matter 2020, 2, 1148–1165. [Google Scholar] [CrossRef]
- Wågberg, L.; Erlandsson, J. The Use of Layer-by-Layer Self-Assembly and Nanocellulose to Prepare Advanced Functional Materials. Adv. Mater. 2021, 33, 2001474. [Google Scholar] [CrossRef]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A Closer Physico-Chemical Look to the Layer-by-Layer Electrostatic Self-Assembly of Polyelectrolyte Multilayers. Adv. Colloid. Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef]
- Fernandes, D.S.; do Carmo, D.R. Silsesquioxane Modified with PAMAM Dendrimer and a Bimetallic Complex for Electrochemical Detection of Ascorbic Acid. Electroanalysis 2021, 33, 365–374. [Google Scholar] [CrossRef]
- Lima, D.; Mendes Hacke, A.C.; Ulmer, B.; Kuss, S. Electrochemical Sensing of Trypanosome- and Flavivirus-Related Neglected Tropical Diseases. Curr. Opin. Electrochem. 2021, 30, 100838. [Google Scholar] [CrossRef]
- Kimmel, D.W.; Leblanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef]
- Sultana, S.; Noroozifar, M.; Kerman, K. Ruthenium Red-Functionalized Sol-Gel and Multi-Walled Carbon Nanotubes for Electrochemical Simultaneous Detection of Three Dihydroxybenzene Isomers. J. Electroanal. Chem. 2021, 899, 115644. [Google Scholar] [CrossRef]
- Islam, R.; Le Luu, H.T.; Kuss, S. Review—Electrochemical Approaches and Advances towards the Detection of Drug Resistance. J. Electrochem. Soc. 2020, 167, 045501. [Google Scholar] [CrossRef]
- do Carmo, D.R.; Barbosa, P.F.P.; Cumba, L.R. Electrochemical Behavior of Titanium (IV) Silsesquioxane Occluded in the MCM-41 Cavity and Their Application in the Electro-Oxidation of Sulphite and Dipyrone Compounds. Silicon 2020, 12, 1111–1123. [Google Scholar] [CrossRef]
- Ananthakrishnan, D.; Venkatesvaran, H.; Kannan, A.; Gandhi, S. Simplistic One-Pot Synthesis of an Inorganic-Organic Cubic Caged Material: A New Interface for Detecting Toxic Bisphenol-A Electrochemically. New J. Chem. 2020, 44, 20192–20202. [Google Scholar] [CrossRef]
- do Carmo, D.R.; Maraldi, V.A.; Cumba, L.R. Voltammetric Properties of Nickel Hexacyanoferrate (III) Obtained on the Titanium (IV) Silsesquioxane Occluded into the H-FAU Zeolite for Detection of Sulfite. Silicon 2019, 11, 267–276. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angtrom Pores. Science 1998, 5350, 448–552. [Google Scholar]
- do Carmo, D.R.; de Oliveira, D.R.; Barbosa, P.F.P.; Godoi, N.M.I. A Cubic Silsesquioxane Chemically Modified with a PAMAM Dendrimer G0: An Application in Electro-Oxidation of Ascorbic Acid. Silicon 2019, 11, 2961–2974. [Google Scholar] [CrossRef]
- Fernandes, D.S.; Maraldi, V.A.; Filho, N.L.D.; do Carmo, D.R. Reactivity of a Silsesquioxane Organofunctionalized with 4-Amino-5-Phenyl-4H-[1,2,4]-Triazole-3-Thiol: Complementary Characterization and an Application to Chronoamperometric Detection of L-Dopamine. Silicon 2019, 11, 1131–1142. [Google Scholar] [CrossRef]
- Dong, W.; Ma, Z.; Duan, Q. Preparation of Stable Crosslinked Polyelectrolyte and the Application for Humidity Sensing. Sens. Actuators B Chem. 2018, 272, 14–20. [Google Scholar] [CrossRef]
- Dai, J.; Qi, R.; Tu, J.; Zhao, H.; Liu, S.; Fei, T.; Zhang, T. Humidity Sensor Preparation by In Situ Click Polymerization. IEEE Electron. Device Lett. 2018, 39, 1234–1237. [Google Scholar] [CrossRef]

| MNPs | Metallic Precursor | SSQ | Reducing Agent | Diameter (nm) | Reference |
|---|---|---|---|---|---|
| AgNPs | AgNO3 | 3-n-propylpyridinium chloride | NaBH4 | 21.0 | [63] |
| AgNPs | AgNO3 | 3-n-propyl(4-methylpyridinium) silsesquioxane | NaBH4 | 3.7 | [57] |
| AgNPs | AgNO3 | 1,4-diazoniabicyclo-[2.2.2]octane silsesquioxane | NaBH4 | – | [64] |
| AuNPs | HAuCl4 | octamercaptopropyl-POSS | NaBH4 | – | [65] |
| AuNPs | HAuCl4 | 3-n-propyl (4-methylpyridinium) silsesquioxane chloride | NaBH4 | 45.0 | [10] |
| AuNPs | HAuCl4 | 3-n-propyl-4-picolinium silsesquioxane chloride | NaBH4 | 50.0 | [59] |
| AuNPs | HAuCl4 | 3-n-propylpyridinium silsesquioxane chloride | NaBH4 | 12.3 | [45] |
| AuNPs | HAuCl4 | 3-n-propyl(2-amino-4-methyl)pyridinium silsesquioxane chloride | NaBH4 | 5.8 | [50] |
| PtNPs | H2PtCl6 | 3-n-propyl (4-methylpyridinium) silsesquioxane chloride | NaBH4 | 2.5 | [58] |
| PtNPs | H2PtCl6 | 3-n-propylpyridinium silsesquioxane chloride | Formic acid | 3.0–4.0 | [66] |
| FeO3NPs | Fe3O4 | thiol-rich polyhedral oligomeric silsesquioxane (POSS-SH) | Solvothermal method | 350–450 | [67] |
| Prussian blue | K3[Fe(CN)6] | octa(aminopropyl) silsesquioxane | – | 200 | [61] |
| PdNPs | Pd(OAc)2 | 3-n-propyl(4-methylpyridinium) silsesquioxane nitrate | NaBH4 | 1.3 | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahrebelnei, F.; Ribicki, A.C.; Martins Duboc Natal, A.; Fujiwara, S.T.; Wohnrath, K.; Lima, D.; Pessôa, C.A. Silsesquioxanes as Promising Materials for the Development of Electrochemical (Bio)Sensors. Chemosensors 2024, 12, 259. https://doi.org/10.3390/chemosensors12120259
Zahrebelnei F, Ribicki AC, Martins Duboc Natal A, Fujiwara ST, Wohnrath K, Lima D, Pessôa CA. Silsesquioxanes as Promising Materials for the Development of Electrochemical (Bio)Sensors. Chemosensors. 2024; 12(12):259. https://doi.org/10.3390/chemosensors12120259
Chicago/Turabian StyleZahrebelnei, Felipe, Ariane Caroline Ribicki, Aline Martins Duboc Natal, Sérgio Toshio Fujiwara, Karen Wohnrath, Dhésmon Lima, and Christiana Andrade Pessôa. 2024. "Silsesquioxanes as Promising Materials for the Development of Electrochemical (Bio)Sensors" Chemosensors 12, no. 12: 259. https://doi.org/10.3390/chemosensors12120259
APA StyleZahrebelnei, F., Ribicki, A. C., Martins Duboc Natal, A., Fujiwara, S. T., Wohnrath, K., Lima, D., & Pessôa, C. A. (2024). Silsesquioxanes as Promising Materials for the Development of Electrochemical (Bio)Sensors. Chemosensors, 12(12), 259. https://doi.org/10.3390/chemosensors12120259





