MOX-Based Resistive Gas Sensors with Different Types of Sensitive Materials (Powders, Pellets, Films), Used in Environmental Chemistry
Abstract
1. Introduction
2. Materials and Methods
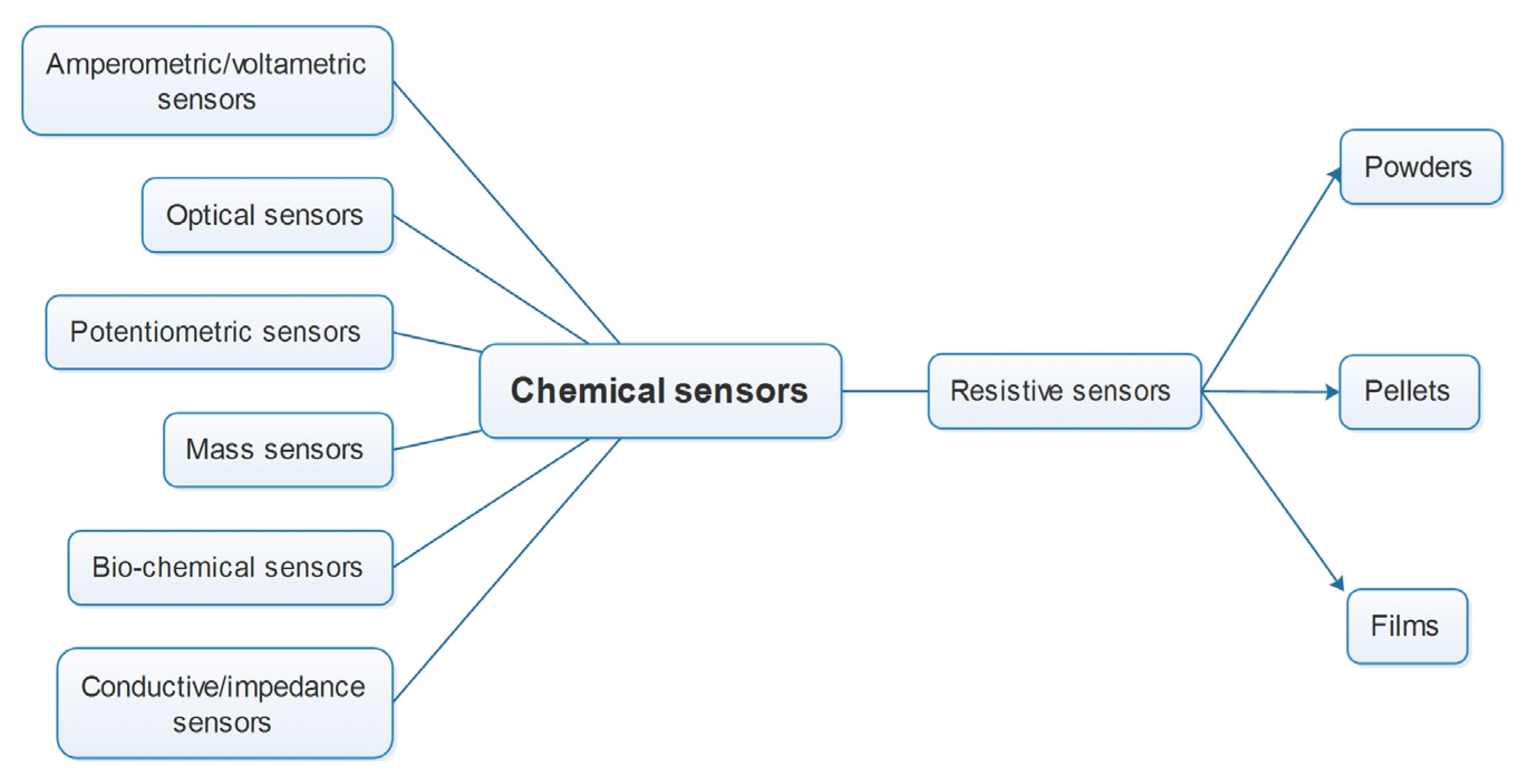
2.1. Types of Chemosensors and Practical Use
2.2. Sensor Characteristics
2.3. Use of Gas Sensors along History
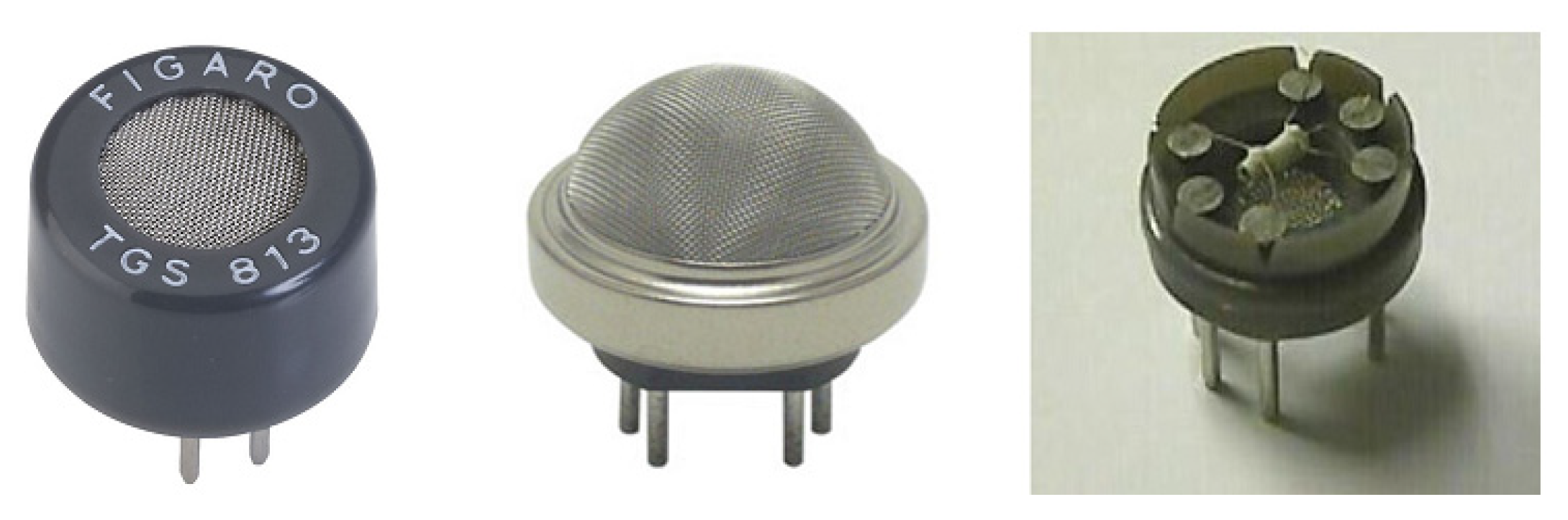
2.4. Modern Gas Sensors
2.5. Sensitive Material Deposition Techniques Used in Modern Practice and Solutions Considered for Sensor Improvement
- chemical vapor deposition (CVD), achieved by laser induction, plasma activation, photon activation, laser pyrolysis, etc;
- physical vapor deposition (PVD), achieved by thermal evaporation, electron or ion beam evaporation, and reactive evaporation;
- spray pyrolysis—involves a sequence of processes that take place sequentially or simultaneously including the generation of aerosols, evaporation of the solvent, deposition in uniform layer of droplets, and decomposition of precursors.
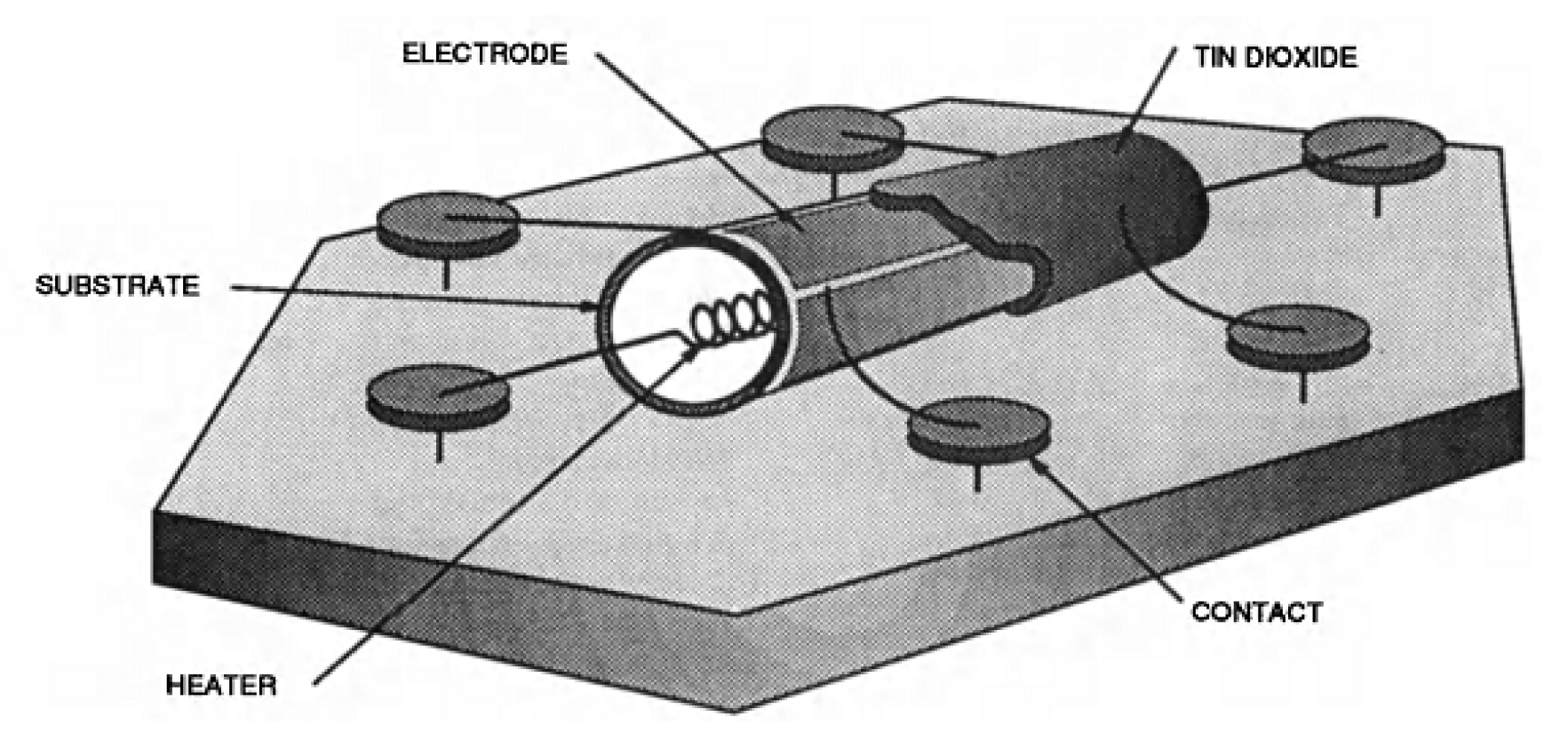
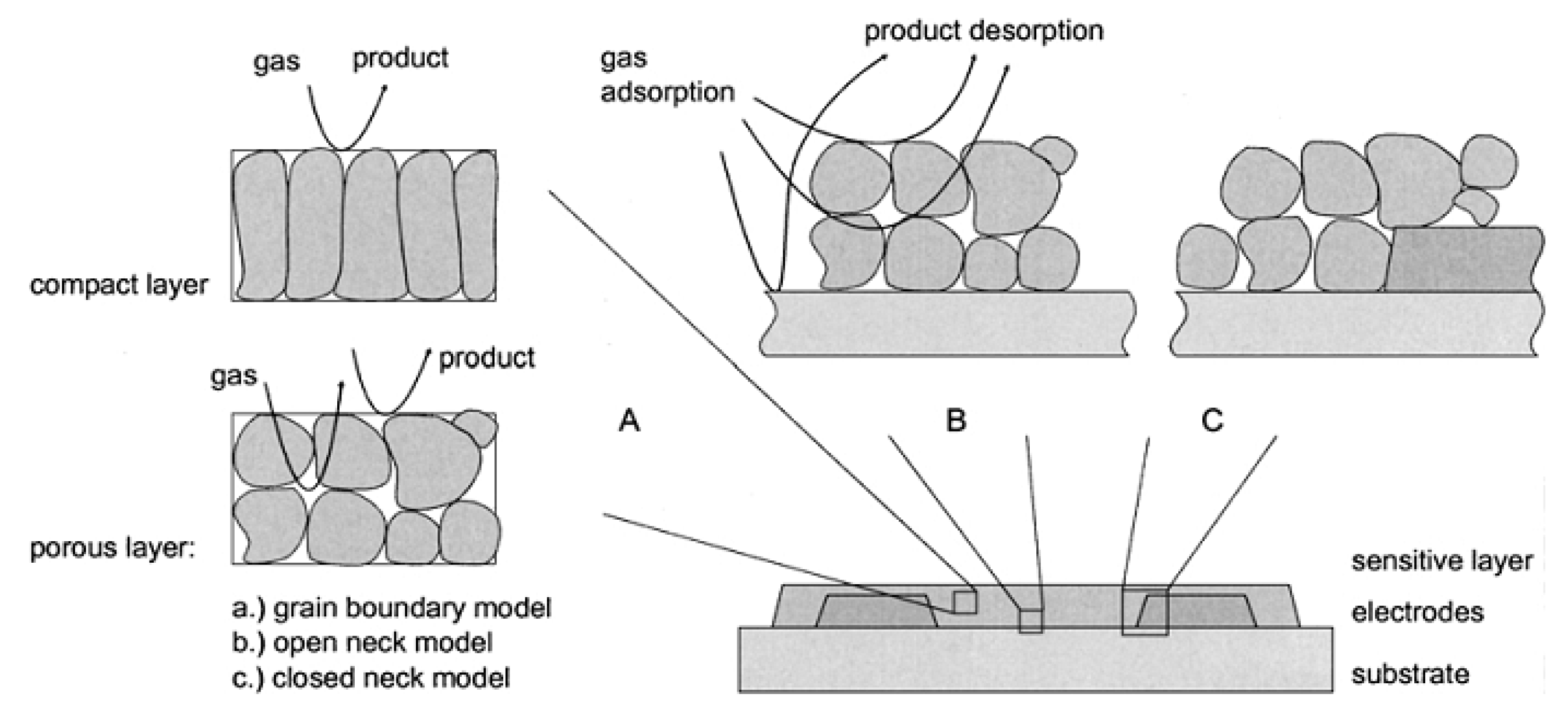
2.6. The Detection Principle for Resistive Gas Sensors
- processes that take place on the surface of sensitive material;
- processes that take place at the intergranular barrier;
- processes that take place between the sensitive layer and the sensor electrodes.
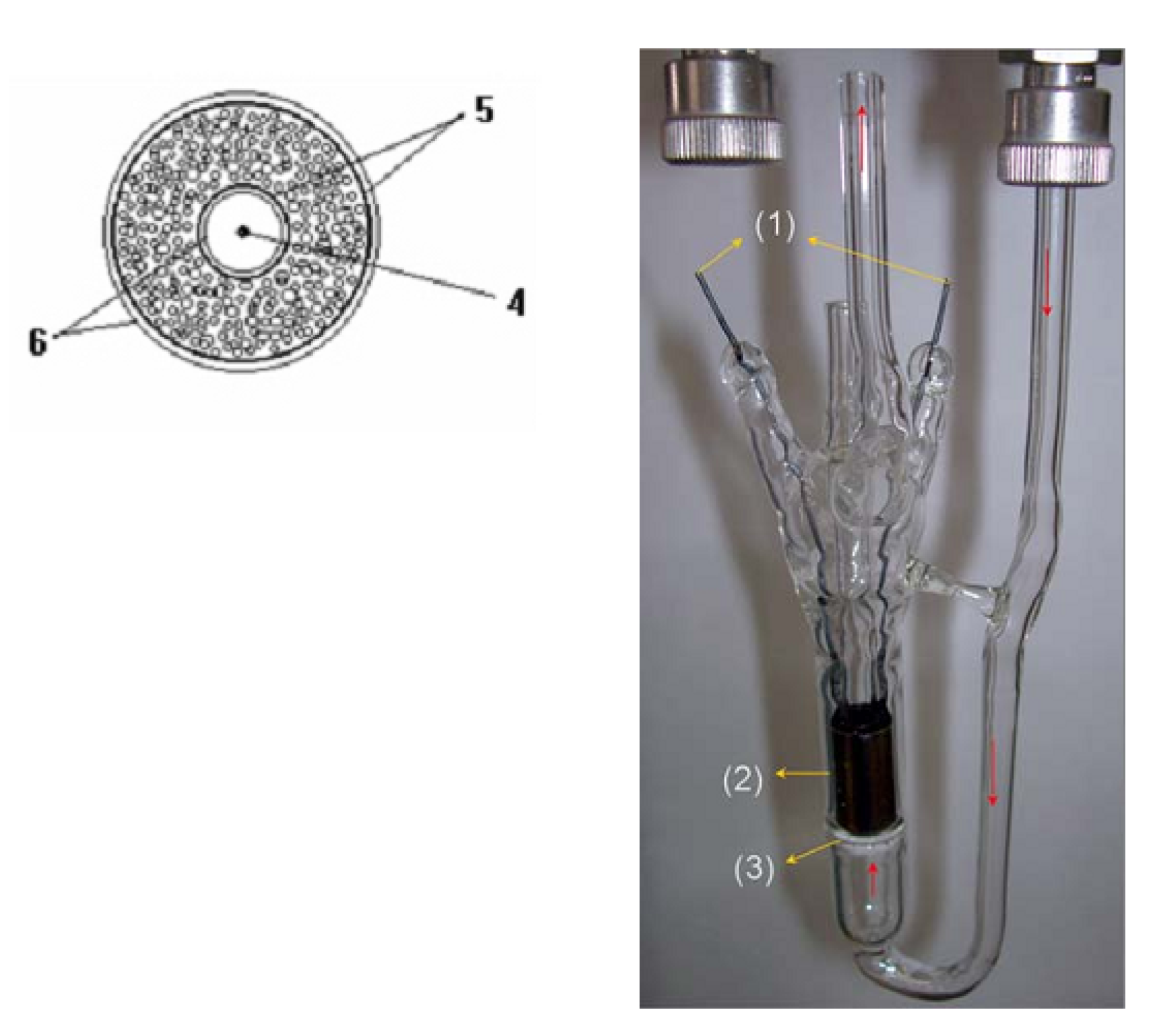
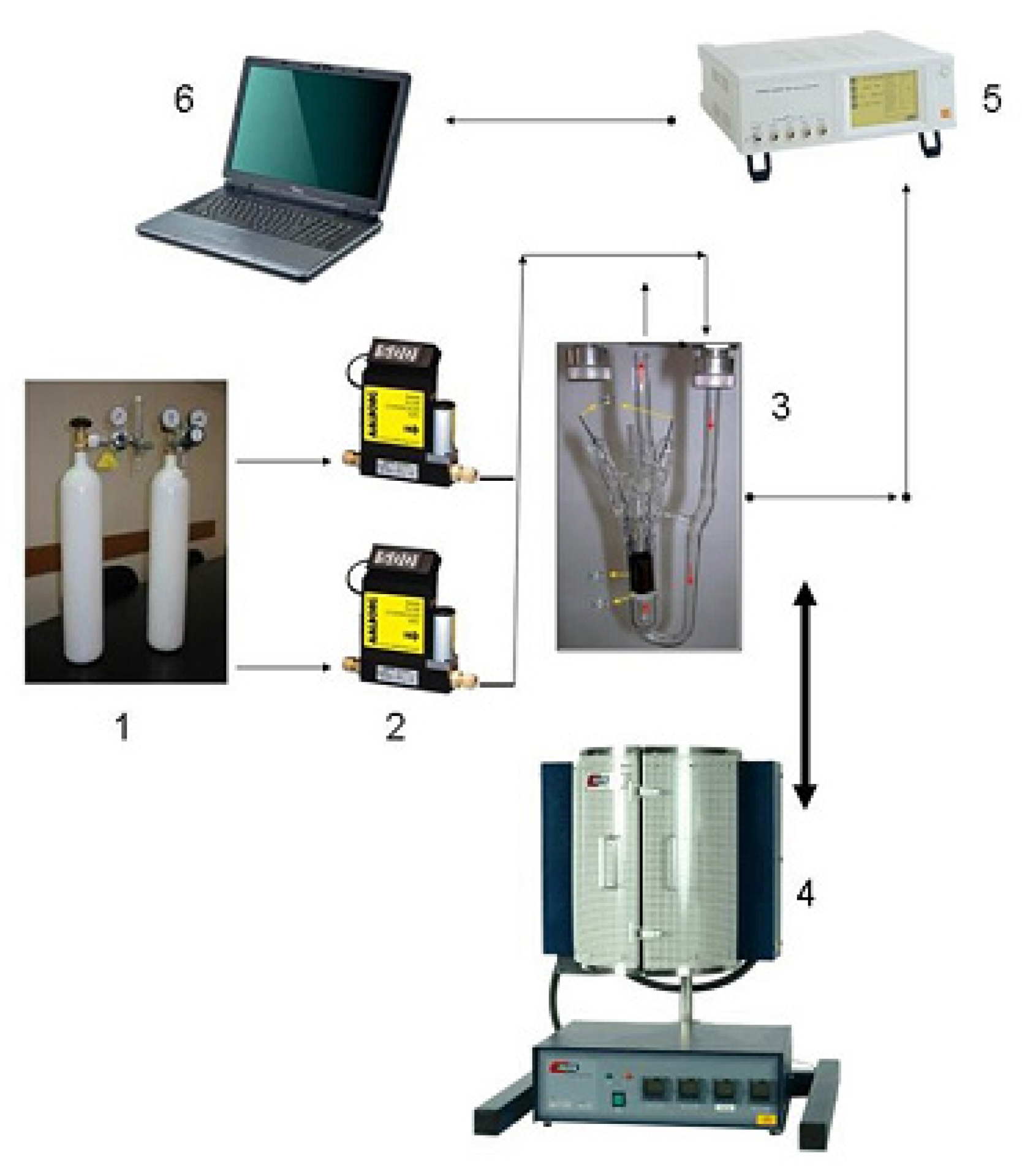
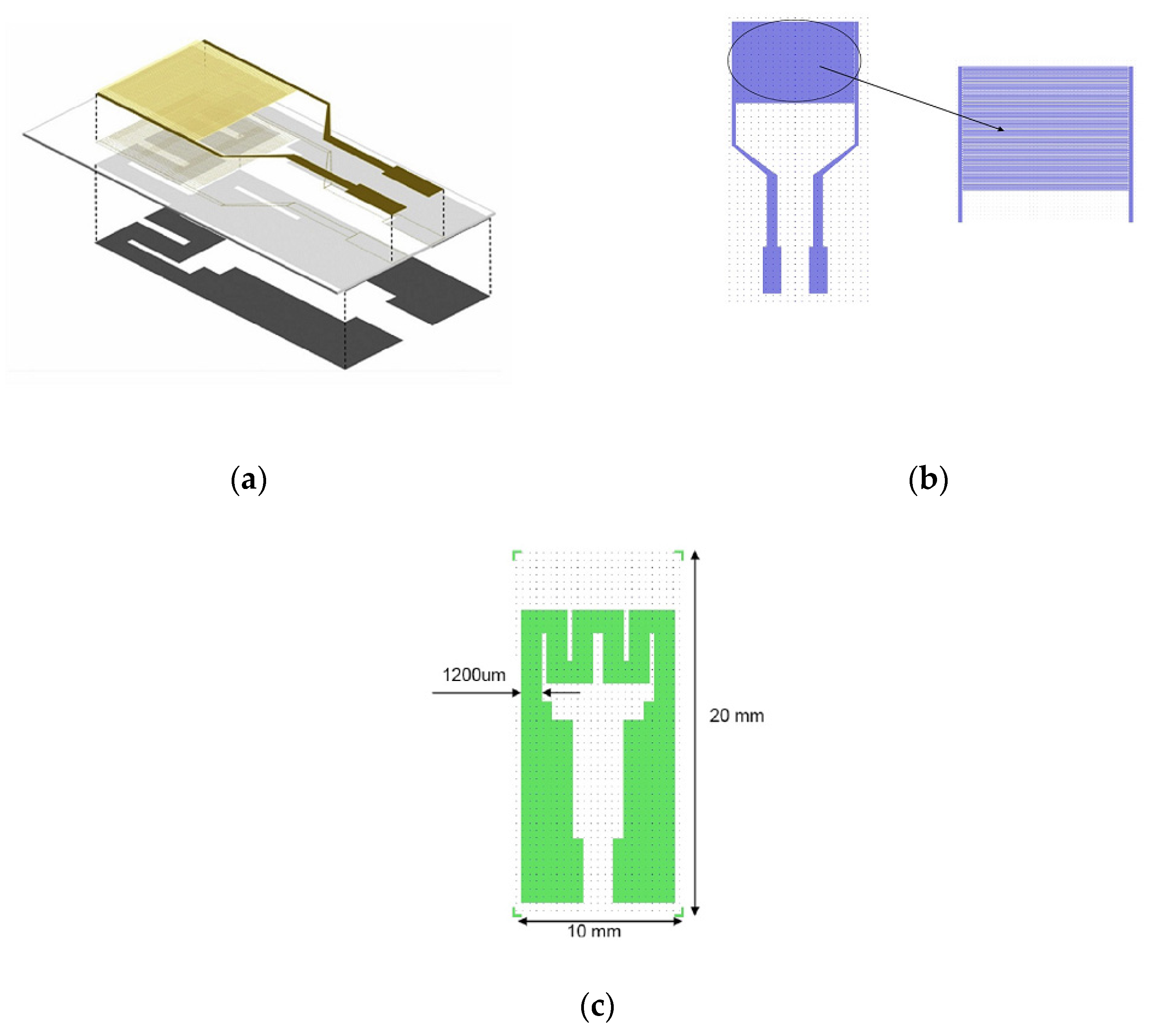
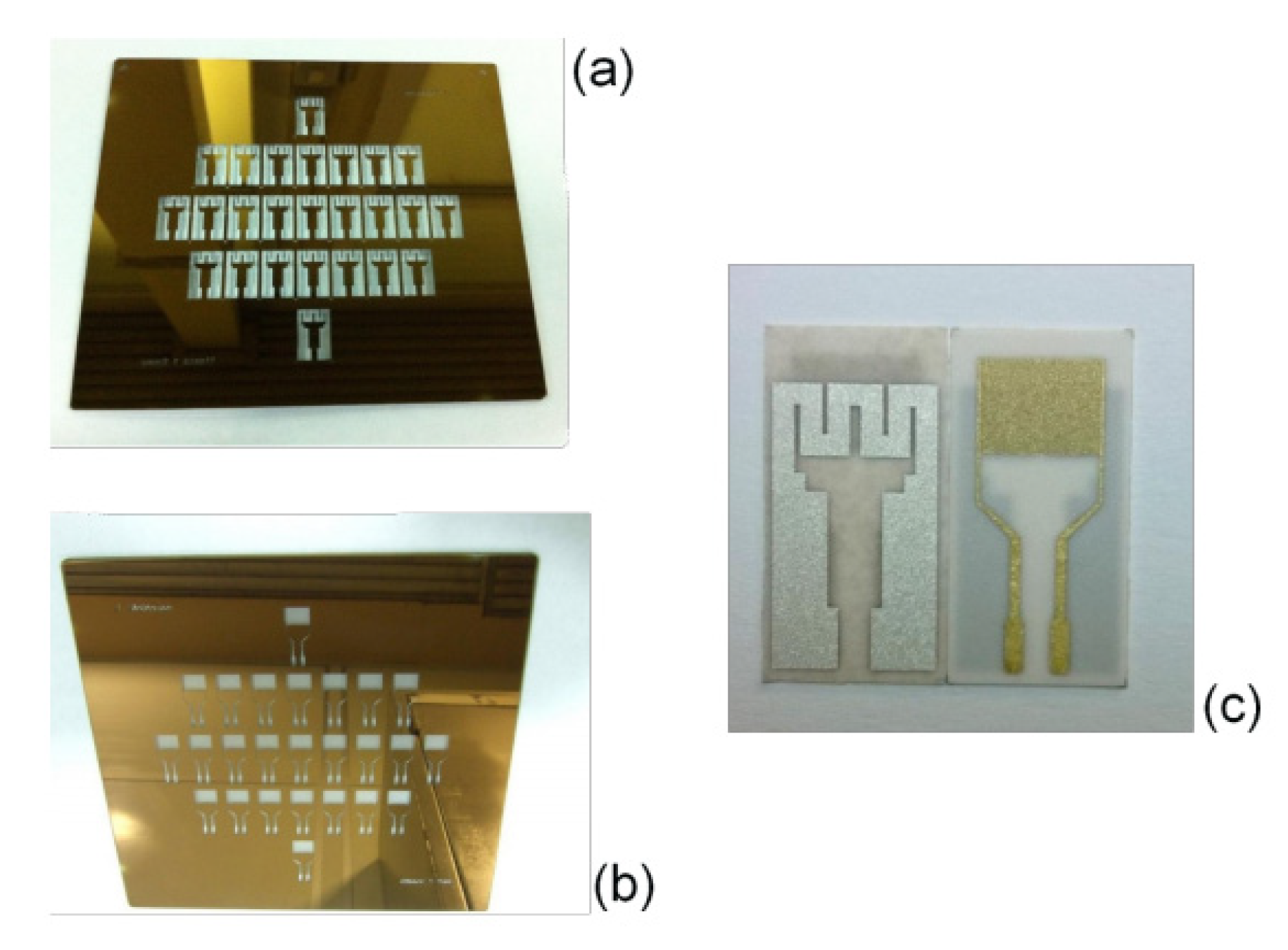
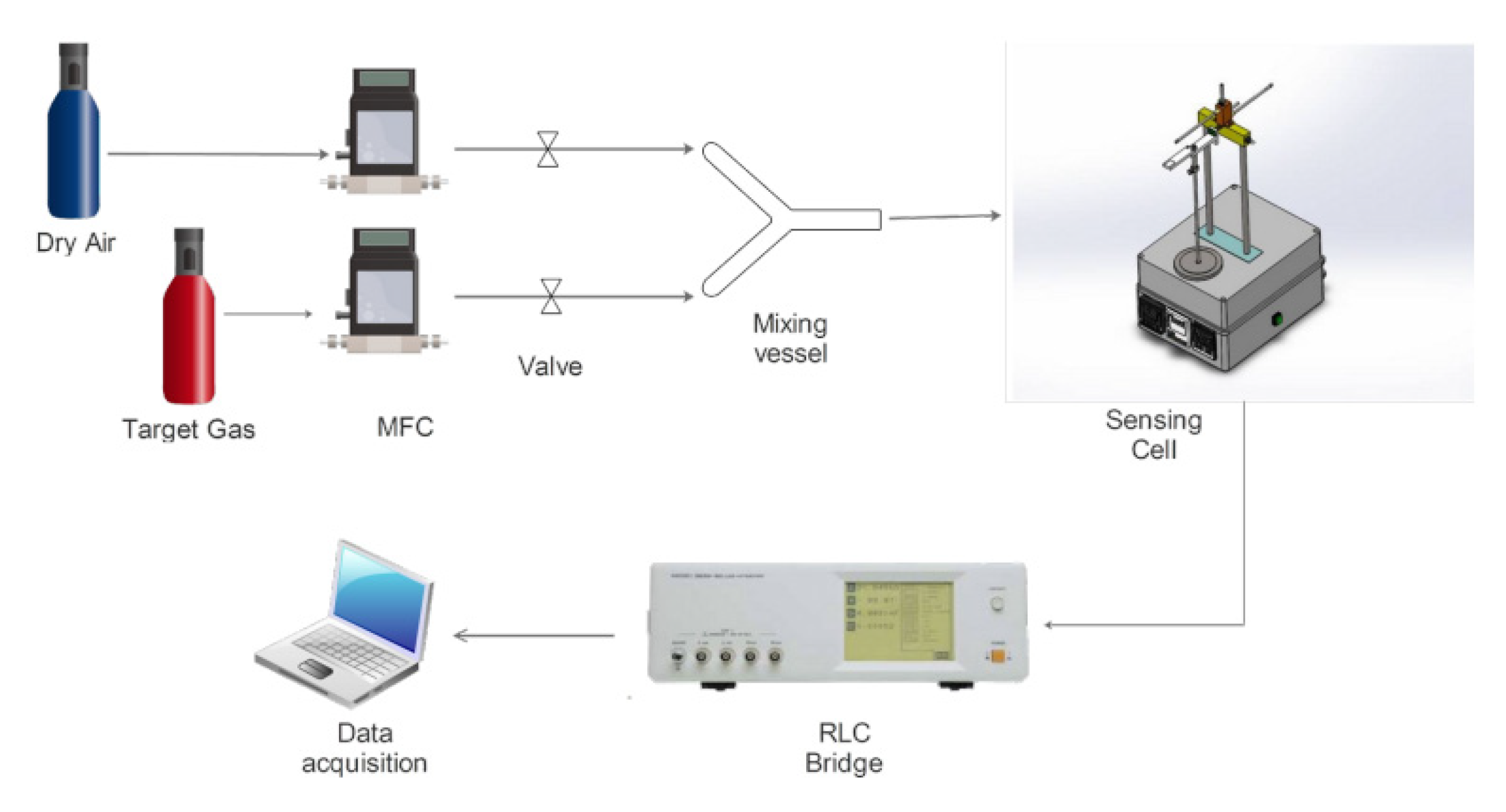
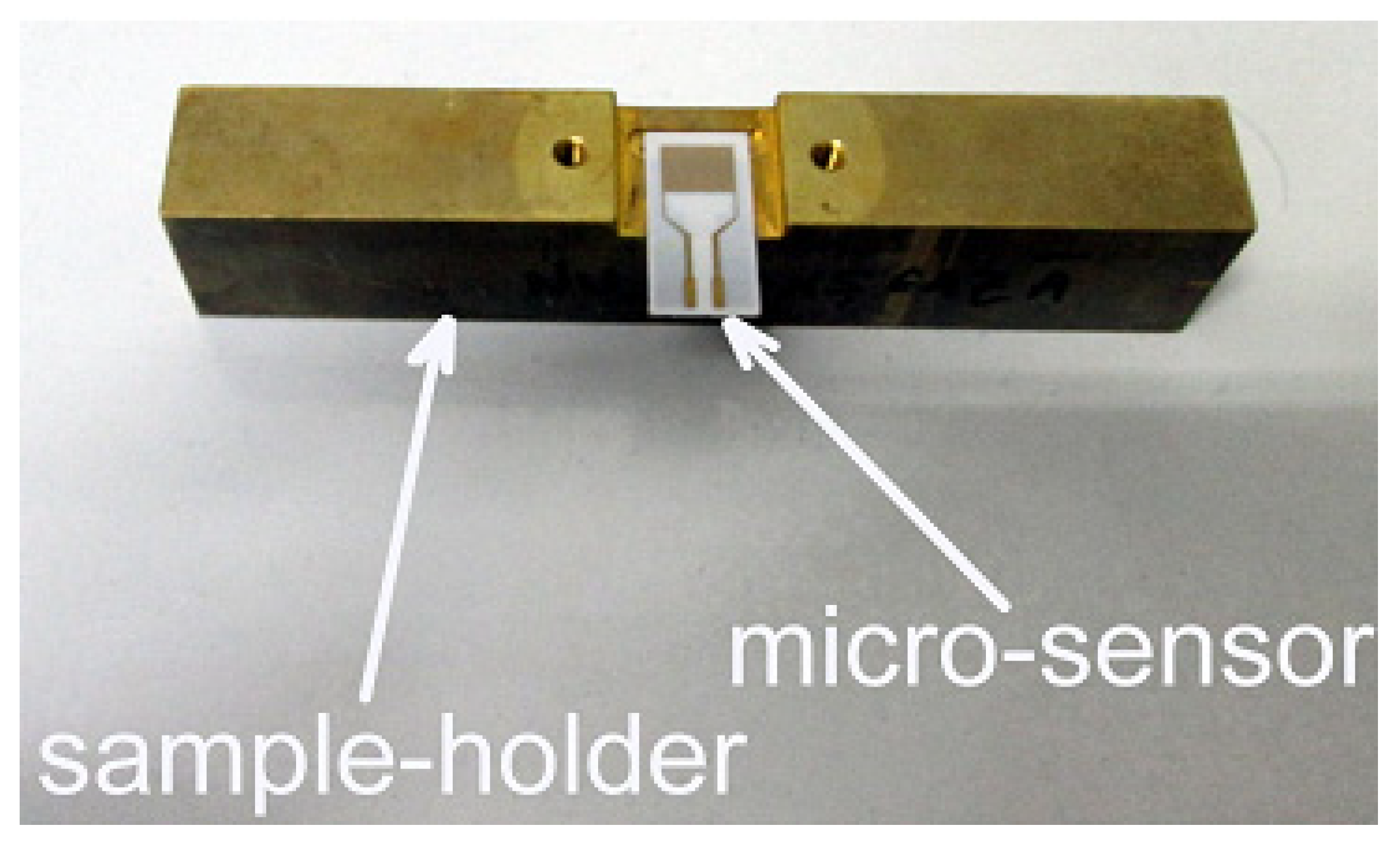
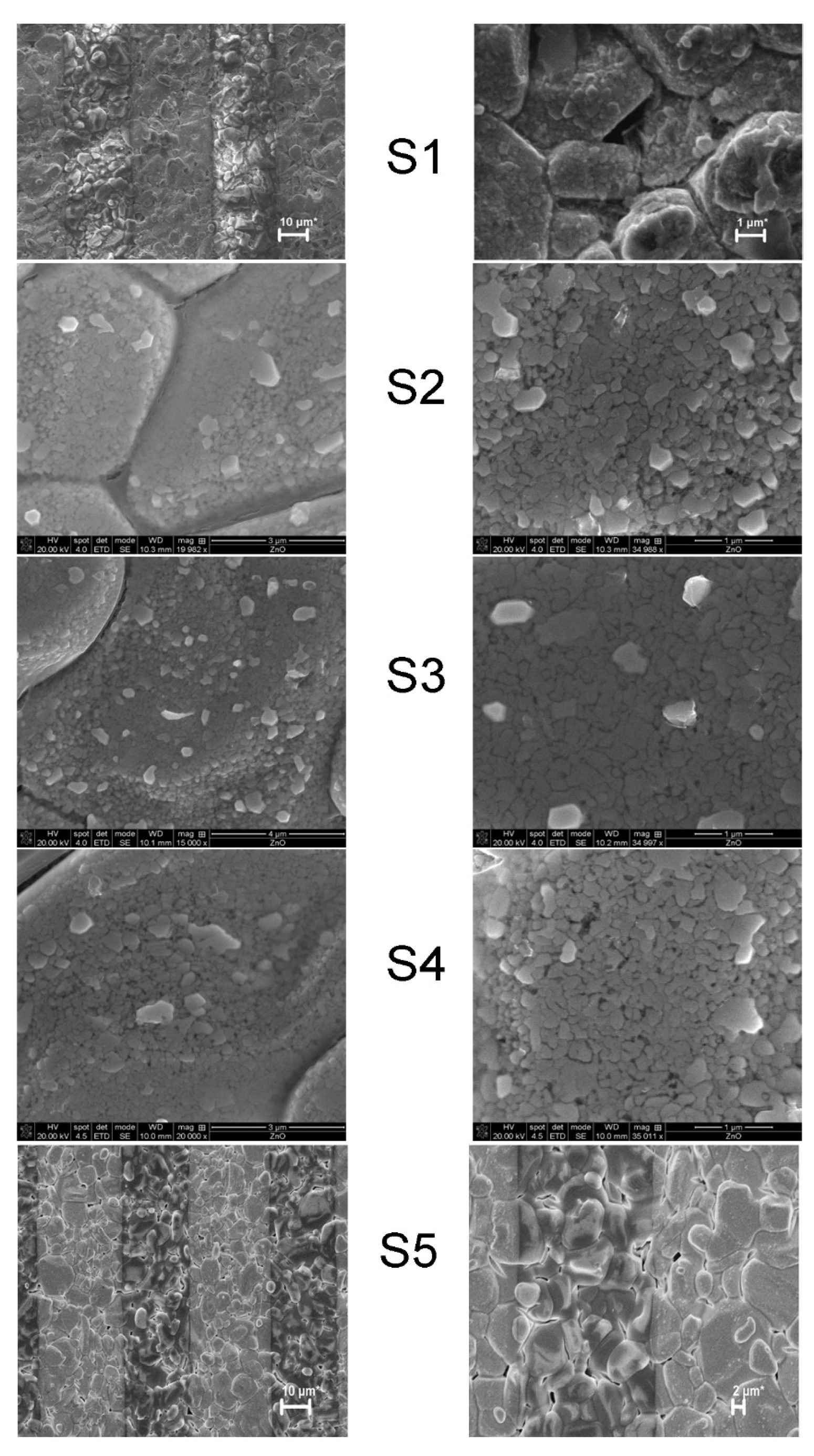
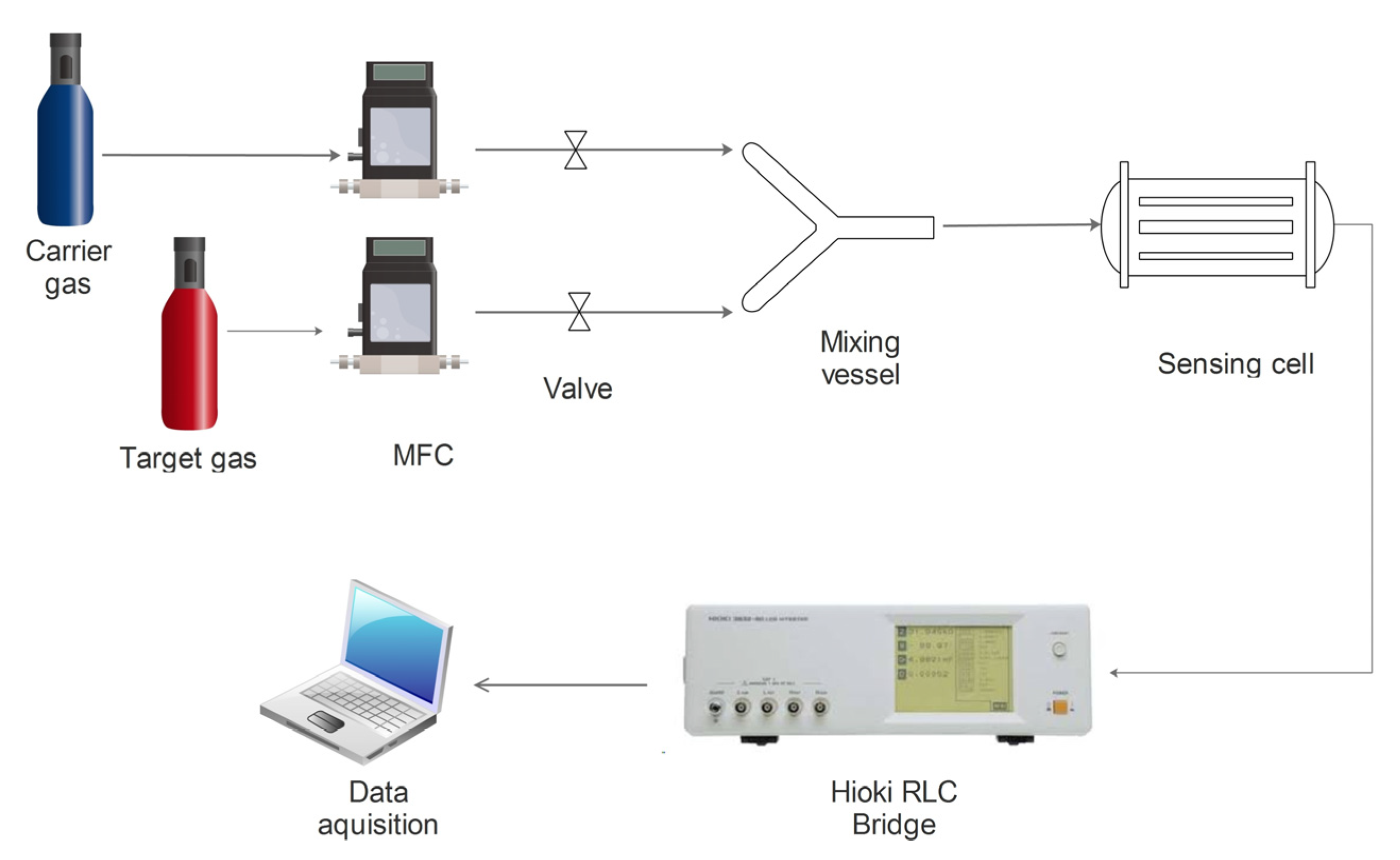
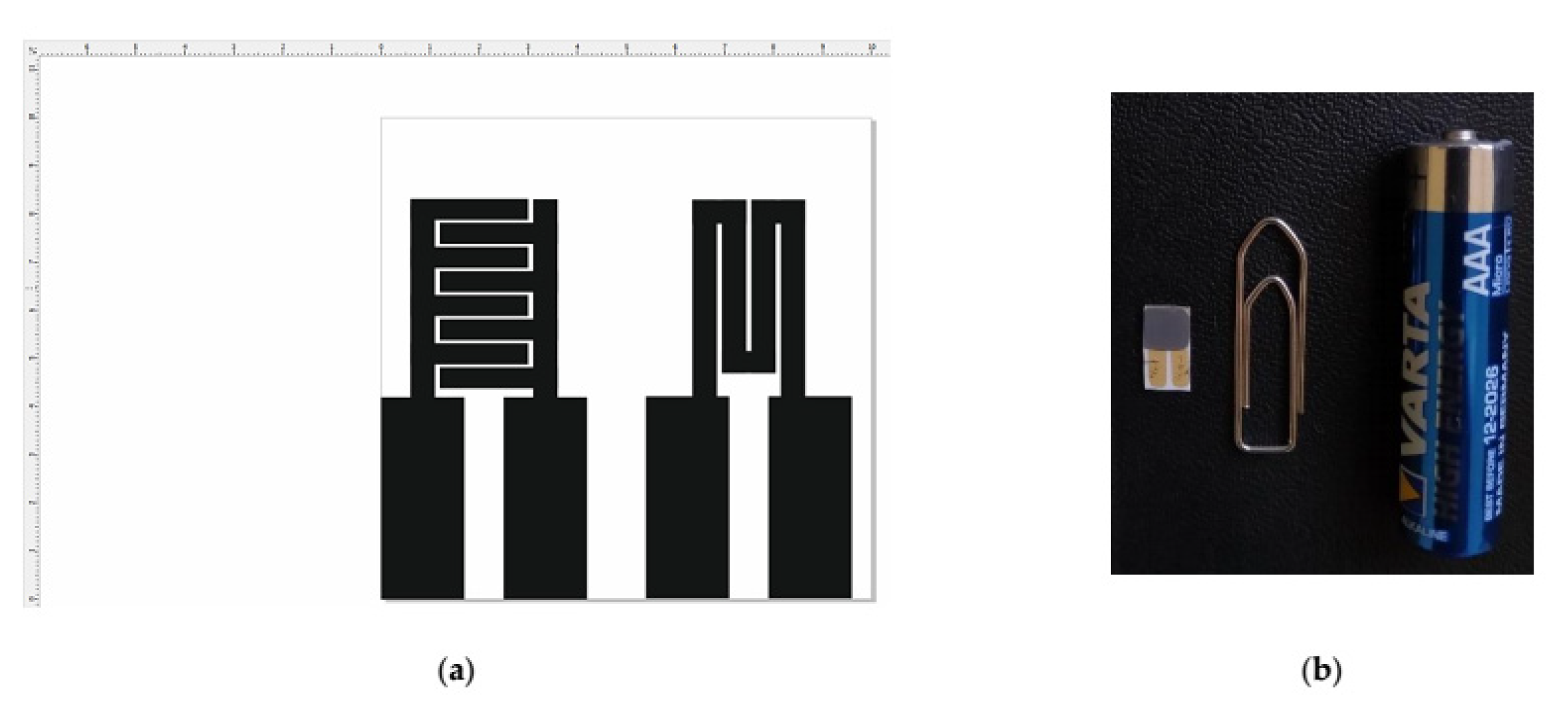
2.7. MOX-Based Sensitive Materials Used in Own-Design Gas Sensor Prototypes
3. Results and Discussion
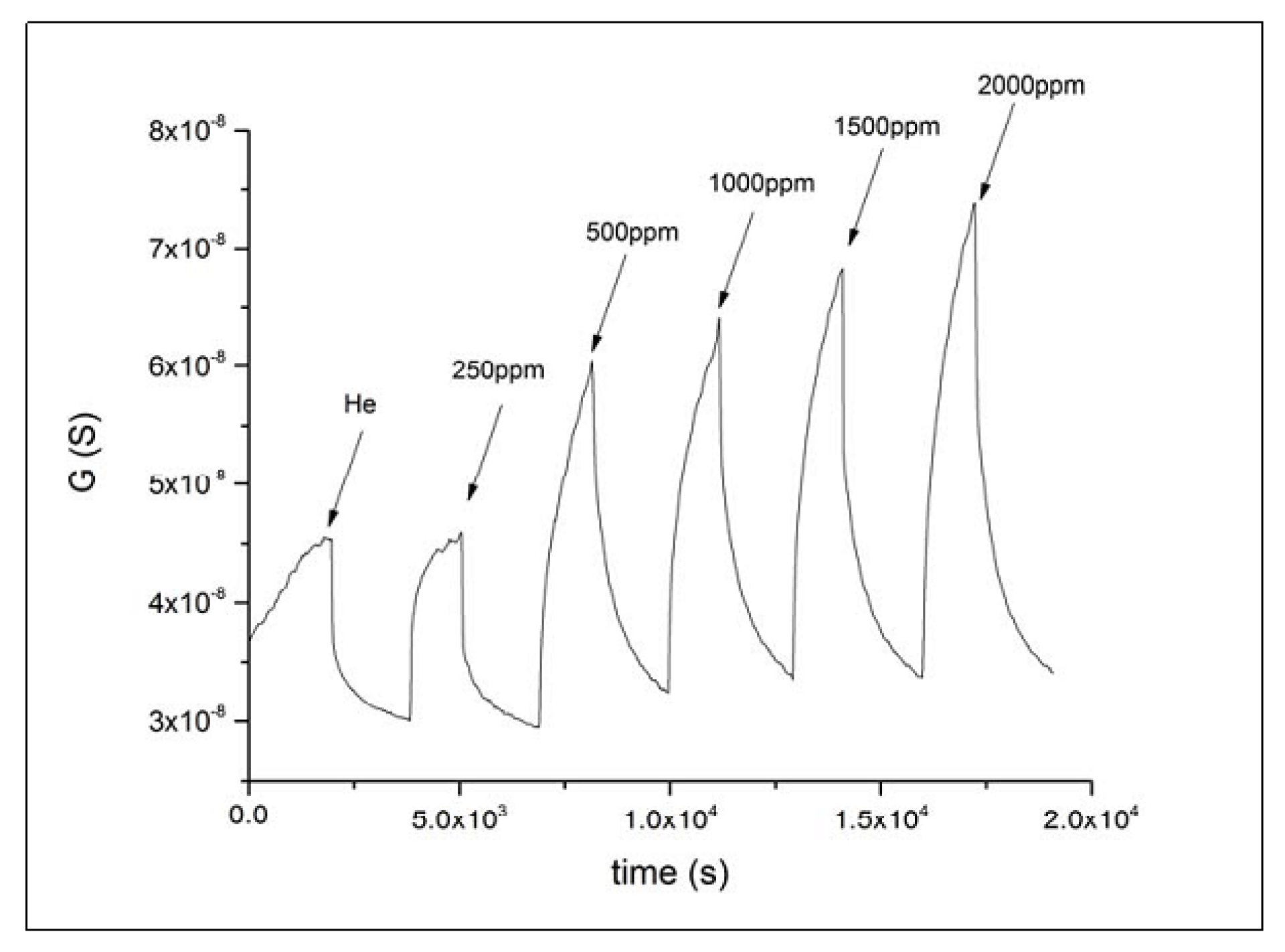
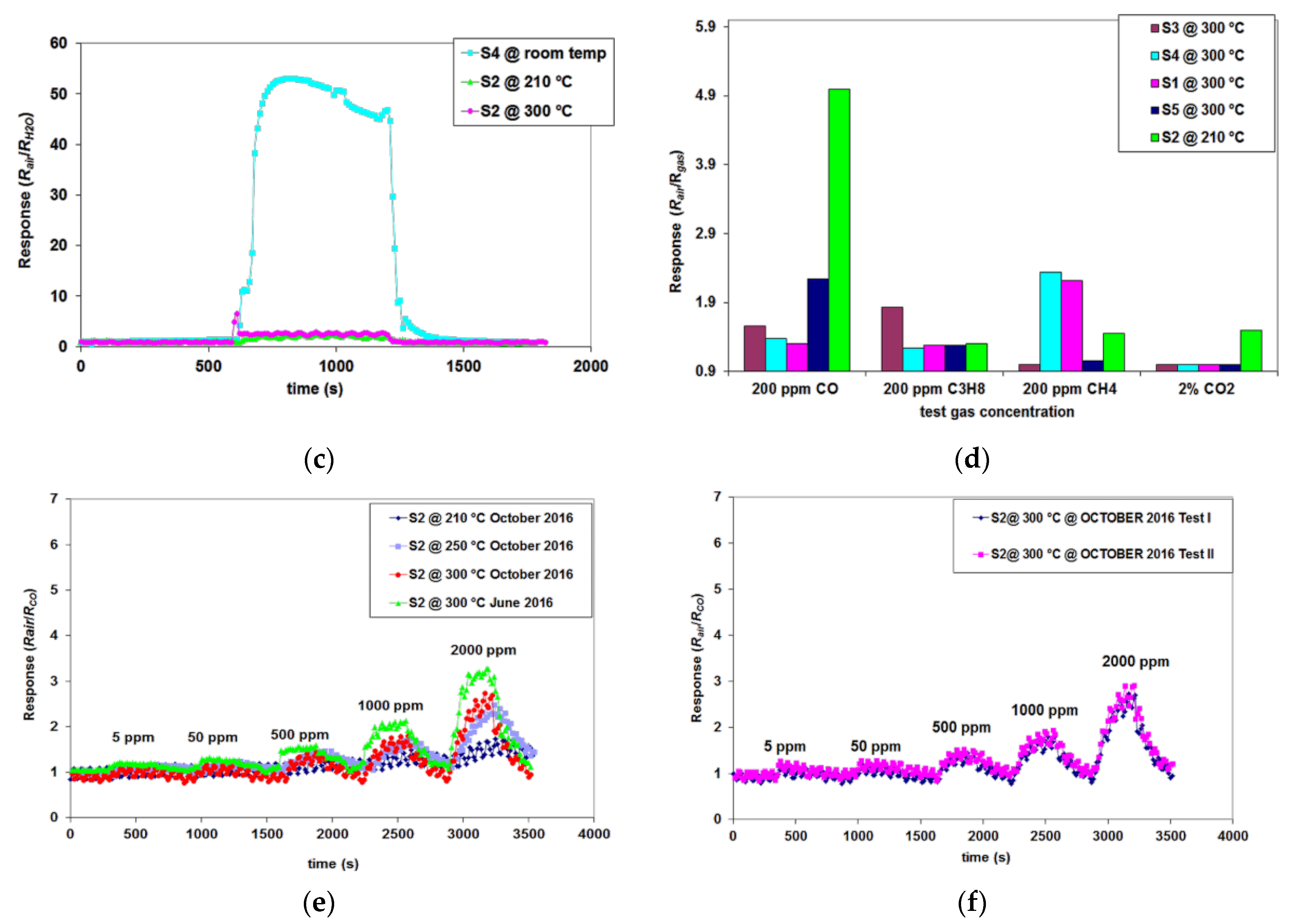
3.1. Powder Type-Sensitive Materials Used for CO Detection
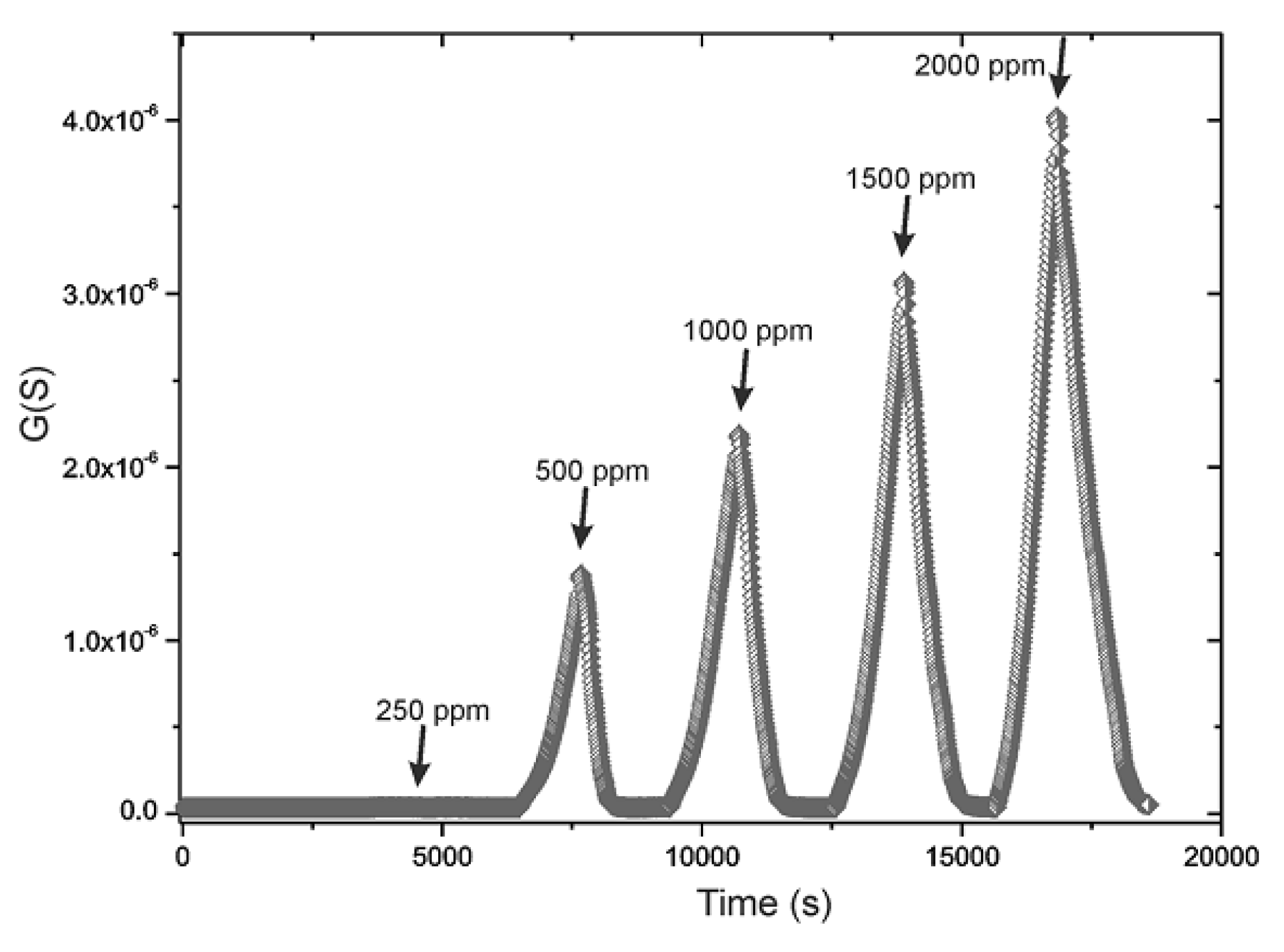
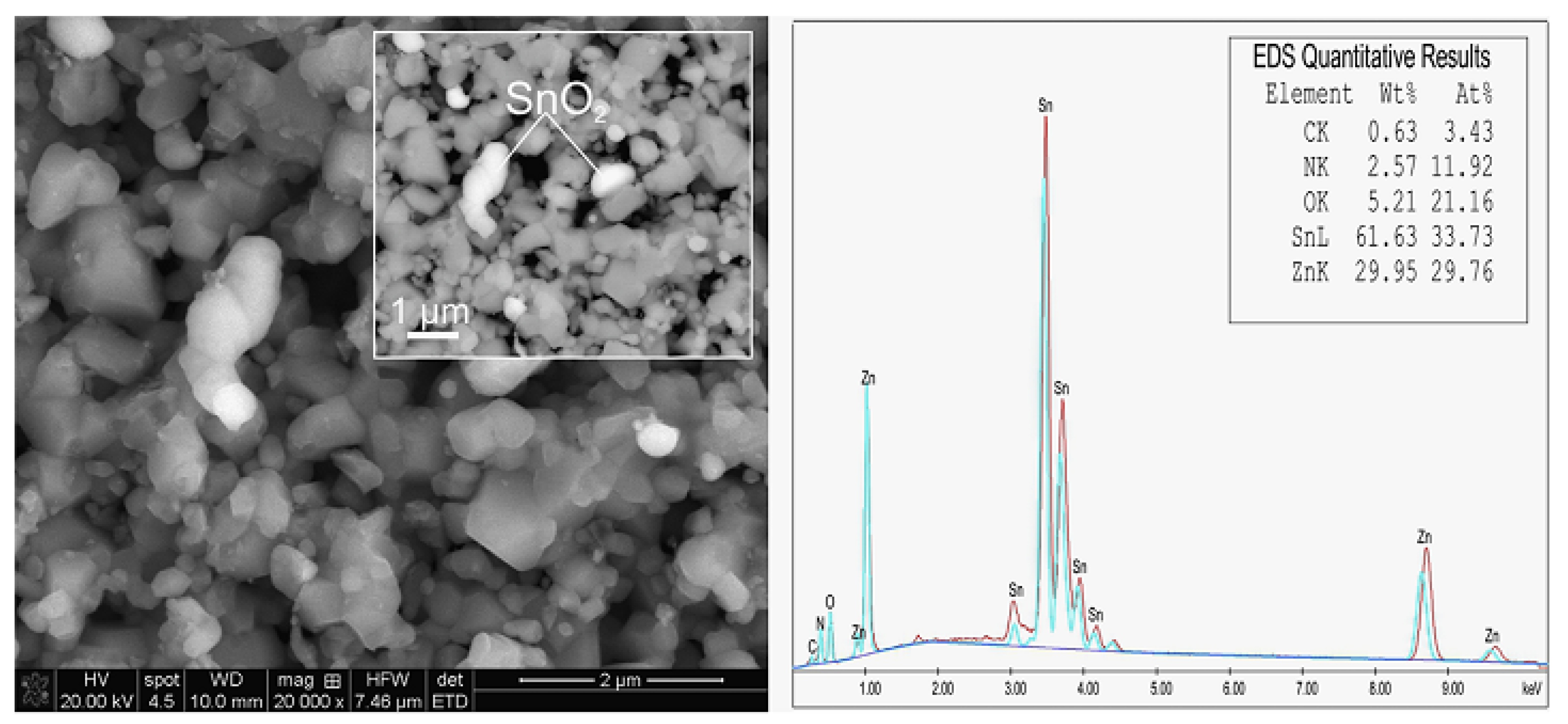
3.2. SnO2-ZnO-Based Pellet-Type Composite Chemiresistors Used for Selective Detection of CO
- for C3H8, it is 2.8 times lower than the sensor response to CO;
- for CH4, it is 5 times lower than the sensor response to CO.
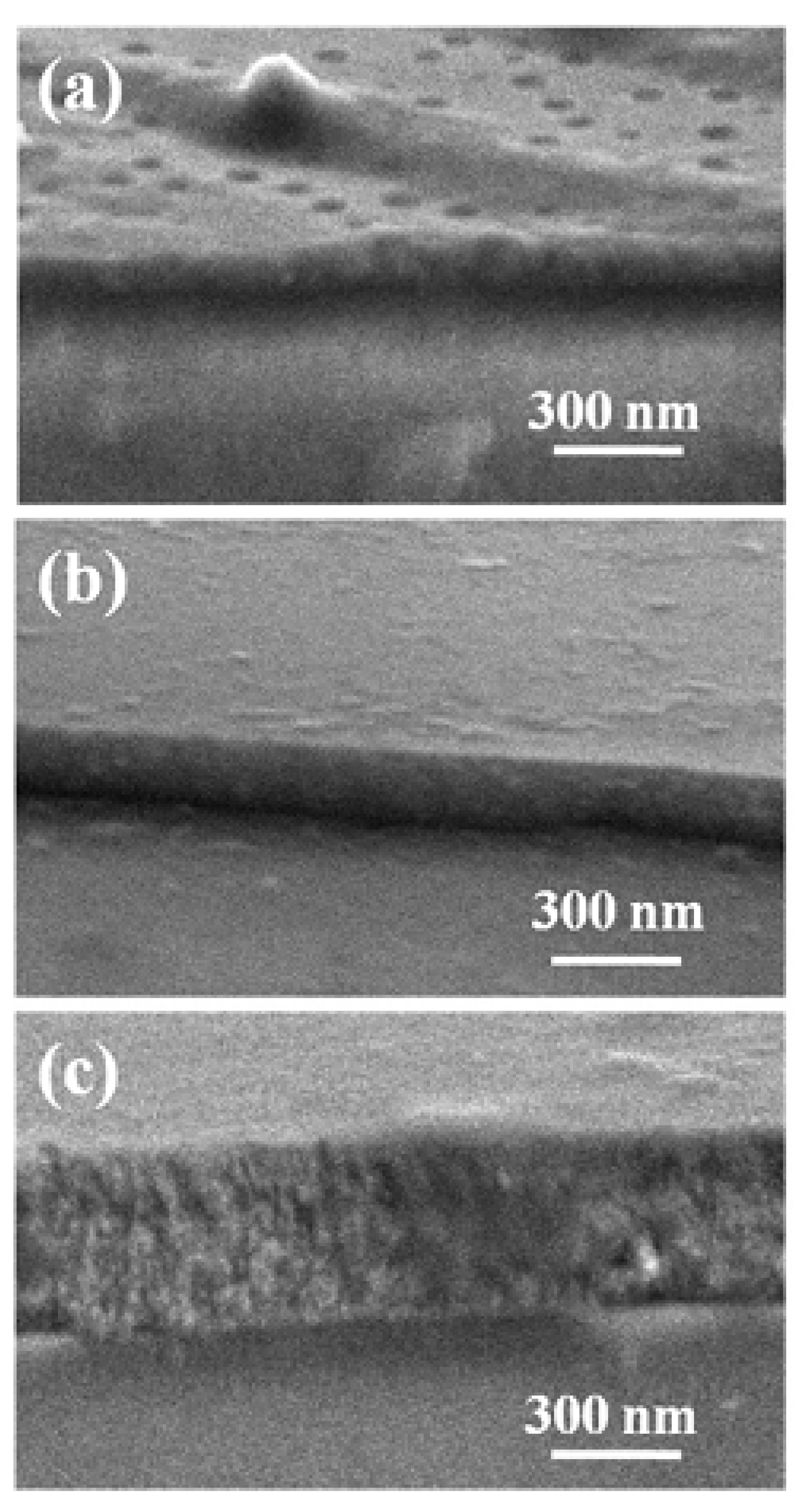
3.3. Sol-Gel Based Thin Film Sensitive Materials Used in Gas Sensors
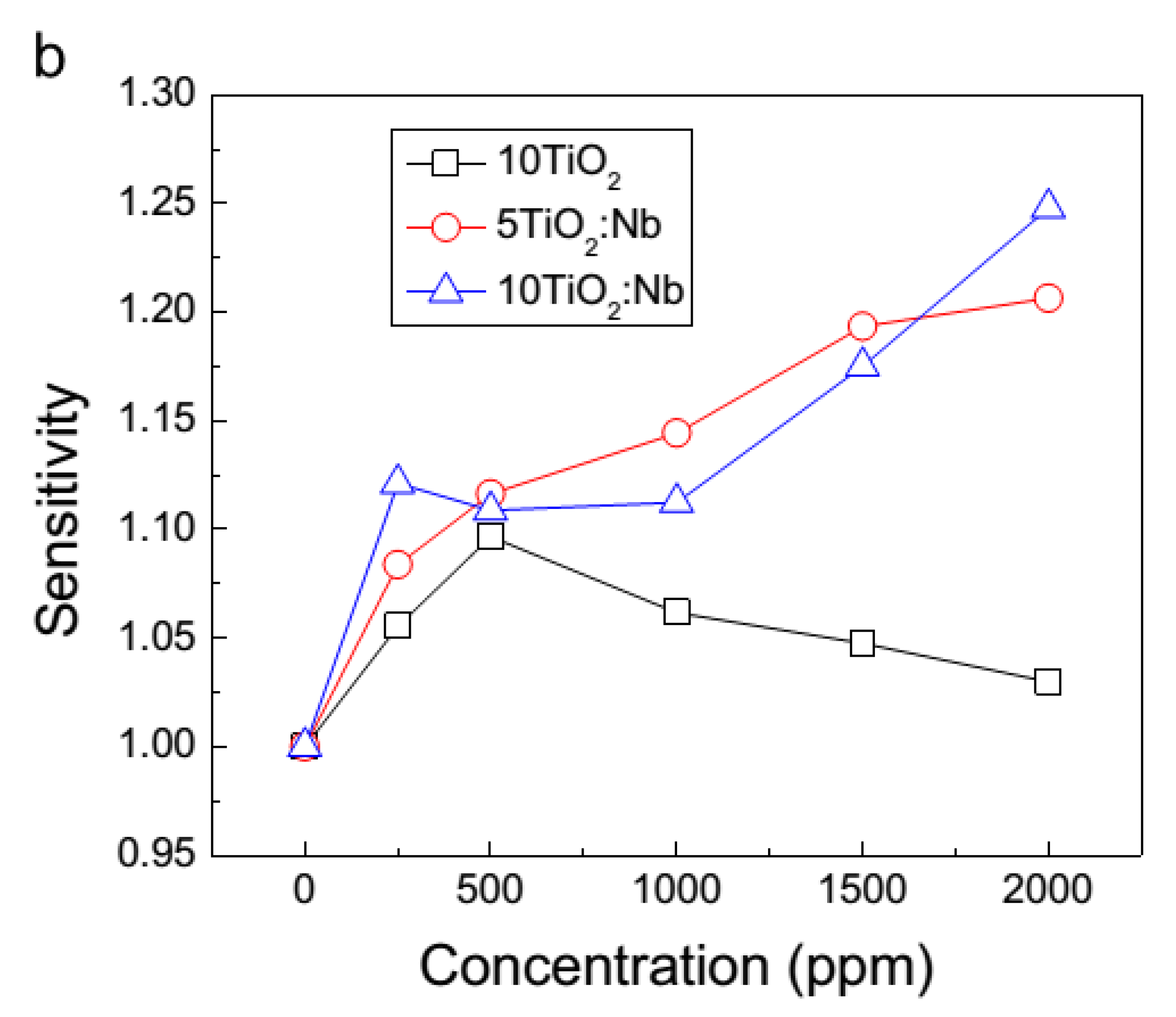
3.3.1. Nb-Doped TiO2 Thin Film Sensors Used for Detection of CO
3.3.2. SnO2-ZnO-Based Thin Film Composite Chemiresistors for Selective Detection of CO
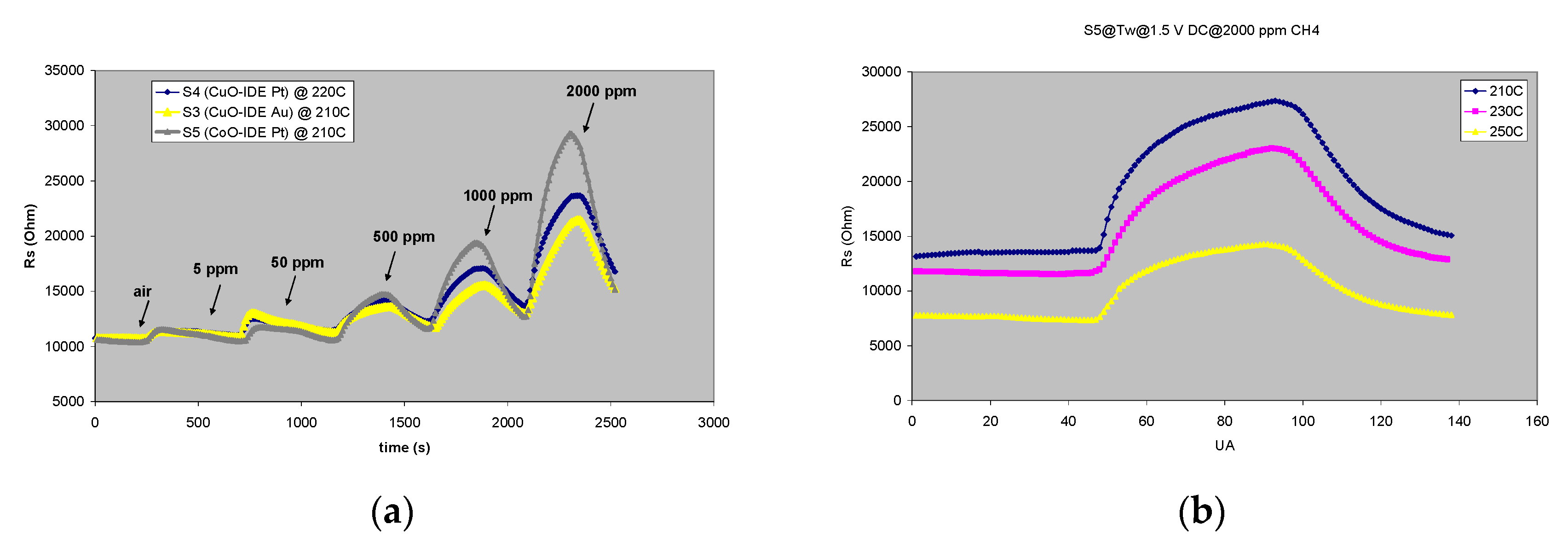
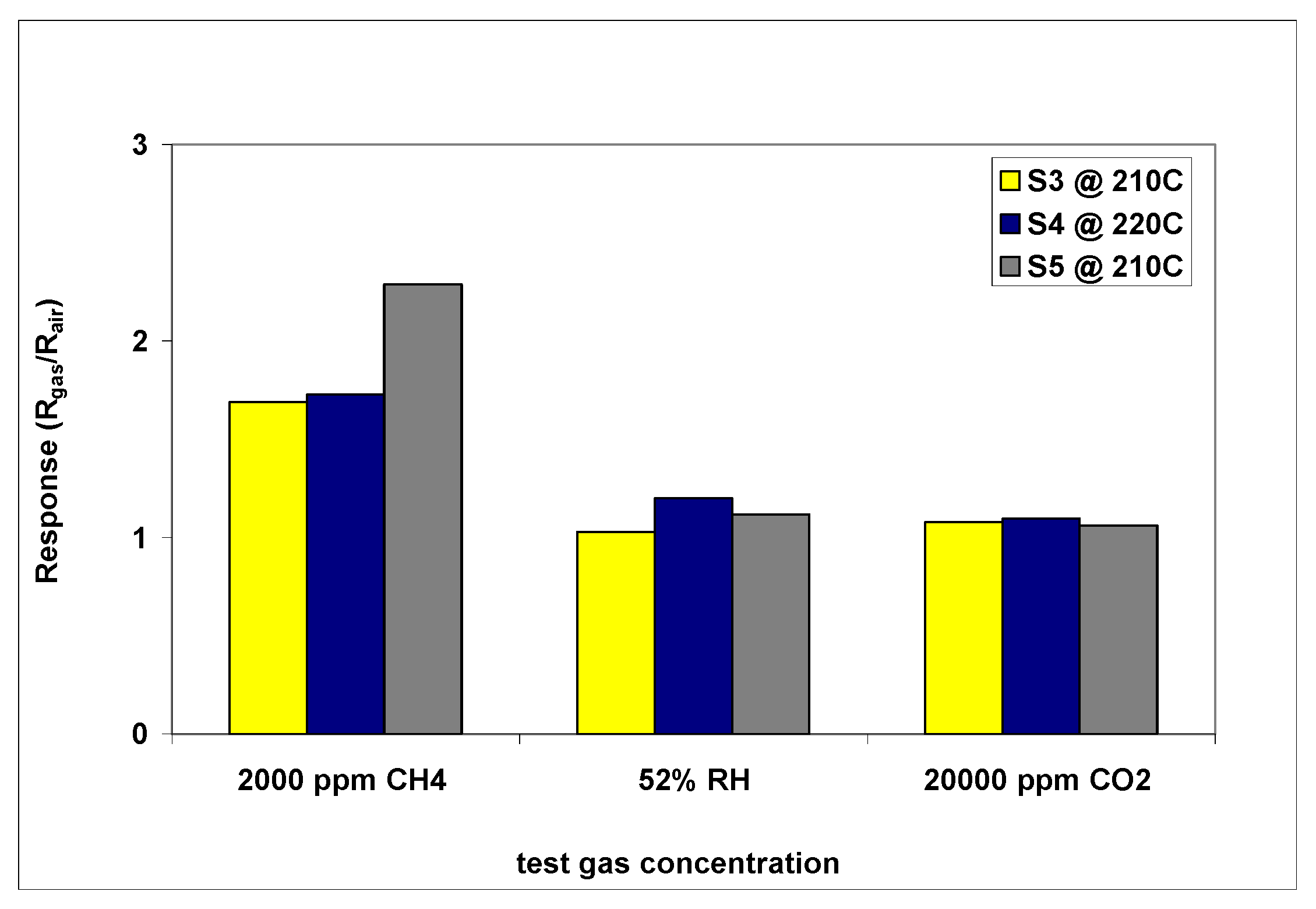
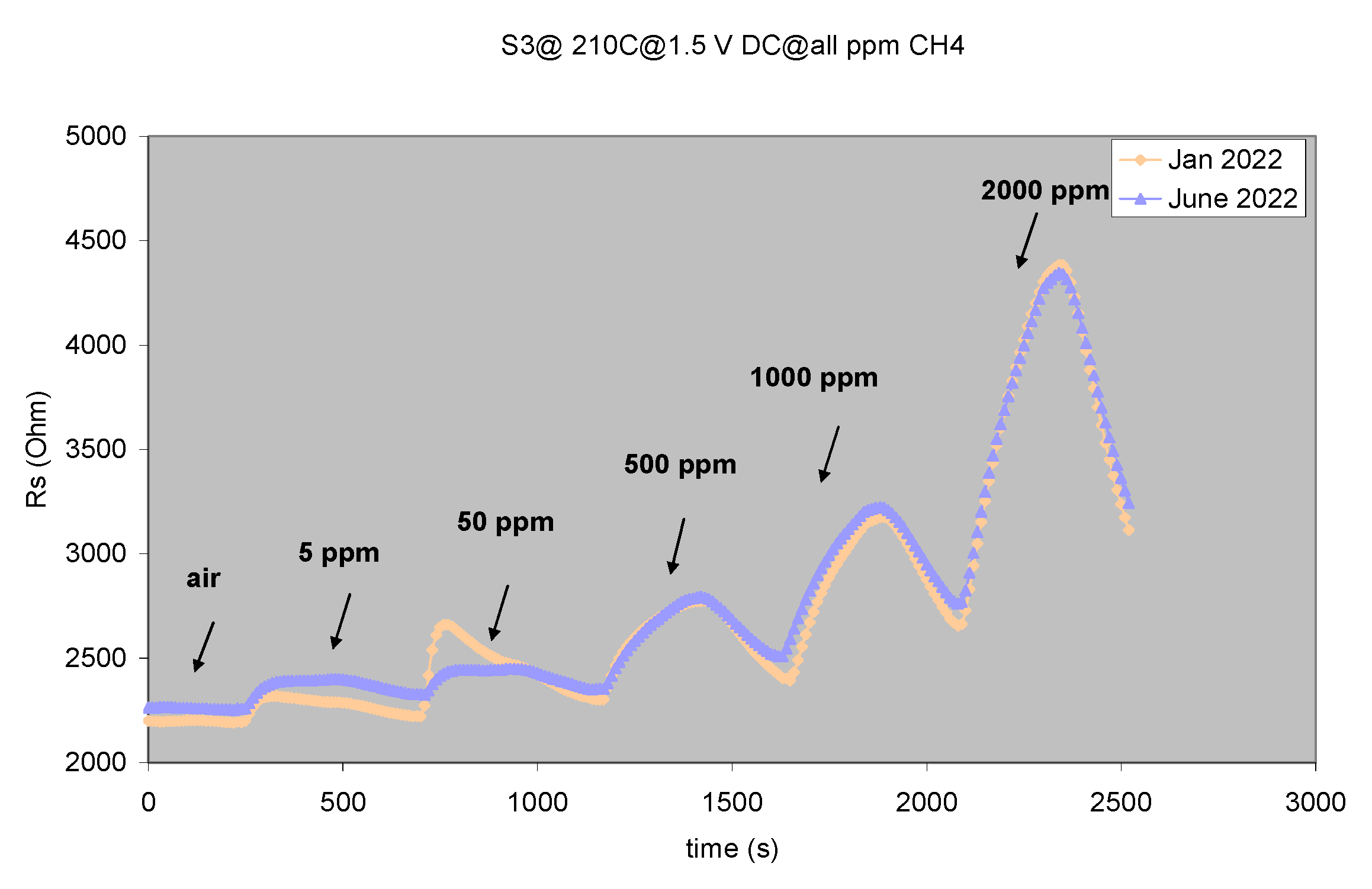
3.3.3. CoO Thin Film Chemiresistors Used for Selective Detection of CH4
3.4. Real-Life Applications of Chemiresistors
- high sensitivity, selectivity, and stability;
- fast response/recovery characteristics;
- a low power consumption;
- a low operating temperature.
3.4.1. Automotive Sensors
3.4.2. Environmental Sensors
- “PVD is an expensive and time consuming method, due to the high vacuum conditions and evaporation/sputtering equipment, therefore economic factors restrict the commercial applications for sensors obtained in such way;
- CVD produces dense films which is a disadvantage for gas sensors as the contribution from the bulk resistance is substantially increased;
- Sol-gel methods are straightforward to operate, but the time required to establish the sol is important for obtaining the desired product, thus can be a slow multi-step process. Full coverage of the substrate with moderately even thickness can be achieved, by using readily available precursors, although these are often expensive. Dopants may be easily introduced and the sol-gel process has low processing temperatures;
- Screen-printing is an industry standard technique. Thick films leads to much more reliable sensor devices than thin film types. Thin film devices are often more sensitive, although not necessarily more selective, but suffer from high baseline resistance due to a lower overall number of charge carriers whether in the bulk or on the surface of the material”.
3.4.3. Medical Sensors
3.5. Latest Concepts and Developments in Gas Sensor Design
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atmosphere of Earth. Available online: https://en.wikipedia.org/wiki/Atmosphere_of_Earth (accessed on 23 December 2022).
- CO Health Risks. Available online: https://www.detectcarbonmonoxide.com/co-health-risks (accessed on 23 December 2022).
- Fraden, J. Handbook of Modern Sensors; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-1-4419-6465-6. [Google Scholar]
- Kappler, J.T. Characterisation of High-Performance SnO2 Gas Sensors for CO Detection by In Situ Techniques; Shaker: Düren, Germany, 2001; ISBN 3826590406. [Google Scholar]
- Ripka, P.; Tipek, A. Modern Sensors Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 111861478X. [Google Scholar]
- Shirke, A.G. Sub-Femtomolar Isothermal Desorption and Reaction Kinetics on Microhotplate Sensor Platforms. Ph.D. Dissertation, The University of Maine, Orono, ME, USA, 2007. [Google Scholar]
- Lipert, R.J.; Shinar, R.; Vaidya, B.; Pris, A.D.; Porter, M.D.; Liu, G.; Grabau, T.D.; Dilger, J.P. Thin Films of Block Copolymer Blends for Enhanced Performance of Acoustic Wave-Based Chemical Sensors. Anal. Chem. 2002, 74, 6383–6391. [Google Scholar] [CrossRef] [PubMed]
- Chesler, P.; Vladut, A. Senzori Chimici Rezistivi; Editura Berg: Sălaj, Romania, 2020; ISBN 978-606-9036-60-0. CIP 2020-14467. [Google Scholar]
- Chesler, P. Oxizi cu Proprietăţi de Senzori de Gaze. Doctoral Dissertation, Institutul de Chimie Fizică Ilie Murgulescu, Bucureşti, Romania, 2017. [Google Scholar] [CrossRef]
- Rydosz, A. The Use of Copper Oxide Thin Films in Gas-Sensing Applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef]
- Wagner, C.; Hauffe, K. The Stationary State of Catalysts in Homogeneous Reactions. Ztschr. Elektrochem. 1938, 33, 172. [Google Scholar]
- Ciureanu, P.; Middelhoek, S. Thin Film Resistive Sensors; CRC Press: Boca Raton, FL, USA, 1992; ISBN 0750301732. [Google Scholar]
- Brattain, W.H.; Bardeen, J. Surface Properties of Germanium. Bell Syst. Tech. J. 1953, 32, 1–41. [Google Scholar] [CrossRef]
- Heiland, G. Zum Einfluß von Adsorbiertem Sauerstoff Auf Die Elektrische Leitfähigkeit von Zinkoxydkristallen. Zeitschrift Phys. 1954, 138, 459–464. [Google Scholar] [CrossRef]
- Morrison, S.R. Surface Barrier Effects in Adsorption, Illustrated by Zinc Oxide. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1955; Volume 7, pp. 259–301. ISBN 0360-0564. [Google Scholar]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A New Detector for Gaseous Components Using Semiconductive Thin Films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Shaver, P.J. Activated Tungsten Oxide Gas Detectors. Appl. Phys. Lett. 1967, 11, 255–257. [Google Scholar] [CrossRef]
- Taguchi, N. Method for Making a Gas-Sensing Element. U.S. Patent No. 3,625,756, 07 December 1971. [Google Scholar]
- Madou, M.J.; Morrison, S.R. Chemical Sensing with Solid State Devices; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 032313985X. [Google Scholar]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- TGS 813 Figaro. Available online: https://www.soselectronic.ro/products/figaro/tgs-813-483 (accessed on 23 December 2022).
- TGS 826-A00 Figaro. Available online: https://www.soselectronic.ro/products/figaro/tgs-826-a00-53106 (accessed on 23 December 2022).
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide Materials for Development of Integrated Gas Sensors—A Comprehensive Review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Mondal, B.; Basumatari, B.; Das, J.; Roychaudhury, C.; Saha, H.; Mukherjee, N. ZnO–SnO2 Based Composite Type Gas Sensor for Selective Hydrogen Sensing. Sens. Actuators B Chem. 2014, 194, 389–396. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly Sensitive and Selective Gas Sensors Using p-Type Oxide Semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Šutka, A.; Gross, K.A. Spinel Ferrite Oxide Semiconductor Gas Sensors. Sens. Actuators B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Li, Y.X.; Wlodarski, W.; Ghantasala, M.K. Sensitivity Enhancement towards Ethanol and Methanol of TiO2 Films Doped with Pt and Nb. Sens. Actuators B Chem. 2000, 64, 169–174. [Google Scholar] [CrossRef]
- Caldararu, M.; Munteanu, C.; Chesler, P.; Carata, M.; Hornoiu, C.; Ionescu, N.I.; Postole, G.; Bratan, V. Supported Oxides as Combustion Catalysts and as Humidity Sensors. Tuning the Surface Behavior by Inter-Phase Charge Transfer. Microporous Mesoporous Mater. 2007, 99, 126–131. [Google Scholar] [CrossRef]
- Caldararu, M.; Scurtu, M.; Hornoiu, C.; Munteanu, C.; Blasco, T.; López Nieto, J.M. Electrical Conductivity of a MoVTeNbO Catalyst in Propene Oxidation Measured in Operando Conditions. Catal. Today 2010, 155, 311–318. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C.; Bratan, V.; Munteanu, C.; Gartner, M.; Ionescu, N.I. Carbon Monoxide Sensing Properties of TiO2. Rev. Roum. Chim. 2015, 60, 227–232. [Google Scholar]
- Prockop, L.D.; Chichkova, R.I. Carbon Monoxide Intoxication: An Updated Review. J. Neurol. Sci. 2007, 262, 122–130. [Google Scholar] [CrossRef]
- Cobb, N.; Ra, E. UNintentional Carbon Monoxide—Related Deaths in the United States, 1979 through 1988. JAMA 1991, 266, 659–663. [Google Scholar] [CrossRef]
- Runyan, C.W.; Johnson, R.M.; Yang, J.; Waller, A.E.; Perkis, D.; Marshall, S.W.; Coyne-Beasley, T.; McGee, K.S. Risk and Protective Factors for Fires, Burns, and Carbon Monoxide Poisoning in US Households. Am. J. Prev. Med. 2005, 28, 102–108. [Google Scholar] [CrossRef]
- Varon, J.; Marik, P.E.; Fromm, R.E., Jr.; Gueler, A. Carbon Monoxide Poisoning: A Review for Clinicians. J. Emerg. Med. 1999, 17, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Ginwalla, A.; Hogg, B.; Patton, B.R.; Chwieroth, B.; Liang, Z.; Gouma, P.; Mills, M.; Akbar, S. Interaction of Carbon Monoxide with Anatase Surfaces at High Temperatures: Optimization of a Carbon Monoxide Sensor. J. Phys. Chem. B 1999, 103, 4412–4422. [Google Scholar] [CrossRef]
- Bratan, V.; Chesler, P.; Hornoiu, C.; Scurtu, M.; Postole, G.; Pietrzyk, P.; Gervasini, A.; Auroux, A.; Ionescu, N.I. In Situ Electrical Conductivity Study of Pt-Impregnated VOx/Gamma-Al2O3 Catalysts in Propene Deep Oxidation. J. Mater. Sci. 2020, 55, 10466–10481. [Google Scholar] [CrossRef]
- Budiman, H.; Wibowo, R.; Zuas, O.; Gunlazuardi, J. Effect of Annealing Temperature on the Characteristic of Reduced Highly Ordered TiO2 nanotube Arrays and Their CO Gas-Sensing Performance. Process. Appl. Ceram. 2021, 15, 417–427. [Google Scholar] [CrossRef]
- Vasile, A.; Bratan, V.; Hornoiu, C.; Caldararu, M.; Ionescu, N.I.; Yuzhakova, T.; Rédey, Á. Electrical and Catalytic Properties of Cerium–Tin Mixed Oxides in CO Depollution Reaction. Appl. Catal. B Environ. 2013, 140–141, 25–31. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C.; Bratan, V.; Munteanu, C.; Postole, G.; Ionescu, N.I.; Juzsakova, T.; Redey, A.; Gartner, M. CO Sensing Properties of SnO2–CeO2 Mixed Oxides. React. Kinet. Mech. Catal. 2016, 117, 551–563. [Google Scholar] [CrossRef]
- Harrison, P.G.; Ball, I.K.; Azelee, W.; Daniell, W.; Goldfarb, D. Nature and Surface Redox Properties of Copper(II)-Promoted Cerium(IV) Oxide CO-Oxidation Catalysts. Chem. Mater. 2000, 12, 3715–3725. [Google Scholar] [CrossRef]
- Stošić, D.; Bennici, S.; Rakić, V.; Auroux, A. CeO2–Nb2O5 Mixed Oxide Catalysts: Preparation, Characterization and Catalytic Activity in Fructose Dehydration Reaction. Catal. Today 2012, 192, 160–168. [Google Scholar] [CrossRef]
- Yuzhakova, T.; Rakić, V.; Guimon, C.; Auroux, A. Preparation and Characterization of Me2O3−CeO2 (Me = B, Al, Ga, In) Mixed-Oxide Catalysts. Chem. Mater. 2007, 19, 2970–2981. [Google Scholar] [CrossRef]
- Vasile, A.; Caldararu, M.; Hornoiu, C.; Bratan, V.; Ionescul, N.I.; Yuzhakova, T.; Redey, A. Elimination of Gas Pollutants Using SnO2-CeO2 Catalysts. Environ. Eng. Manag. J. 2012, 11, 481–486. [Google Scholar] [CrossRef]
- Yuzhakova, T.; Rédey, Á.; Vasile, A.; Hornoiu, C.; Bratan, V.; Utasi, A.; Kovacs, J. Thermal Stability and Surface Structure of SnO2-CeO2 Impregnated Catalysts. Environ. Eng. Manag. J. 2012, 11, 225–230. [Google Scholar] [CrossRef]
- Durrani, S.M.A.; Al-Kuhaili, M.F.; Bakhtiari, I.A. Carbon Monoxide Gas-Sensing Properties of Electron-Beam Deposited Cerium Oxide Thin Films. Sens. Actuators B Chem. 2008, 134, 934–939. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem. 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Barreca, D.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E.; Comini, E.; Sberveglieri, G. Columnar CeO2 Nanostructures for Sensor Application. Nanotechnology 2007, 18, 125502. [Google Scholar] [CrossRef]
- Khawaja, E.E.; Durrani, S.M.A.; Al-Kuhaili, M.F. Determination of Average Refractive Index of Thin CeO2 Films with Large Inhomogeneities. J. Phys. D Appl. Phys. 2003, 36, 545. [Google Scholar] [CrossRef]
- Izu, N.; Shin, W.; Murayama, N.; Kanzaki, S. Resistive Oxygen Gas Sensors Based on CeO2 Fine Powder Prepared Using Mist Pyrolysis. Sensors Actuators B Chem. 2002, 87, 95–98. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Beie, H.-J.; Gnörich, A. Oxygen Gas Sensors Based on CeO2 Thick and Thin Films. Sens. Actuators B Chem. 1991, 4, 393–399. [Google Scholar] [CrossRef]
- Sharma, R.K.; Chan, P.C.H.; Tang, Z.; Yan, G.; Hsing, I.-M.; Sin, J.K.O. Sensitive, Selective and Stable Tin Dioxide Thin-Films for Carbon Monoxide and Hydrogen Sensing in Integrated Gas Sensor Array Applications. Sens. Actuators B Chem. 2001, 72, 160–166. [Google Scholar] [CrossRef]
- Mandayo, G.G.; Castaño, E.; Gracia, F.J.; Cirera, A.; Cornet, A.; Morante, J.R. Strategies to Enhance the Carbon Monoxide Sensitivity of Tin Oxide Thin Films. Sens. Actuators B Chem. 2003, 95, 90–96. [Google Scholar] [CrossRef]
- Park, S.-S.; Mackenzie, J.D. Thickness and Microstructure Effects on Alcohol Sensing of Tin Oxide Thin Films. Thin Solid Films 1996, 274, 154–159. [Google Scholar] [CrossRef]
- Fang, G.; Liu, Z.; Liu, C.; Yao, K. Room Temperature H2S Sensing Properties and Mechanism of CeO2–SnO2 Sol–Gel Thin Films. Sens. Actuators B Chem. 2000, 66, 46–48. [Google Scholar] [CrossRef]
- Figaj, M.; Becker, K.D. An Electron Paramagnetic Resonance Study of Impurities in Ceria, CeO2. Solid State Ion. 2001, 141–142, 507–512. [Google Scholar] [CrossRef]
- Taylor, K.C. Nitric Oxide Catalysis in Automotive Exhaust Systems. Catal. Rev. Eng. 1993, 35, 457–481. [Google Scholar] [CrossRef]
- Yuzhakova, T.; Caldararu, M.; Kovács, J.; Postole, G.; Hornoiu, C.; Vasile, A.; Domokos, E. SnO2-CeO2 Oxidation Catalysts. Environ. Eng. Manag. J. 2009, 8, 1403–1406. [Google Scholar] [CrossRef]
- Munteanu, C.; Caldararu, M.; Bratan, V.; Yetisemiyen, P.; Karakas, G.; Ionescu, N.I. Electrical and Catalytic Properties of SnO2/TiO2 Measured in Operando Conditions. React. Kinet. Mech. Catal. 2012, 105, 13–22. [Google Scholar] [CrossRef]
- Dascalu, I.; Culita, D.; Calderon-Moreno, J.M.; Osiceanu, P.; Hornoiu, C.; Anastasescu, M.; Somacescu, S.; Gartner, M. Structural, Textural, Surface Chemistry and Sensing Properties of Mesoporous Pr, Zn Modified SnO2–TiO2 Powder Composites. Ceram. Int. 2016, 42, 14992–14998. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Zhang, S.; Yan, S.; Cao, B.; Wang, Z.; Fu, Y. Enhanced NH3 Gas-Sensing Performance of Silica Modified CeO2 Nanostructure Based Sensors. Sens. Actuators B Chem. 2018, 255, 862–870. [Google Scholar] [CrossRef]
- Gu, C.; Qi, R.; Wei, Y.; Zhang, X. Preparation and Performances of Nanorod-like Inverse CeO2-CuO Catalysts Derived from Ce-1,3,5-Benzene Tricarboxylic Acid for CO Preferential Oxidation. React. Kinet. Mech. Catal. 2018, 124, 651–667. [Google Scholar] [CrossRef]
- Zhao, R.; Tang, D.; Wu, Q.; Li, W.; Zhang, X.; Guo, R.; Chen, M.; Diao, G. Double-Shell SnO2/CeO2: Yb, Er Hollow Nanospheres as an Assistant Layer That Suppresses Charge Recombination in Dye-Sensitized Solar Cells. New J. Chem. 2018, 42, 14453–14458. [Google Scholar] [CrossRef]
- Khodadadi, A.; Mohajerzadeh, S.S.; Mortazavi, Y.; Miri, A.M. Cerium Oxide/SnO2-Based Semiconductor Gas Sensors with Improved Sensitivity to CO. Sens. Actuators B Chem. 2001, 80, 267–271. [Google Scholar] [CrossRef]
- Vendrell, X.; Kubyshin, Y.; Mestres, L.; Llorca, J. CO Oxidation on Ceria Studied by Electrochemical Impedance Spectroscopy. ChemCatChem 2020, 12, 59265931. [Google Scholar] [CrossRef]
- Leangtanom, P.; Wisitsoraat, A.; Jaruwongrungsee, K.; Chanlek, N.; Phanichphant, S.; Kruefu, V. Highly Sensitive and Selective Ethylene Gas Sensors Based on CeOx-SnO2 Nanocomposites Prepared by a Co-Precipitation Method. Mater. Chem. Phys. 2020, 254, 123540. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, L.X.; Xing, Y.; Zhang, P.; Chen, J.J.; Yin, J.; Bie, L.J. Morphology-Controlled Synthesis of CeO2 Nanocrystals and Their Facet-Dependent Gas Sensing Properties. Sens. Actuators B Chem. 2021, 330, 129374. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C.; Mihaiu, S.; Munteanu, C.; Gartner, M. Tin–Zinc Oxide Composite Ceramics for Selective CO Sensing. Ceram. Int. 2016, 42, 16677–16684. [Google Scholar] [CrossRef]
- Lee, D.-D.; Sohn, B.-K.; Ma, D.-S. Low Power Thick Film CO Gas Sensors. Sens. Actuators 1987, 12, 441–447. [Google Scholar] [CrossRef]
- Li, G.-J.; Zhang, X.-H.; Kawi, S. Relationships between Sensitivity, Catalytic Activity, and Surface Areas of SnO2 Gas Sensors. Sens. Actuators B Chem. 1999, 60, 64–70. [Google Scholar] [CrossRef]
- NIOSH. NIOSH Recommendations for Occupational Safety and Health; US Department of Health and Human Services, Public Health Service Centers: Washington, DC, USA, 1992.
- Yu, J.H.; Choi, G.M. Electrical and CO Gas Sensing Properties of ZnO–SnO2 Composites. Sens. Actuators B Chem. 1998, 52, 251–256. [Google Scholar]
- Hemmati, S.; Anaraki Firooz, A.; Khodadadi, A.A.; Mortazavi, Y. Nanostructured SnO2–ZnO Sensors: Highly Sensitive and Selective to Ethanol. Sens. Actuators B Chem. 2011, 160, 1298–1303. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Jones, P.R.H.; Ratcliffe, N.M.; Wat, R.K.M. Study of the Catalytic and Vapour-Sensing Properties of Zinc Oxide and Tin Dioxide in Relation to 1-Butanol and Dimethyldisulphide. Sens. Actuators B Chem. 1999, 61, 199–207. [Google Scholar] [CrossRef]
- Sonker, R.K.; Sharma, A.; Shahabuddin, M.; Tomar, M.; Gupta, V. Low Temperature Sensing of NO2 Gas Using SnO2-ZnO Nanocomposite Sensor. Adv. Mat. Lett 2013, 4, 196–201. [Google Scholar]
- Nanto, H.; Morita, T.; Habara, H.; Kondo, K.; Douguchi, Y.; Minami, T. Doping Effect of SnO2 on Gas Sensing Characteristics of Sputtered ZnO Thin Film Chemical Sensor. Sens. Actuators B Chem. 1996, 36, 384–387. [Google Scholar] [CrossRef]
- Raju, A.R.; Rao, C.N.R. Gas-Sensing Characteristics of ZnO and Copper-Impregnated ZnO. Sens. Actuators B Chem. 1991, 3, 305–310. [Google Scholar] [CrossRef]
- Yu, J.H.; Choi, G.M. Electrical and CO Gas-Sensing Properties of ZnO/SnO2 Hetero-Contact. Sens. Actuators B Chem. 1999, 61, 59–67. [Google Scholar] [CrossRef]
- Egashira, M.; Shimizu, Y.; Takao, Y.; Sako, S. Variations in I–V Characteristics of Oxide Semiconductors Induced by Oxidizing Gases. Sens. Actuators B Chem. 1996, 35, 62–67. [Google Scholar] [CrossRef]
- Yoon, D.H.; Yu, J.H.; Choi, G.M. CO Gas Sensing Properties of ZnO–CuO Composite. Sens. Actuators B Chem. 1998, 46, 15–23. [Google Scholar] [CrossRef]
- Mao, W.; Li, Z.; Bao, K.; Zhang, K.; Wang, W.; Li, B. Nanowire-Based Zinc-Doped Tin Oxide Microtubes for Enhanced Solar Energy Utilization Efficiency. Ceram. Int. 2017, 43, 6822–6830. [Google Scholar] [CrossRef]
- Dascalu, I.; Somacescu, S.; Hornoiu, C.; Calderon-Moreno, J.M.; Stanica, N.; Stroescu, H.; Anastasescu, M.; Gartner, M. Sol-Gel Zn, Fe Modified SnO2 Powders for CO Sensors and Magnetic Applications. Process Saf. Environ. Prot. 2018, 117, 722–729. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Li, T.; Yu, H.; Yang, Y.; Yang, H.; Dong, X. Enhanced NOx Gas Sensing Properties of Cr2O3 Film Modified Ordered Porous ZnO Gas Sensors. Solid State Ion. 2018, 326, 173–182. [Google Scholar] [CrossRef]
- Jiang, R.; Jia, L.; Guo, X.; Zhao, Z.; Du, J.; Wang, X.; Wang, P.; Xing, F. Dimethyl Sulfoxide-Assisted Hydrothermal Synthesis of Co3O4-Based Nanorods for Selective and Sensitive Diethyl Ether Sensing. Sens. Actuators B Chem. 2019, 290, 275–284. [Google Scholar] [CrossRef]
- Jutimoosik, J.; Jantaratana, P.; Yimnirun, R.; Prasatkhetragarn, A. Phase Formation, Morphology and Magnetic Properties of PbTiO3-Fe2O3 Heterostructure Ceramics. Integr. Ferroelectr. 2021, 214, 19–26. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, S.; Yang, Y.; Li, Q.; Li, T.; Zhang, D. Adsorption of Gas Molecules (NH3, C2H6O, C3H6O, CO, H2S) on a Noble Metal (Ag, Au, Pt, Pd, Ru)-Doped MoSe2 Monolayer: A First-Principles Study. New J. Chem. 2021, 45, 12367–12376. [Google Scholar] [CrossRef]
- Huang, C.X.; Chen, G.R.; Nashalian, A.; Chen, J. Advances in Self-Powered Chemical Sensing via a Triboelectric Nanogenerator. Nanoscale 2021, 13, 2065–2081. [Google Scholar] [CrossRef]
- Duta, M.; Predoana, L.; Calderon-Moreno, J.M.; Preda, S.; Anastasescu, M.; Marin, A.; Dascalu, I.; Chesler, P.; Hornoiu, C.; Zaharescu, M.; et al. Nb-Doped TiO2 Sol–Gel Films for CO Sensing Applications. Mater. Sci. Semicond. Process. 2016, 42, 397–404. [Google Scholar] [CrossRef]
- Moon, H.G.; Jang, H.W.; Kim, J.-S.; Park, H.-H.; Yoon, S.-J. Size Effects in the CO Sensing Properties of Nanostructured TiO2 Thin Films Fabricated by Colloidal Templating. Electron. Mater. Lett. 2010, 6, 31–34. [Google Scholar] [CrossRef]
- Seo, M.-H.; Yuasa, M.; Kida, T.; Huh, J.-S.; Shimanoe, K.; Yamazoe, N. Gas Sensing Characteristics and Porosity Control of Nanostructured Films Composed of TiO2 Nanotubes. Sens. Actuators B Chem. 2009, 137, 513–520. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C.; Wang, X. Ni-Doped TiO2 Nanotubes for Wide-Range Hydrogen Sensing. Nanoscale Res. Lett. 2014, 9, 118. [Google Scholar] [CrossRef]
- Ferroni, M.; Carotta, M.C.; Guidi, V.; Martinelli, G.; Ronconi, F.; Richard, O.; Van Dyck, D.; Van Landuyt, J. Structural Characterization of Nb–TiO2 Nanosized Thick-Films for Gas Sensing Application. Sens. Actuators B Chem. 2000, 68, 140–145. [Google Scholar] [CrossRef]
- Furubayashi, Y.; Hitosugi, T.; Yamamoto, Y.; Inaba, K.; Kinoda, G.; Hirose, Y.; Shimada, T.; Hasegawa, T. A Transparent Metal: Nb-Doped Anatase TiO2. Appl. Phys. Lett. 2005, 86, 2101. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z. Impact of Nb Doping on Gas-Sensing Performance of TiO2 Thick-Film Sensors. Sens. Actuators B Chem. 2012, 166–167, 141–149. [Google Scholar] [CrossRef]
- Anukunprasert, T.; Saiwan, C.; Traversa, E. The Development of Gas Sensor for Carbon Monoxide Monitoring Using Nanostructure of Nb–TiO2. Sci. Technol. Adv. Mater. 2005, 6, 359–363. [Google Scholar] [CrossRef]
- Ruiz, A.; Calleja, A.; Espiell, F.; Cornet, A.; Morante, J.R. Nanosized Nb-TiO2 Gas Sensors Derived from Alkoxides Hydrolization. IEEE Sens. J. 2003, 3, 189–194. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, H.; Singh, V.N.; Jain, K.; Senguttuvan, T.D. Highly Sensitive and Pulse-like Response toward Ethanol of Nb Doped TiO2 Nanorods Based Gas Sensors. Sens. Actuators B Chem. 2012, 171, 899–906. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bhatnagar, M.C.; Sharma, G.L. Mechanism in Nb Doped Titania Oxygen Gas Sensor. Sens. Actuators B Chem. 1998, 46, 194–201. [Google Scholar] [CrossRef]
- Gan, L.; Wu, C.; Tan, Y.; Chi, B.; Pu, J.; Jian, L. Oxygen Sensing Performance of Nb-Doped TiO2 Thin Film with Porous Structure. J. Alloys Compd. 2014, 585, 729–733. [Google Scholar] [CrossRef]
- Teleki, A.; Bjelobrk, N.; Pratsinis, S.E. Flame-Made Nb- and Cu-Doped TiO2 Sensors for CO and Ethanol. Sens. Actuators B Chem. 2008, 130, 449–457. [Google Scholar] [CrossRef]
- Zheng, X.W.; Li, Z.Q. Low-Temperature Magnetoresistance of Nb-Doped Transparent Conducting Films. Solid State Commun. 2010, 150, 1625–1628. [Google Scholar] [CrossRef]
- Yamada, N.; Hitosugi, T.; Kasai, J.; Hoang, N.L.H.; Nakao, S.; Hirose, Y.; Shimada, T.; Hasegawa, T. Transparent Conducting Nb-Doped Anatase TiO2 (TNO) Thin Films Sputtered from Various Oxide Targets. Thin Solid Films 2010, 518, 3101–3104. [Google Scholar] [CrossRef]
- Tavares, C.J.; Castro, M.V.; Marins, E.S.; Samantilleke, A.P.; Ferdov, S.; Rebouta, L.; Benelmekki, M.; Cerqueira, M.F.; Alpuim, P.; Xuriguera, E.; et al. Effect of Hot-Filament Annealing in a Hydrogen Atmosphere on the Electrical and Structural Properties of Nb-Doped TiO2 Sputtered Thin Films. Thin Solid Films 2012, 520, 2514–2519. [Google Scholar] [CrossRef]
- Tucker, R.T.; Beckers, N.A.; Fleischauer, M.D.; Brett, M.J. Electron Beam Deposited Nb-Doped TiO2 toward Nanostructured Transparent Conductive Thin Films. Thin Solid Films 2012, 525, 28–34. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Yamauchi, H.; Karppinen, M. Conducting Nb-Doped TiO2 Thin Films Fabricated with an Atomic Layer Deposition Technique. Thin Solid Films 2014, 551, 19–22. [Google Scholar] [CrossRef]
- Yu, C.-F.; Sun, S.-J.; Chen, J.-M. Magnetic and Electrical Properties of TiO2:Nb Thin Films. Appl. Surf. Sci. 2014, 292, 773–776. [Google Scholar] [CrossRef]
- Le Boulbar, E.; Millon, E.; Mathias, J.; Boulmer-Leborgne, C.; Nistor, M.; Gherendi, F.; Sbaï, N.; Quoirin, J.B. Pure and Nb-Doped TiO1.5 Films Grown by Pulsed-Laser Deposition for Transparent p–n Homojunctions. Appl. Surf. Sci. 2011, 257, 5380–5383. [Google Scholar] [CrossRef]
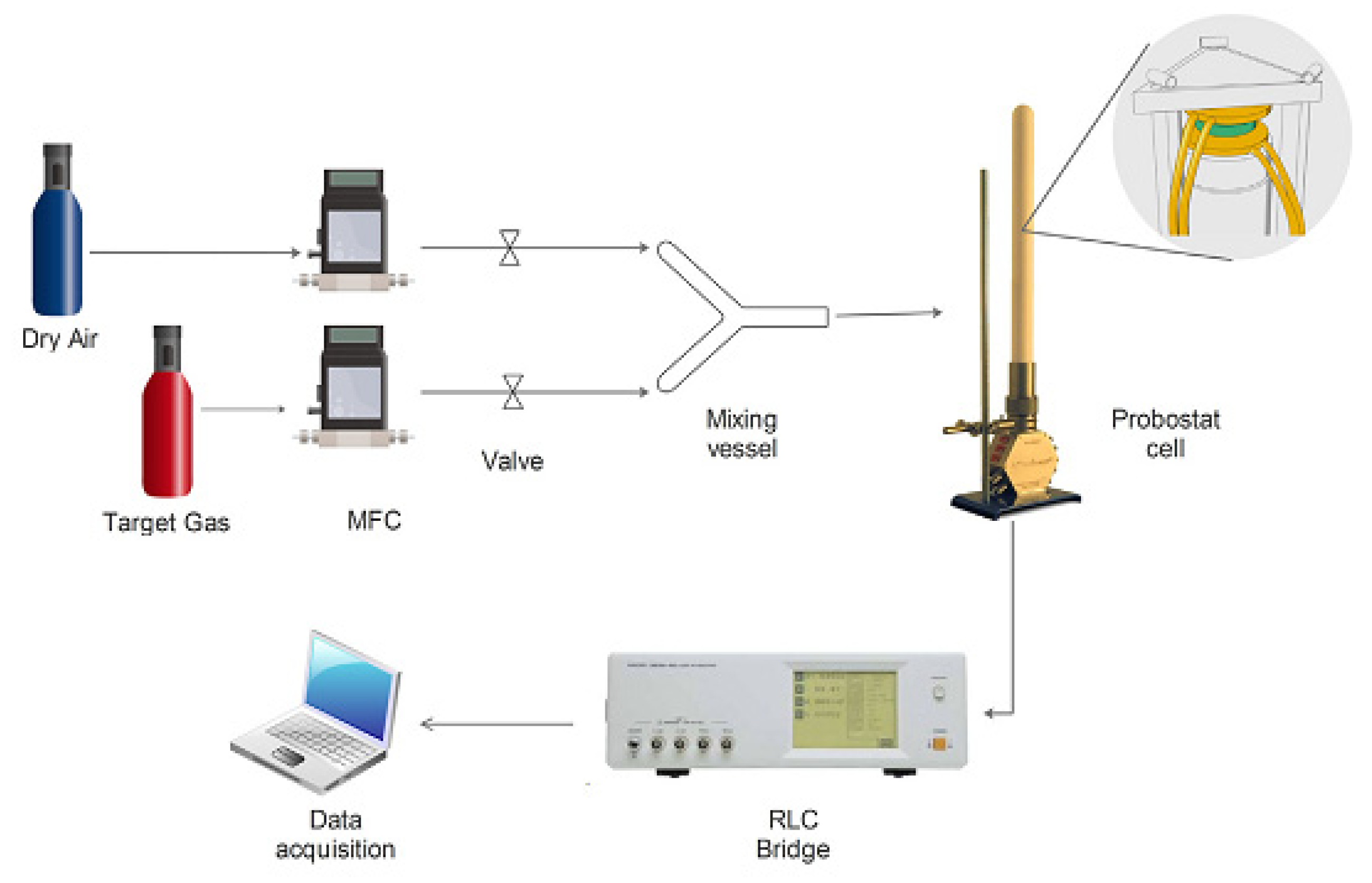
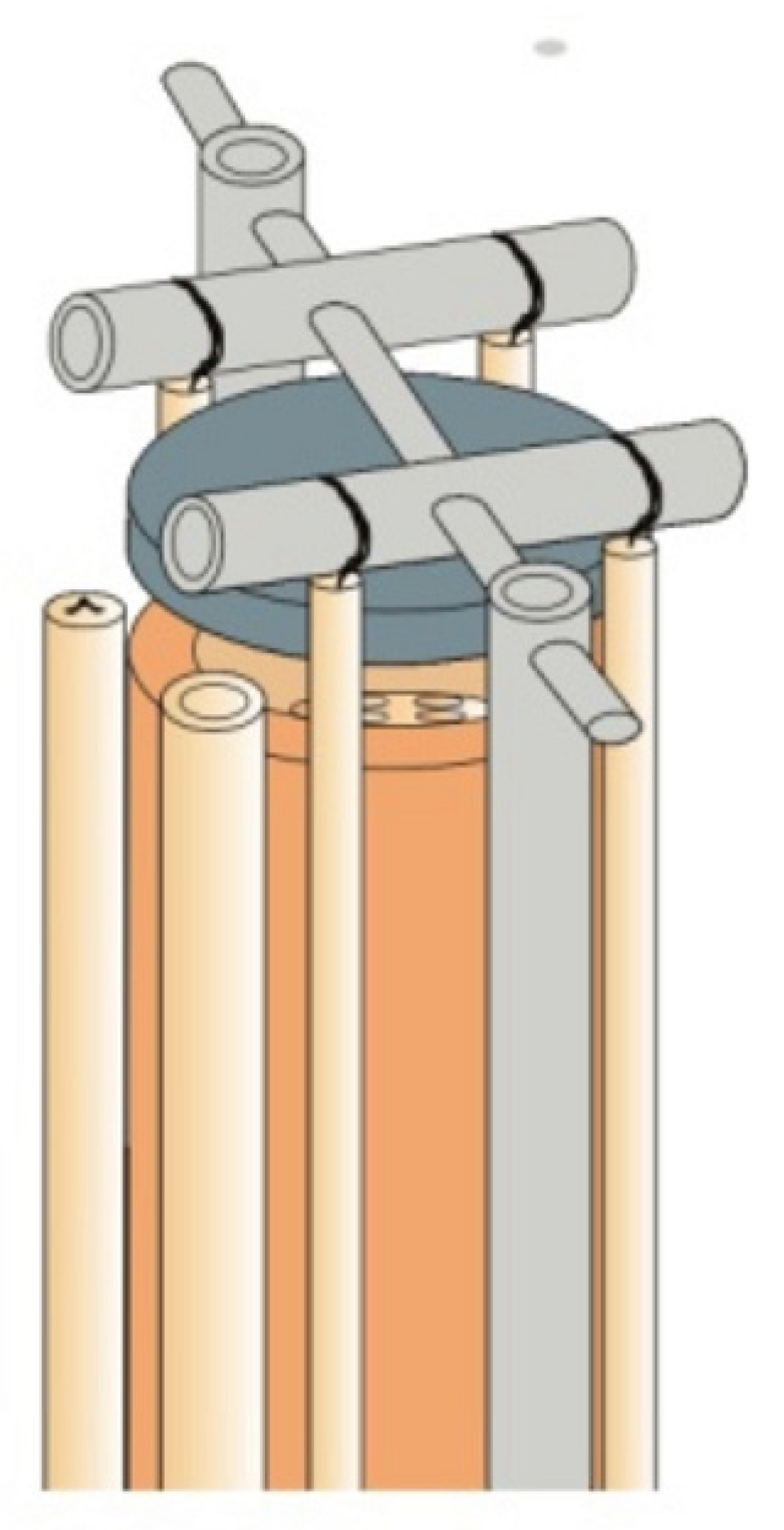
- ProboStat, Overview. Available online: http://www.norecs.com/index.php?page=Overview (accessed on 23 December 2022).
- Nechita, V.; Schoonman, J.; Musat, V. Ethanol and Methanol Sensing Characteristics of Nb-doped TiO2 Porous Thin Films. Phys. Status Solidi 2012, 209, 153–159. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology. IUPAC Recommendations; Blackwell: Oxford, UK, 1997. [Google Scholar]
- Bai, J.; Huang, Y.; Wei, D.; Fan, Z.; Seo, H.J. Synthesis and Characterization of Semiconductor Heterojunctions Based on Zr6Nb2O17 Nanoparticles. Mater. Sci. Semicond. Process. 2020, 112, 105010. [Google Scholar] [CrossRef]
- Gartner, M.; Stoica, M.; Nicolescu, M.; Stroescu, H. The Ellipsometry Versatility in the Study of Sol-Gel Films. J. Sol-Gel Sci. Technol. 2021, 98, 1–23. [Google Scholar] [CrossRef]
- Wategaonkar, S.B.; Parale, V.G.; Pawar, R.P.; Mali, S.S.; Hong, C.K.; Powar, R.R.; Moholkar, A.V.; Park, H.H.; Sargar, B.M.; Mane, R.K. Structural, Morphological, and Optical Studies of Hydrothermally Synthesized Nb-Added TiO2 for DSSC Application. Ceram. Int. 2021, 47, 25580–25592. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Gomes, G.H.M.; de Jesus, M.; Ferlauto, A.S.; Viana, M.M.; Mohallem, N.D.S. Characterization and Application of Niobium-Doped Titanium Dioxide Thin Films Prepared by Sol-Gel Process. Appl. Phys. A Mater. Sci. Process. 2021, 127, 641. [Google Scholar] [CrossRef]
- Khamfoo, K.; Wisitsoraat, A.; Punginsang, M.; Tuantranont, A.; Liewhiran, C. Selectivity towards Acetylene Gas of Flame-Spray-Made Nb-Substituted SnO2 Particulate Thick Films. Sens. Actuators B Chem. 2021, 349, 130808. [Google Scholar] [CrossRef]
- Zhao, S.; Lin, D.X.; Wang, Y.X. The Synthesis and Optical Properties of Zinc-Nitrogen Co-Doped TiO2 Thin Films Using Sol-Gel Derived Spin-Coating Method. J. Nanoelectron. Optoelectron. 2021, 16, 967–973. [Google Scholar] [CrossRef]
- Mathew, S.; John, B.K.; Abraham, T.; Mathew, B. Metal-Doped Titanium Dioxide for Environmental Remediation, Hydrogen Evolution and Sensing: A Review. ChemistrySelect 2021, 6, 12742–12751. [Google Scholar] [CrossRef]
- Solis-Cortazar, J.C.; Zamudio-Torres, I.; Rojas-Blanco, L.; Perez-Hernandez, G.; Arellano-Cortaza, M.; Castillo-Palomera, R.; de los Monteros, A.E.; Ramirez-Morales, E. Photoresponse Enhancement in TiO2 Thin Films by Incorporation Ni and Cr Nanoparticles Using Sol–Gel Method. J. Mater. Sci. Mater. Electron. 2022, 33, 7668–7678. [Google Scholar] [CrossRef]
- Haverkamp, R.G.; Kappen, P.; Sizeland, K.H.; Wallwork, K.S. Niobium K-Edge X-Ray Absorption Spectroscopy of Doped TiO2 Produced from Ilmenite Digested in Hydrochloric Acid. ACS Omega 2022, 7, 28258–28264. [Google Scholar] [CrossRef]
- Gartner, M.; Anastasescu, M.; Calderon-Moreno, J.M.; Nicolescu, M.; Stroescu, H.; Hornoiu, C.; Preda, S.; Predoana, L.; Mitrea, D.; Covei, M. Multifunctional Zn-Doped ITO Sol–Gel Films Deposited on Different Substrates: Application as CO2-Sensing Material. Nanomaterials 2022, 12, 3244. [Google Scholar] [CrossRef] [PubMed]
- Kuranov, D.; Platonov, V.; Khmelevsky, N.; Bozhev, I.; Maksimov, S.; Rumyantseva, M.; Krivetskiy, V. Effect of Nb (V) Doping on the Structure and Oxygen Chemisorption on Nanocrystalline TiO2. ChemistrySelect 2022, 7, e202202644. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Lee, S.Y.; Gwon, J.G.; Vijayakumar, E.; Lee, H.G.; Lee, W.H. Effects of Hydrothermal Treatment of Cellulose Nanocrystal Templated TiO2 Films on Their Photodegradation Activity of Methylene Blue, Methyl Orange, and Rhodamine B. Ceram. Int. 2023, 49, 2911–2922. [Google Scholar] [CrossRef]
- Vega, F.L.A.; Olaya, J.J.; Ruiz, J.B. Synthesis and Corrosion Resistance of SiO2-TiO2-ZrO2-Bi2O3 Coatings Spin-Coated on Ti6Al4V Alloy. Ceram. Int. 2018, 44, 2123–2131. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C.; Mihaiu, S.; Vladut, C.; Moreno, J.M.C.; Anastasescu, M.; Moldovan, C.; Firtat, B.; Brasoveanu, C.; Muscalu, G.; et al. Nanostructured SnO2-ZnO Composite Gas Sensors for Selective Detection of Carbon Monoxide. Beilstein J. Nanotechnol. 2016, 7, 2045–2056. [Google Scholar] [CrossRef]
- Firtat, B.; Moldovan, C.; Brasoveanu, C.; Muscalu, G.; Gartner, M.; Zaharescu, M.; Chesler, P.; Hornoiu, C.; Mihaiu, S.; Vladut, C.; et al. Miniaturised MOX Based Sensors for Pollutant and Explosive Gases Detection. Sens. Actuators B Chem. 2017, 249, 647–655. [Google Scholar] [CrossRef]
- Park, C.O.; Akbar, S.A. Ceramics for Chemical Sensing. J. Mater. Sci. 2003, 38, 4611–4637. [Google Scholar] [CrossRef]
- Huang, X.-J.; Choi, Y.-K. Chemical Sensors Based on Nanostructured Materials. Sens. Actuators B Chem. 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Choi, S.-W.; Katoch, A.; Zhang, J.; Kim, S.S. Electrospun Nanofibers of CuOSnO2 Nanocomposite as Semiconductor Gas Sensors for H2S Detection. Sens. Actuators B Chem. 2013, 176, 585–591. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, I.-D.; Lee, J.-H. Selective and Sensitive Detection of Trimethylamine Using ZnO–In2O3 Composite Nanofibers. Sens. Actuators B Chem. 2013, 181, 463–470. [Google Scholar] [CrossRef]
- Tharsika, T.; Haseeb, A.; Akbar, S.A.; Sabri, M.F.M.; Hoong, W.Y. Enhanced Ethanol Gas Sensing Properties of SnO2-Core/ZnO-Shell Nanostructures. Sensors 2014, 14, 14586–14600. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, S.; Li, Y.; Yang, G.; Mao, Y.; Luo, J.; Gengzang, D.; Xu, X.; Yan, S. Enhanced Ethanol Sensing Performance of Hollow ZnO–SnO2 Core–Shell Nanofibers. Sens. Actuators B Chem. 2015, 211, 392–402. [Google Scholar] [CrossRef]
- Mihaiu, S.; Toader, A.; Atkinson, I.; Mocioiu, O.C.; Hornoiu, C.; Teodorescu, V.S.; Zaharescu, M. Advanced Ceramics in the SnO2–ZnO Binary System. Ceram. Int. 2015, 41, 4936–4945. [Google Scholar] [CrossRef]
- Chelu, M.; Chesler, P.; Anastasescu, M.; Hornoiu, C.; Mitrea, D.; Atkinson, I.; Brasoveanu, C.; Moldovan, C.; Craciun, G.; Gheorghe, M.; et al. ZnO/NiO Heterostructure-Based Microsensors Used in Formaldehyde Detection at Room Temperature: Influence of the Sensor Operating Voltage. J. Mater. Sci. Mater. Electron. 2022, 33, 19998–20011. [Google Scholar] [CrossRef]
- Vladut, C.M.; Mihaiu, S.; Tenea, E.; Preda, S.; Calderon-Moreno, J.M.; Anastasescu, M.; Stroescu, H.; Atkinson, I.; Gartner, M.; Moldovan, C.; et al. Optical and Piezoelectric Properties of Mn-Doped ZnO Films Deposited by Sol-Gel and Hydrothermal Methods. J. Nanomater. 2019, 2019, 6269145. [Google Scholar] [CrossRef]
- Das, A.; Panda, D. SnO2 Tailored by CuO for Improved CH4 Sensing at Low Temperature. Phys. Status Solidi B Basic Solid State Phys. 2019, 256, 1800296. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, M.D.; Wang, Y.G.; Feng, J.H.; Zhang, Y.; Sun, X.; Du, B.; Wei, Q. Label-Free Photoelectrochemical Immunosensor for Amyloid Beta-Protein Detection Based on SnO2/CdCO3/CdS Synthesized by One-Pot Method. Biosens. Bioelectron. 2019, 126, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, K.K.; Cabibihan, J.J.; Deshmukh, K.; Goutham, S.; Abubasha, M.K.; Gogoi, J.P.; Klemenoks, I.; Sakale, G.; Sekhar, B.S.; Sreekanth, P.S.R.; et al. A Review on Porous Polymer Composite Materials for Multifunctional Electronic Applications. Polym. Technol. Mater. 2019, 58, 1253–1294. [Google Scholar] [CrossRef]
- Lai, T.Y.; Fang, T.H.; Hsiao, Y.J.; Chan, C.A. Characteristics of Au-Doped SnO2-ZnO Heteronanostructures for Gas Sensing Applications. Vacuum 2019, 166, 155–161. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Eker, Y.R.; Yilmaz, M. Structural, Optical, Electrical and Room Temperature Gas Sensing Characterizations of Spin Coated Multilayer Cobalt-Doped Tin Oxide Thin Films. Superlattices Microstruct. 2020, 140, 106465. [Google Scholar] [CrossRef]
- Yang, W.J.; Liu, J.J.; Guan, Z.Y.; Liu, Z.H.; Chen, B.H.; Zhao, L.T.; Li, Y.X.; Cao, X.B.; He, X.; Zhang, C.; et al. Morphology, Electrical and Optical Properties of Magnetron Sputtered Porous ZnO Thin Films on Si(100) and Si(111) Substrates. Ceram. Int. 2020, 46, 6605–6611. [Google Scholar] [CrossRef]
- Mousavi, H.; Mortazavi, Y.; Khodadadi, A.A.; Saberi, M.H.; Alirezaei, S. Enormous Enhancement of Pt/SnO2 Sensors Response and Selectivity by Their Reduction, to CO in Automotive Exhaust Gas Pollutants Including CO, NOx and C3H8. Appl. Surf. Sci. 2021, 546, 149120. [Google Scholar] [CrossRef]
- Pandit, N.; Ahmad, T. Tin Oxide Based Hybrid Nanostructures for Efficient Gas Sensing. Molecules 2022, 27, 7038. [Google Scholar] [CrossRef]
- Zaharescu, M.; Anastasescu, M.; Stroescu, H.; Calderon-Moreno, J.M.; Apostol, N.; Preda, S.; Vladut, C.M.; Mihaiu, S.; Petrik, P.; Gartner, M. Comparative Study of the Dopants (Mn vs. V) Influence on the Properties of Sol-Gel ZnO Films. J. Sol-Gel Sci. Technol. 2022, 104, 67–77. [Google Scholar] [CrossRef]
- Nicolescu, M.; Mitrea, D.; Hornoiu, C.; Preda, S.; Stroescu, H.; Anastasescu, M.; Calderon-Moreno, J.; Predoana, L.; Teodorescu, V.; Maraloiu, V.-A.; et al. Structural, Optical, and Sensing Properties of Nb-Doped ITO Thin Films Deposited by the Sol–Gel Method. Gels 2022, 8, 717. [Google Scholar] [CrossRef]
- Kakoty, P.; Das, K. Performance Enhancement of Linalool Sensor with Pt Decorated Composite of ZnO Nanorod and SnO. IEEE Sens. J. 2022, 22, 20223–20229. [Google Scholar] [CrossRef]
- Torres-Tello, J.; Guaman, A.V.; Ko, S.B. Improving the Detection of Explosives in a MOX Chemical Sensors Array with LSTM Networks. IEEE Sens. J. 2020, 20, 14302–14309. [Google Scholar] [CrossRef]
- Wasilewski, T.; Gebicki, J. Emerging Strategies for Enhancing Detection of Explosives by Artificial Olfaction. Microchem. J. 2021, 164, 106025. [Google Scholar] [CrossRef]
- Djedidi, O.; Djeziri, M.A.; Morati, N.; Seguin, J.L.; Bendahan, M.; Contaret, T. Accurate Detection and Discrimination of Pollutant Gases Using a Temperature Modulated MOX Sensor Combined with Feature Extraction and Support Vector Classification. Sens. Actuators B Chem. 2021, 339, 129817. [Google Scholar] [CrossRef]
- Sui, R.H.; Charpentier, P.A.; Marriott, R.A. Metal Oxide-Related Dendritic Structures: Self-Assembly and Applications for Sensor, Catalysis, Energy Conversion and Beyond. Nanomaterials 2021, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Olimov, D.; Yin, L. Semiconductor-Type Gas Sensors Based on γ-Fe2O3 Nanoparticles and Its Derivatives in Conjunction with SnO2 and Graphene. Chemosensors 2022, 10, 267. [Google Scholar] [CrossRef]
- Fioravanti, A.; Morandi, S.; Carotta, M.C. Spectroscopic–Electrical Combined Analysis to Assess the Conduction Mechanisms and the Performances of Metal Oxide Gas Sensors. Chemosensors 2022, 10, 447. [Google Scholar] [CrossRef]
- Khrissi, S.; Lifi, H.; Lifi, M.; Nossir, N.; Tabbai, Y.; Hnawi, S.K. Piezoelectric Vibration Energy Harvesters with Distinct Interdigital Electrodes Used for Toxic Gas Detection and in a Numerical Simulation for a Glucose Sensor Application. Int. J. Sensors Wirel. Commun. Control 2022, 12, 272–280. [Google Scholar]
- Chesler, P.; Hornoiu, C.; Anastasescu, M.; Calderon-Moreno, J.M.; Gheorghe, M.; Gartner, M. Cobalt- and Copper-Based Chemiresistors for Low Concentration Methane Detection, a Comparison Study. Gels 2022, 8, 721. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Abdel-Rahim, M.A. CuO Nanoparticles Synthesized by Microwave-Assisted Method for Methane Sensing. Opt. Quantum Electron. 2016, 48, 531. [Google Scholar] [CrossRef]
- Jayatissa, A.H.; Samarasekara, P.; Kun, G. Methane Gas Sensor Application of Cuprous Oxide Synthesized by Thermal Oxidation. Phys. Status Solidi 2009, 206, 332–337. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Mehrabadi, Z.S.; Esfandyari, J.R.; Koolivand-Salooki, M. Modeling of Cu Doped Cobalt Oxide Nanocrystal Gas Sensor for Methane Detection: ANFIS Approach. J. Chem. Eng. Process Technol. 2012, 3, 124. [Google Scholar]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Pijolat, C.; Pupier, C.; Sauvan, M.; Tournier, G.; Lalauze, R. Gas Detection for Automotive Pollution Control. Sens. Actuators B Chem. 1999, 59, 195–202. [Google Scholar] [CrossRef]
- Cederquist, A.L.; Gibbons, E.F.; Meitzler, A.H. Characterization of Zirconia and Titania Engine Exhaust Gas Sensors for Air/Fuel Feedback Control Systems; SAE Technical Paper; SAE: Pittsburgh, PA, USA, 1976. [Google Scholar]
- Holt, C.T.; Azad, A.-M.; Swartz, S.L.; Rao, R.R.; Dutta, P.K. Carbon Monoxide Sensor for PEM Fuel Cell Systems. Sens. Actuators B Chem. 2002, 87, 414–420. [Google Scholar] [CrossRef]
- Lee, D.-D.; Lee, D.-S. Environmental Gas Sensors. IEEE Sens. J. 2001, 1, 214–224. [Google Scholar]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Marsal, A.; Dezanneau, G.; Cornet, A.; Morante, J.R. A New CO2 Gas Sensing Material. Sens. Actuators B Chem. 2003, 95, 266–270. [Google Scholar] [CrossRef]
- D’Amico, A.; Di Natale, C.; Paolesse, R.; Macagnano, A.; Martinelli, E.; Pennazza, G.; Santonico, M.; Bernabei, M.; Roscioni, C.; Galluccio, G. Olfactory Systems for Medical Applications. Sens. Actuators B Chem. 2008, 130, 458–465. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath Analysis by Nanostructured Metal Oxides as Chemo-Resistive Gas Sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Marczin, N.; Kharitonov, S.; Yacoub, M.; Barnes, P.J. Disease Markers in Exhaled Breath; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Gardner, J.W.; Guha, P.K.; Udrea, F.; Covington, J.A. CMOS Interfacing for Integrated Gas Sensors: A Review. IEEE Sens. J. 2010, 10, 1833–1848. [Google Scholar] [CrossRef]
- Bosch MEMS Sensors. Available online: https://www.bosch-mobility-solutions.com/en/solutions/electronic-components/mems-sensors/ (accessed on 13 January 2023).










| Sensor Applications | |
|---|---|
| Automotive: | Medical: |
|
|
| Food: | Safety: |
|
|
| Environmental: | Industry: |
|
|
| Risc Assessment | CO Concentration | NO2 Concentration |
|---|---|---|
| Low | <10 ppm * | <150 ppb ** |
| Medium | 10–14 ppm | 150–299 ppb |
| High | 15–19 ppm | 300–399 ppb |
| Very high | >20 ppm | >400 ppb |
| Maximum allowed concentration (workspace) | 50 ppm | 5 ppm |
| Imminent life threat | 1200 ppm | 20 ppm |
| Sample | Abbreviation | SBET (m2/g) | Reference |
|---|---|---|---|
| TiO2 | TiO2 | 108.3 | [60] |
| SnO2 | SnO2 | 13 | [40] |
| 5% SnO2-CeO2 | Sn5Ce | 105 | [40] |
| 10% SnO2-CeO2 | Sn10Ce | 99 | [40] |
| 20% SnO2-CeO2 | Sn20Ce | 93 | [40] |
| Sample * | ZnO (wt%) | SnO2 (wt%) |
|---|---|---|
| S1 | 100 | - |
| S2 | 98 | 2 |
| S3 | 50 | 50 |
| S4 | 2 | 98 |
| S5 | - | 100 |
| Sample | Sensitive Film | Transducer Components (IDE/wafer) |
|---|---|---|
| S3 | CuO | Au/Al2O3 |
| S4 | CuO | Pt/Al2O3 |
| S5 | CoO | Pt/Al2O3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chesler, P.; Hornoiu, C. MOX-Based Resistive Gas Sensors with Different Types of Sensitive Materials (Powders, Pellets, Films), Used in Environmental Chemistry. Chemosensors 2023, 11, 95. https://doi.org/10.3390/chemosensors11020095
Chesler P, Hornoiu C. MOX-Based Resistive Gas Sensors with Different Types of Sensitive Materials (Powders, Pellets, Films), Used in Environmental Chemistry. Chemosensors. 2023; 11(2):95. https://doi.org/10.3390/chemosensors11020095
Chicago/Turabian StyleChesler, Paul, and Cristian Hornoiu. 2023. "MOX-Based Resistive Gas Sensors with Different Types of Sensitive Materials (Powders, Pellets, Films), Used in Environmental Chemistry" Chemosensors 11, no. 2: 95. https://doi.org/10.3390/chemosensors11020095
APA StyleChesler, P., & Hornoiu, C. (2023). MOX-Based Resistive Gas Sensors with Different Types of Sensitive Materials (Powders, Pellets, Films), Used in Environmental Chemistry. Chemosensors, 11(2), 95. https://doi.org/10.3390/chemosensors11020095