Er:Y2O3 and Nd:Y2O3 Nanoparticles: Synthesis, Pegylation, Characterization and Study of Their Luminescence Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Er:Y2O3 and Nd:Y2O3
2.3. Preparation of PEGylated Er:Y2O3 and Nd:Y2O3 NPs
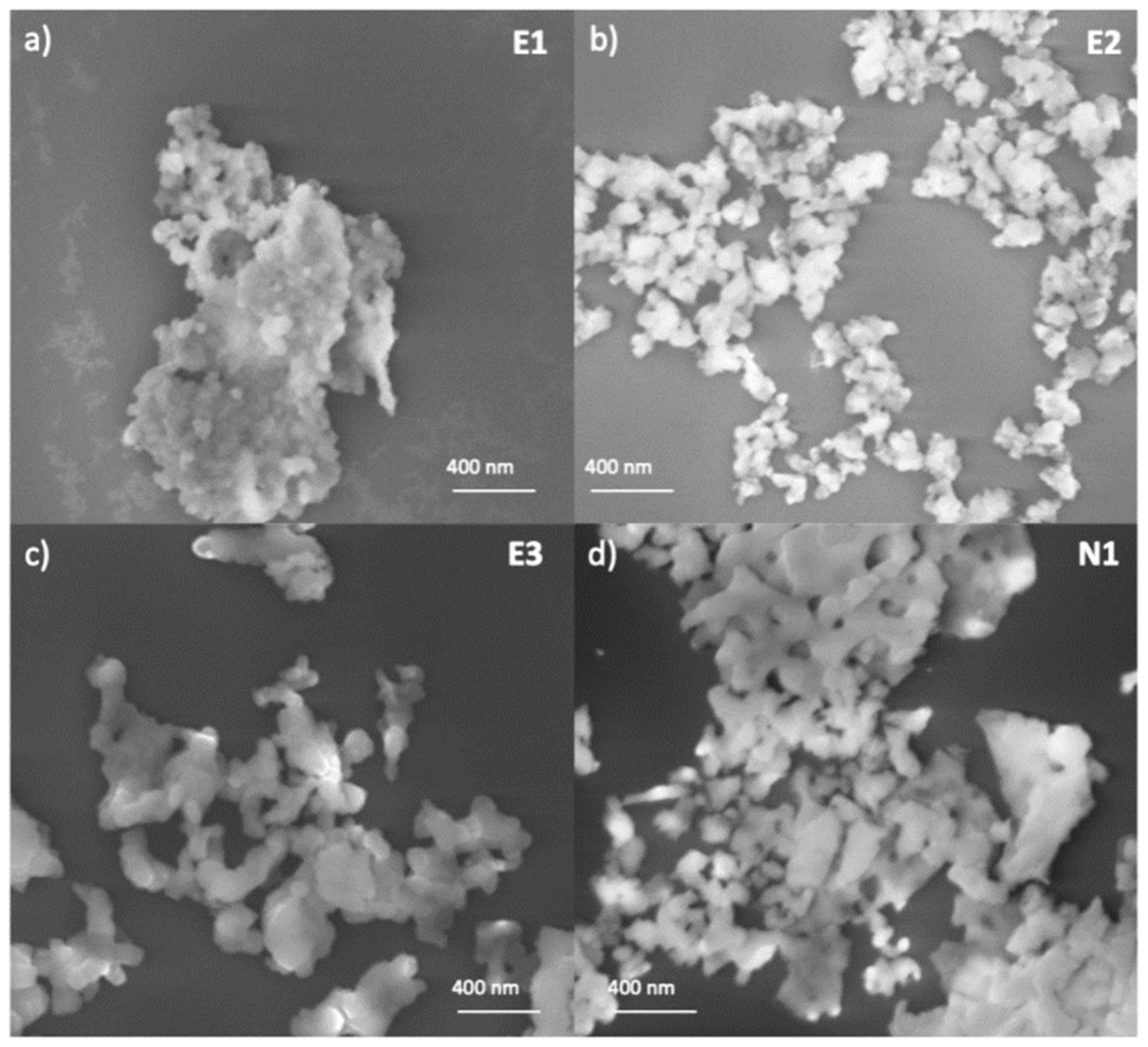
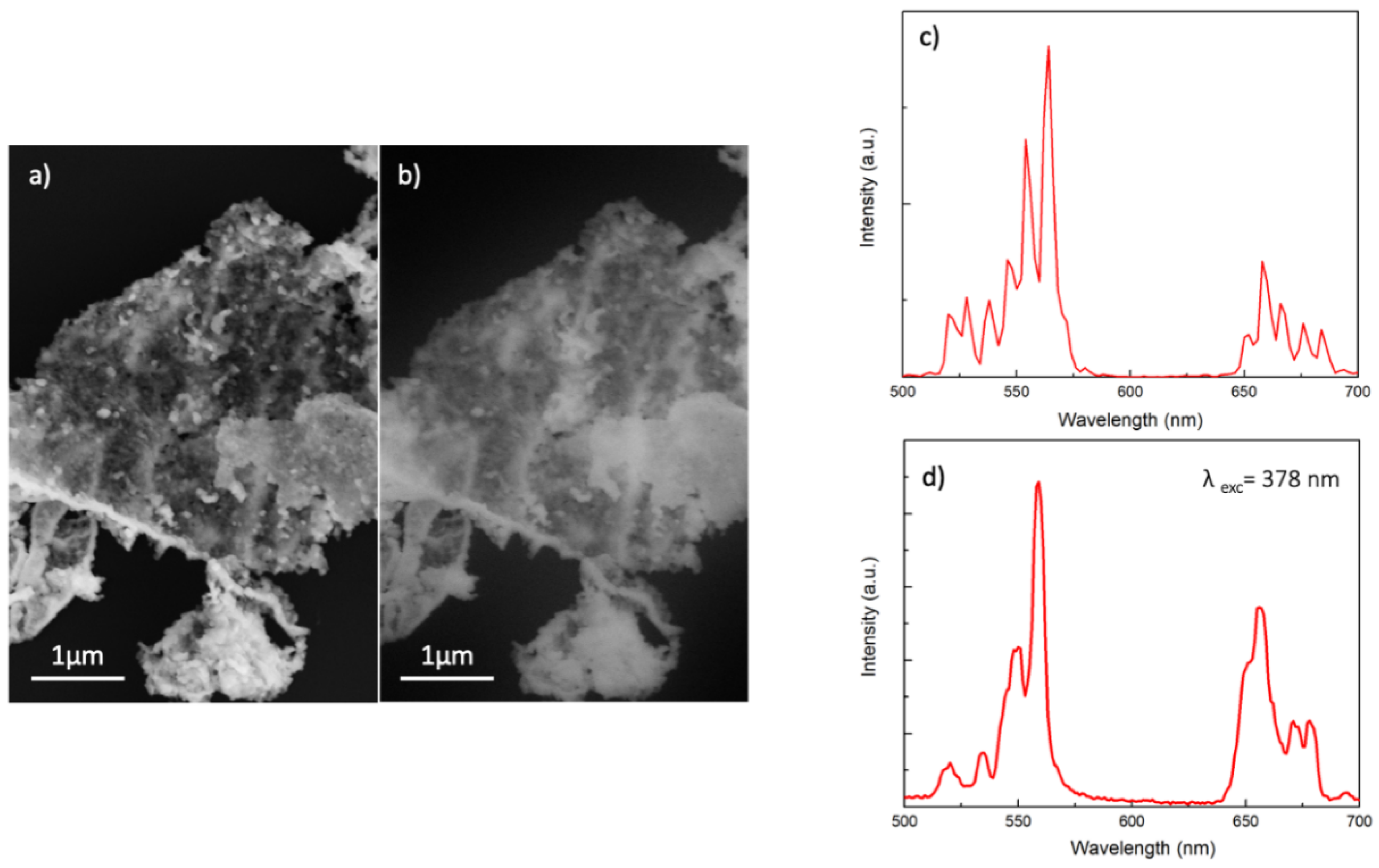
2.4. SEM Analyses
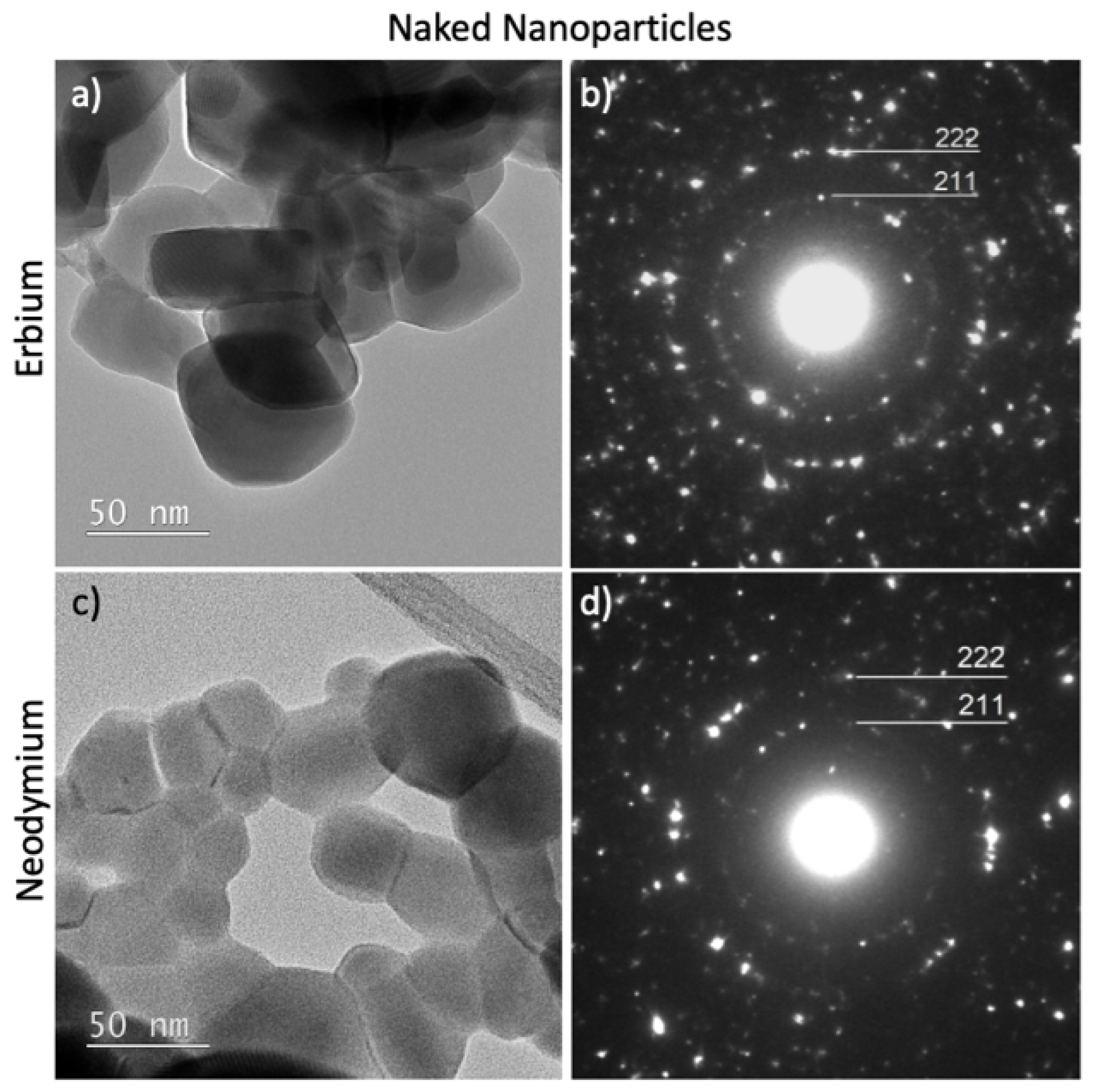
2.5. TEM Analyses
2.6. XRD Characterization
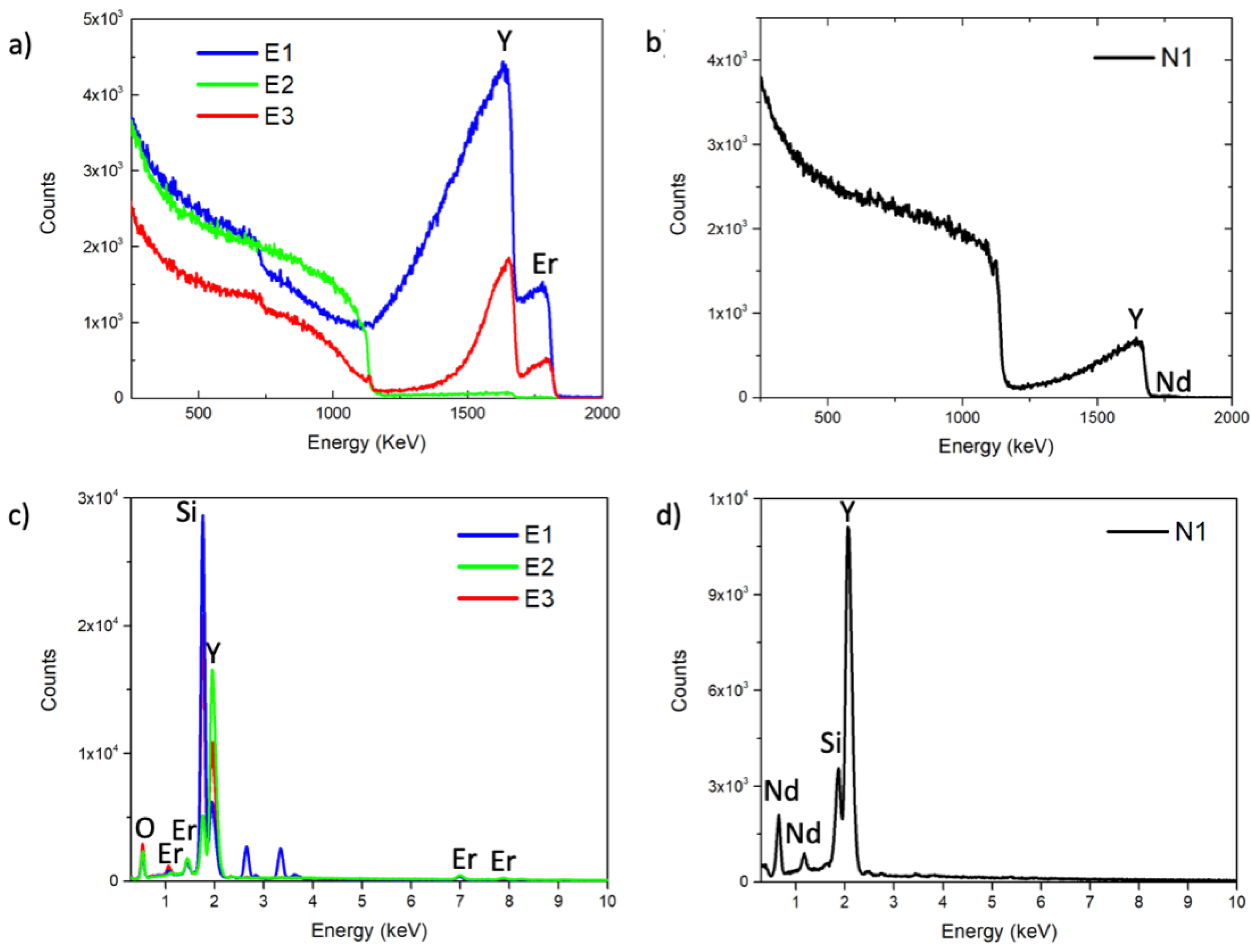
2.7. Rutherford Backscattering Spectrometry
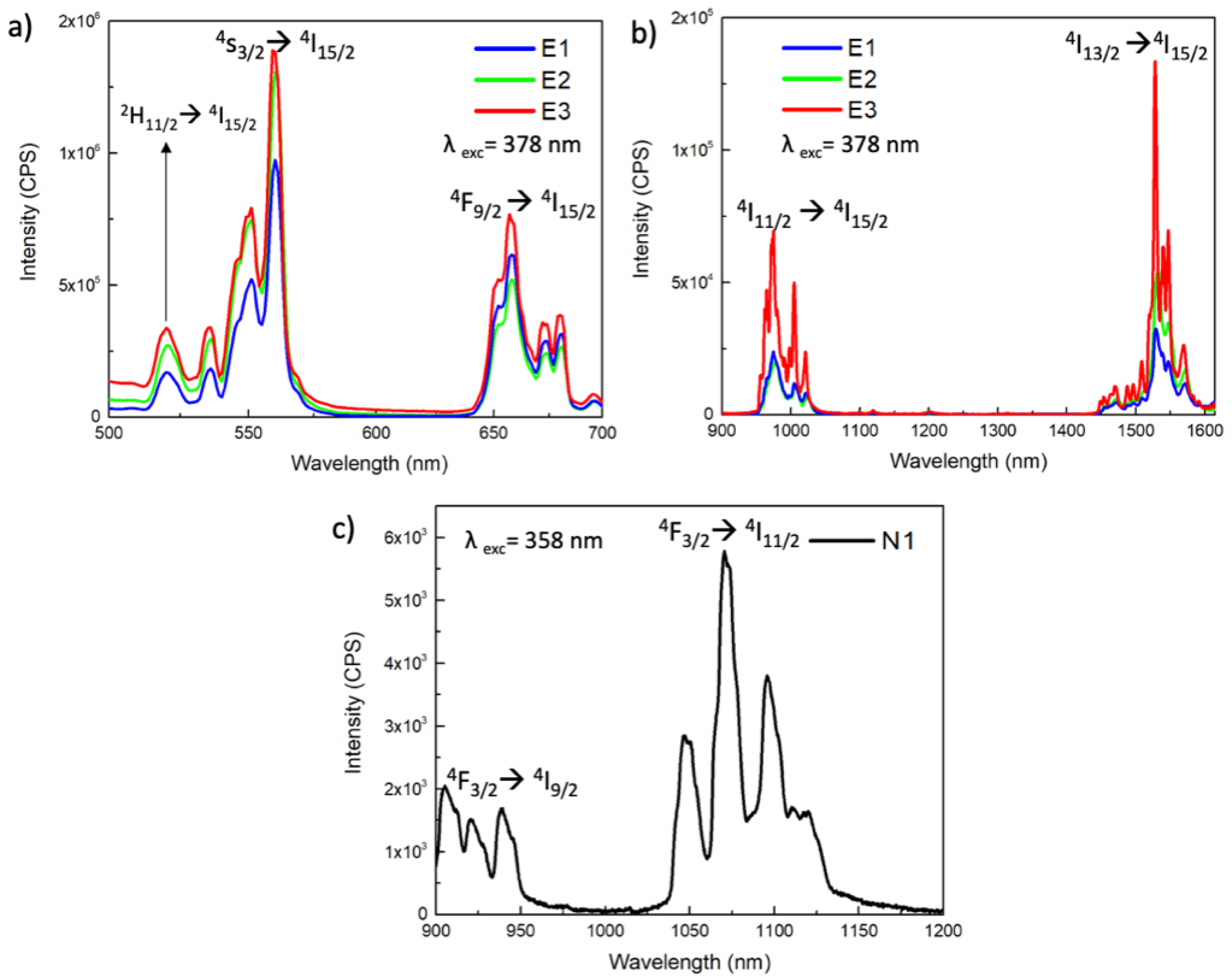
2.8. Photoluminescence
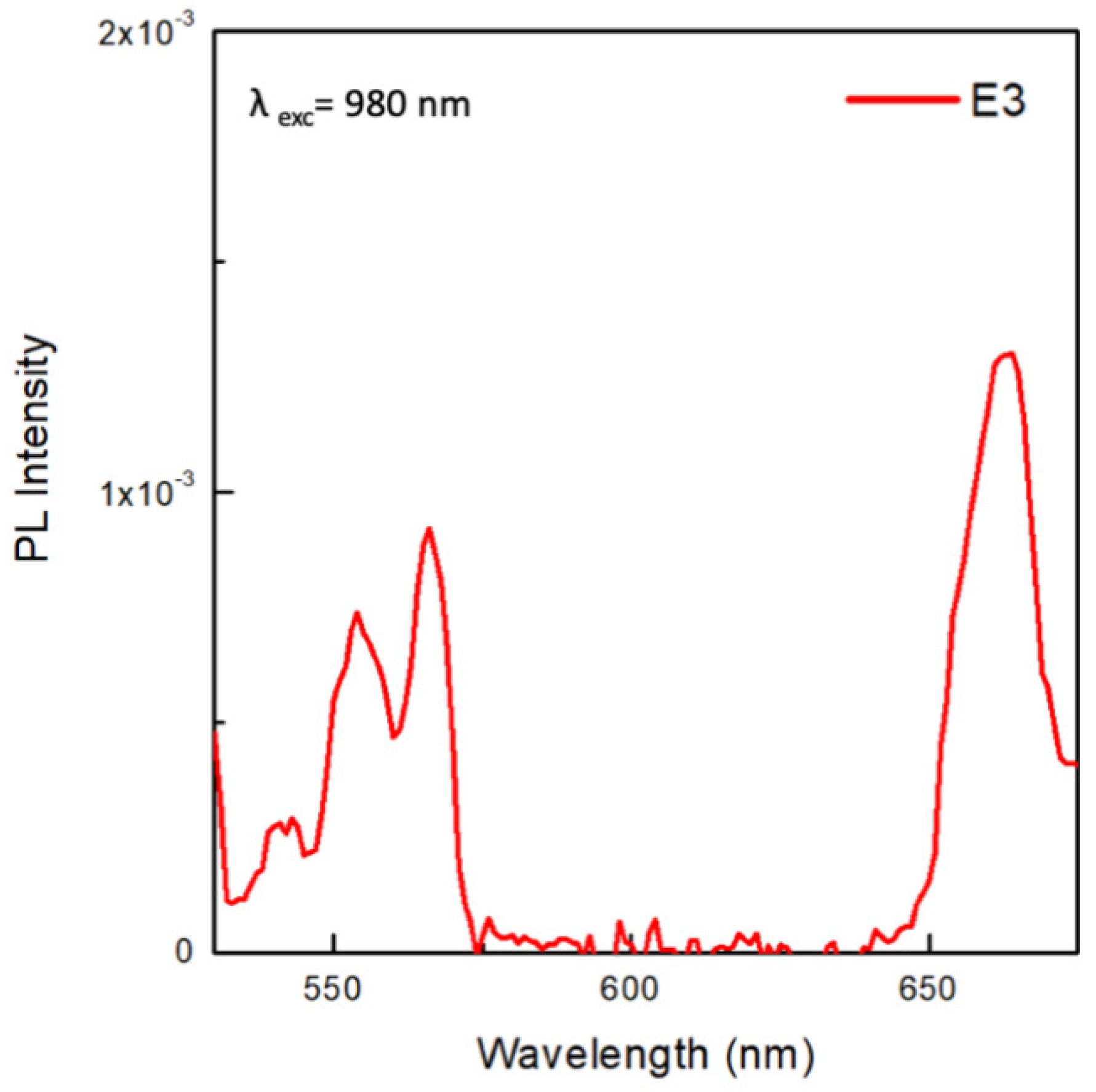
2.9. Upconversion
2.10. ζ-Potential Measurements
2.11. Cathodoluminescence
3. Results and Discussion
3.1. Synthesis of NPs
3.2. Structural and Compositional Characterization of NPs
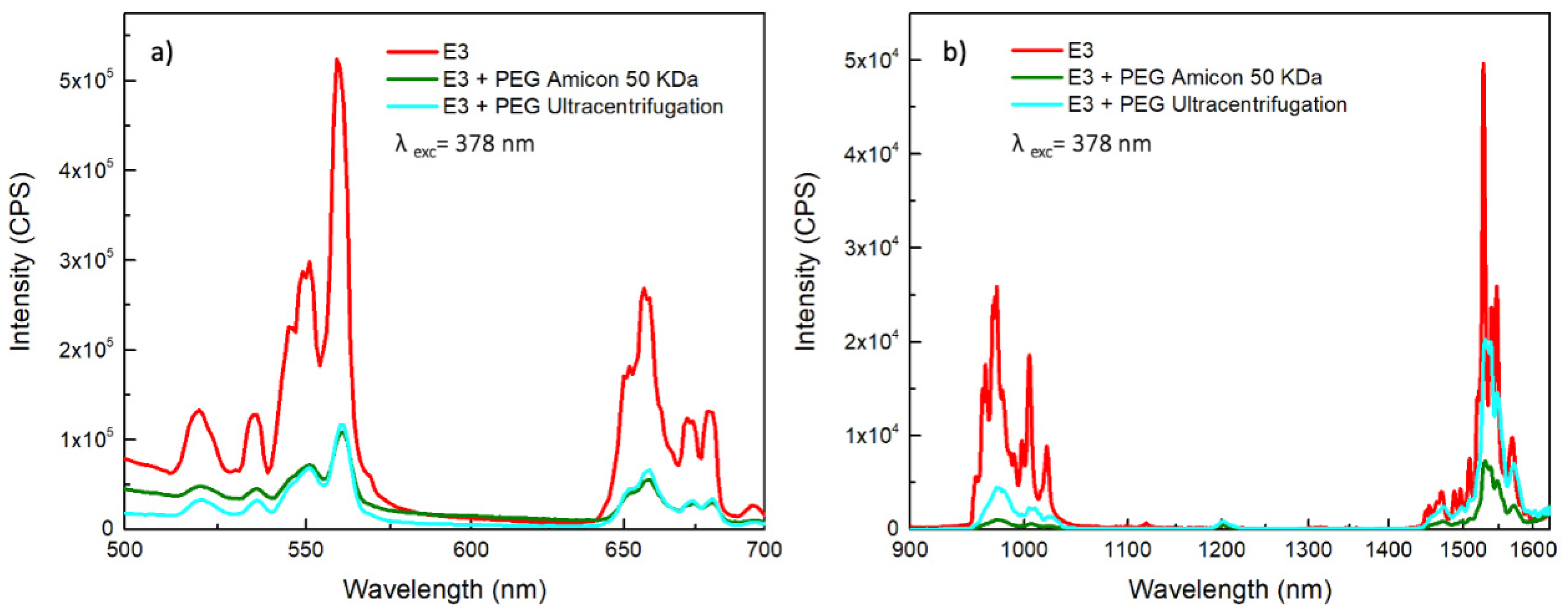
3.3. Pegylation of Er:Y2O3 and Nd:Y2O3 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Chen, Q.; Pan, X.; Lu, W.; Zhang, J. Development and Challenge of Fluorescent Probes for Bioimaging Applications: From Visualization to Diagnosis. Top. Curr. Chem. 2022, 380, 22. [Google Scholar] [CrossRef]
- Harrison, V.S.R.; Carney, C.E.; MacRenaris, K.W.; Waters, E.A.; Meade, T.J. Multimeric Near IR–MR Contrast Agent for Multimodal In Vivo Imaging. J. Am. Chem. Soc. 2015, 137, 9108–9116. [Google Scholar] [CrossRef]
- Thi Kim Dung, D.; Umezawa, M.; Nigoghossian, K.; Yeroslavsky, G.; Okubo, K.; Kamimura, M.; Yamaguchi, M.; Fujii, H.; Soga, K. Development of Molecular Imaging Probe for Dual NIR/MR Imaging. J. Photopolym. Sci. Technol. 2020, 33, 117–122. [Google Scholar] [CrossRef]
- Yang, Z.; Sharma, A.; Qi, J.; Peng, X.; Lee, D.Y.; Hu, R.; Lin, D.; Qu, J.; Kim, J.S. Super-Resolution Fluorescent Materials: An Insight into Design and Bioimaging Applications. Chem. Soc. Rev. 2016, 45, 4651–4667. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Ji, S.; Liu, Q.; Ding, D.; Zhao, D.; Liu, B. Long Wavelength Excitable Near-Infrared Fluorescent Nanoparticles with Aggregation-Induced Emission Characteristics for Image-Guided Tumor Resection. Chem. Sci. 2017, 8, 2782–2789. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, L.; Zhu, Q.; Feng, Y.; Wu, M. Recent Advances in Second Near-Infrared Region (NIR-II) Fluorophores and Biomedical Applications. Front. Chem. 2021, 9, 750404. [Google Scholar] [CrossRef]
- Camargo, K.C.; Pereira, R.R.; dos Santos, L.F.; de Oliveira, S.R.; Gonçalves, R.R.; de S. Menezes, L. Continuous Wave Near-Infrared Phonon-Assisted Upconversion in Single Nd3+-Doped Yttria Nanoparticles. J. Lumin. 2017, 192, 963–968. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sudarshan, K.; Kadam, R.M. Optical Nanomaterials with Focus on Rare Earth Doped Oxide: A Review. Mater. Today Commun. 2021, 27, 102277. [Google Scholar] [CrossRef]
- Bouzigues, C.; Gacoin, T.; Alexandrou, A. Biological Applications of Rare-Earth Based Nanoparticles. ACS Nano 2011, 5, 8488–8505. [Google Scholar] [CrossRef]
- Liu, S.; De, G.; Xu, Y.; Wang, X.; Liu, Y.; Cheng, C.; Wang, J. Size, Phase-Controlled Synthesis, the Nucleation and Growth Mechanisms of NaYF4:Yb/Er Nanocrystals. J. Rare Earths 2018, 36, 1060–1066. [Google Scholar] [CrossRef]
- Rajakumar, G.; Mao, L.; Bao, T.; Wen, W.; Wang, S.; Gomathi, T.; Gnanasundaram, N.; Rebezov, M.; Shariati, M.A.; Chung, I.-M.; et al. Yttrium Oxide Nanoparticle Synthesis: An Overview of Methods of Preparation and Biomedical Applications. Appl. Sci. 2021, 11, 2172. [Google Scholar] [CrossRef]
- Keevend, K.; Puust, L.; Kurvits, K.; Gerken, L.R.H.; Starsich, F.H.L.; Li, J.-H.; Matter, M.T.; Spyrogianni, A.; Sotiriou, G.A.; Stiefel, M.; et al. Ultrabright and Stable Luminescent Labels for Correlative Cathodoluminescence Electron Microscopy Bioimaging. Nano Lett. 2019, 19, 6013–6018. [Google Scholar] [CrossRef]
- Battaglia, R.; Musumeci, P.; Ragusa, M.; Barbagallo, D.; Scalia, M.; Zimbone, M.; Lo Faro, J.M.; Borzì, P.; Scollo, P.; Purrello, M.; et al. Ovarian Aging Increases Small Extracellular Vesicle CD81+ Release in Human Follicular Fluid and Influences MiRNA Profiles. Aging 2020, 12, 12324–12341. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, Y.; Wen, S.; Mei, H.; Liu, Y.; Wang, D.; Maddahfar, M.; Su, Q.P.; Lin, G.; Chen, Y.; et al. Single Small Extracellular Vesicle (SEV) Quantification by Upconversion Nanoparticles. Nano Lett. 2022, 22, 3761–3769. [Google Scholar] [CrossRef]
- Bogdan, N.; Vetrone, F.; Ozin, G.A.; Capobianco, J.A. Synthesis of Ligand-Free Colloidally Stable Water Dispersible Brightly Luminescent Lanthanide-Doped Upconverting Nanoparticles. Nano Lett. 2011, 11, 835–840. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Stein, N.C.; Mulac, D.; Herrmann, F.C.; Humpf, H.-U.; Langer, K. Effect of Nanoparticle Size and PEGylation on the Protein Corona of PLGA Nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80. [Google Scholar] [CrossRef]
- Fukushima, S.; Furukawa, T.; Niioka, H.; Ichimiya, M.; Sannomiya, T.; Tanaka, N.; Onoshima, D.; Yukawa, H.; Baba, Y.; Ashida, M.; et al. Correlative Near-Infrared Light and Cathodoluminescence Microscopy Using Y2O3:Ln, Yb (Ln = Tm, Er) Nanophosphors for Multiscale, Multicolour Bioimaging. Sci. Rep. 2016, 6, 25950. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Saito, Y.; Soga, K. Synthesis of Er3+ Doped Y2O3 Nanophosphors. J. Am. Ceram. Soc. 2009, 92, 1006–1010. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Hemmer, E.; Yamano, T.; Hyodo, H.; Kishimoto, H.; Soga, K. Synthesis and Toxicity Assay of Ceramic Nanophosphors for Bioimaging with Near-Infrared Excitation. Prog. Cryst. Growth Charact. Mater. 2012, 58, 121–134. [Google Scholar] [CrossRef]
- Kamimura, M.; Miyamoto, D.; Saito, Y.; Soga, K.; Nagasaki, Y. Design of Poly(Ethylene Glycol)/Streptavidin Coimmobilized Upconversion Nanophosphors and Their Application to Fluorescence Biolabeling. Langmuir 2008, 24, 8864–8870. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M. SIMNRA, a Simulation Program for the Analysis of NRA, RBS and ERDA. In AIP Conference Proceedings; AIP: Denton, TX, USA, 1999; pp. 541–544. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, W.; Li, Y.; Guo, X.; Wang, N.; Song, Q.; Wang, X.; Han, Y.; Wang, L. Hydrothermal Method for Y2O3:Eu3+ Uniform Nanophosphors with Different Templates. Mod. Phys. Lett. B 2019, 33, 1950227. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Wang, S.; Liang, D.; Cai, X.; Wang, D.; Han, Y.; Jia, G. Preparation of Lanthanide Ions-Doped BiPO4 Nanoparticles and Fe3+ Ions Assay. J. Nanosci. Nanotechnol. 2018, 18, 4000–4005. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Musumeci, P.; Contino, A.; Maccarrone, G.; Calcagno, L. DNA-Triggered Asymmetric ZnO Nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2016, 489, 336–342. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; De Giorgi, M.L.; Nobile, C.; Rinaldi, R.; Leporatti, S. Control of Colloidal CaCO3 Suspension by Using Biodegradable Polymers during Fabrication. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 60–70. [Google Scholar] [CrossRef]
- Hanafy, N.; Kemary, M.; Leporatti, S. Reduction Diameter of CaCO3 Crystals by Using Poly Acrylic Acid Might Improve Cellular Uptake of Encapsulated Curcumin in Breast Cancer. J. Nanomed. Res. 2018, 7, 235–239. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Martin, D.A.; Alvarez, V.A.; Gonzalez, J.S. Polyacrylic Acid-Coated Iron Oxide Magnetic Nanoparticles: The Polymer Molecular Weight Influence. Colloids Surf. Physicochem. Eng. Asp. 2018, 543, 28–37. [Google Scholar] [CrossRef]
- Panáček, A.; Prucek, R.; Hrbáč, J.; Nevečná, T.; Šteffková, J.; Zbořil, R.; Kvítek, L. Polyacrylate-Assisted Size Control of Silver Nanoparticles and Their Catalytic Activity. Chem. Mater. 2014, 26, 1332–1339. [Google Scholar] [CrossRef]
- Alrowaili, Z.A.; Taha, T.A.; El-Nasser, K.S.; Donya, H. Significant Enhanced Optical Parameters of PVA-Y2O3 Polymer Nanocomposite Films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3101–3110. [Google Scholar] [CrossRef]
- Laricchiuta, G.; Vandervorst, W.; Vickridge, I.; Mayer, M.; Meersschaut, J. Rutherford Backscattering Spectrometry Analysis of InGaAs Nanostructures. J. Vac. Sci. Technol. A 2019, 37, 020601. [Google Scholar] [CrossRef]
- Kamimura, M.; Kanayama, N.; Tokuzen, K.; Soga, K.; Nagasaki, Y. Near-Infrared (1550 Nm) in Vivo Bioimaging Based on Rare-Earth Doped Ceramic Nanophosphors Modified with PEG-b-Poly(4-Vinylbenzylphosphonate). Nanoscale 2011, 3, 3705. [Google Scholar] [CrossRef] [PubMed]

| Calculated | Obtained | Yield | |
|---|---|---|---|
| E1 | 0.2703 g | 0.0157 g | 5.6% |
| E2 | 0.2703 g | 0.1006 g | 37.0% |
| E3 | 0.2703 g | 0.0637 g | 23.6% |
| N1 | 0.2703 g | 0.1152 g | 42.6% |
| RBS | ||||
|---|---|---|---|---|
| Y 1015 at/cm2 | Er 1015 at/cm2 | Er / (Er + Y) | Er % in NPs | |
| E1 | 535 ± 27 | 70 ± 4 | 0.12 ± 0.01 | 4.6 ± 0.3% |
| E2 | 8.0 ± 0.4 | 0.75 ± 0.04 | 0.09 ± 0.01 | 3.4 ± 0.2% |
| E3 | 229 ± 11 | 22 ± 1 | 0.09 ± 0.01 | 3.5 ± 0.2% |
| Y 1015at/cm2 | Nd 1015at/cm2 | Nd / (Nd + Y) | Nd % in NPs | |
| N1 | 63 ± 3.14 | 0.84 ± 0.04 | 0.010 ± 0.001 | 0.5 ± 0.1% |
| EDX | ||||
| Y (At %) | Er (At %) | Er / (Er + Y) | Er % in NPs | |
| E1 | 89.50 ± 0.11 | 10.50 ± 0.71 | 0.110 ± 0.008 | 4.2 ± 0.3% |
| E2 | 93.50 ± 0.03 | 6.50 ± 0.39 | 0.060 ± 0.003 | 2.6 ± 0.2% |
| E3 | 92.40 ± 0.05 | 7.60 ± 0.46 | 0.080 ± 0.005 | 3.2 ± 0.2% |
| Y (At %) | Nd (At %) | Nd / (Nd + Y) | Nd % in NPs | |
| N1 | 98.60 ± 0.46 | 1.4 ± 31.28 | 0.01 ± 0.22 | 0.6 ± 13.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiechio, R.M.; Battaglia, R.; Caponnetto, A.; Butera, E.; Franzò, G.; Reitano, R.; Purrello, M.; Ragusa, M.; Barbagallo, D.; Barbagallo, C.; et al. Er:Y2O3 and Nd:Y2O3 Nanoparticles: Synthesis, Pegylation, Characterization and Study of Their Luminescence Properties. Chemosensors 2023, 11, 20. https://doi.org/10.3390/chemosensors11010020
Chiechio RM, Battaglia R, Caponnetto A, Butera E, Franzò G, Reitano R, Purrello M, Ragusa M, Barbagallo D, Barbagallo C, et al. Er:Y2O3 and Nd:Y2O3 Nanoparticles: Synthesis, Pegylation, Characterization and Study of Their Luminescence Properties. Chemosensors. 2023; 11(1):20. https://doi.org/10.3390/chemosensors11010020
Chicago/Turabian StyleChiechio, Regina Maria, Rosalia Battaglia, Angela Caponnetto, Ester Butera, Giorgia Franzò, Riccardo Reitano, Michele Purrello, Marco Ragusa, Davide Barbagallo, Cristina Barbagallo, and et al. 2023. "Er:Y2O3 and Nd:Y2O3 Nanoparticles: Synthesis, Pegylation, Characterization and Study of Their Luminescence Properties" Chemosensors 11, no. 1: 20. https://doi.org/10.3390/chemosensors11010020
APA StyleChiechio, R. M., Battaglia, R., Caponnetto, A., Butera, E., Franzò, G., Reitano, R., Purrello, M., Ragusa, M., Barbagallo, D., Barbagallo, C., Di Pietro, C., Marchi, V., Lo Faro, M. J., Contino, A., Maccarrone, G., & Musumeci, P. (2023). Er:Y2O3 and Nd:Y2O3 Nanoparticles: Synthesis, Pegylation, Characterization and Study of Their Luminescence Properties. Chemosensors, 11(1), 20. https://doi.org/10.3390/chemosensors11010020











