Abstract
Extra virgin olive oil (EVOO) is highly appreciated by consumers for its unique sensory characteristics that are directly related to its volatile composition. The objective of this study was to investigate the effect of cultivar and geographical origin on the volatile composition of Greek monovarietal EVOOs. Samples of three local cultivars (Koroneiki, Kolovi and Adramytini) originating from three areas of Greece (Crete, Lesvos and the Peloponnese), spanning two consecutive harvesting periods, were selected. Their volatile components were determined using headspace solid-phase microextraction combined with gas chromatography–mass spectrometry. More than 70 volatile compounds were identified. Alcohols were the dominant class (43–50%), followed by ketones (12–24%), esters (12–18%) and aldehydes (4–12%). The most prominent volatile compounds were (Z)-3-hexen-1-ol (6–11%), 1-penten-3-ol (7–11%), (E)-3-hexenyl acetate (0.5–11%) and 3-pentanone (8–16%). Significant differences were observed and highlighted. Clear separations between samples from different cultivars and geographic provenances were achieved using multivariate analysis and the most discriminating volatiles were identified. Additionally, using multivariate receiver operating characteristic (ROC) curve analysis, a combination of five chemical markers was found superior (area under the curve, AUC: 1.00; predictive accuracy: 100%) for the correct classification of Koroneiki EVOOs according to geographical origin.
Keywords:
extra virgin olive oil; volatiles; authentication; cultivar; geographical origin; SPME; GC-MS; chemometrics 1. Introduction
Extra virgin olive oil (EVOO) is at the forefront of research due to its high consumption in the Mediterranean and worldwide. Its consumption is associated with lower risk of cancer, cardiovascular disease and other diseases [1]. Apart from its nutritional value, olive oil is highly appreciated by consumers for its unique sensory characteristics that are related to the volatile composition, the high content of oleic acid and the presence of phenolic compounds [2,3].
There is also a very significant economic and social role played by olive oil production in the Mediterranean. According to the International Olive Oil Council, 2022 [4], world olive oil production has tripled in the last 60 years. Specifically for the crop year 2020/21, the production reached 3010,000 tons, of which Spain produced 46.15%, Greece ranks second with 9.14% of the world olive oil production and Italy third with 9.09%. Since the Greek olive oil industry exports approximately 35% of its annual production, characterization and standardization of its olive oil are crucial in order to benefit from significant price increases [5].
Volatile compounds are low-molecular-mass compounds that easily vaporize at ambient temperature, and when they reach the olfactory receptors, they cause an odor sensation [6]. A great number of volatile compounds have been reported in the volatile fraction of EVOOs belonging to several chemical classes, especially carbonyl compounds, alcohols, esters, hydrocarbons and other compounds such as acids, ethers, terpenes and furan derivatives [7]. The C6 and C5 compounds, especially C6 unsaturated and saturated aldehydes, represent the most important fraction of volatile compounds that contribute to green odor notes and are enzymatically produced from polyunsaturated fatty acids through the lipoxygenase (LOX) pathway [8]. Thus, the positive aroma perceptions in olive oil are attributed to the action of the endogenous plant enzymes, whereas the negative notes result are from chemical oxidation and the microbial activity [6]. During the olive fruit storage, the concentration of aldehydes and esters decreases and off-flavor compounds with low odor thresholds are generated [9]. Cultivar, geographic region, climatic and agronomic conditions (e.g., irrigation and fertilization), fruit maturity and processing method (e.g., malaxation time and temperature) influence the volatile composition of olive oil [10,11,12].
Since consumers are increasingly showing the need to purchase certified EVOOs produced in certain regions and avoid food fraud, quality assessment of olive oil and authenticity is at the core of scientific research [13,14,15]. Various instrumental techniques have been applied regarding the non-volatile and volatile fraction of olive oil to prevent adulteration and false labeling. The determination of fatty acid and sterol composition via gas chromatography equipped with a flame ionization detector has been widely used [16], as well as spectroscopic techniques, including nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FT-IR), fluorescence spectroscopy, near infrared spectroscopy (NIR) and mass spectrometry [13].
The application of chemometrics for the characterization and authentication of EVOOs using volatile composition data is quite common in the literature [13,14,17,18]. In comparison to other methods that are widely used for the determination of volatile compounds, headspace solid-phase microextraction (HS-SPME) combined with gas chromatography–mass spectrometry (GC-MS) is generally considered more sensitive, faster, fully automatable and cost effective, while providing the advantages of an untargeted approach [18]. To fully utilize the large amount of data produced from olive oil profiling, chemometric tools are required. Principal component analysis (PCA), linear and partial least squares discriminant analysis (LDA, PLS-DA) [13] and orthogonal PLS-DA (OPLS-DA) [19] are most commonly used.
A number of researchers have examined olive oil discrimination based on its volatile composition regarding Spanish [20,21,22], Italian [6,13], Tunisian [23] and Greek EVOO samples [5,12,24]. Undoubtedly, promising results have been achieved in the field of olive oil varietal and geographical origin assessment. However, few studies [25,26,27] report the use of receiver operating characteristic (ROC) curve analysis, which is considered the standard method for robust marker selection [28].
Based on the above, the purpose of the current study was to examine how cultivar and geographical origin could affect the volatile composition of Greek monovarietal EVOOs. To achieve that, olive oil samples of Koroneiki, Kolovi and Adramytini cultivars were collected over two consecutive harvesting seasons. The samples derived from the island of Crete, the island of Lesvos and the Peloponnese, the three major olive oil producing areas in Greece. Multivariate and univariate data analysis were used to find significant chemical markers typical of certain cultivars and regions that could correctly classify EVOOs. Special focus was given to the selection of a small number of regional markers by applying multivariate ROC analysis that could potentially be used for routine analysis.
2. Materials and Methods
2.1. Olive Oil Samples
A total of 129 monovarietal EVOOs were obtained from local producers during the harvesting periods 2019–2020 and 2020–2021. Sampling was carried out from October to the end of January. The samples were produced from three cultivars (Koroneiki, Kolovi and Adramytini) in three different geographical regions of Greece (Crete, the Peloponnese, and Lesvos). Specifically, olive oils of Adramytini (n = 7) and Kolovi (n = 32) cultivars derived from Lesvos (n = 39), while olive oils of Koroneiki (n = 90) from various prefectures of the Peloponnese (n = 50) and Crete (n = 40). Note that due to the different cultivation practices (conventional, integrated and biological farming) and production procedures (two-phase/three-phase system) employed by each local producer, the set of samples had high inherent variability, which is considered positive for the purposes of our study. All the collected EVOOs were documented by filling a form with the appropriate information for each sample, such as olive tree cultivar, region, cultivation practice, pedoclimatic conditions, production procedure and many other parameters. A detailed list of the samples is provided in Table S1. The oil samples were packaged in amber glass bottles (200 mL, the headspace was kept minimum and purged with nitrogen) and kept at 4 °C until analysis, which was performed after the end of each harvesting period. Before analysis, the oils were reconditioned at room temperature for 24 h.
2.2. Headspace Solid-Phase Microextraction (SPME)
The volatile compounds were isolated according to the method applied in previous studies [21,29] with slight modifications. An aliquot (2 g) of olive oil sample was accurately weighed (±0.0001 g) into a 20 mL glass vial and hermetically closed with a polytetrafluoroethylene (PTFE)/silicone septum. The vial was left for 5 min at 40 °C under agitation (250 rpm) to allow for equilibration of the volatile compounds in the headspace. Then, the SPME fiber (Divinylbenzene [DVB]/Carboxen [CAR]/Polydimethylsiloxane [PDMS], 50/30 μm, length 2 cm; Sigma Aldrich, Darmstadt, Germany) was exposed to the headspace for 40 min to isolate the volatile compounds, under the same conditions. Before use, the fiber was thermally cleaned according to the instructions of the manufacturer and after each sample analysis at 260 °C for 5 min.
2.3. Gas Chromatography–Mass Spectrometry
The extracted volatiles were desorbed in the inlet of a GCMS QP-2010 Ultra (Shimadzu Inc., Kyoto, Japan) system equipped with an SPME liner (0.7 mm i.d.; Sigma Aldrich) at 240 °C for 5 min using a split ratio of 1/5 to avoid saturated peaks. Subsequently, the volatiles were separated in a DB-Wax fused silica capillary column (30 m, I.D. 0.25 mm, film thickness 0.25 μm; Agilent Technologies Inc., Santa Clara, CA, USA) using helium as a carrier gas. Its linear velocity was held constant (36 cm/s) during analysis. The oven temperature was set initially at 40 °C for 5 min and then increased to 180 °C by 5 °C/min. A cleaning step followed, by increasing the temperature to 240 °C (30°C/min), at which it was kept for 5 min. The operation mode of the mass spectrometer was electron ionization (70 eV) using a scan range of 40–300 m/z. The temperature of the source and the interface were kept at 230 °C and 240 °C, respectively. Data acquisition was accomplished using vendor’s software (GCMS Solution, ver. 4.30, Shimadzu Inc., Kyoto, Japane), whereas the identification of the compounds was performed by AMDIS (ver. 2.72; NIST, Gaithersburg, MD, USA) and NIST MS Search (ver. 2.2; NIST, USA) software according to Mikrou et al. [20]. The relative content of each compound was calculated by determining the ratio of the area corresponding to Amdis component to the sum of all components.
2.4. Statistical Analysis
Multivariate and univariate analysis was performed using MetaboAnalyst 5.0 web-based tool suite (http://www.metaboanalyst.ca, accessed on 1 August 2022) [30,31] and The Unscrambler X (CAMO Software AS, Oslo, Norway) software. Non-parametric one-way analysis of variance (Kruskal Wallis test) was used to test for significant differences (p < 0.05) on the relative content of individual compounds and chemical categories between the cultivars and geographical regions independently. The multivariate analysis was carried out as follows. First, the original full dataset was split into two subsets using the Kennard–Stone method. One subset (approximately 75% of all samples) was used for developing (calibration) and testing the multivariate models by the full cross-validation approach, whereas the other subset (approximately 25% of all samples) was used exclusively for the external validation of the models. The peak area values were used as variable X (predictors) after log transformation and autoscaling. A PLS-DA model was built to discriminate the olive oils from Koroneiki and Kolovi cultivars (Adramytini was left out due to the low number of samples). A second PLS-DA model was built using the Koroneiki samples as variable Y (responses) to discriminate them according to geographical origin. Due to partial overlap of the groups, OPLS-DA was applied because it maximizes the between-class separation by removing the uncorrelated variation. Volatile compounds with variable importance in projection (VIP) score > 1 were selected as important variables for class separation and further used in multivariate ROC curve analysis for the discovery of a combination subset with the minimum number of potential biomarkers. For this purpose, the module “Biomarker Analysis” of MetaboAnalyst was used. Multiple classification models were built using the Monte Carlo cross-validation approach with balanced sub-sampling. The classification and feature ranking method were based on the linear support vector machine algorithm. The optimal model was selected by comparison of the AUC (area under the curve) values as well as the predictive accuracies and further validated by two approaches (cross-validation and external validation).
3. Results and Discussion
3.1. Volatile Composition of Olive Oil
A total of 72 volatile compounds were identified for both harvesting periods. The volatiles were classified to the appropriate chemical class and consisted of nine acids, nineteen alcohols, thirteen aldehydes, seven esters, six hydrocarbons, seven ketones, six terpenoids and five miscellaneous compounds. The comparison of the volatile composition was studied within samples according to their growing area (Table 1) and according to their variety (Table S2). The identified compounds have already been reported by other researchers for olive oils from Spain [20,21], Italy [32], Greece [12] and Tunisia [23].

Table 1.
Volatile composition of EVOOs according to the growing area of olive tree. The values represent the mean content (%) of the samples in each region. Data in the same row with different lowercase letters differ significantly (p < 0.05). CRE: Crete; PEL: Peloponnese; LES: Lesvos.
Alcohols were the most numerous chemical class of volatile compounds detected, including mainly C5 and C6 compounds. 1-Penten-3-ol was the most prominent alcohol for olive oil samples from CRE and PEL (10.56% and 10.96%, respectively), whereas (Z)-3-hexen-1-ol was the dominant alcohol (10.42%) in LES samples. Other alcohols found in relatively high levels were ethanol, 2-penten-1-ol, 1-hexanol and 2-hexen-1-ol, responsible for the green, fruity and floral notes of olive oil [8]. The most quantitatively important aldehydes were (E)-3-hexenal followed by (E)-2-hexenal and hexanal, which provide the typical “green note” of olive oil aroma [12].
According to Kiritsakis et al. [33], hexanal, (E)-2-hexenal and 1-hexanol are the major volatile compounds of olive oil, which is in agreement with our results. 2,4-Hexadienal and nonanal are among the aldehydes identified and have been associated with the oxidative status of EVOO [12]. Small amounts of C5 ketones and pentene dimers also positively affect olive oil aroma [34] that is characterized as sweet. In the current study, 3-pentanone was identified in abundance (CRE: 8.10%, PEL: 12.27%, LES: 15.75%, p < 0.05) and 1-penten-3-one in lower levels but significantly higher in LES samples (6.06%, p < 0.05%). (Z)-3-Hexenyl acetate, which derives from LOX pathway [8,35,36], was the most prominent ester in the olive oil samples from the CRE and PEL regions (10.12% and 10.80%, respectively). Relatively high concentration of this ester has also been reported by other researchers [12,23], and it contributes to the pleasant green and banana notes of olive oil [37].
On the contrary, samples from LES displayed lower levels of (Z)-3-hexenyl-acetate (3.77%, p < 0.05). This could be attributed to a reduced activity of alcohol acetyl transferase (AAT), an enzyme that catalyzes the transformation of alcohols to esters [35], a process which seems to be affected by environmental growth conditions, cultivar and oil extraction processing parameters [24,35]. Ethyl acetate was the most prominent ester (5.08%, p < 0.05) in the LES samples. It is commonly found in various olive oils [11,38] and related to aerobic fermentation [36]. Among the carboxylic acids, acetic acid was the dominant one in our samples (CRE: 4.62%, PEL: 3.04%, LES: 5.43%), out of nine acids identified. Carboxylic acids, such as acetic acid, have been suggested to derive from sugar fermentation [38] but are also linked with sensory defects (pungent, like acetic acid) resulting from bacterial growth during olive fruit storage [8]. As far as hydrocarbons are concerned, 4-methyl-2,6-octadiene was the most abundant one displaying slightly lower levels in samples from PEL (CRE: 4.71%, PEL: 2.75% and LES: 3.89%, p < 0.05), followed by 3-ethyl-1,5-octadiene. It is noteworthy that (E)-4,8-dimethylnona-1,3,7-triene, a monoterpene, was present in relatively high levels in samples deriving from CRE. This compound has been reported by only a few researchers [12,34]. Miscellaneous compounds were generally found in low levels (<0.12%). The total relative content (%) of each chemical class according to geographical origin and cultivar is presented in Figure 1 and Figure 2, respectively.
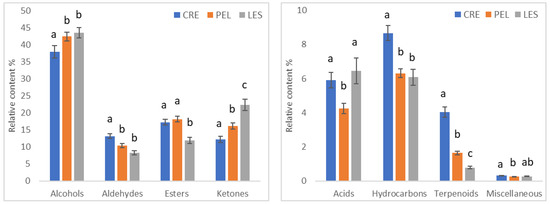
Figure 1.
Comparison of the relative content (%) of the chemical classes of volatile compounds identified in EVOOs originating from various growing locations (CRE: Crete; PEL: Peloponnese; LES: Lesvos). The error bars indicate standard errors at 95% confidence level. Data in the same class with different lowercase letters differ significantly (p < 0.05).
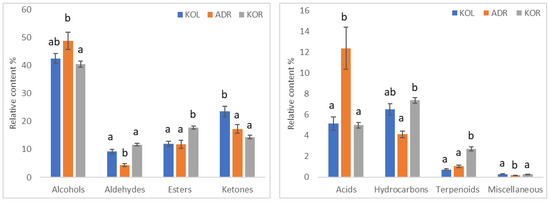
Figure 2.
Comparison of the relative content (%) of the chemical classes of volatile compounds identified in EVOOs originated from various cultivars. KOL: Kolovi; ADR: Adramytini; KOR: Koroneiki. The error bars indicate standard errors at 95% confidence level. Data in the same class with different lowercase letters differ significantly (p < 0.05).
It is evident from Figure 1 that alcohols were the dominant chemical class within samples of the three geographical origins (~40–45%), followed by ketones (~12–22%), esters (~12–18%) and aldehydes (~8–12%). The content of the remaining chemical classes was lower (<9%). The content of alcohols was similar (p > 0.05) between samples from PEL and LES (43.9% and 44.5%, respectively), whereas a lower value (39.5%, p < 0.05) was observed in CRE samples. The opposite trend was evident when the content of aldehydes was examined. Samples from PEL and LES did not differ significantly (9.29% and 7.60%, respectively; p > 0.05), and samples from CRE displayed higher levels of aldehydes (12.1%, p < 0.05). The highest and lowest content of ketones (p < 0.05) was observed in the LES (22.4%) and CRE (12.3%) samples, respectively, whereas the LES samples displayed a lower value of esters (11.9%, p < 0.05) in comparison with samples from the other regions. Significant differences were also found in the minor chemical classes. Specifically, the acid content ranged from 4.3% to 6.5% (PEL and LES, respectively; p < 0.05). As far as hydrocarbons and terpenoids are concerned, the CRE samples displayed higher content (p < 0.05) than the samples from the other regions (8.7% for hydrocarbons and 4.0% for terpenoids), while the lowest content was observed in samples from LES (6.1% for hydrocarbons and 0.8% for terpenoids).
When the samples were studied according to their cultivar (Figure 2), the content of alcohols varied from 43% to 50%, ketones from 14% to 24%, esters from 12% to 18% and aldehydes between 4% and 11%. ADR and KOR cultivars differed significantly due to their alcohol content (49.5% and 41.9%, respectively; p < 0.05), with samples from ADR cultivar displaying higher levels. The highest and lowest content of ketones (p < 0.05) was observed in the KOL (23.5%) and KOR (14.4%) samples. On the contrary, the highest value of aldehyde content was evident in the KOR samples (11.5%) and the lowest value in the ADR samples (3.8%, p < 0.05). The KOR samples displayed a higher content of esters (17.8, p < 0.05), whereas the samples of KOL and ADR presented similar levels (12.0% and 11.8%). The same pattern was observed for terpenoids (KOL: 0.73%, ADR: 1.1% and KOR: 2.7%). The ADR samples displayed a much higher content of acids (p < 0.05) than KOL and KOR (12.4%, 5.16% and 5.0%, respectively) and the lowest content of hydrocarbons (4.13%, p < 0.05).
Similar studies regarding EVOO classification of Greek cultivars from various regions have shown that the most prominent volatile compounds are C6 aldehydes and alcohols, deriving from the LOX pathway [12], which was also confirmed in the present study. Kosma et al. [24] studied olive oil samples belonging to the Koroneiki cultivar from regions of the Peloponnese (Messenia and Lakonia) and Crete (Heraklion). In accordance with our results, they reported that the alcohol content of the samples from Crete was slightly lower than that of the samples from the Peloponnese and contained higher levels of hydrocarbons. However, an opposite trend than the one found in our study was reported for ketones and esters, which were present at slightly higher levels in Crete samples. Kosma et al. [5] also studied olive oil samples from various Greek regions and different cultivars, including Koroneiki and Adramytini. As in our study, aldehydes and esters were present at higher levels in the Koroneiki samples. On the contrary, alcohols and ketones were found in slightly higher levels in the Adramytini samples.
3.2. Classification of EVOOs According to Cultivar
Multivariate analysis was also performed to differentiate EVOOs according to their cultivar. Samples were grouped into two classes: KOR (Koroneiki) and KOL (Kolovi), whereas the olive oils from the Adramytini variety were excluded from the analysis due to the very low number of samples. Partial least squares discriminant analysis (PLS-DA) resulted in a clear discrimination between the samples from KOR and KOL cultivars according to the scores plot shown in Figure 3a. The two factors explained the 89% (85%) of calibration (validation) variation in Y-variable (response). The volatile compounds (X-variables) that play an important role in the regression model were identified from the weighted regression coefficients (Bw) plot (Figure S1). Specifically, with a decreasing order of Bw magnitude, 4,8-dimethyl-1,3,7-nonatriene, 2-methylfuran, methyl benzoate, 6-methyl-5-hepten-2-one, 2-phenylethanol, heptanal, (E)-3-hexen-1-ol and (E & Z)-3-hexenal correlated positively with the KOR samples, whereas α-farnesene, methyl salicylate, 3-methyl-1-butanol, hexanal, 1-hexanol, 2-methyl-1-propanol, 2-ethylfuran, 2-ethyl-1-hexanol, D-limonene, 2-octanone and 2-methybutanal correlated positively with the KOL samples.
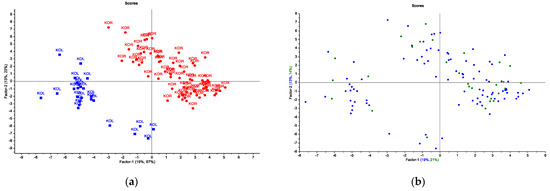
Figure 3.
Scores plot of EVOO samples obtained from partial least squares discriminant analysis (PLS−DA) using (a) full−cross validation (red circles: Koroneiki; blue squares: Kolovi cultivar) and (b) external validation (blue dots: calibration samples; green dots: validation samples).
To investigate how the prediction model will behave on unknown samples, a set of samples (25% of the total data set) was used to perform external validation and the results are presented in Figure 3b. It is evident that all the predicted samples (green) fall within the two groups defined by the model samples (blue). The same conclusion can be deduced by observing the plot of predicted values with their estimated uncertainties (Figure S2). The reliability of the prediction was checked by studying the Inliers vs. Hotelling’s T2 plot (Figure S3). In our case, the predicted samples fall inside the critical limit lines, and thus, the prediction is valid.
Clear classification between Greek olive oils from different cultivars has also been achieved by other researchers. Pouliarekou et al. [12] characterized olive oil samples of five cultivars from western Greece by volatile analysis combined with chemometrics. Some discriminant volatiles were like those of the present study: 3-pentanone, 3-ethyl-1,5-octadiene, hexyl acetate and 4,8-dimethyl-1,3,7-nonatriene. Kosma et al. [5] also managed to discriminate olive oil samples of Samothraki and Galano cultivars from those of Adramytini, Koroneiki, Ladolia Corfu and Athinolia with an overall classification rate 97%. Most of the chemical markers identified are also proposed by the current study.
Lukić et al. [39] managed to differentiate Croatian virgin olive oils according to variety based on volatile composition. Using multivariate analysis, 21 volatile compounds were proposed as markers of varietal origin. These included non-LOX-originating alcohols, hydrocarbons (such as 3-ethyl-1,5-octadiene and pentadiene isomers) as well as 1-penten-3-one and (Z)-2-hexenal. A clear discrimination between samples from different Spanish cultivars emerged from the research of Tomé-Rodriguez et al. [22]. The two first components of the PCA model explained 59.6% of the total variance and the most discriminating volatiles were 2-pentenal, (E)-2-hexenal, (E)-2-hexen-1-ol and 1-penten-3-ol.
3.3. Classification of EVOOs According to Geographical Origin
A second classification model was built by using only the Koroneiki samples to discriminate them according to their region of origin (Crete and the Peloponnese). The scores plot of the PLS-DA model is presented in Figure 4.
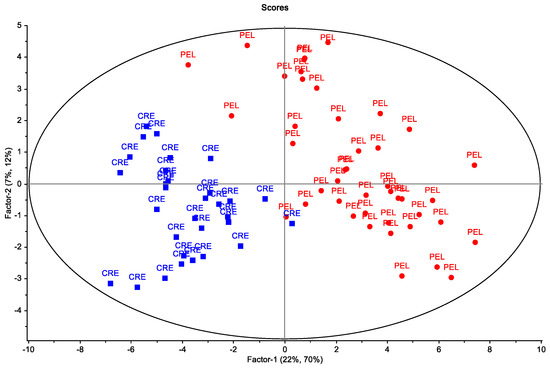
Figure 4.
Multivariate analysis of EVOO samples of Koroneiki cultivar from the Peloponnese (PEL) and Crete (CRE). PLS−DA scores plot (red dots—PEL; blue squares—CRE). The ellipse represents the Hotelling’s T2 critical limit.
It is evident that the samples from the two regions are separated satisfactorily in the two first factors, although there is a partial overlap. To overcome this problem, OPLS-DA was performed (Figure 5a). The advantage of OPLS-DA compared to PLS-DA is that the between-class separation is found only in the first predictive component (x-axis), whereas the uncorrelated variation (which represents the within-class variation) is indicated in the orthogonal components [19]. The OPLS-DA model can classify the samples into the correct geographic region. The goodness-of-fit parameters for the OPLS model, R2X, R2Y and Q2Y, were equal to 0.577, 0.889 and 0.745, respectively. R2X and R2Y represent the proportion of the variance of X and Y variables explained by the model, whereas Q2Y indicates the predictive ability of the model. The closeness of R2Y and Q2Y values to one suggests a more stable and reliable model. The significance of class discrimination was verified by performing a permutation test (p < 0.01; 0/100) (Figure S4). According to the VIP (variable in projection) list of S-plot, a total of 28 volatiles with VIP > 1 were selected as the most contributing variables to class separation (Figure 5b). The nonparametric Kruskal–Wallis rank sum test was also run to confirm their significance (FDR < 0.05).
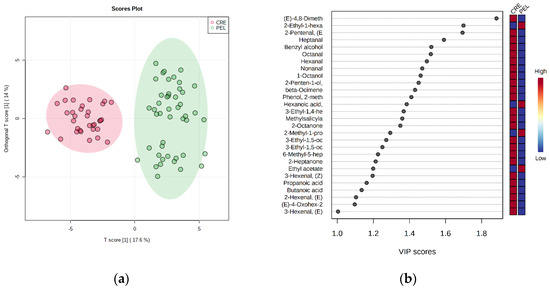
Figure 5.
(a) OPLS−DA scores plot (green dots—PEL; red dots—CRE). The colored ellipses represent the 95% confidence region. (b) Volatile compounds with VIP score > 1. The color bars on the right indicate the normalized content of each volatile compound in the respective class.
Subsequently, multivariate ROC curve analysis was performed to reduce the number of important volatile compounds and evaluate the performance of the models.
According to the ROC curves plot (Figure S5a) and the predictive accuracies plot (Figure S5b), the models with 5, 10, 20 and 28 volatile compounds have similar AUC (area under the curve) values, ranging from 0.920 to 0.934, and similar predictive accuracies, ranging from 86.5% to 87.8%. Thus, we selected the five-compound model, consisting of ethyl acetate, 2-methoxyphenol, 2-methyl-1-propanol, 4,8-dimethylnona-1,3,7-triene and 3-ethyl-1,5-octadiene (Figure S5c) for further ROC analysis. Then, two-thirds of samples were selected as a training–cross-validation set, and the remaining samples were used for external validation (hold-out set) purposes. The combination of the five selected volatile compounds could correctly predict the 88.3% of samples in the cross-validation (CV) set with the corresponding AUC equal to 0.955 and could correctly predict the 100% of samples in the hold-out set with the corresponding AUC equal to 1.00 (Figure 6).
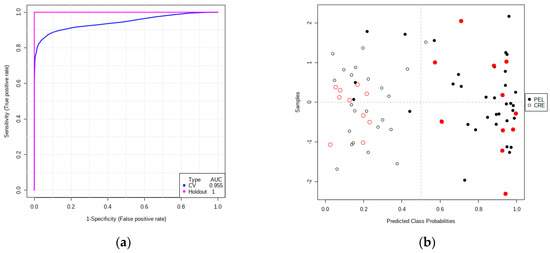
Figure 6.
(a) ROC curves from the combination of the potential biomarkers for the cross−validation (blue—CV) and external validation set (pink—hold-out). (b) Plot of the average predicted class probabilities for each olive oil sample from the Peloponnese (solid dots) and Crete (open dots). The red dots (solid and open) denote samples belonging to the external validation set. The classification boundary is located at the center (x = 0.5, the dotted line) because the algorithm uses a balanced sub-sampling approach.
Thus, the combination of the prospective biomarkers appears to have the potential to distinguish the Koroneiki EVOOs from the two geographic regions studied. However, a larger number of samples is required to confirm this finding.
Classification of Greek EVOOs according to geographical origin has also been reported in the literature. Kosma et al. [24], using multivariate analysis (LDA), differentiated olive oil samples from Heraklion (Crete) and Etoloakarnania from those originating from Lakonia and Messinia (Peloponnese) with an overall classification rate 93.2%. Pouliarekou et al. [12] discriminated satisfactorily olive oils from the Ionian Islands (Zakynthos, Kefalonia, Lefkada and Kerkyra) of western Greece. Some of the most discriminant compounds selected for the classification of the samples according to geographical origin, coinciding with those that emerged from the current study: 3-ethyl-1,5-octadiene and 4,8-dimethyl-1,3,7-nonatriene.
Apart from olive oil samples from Greek growing areas, the results occurring from other oil-producing countries are also promising. A chemometric approach for the discrimination of virgin olive oils produced in Italy, Spain, Greece, Portugal, Tunisia and others has been proposed from Cecchi et al. [32] with a very good prediction capability. Cajka et al. [13] successfully classified Ligurian olive oil samples from samples from other regions in Italy, Spain, France, Greece, Cyprus and Turkey. Nonetheless, a large inter-annual variability in volatile composition from specific regions was observed. Youssef et al. [23] also detected significant differences in the volatile fraction from oils of different geographical regions of Tunisia. Nonanal and octanal were correlated with specific classes as it was also observed in the study, but (Z)-3-hexenyl acetate and 2,4-heptadienal were also among the volatile compounds that played an important role in the classification model.
4. Conclusions
The application of SPME-GC/MS combined with chemometric analysis of EVOOs resulted in the detection of significant differences in the volatile composition from samples of different cultivar and geographical origin of two consecutive agronomical seasons. The differences were mainly quantitative and the association of C5 and C6 volatiles deriving from the LOX pathway in the discrimination of monovarietal EVOOs was obvious. The chemometric models developed were satisfactory in terms of performance and could correctly classify all samples of the external validation set. In addition, multivariate ROC curve analysis resulted in the selection of a model combining only five volatile compounds that could be useful for routine geographical origin assessment of Greek EVOOs. It is noteworthy that the high performance of the model was achieved using oil samples produced under a real scenario, which implies the model can cope with the wide range of different cultivation and oil extraction practices employed by the local producers.
It is important to highlight that factors other than cultivar and geographic origin could also affect the discrimination of EVOOs, but the results clearly showed that these two factors played a key role. The approach and the results presented in this study have the capacity to deliver impact directly to Greek and Mediterranean society as it is focused on olive oil authenticity and traceability, an issue relevant for the majority of the food industry. The work aims to attribute an added value to EVOO, as an emblematic product of Greek and Mediterranean countries for its unique taste and properties. However, more systematic research consisting of a larger set of samples from various cultivars, geographical origin and harvesting periods would further support the results acquired from the current work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11020080/s1, Table S1: Information about olive variety, region and sub-region of production, harvesting year for all the samples collected and analyzed. Table S2: Volatile composition of EVOOs according to the olive tree cultivar. The values represent the mean content (%) of the samples in each variety. Data in the same row with different lowercase letters differ significantly (p < 0.05). ADR: Adramytini variety; KOL: Kolovi variety; KOR: Koroneiki variety. Figure S1: Weighted regression coefficients (Bw) plot of volatile compounds identified in EVOOs from Koroneiki and Kolovi cultivar. The error bars denote uncertainty limits that correspond to 2 standard deviations. Variables with uncertainty limits that do not cross the zero line are significant (shaded bars) for class (cultivar) separation. Figure S2: Prediction with the external validation data set. The predicted Y value 0 corresponds to the KOR variety and the predicted Y value 1 to KOL variety. The predicted response of the validation samples is shown with a red horizontal line. The vertical red lines indicate the root mean squared error (RMSE). The blue box spans the deviation in both directions and is an estimate of the prediction uncertainty. Figure S3: Inliers vs. Hotelling’s T2 plot of the predicted olive oil samples (blue dots) used in external validation of PLS-DA model. The associated critical limits (with a p-value of 5%) are displayed as horizontal and vertical red lines. Figure S4: Permutation test of the OPLS-DA model. Figure S5: (a) ROC curves of all models resulted from the automated combination of 2, 3, 5, 10, 20 and 28 volatile compounds. The legend presents the AUC value and the corresponding confidence limits (CLs) for each model. (b) Plot of the predicted accuracies of each model with an increasing number of features (volatile compounds). The most accurate model is highlighted with a red dot. (c) Plot of the most important features of the selected model, ranked from most important to least important.
Author Contributions
Conceptualization, A.M., C.G. and M.K.; methodology, A.M.; formal analysis, T.M. and A.M.; investigation, T.M., A.P. and M.L.; data curation, A.M., C.G. and T.M.; writing—original draft preparation, T.M.; writing—review and editing, A.M. and C.G.; supervision, A.M., C.G. and M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been financed by Greek national funds through the Public Investments Program (PIP) of General Secretariat for Research & Technology (GSRT), under the Emblematic Action “The Olive Road” (project code: 2018SE01300000).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Boskou, D. Olive Fruit, Table Olives, and Olive Oil Bioactive Constituents. In Olive and Olive Oil Bioactive Constituents; AOCS Press: Urbana, IL, USA, 2015; pp. 1–30. [Google Scholar]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Beltrán, G.; Ruano, M.T.; Jiménez, A.; Uceda, M.; Aguilera, M.P. Evaluation of virgin olive oil bitterness by total phenol content analysis. Eur. J. Lipid Sci. Technol. 2007, 109, 193–197. [Google Scholar] [CrossRef]
- THE WORLD OF OLIVE OIL. Available online: https://www.internationaloliveoil.org/the-world-of-olive-oil/ (accessed on 3 January 2023).
- Kosma, I.; Badeka, A.; Vatavali, K.; Kontakos, S.; Kontominas, M. Differentiation of Greek extra virgin olive oils according to cultivar based on volatile compound analysis and fatty acid composition: Differentiation of Greek extra virgin olive oils. Eur. J. Lipid Sci. Technol. 2016, 118, 849–861. [Google Scholar] [CrossRef]
- Cecchi, T.; Alfei, B. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME–GC–MS: Newly identified compounds, flavors molecular markers, and terpenic profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The Compounds Responsible for the Sensory Profile in Monovarietal Virgin Olive Oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Angerosa, F. Virgin olive oil odour notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Sacchi, R. Temporal changes of virgin olive oil volatile compounds in a model system simulating domestic consumption: The role of biophenols. Food Res. Int. 2015, 77, 670–674. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Pouliarekou, E.; Badeka, A.; Tasioula-Margari, M.; Kontakos, S.; Longobardi, F.; Kontominas, M.G. Characterization and classification of Western Greek olive oils according to cultivar and geographical origin based on volatile compounds. J. Chromatogr. A 2011, 1218, 7534–7542. [Google Scholar] [CrossRef]
- Cajka, T.; Riddellova, K.; Klimankova, E.; Cerna, M.; Pudil, F.; Hajslova, J. Traceability of olive oil based on volatiles pattern and multivariate analysis. Food Chem. 2010, 121, 282–289. [Google Scholar] [CrossRef]
- Bajoub, A.; Bendini, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Olive oil authentication: A comparative analysis of regulatory frameworks with especial emphasis on quality and authenticity indices, and recent analytical techniques developed for their assessment. A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 832–857. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; Garcia-Gonzalez, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Angerosa, F.; Campestre, C.; Giansante, L. Analysis and authentication. In Olive Oil: Chemistry and Technology; Boscou, D., Ed.; AOCS Publishing: Champaign, IL, USA, 2006; pp. 113–154. [Google Scholar]
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Application of an advanced and wide scope non-target screening workflow with LC-ESI-QTOF-MS and chemometrics for the classification of the Greek olive oil varieties. Food Chem. 2018, 256, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Casas, B.; Bustamante, J.; Guardiola, F.; García-González, D.L.; Barbieri, S.; Bendini, A.; Toschi, T.G.; Vichi, S.; Tres, A. Virgin olive oil volatile fingerprint and chemometrics: Towards an instrumental screening tool to grade the sensory quality. LWT 2020, 121, 108936. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-Based Metabolomics Data for Identification of Biochemically Interesting Compounds Using OPLS Class Models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Pérez, A.G.; de la Rosa, R.; Pascual, M.; Sánchez-Ortiz, A.; Romero-Segura, C.; León, L.; Sanz, C. Assessment of volatile compound profiles and the deduced sensory significance of virgin olive oils from the progeny of Picual×Arbequina cultivars. J. Chromatogr. A 2016, 1428, 305–315. [Google Scholar] [CrossRef]
- Romero, I.; García-González, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Validation of SPME–GCMS method for the analysis of virgin olive oil volatiles responsible for sensory defects. Talanta 2015, 134, 394–401. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Cultivar influence on the volatile components of olive oil formed in the lipoxygenase pathway. LWT 2021, 147, 111485. [Google Scholar] [CrossRef]
- Youssef, O.; Guido, F.; Manel, I.; Youssef, N.B.; Luigi, C.P.; Mohamed, H.; Daoud, D.; Mokhtar, Z. Volatile compounds and compositional quality of virgin olive oil from Oueslati variety: Influence of geographical origin. Food Chem. 2011, 124, 1770–1776. [Google Scholar] [CrossRef]
- Kosma, I.; Vatavali, K.; Kontakos, S.; Kontominas, M.; Kiritsakis, A.; Badeka, A. Geographical Differentiation of Greek Extra Virgin Olive Oil from Late-Harvested Koroneiki Cultivar Fruits. J. Am. Oil Chem. Soc. 2017, 94, 1373–1384. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Marin, M.; Guardiola, F.; García-González, D.L.; Barbieri, S.; Bendini, A.; Gallina Toschi, T.; Vichi, S.; Tres, A. Supporting the Sensory Panel to Grade Virgin Olive Oils: An In-House-Validated Screening Tool by Volatile Fingerprinting and Chemometrics. Foods 2020, 9, 1509. [Google Scholar] [CrossRef] [PubMed]
- Ruisánchez, I.; Jiménez-Carvelo, A.M.; Callao, M.P. ROC curves for the optimization of one-class model parameters. A case study: Authenticating extra virgin olive oil from a Catalan protected designation of origin. Talanta 2021, 222, 121564. [Google Scholar] [CrossRef] [PubMed]
- Stilo, F.; Jiménez-Carvelo, A.M.; Liberto, E.; Bicchi, C.; Reichenbach, S.E.; Cuadros-Rodríguez, L.; Cordero, C. Chromatographic Fingerprinting Enables Effective Discrimination and Identitation of High-Quality Italian Extra-Virgin Olive Oils. J. Agric. Food Chem. 2021, 69, 8874–8889. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; García-González, D.L.; Morales, M.T.; Lobo-Prieto, A.; Romero, I. Comparison of two analytical methods validated for the determination of volatile compounds in virgin olive oil: GC-FID vs GC-MS. Talanta 2018, 187, 133–141. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J.J.; Wishart, D.S. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Rossetti, A.; Cane, A.; Mulinacci, N.; Melani, F. Authentication of the geographical origin of virgin olive oils from the main worldwide producing countries: A new combination of HS-SPME-GC-MS analysis of volatile compounds and chemometrics applied to 1217 samples. Food Control 2020, 112, 107156. [Google Scholar] [CrossRef]
- Kiritsakis, A.K. Flavor components of olive oil—A review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Fernandez, X.; Lizzani-Cuvelier, L.; Loiseau, A.-M. Characterization of volatile compounds of French and Spanish virgin olive oils by HS-SPME: Identification of quality-freshness markers. Food Chem. 2004, 88, 151–157. [Google Scholar] [CrossRef]
- Salas, J.J.; Harwood, J.L.; Martínez-Force, E. Lipid Metabolism in Olive: Biosynthesis of Triacylglycerols and Aroma Components. In Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 97–127. [Google Scholar]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, O.; Guerfel, M.; Baccouri, B.; Cerretani, L.; Bendini, A.; Lercker, G.; Zarrouk, M.; Daoud Ben Miled, D. Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem. 2008, 109, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Castellote, A.I.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; López-Tamames, E. Analysis of virgin olive oil volatile compounds by headspace solid-phase microextraction coupled to gas chromatography with mass spectrometric and flame ionization detection. J. Chromatogr. A 2003, 983, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Carlin, S.; Horvat, I.; Vrhovsek, U. Combined targeted and untargeted profiling of volatile aroma compounds with comprehensive two-dimensional gas chromatography for differentiation of virgin olive oils according to variety and geographical origin. Food Chem. 2019, 270, 403–414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).