HS-SPME-GC/MS Method for the Simultaneous Determination of Trihalomethanes, Geosmin, and 2-Methylisoborneol in Water Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling Procedure
2.3. HS-SPME-GC/MS Method
2.3.1. Chromatographic Conditions
2.3.2. HS-SPME Procedure
2.4. HS-SPME-GC/MS Method Validation
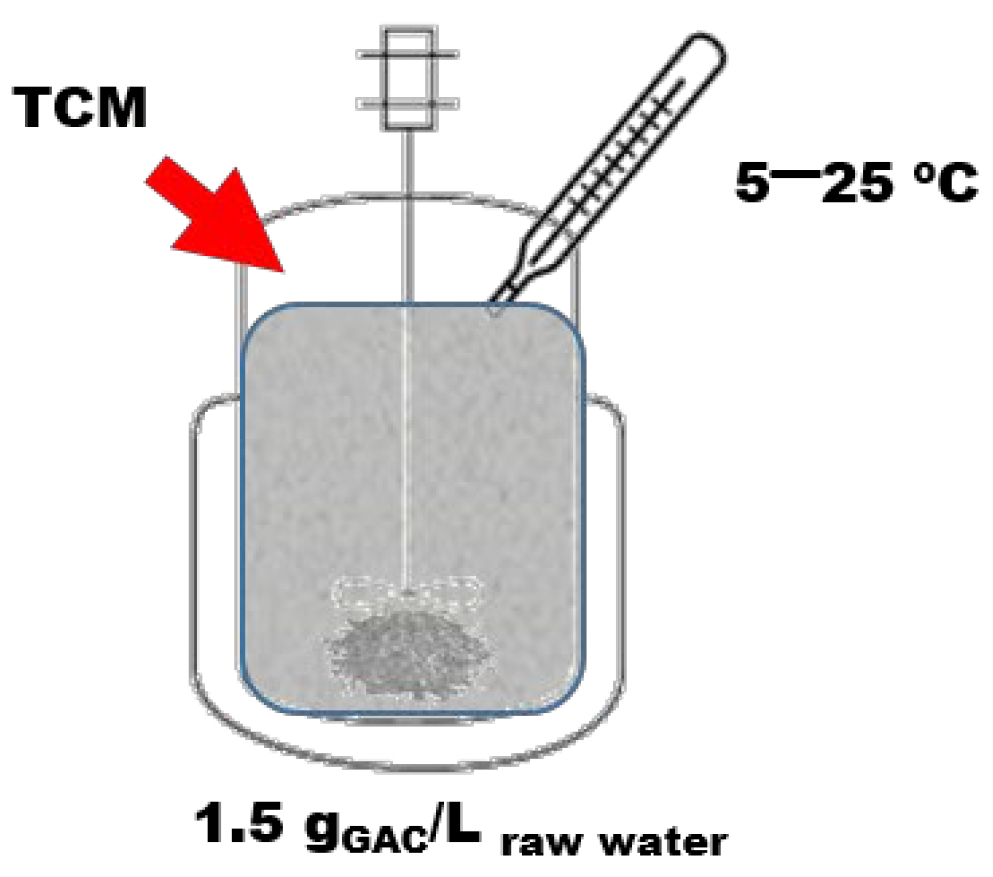
2.5. Parametric Analysis on GAC Sorption Capacity
3. Results and Discussion
3.1. HS-SPME-GC/MS Method
3.2. HS-SPME-GC/MS Method Validation
3.3. Samples Analysis
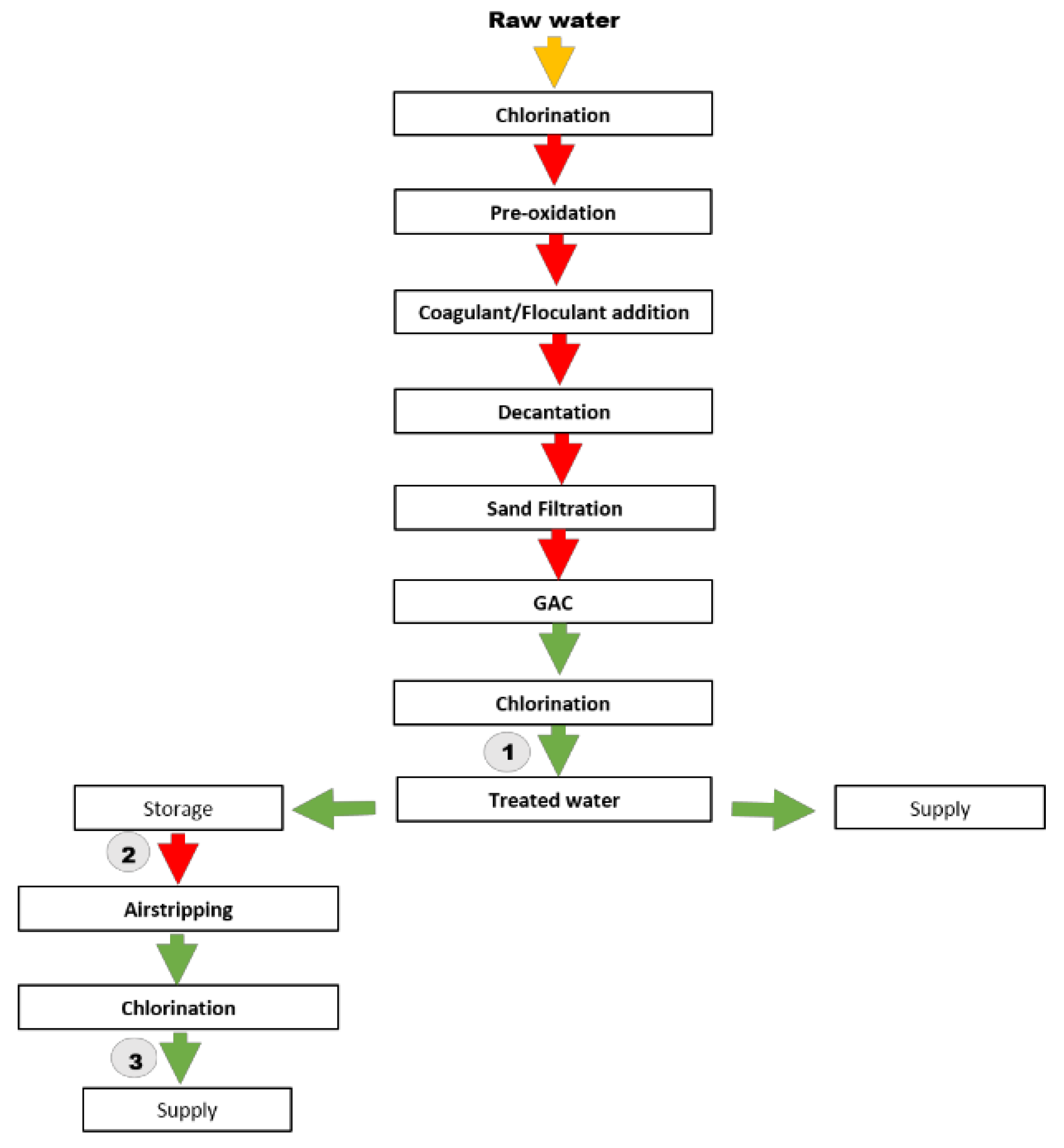
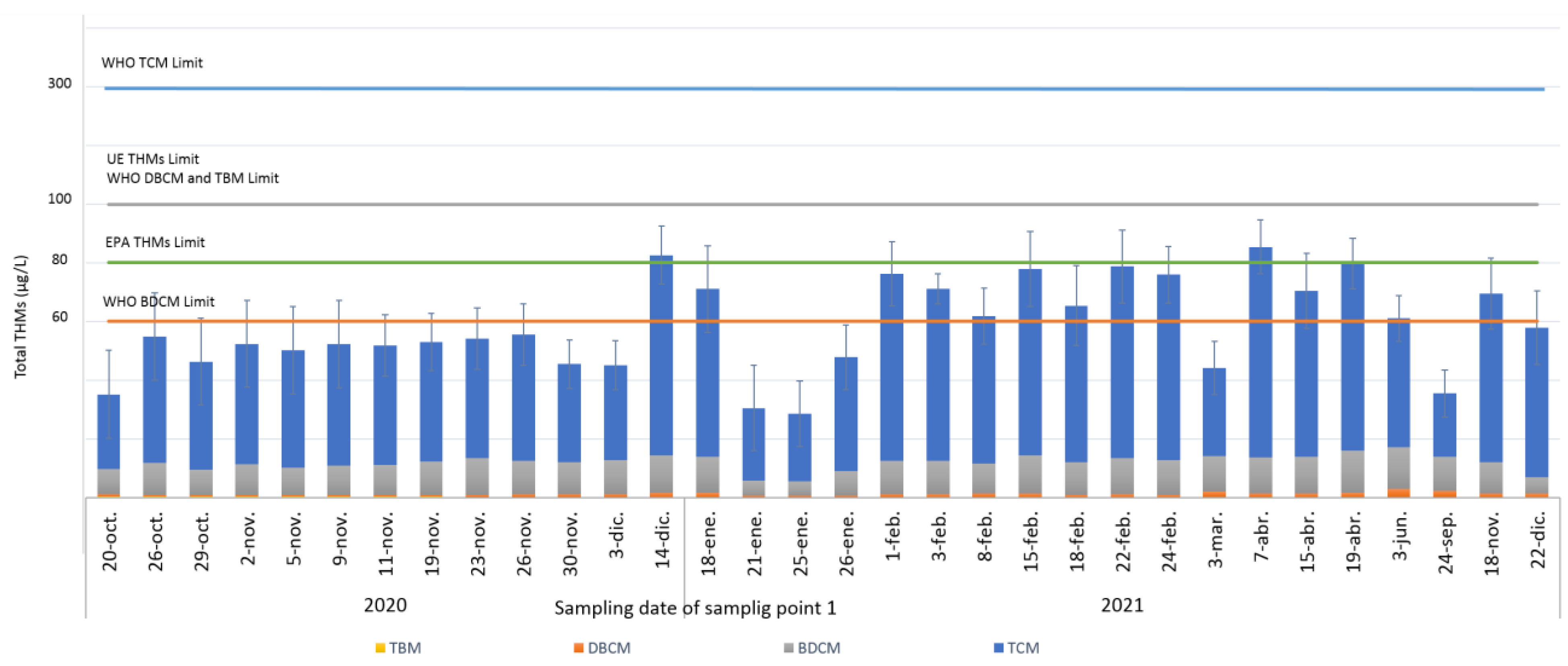
3.3.1. Application of HS-SPME-GC/MS Method to the Analysis of Raw Water
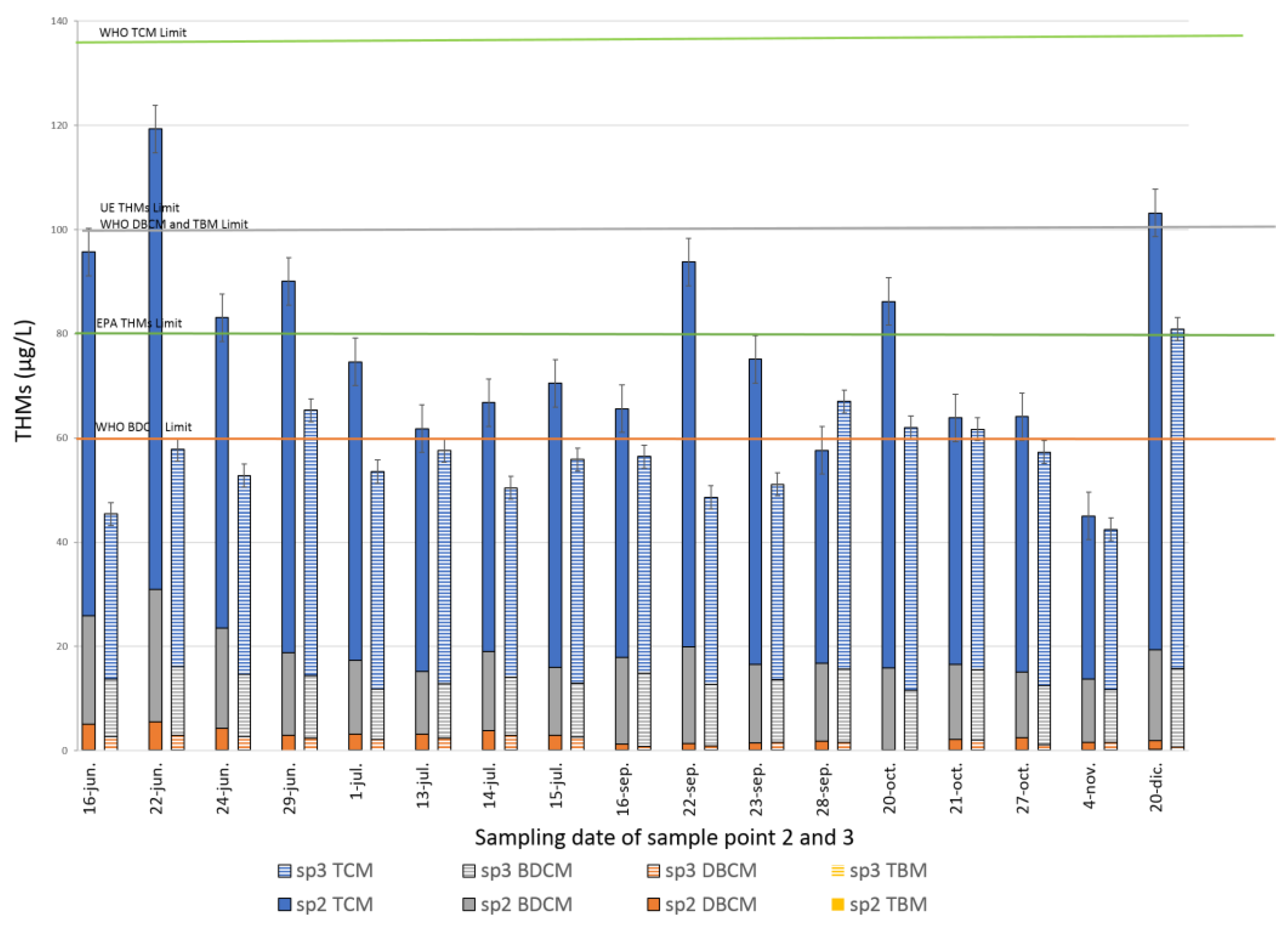
3.3.2. Application of HS-SPME-GC/MS Method to the Analysis of Water from DWTP
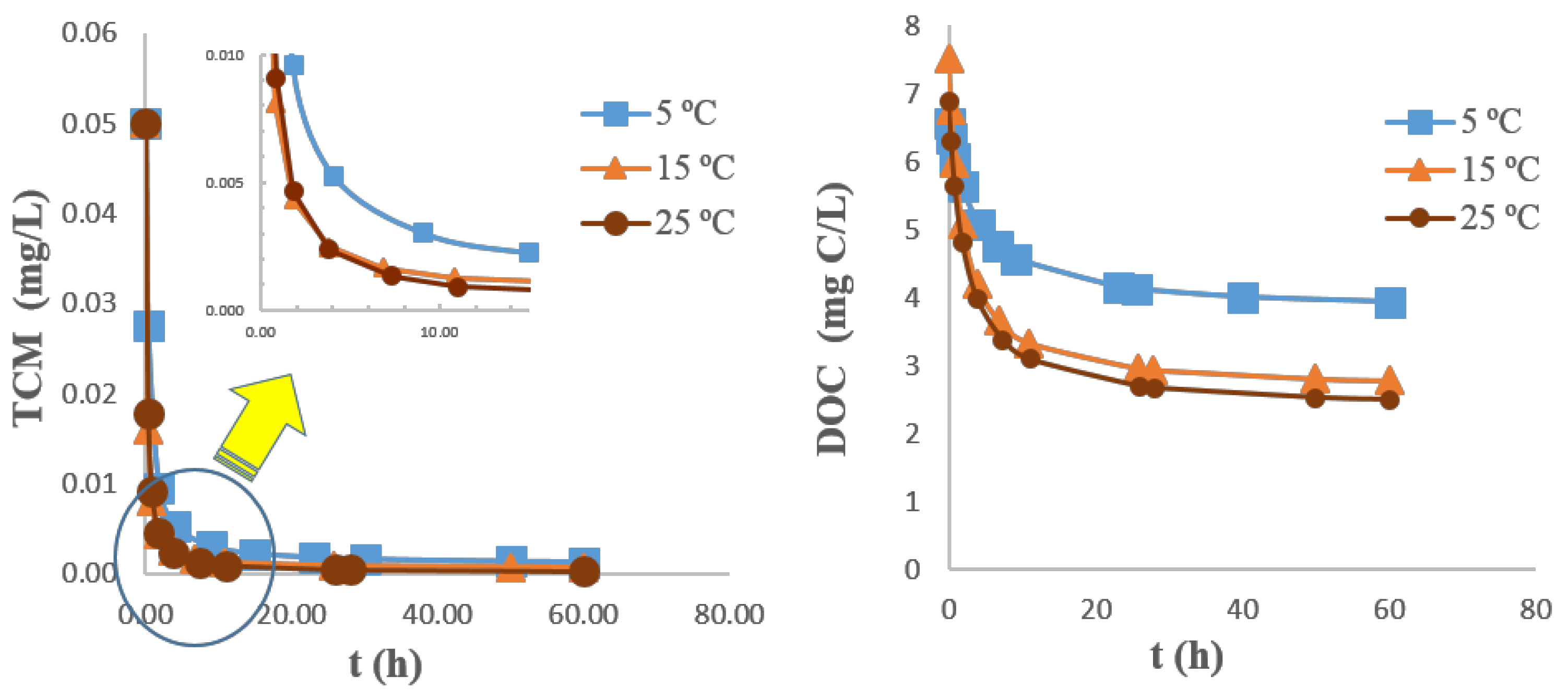
3.3.3. Parametric Analysis on GAC Sorption Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García Vázquez, R.; Astillero Pinilla, M.J.; Onaindia Olalde, C. Contaminantes Procedentes del Tratamiento de las Aguas de Consumo en la CAPV. Análisis de los Tratamientos y de las Variables que Influyen en la Formación de Subproductos en la CAPV. Gob. Vasco. 2011, 1–14. Available online: https://www.euskadi.eus/contenidos/informacion/2012_osteba_publicacion/es_def/adjuntos/D-12-01_WEB%20_%20aguas%20de%20consumo.pdf (accessed on 20 December 2022).
- Pérez Pavón, J.L.; Herrero Martín, S.; García Pinto, C.; Moreno Cordero, B. Determination of trihalomethanes in water samples: A review. Anal. Chim. Acta 2008, 629, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Directiva 98/83/CE del Consejo de 3 de Noviembre de 1998 Relativa a la Calidad de las Aguas Destinadas al Consumo Humano. Diario Oficial de las Comunidades Europeas L 330/1998. pp. 32–54. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2000-82524 (accessed on 20 December 2022).
- United States Environmental Protection Agency. Stage 2 Disinfectants and Disinfection Byproducts Rule (Stage 2 DBPR); EPA: Washington, DC, USA, 71 FR 388; 2006; Volume 71, pp. 388–493.
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 427.
- De Boer, S.; González-Rodríguez, J.; Conde, J.J.; Moreira, M.T. Benchmarking tertiary water treatments for the removal of micropollutants and pathogens based on operational and sustainability criteria. J. Water Process Eng. 2022, 46, 102587. [Google Scholar] [CrossRef]
- Babi, K.G.; Koumenides, K.M.; Nikolaou, A.D.; Makri, C.A.; Tzoumerkas, F.K.; Lekkas, T.D. Pilot study of the removal of THMs, HAAs and DOC from drinking water by GAC adsorption. Desalination 2007, 210, 215–224. [Google Scholar] [CrossRef]
- Hammond, D.; Murri, A.; Mastitsky, S.; Yang, Z.; Foster, R.; Schweitzer, L. Geosmin reduction by algaecide application to drinking water: Field scale efficacy and mechanistic insights. Heliyon 2021, 7, e07706. [Google Scholar] [CrossRef]
- Soyluoglu, M.; Kim, D.; Zaker, Y.; Karanfil, T. Removal mechanisms of geosmin and MIB by oxygen nanobubbles during water treatment. Chem. Eng. J. 2022, 443, 136535. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sorial, G.A. Treatment of taste and odor causing compounds 2-methyl isoborneol and geosmin in drinking water: A critical review. J. Environ. Sci. 2011, 23, 1–13. [Google Scholar] [CrossRef]
- World Health Organization. Management of Cyanobacteria in Drinking-Water Supplies: Information for Regulators and Water Suppliers. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 28 August 2022).
- Department of Economic and Social Affairs. Sustainable Development. Available online: https://www.sdgs.un.org (accessed on 28 August 2022).
- Rahnama Kozani, R.; Assadi, Y.; Shemirani, F.; Milani Hosseini, M.; Jamali, M. Determination of Trihalomethanes in Drinking Water by Dispersive Liquid–Liquid Microextraction then Gas Chromatography with Electron-Capture Detection. Chromatographia 2007, 66, 81–86. [Google Scholar] [CrossRef]
- Sá, C.S.A.; Boaventura, R.A.R.; Pereira, I.B. Analysis of trihalomethanes in water and air from indoor swimming pools using HS-SPME/GC/ECD. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2011, 46, 355–363. [Google Scholar] [CrossRef]
- Alexandrou, L.D.; Spencer, M.J.S.; Morrison, P.D.; Meehan, B.J.; Jones, O.A.H. Micro versus macro solid phase extraction for monitoring water contaminants: A preliminary study using trihalomethanes. Sci. Total Environ. 2015, 512–513, 210–214. [Google Scholar] [CrossRef]
- Franco, E.; Pádua, V.; Giani, A.; Rodríguez, M.; Silva, D.; Ferreira, A.; Júnior, I.; Pereira, M.; Rodrigues, J. Validation of a robust LLE-GC-MS method for determination of trihalomethanes in environmental samples. Environ. Monit. Assess. 2018, 190, 1–9. [Google Scholar] [CrossRef]
- Valencia, S.; Marín, J.; Restrepo, G. Method for trihalomethane analysis in drinking water by solid-phase microextraction with gas chromatography and mass spectrometry detection. Water Supply 2013, 13, 499–506. [Google Scholar] [CrossRef]
- Ruiz-Bevia, F.; Fernandez-Torres, M.J.; Blasco-Alemany, M.P. Purge efficiency in the determination of trihalomethanes in water by purge-and-trap gas chromatography. Anal. Chim. Acta 2009, 632, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Merib, J.; Simão, V.; Dias, A.N.; Carasek, E. Simultaneous determination of trihalomethanes and organochlorine pesticides in water samples by direct immersion-headspace-solid phase microextraction. J. Chromatogr. A 2013, 1321, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.S.; Martendal, E.; Carasek, E. Determination of THMs in soft drink by solid-phase microextraction and gas chromatography. Food Chem. 2011, 127, 290–295. [Google Scholar] [CrossRef]
- Alexandrou, L.D.; Meehan, B.J.; Morrison, P.D.; Jones, O.A.H. A New Method for the Fast Analysis of Trihalomethanes in Tap and Recycled Waters Using Headspace Gas Chromatography with Micro-Electron Capture Detection. Int. J. Environ. Res. Public Health 2017, 14, 527. [Google Scholar] [CrossRef]
- Peverly, A.A.; Peters, D.G. Electrochemical Determination of Trihalomethanes in Water by Means of Stripping Analysis. Anal. Chem. 2012, 84, 6110–6115. [Google Scholar] [CrossRef]
- Zavar, M.H.A.; Rounaghi, G.H.; Chamsaz, M.; Ashraf, N. Determination of trihalomethanes in tap water by UV-Vis spectrophotometry. Asian J. Chem. 2009, 21, 2903–2910. [Google Scholar]
- Ma, X.; Gao, N.; Chen, B.; Li, Q.; Zhang, Q.; Gu, G. Detection of geosmin and 2-methylisoborneol by liquid-liquid extraction-gas chromatograph mass spectrum (LLE-GCMS) and solid phase extraction-gas chromatograph mass spectrum (SPE-GCMS). Front. Environ. Sci. Eng. China 2007, 1, 286–291. [Google Scholar] [CrossRef]
- Cortada, C.; Vidal, L.; Canals, A. Determination of geosmin and 2-methylisoborneol in water and wine samples by ultrasound-assisted dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 17–22. [Google Scholar] [CrossRef]
- Salemi, A.; Lacorte, S.; Bagheri, H.; Barceló, D. Automated trace determination of earthy-musty odorous compounds in water samples by on-line purge-and-trap–gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1136, 170–175. [Google Scholar] [CrossRef]
- Parinet, J.; Rodriguez, M.J.; Serodes, J.; Proulx, F. Automated analysis of geosmin, 2-methyl-isoborneol, 2-isopropyl-3-methoxypyrazine, 2-isobutyl-3-methoxypyrazine and 2,4,6-trichloroanisole in water by SPME-GC-ITDMS/MS. Int. J. Environ. Anal. Chem. 2011, 91, 505–515. [Google Scholar] [CrossRef]
- Saito, K.; Okamura, K.; Kataoka, H. Determination of musty odorants, 2-methylisoborneol and geosmin, in environmental water by headspace solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2008, 1186, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Daishima, S. Simultaneous determination of 22 volatile organic compounds, methyl- tert-butyl ether, 1,4-dioxane, 2-methylisoborneol and geosmin in water by headspace solid phase microextraction-gas chromatography–mass spectrometry. Anal. Chim. Acta 2005, 548, 79–85. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Method 8260D (SW-846): Volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); revision 3; United States Environmental Protection Agency: Washington, DC, USA, 2006.

| Parameter | Procedure | Criteria | |
|---|---|---|---|
| Linearity | 7 calibration points 0.01–300 µg/L for THMs 0.005–0.05 µg/L for T&Os | r2 > 0.99 %RSD < 20 | |
| LOD, LOQ | 10 MilliQ water blanks | %R 80–120 (for LOQ) | SignalLOD = SignalBlank + 3·sBlank SignalLOQ = SignalBlank + 10·sBlank |
| Selectivity | % Analyte chromatographic area in the blank < 5% of its LOQ chromatographic area | ||
| Carry-Over | 1 MilliQ water blank | No contamination from the higher sample concentration Injected before the blank | |
| Accuracy | 5 replicas intra-day 3 replicas inter-day | %R 80–120 | |
| Repeatability | %RSD < 20 | ||
| Matrix effect | Chromatographic area from solvent standards versus matrix matched standards SD < 20% | ||
| Método | Parámetros | |
|---|---|---|
| HS-SPME | Pre-extraction t (s) | 180 |
| Pre-extraction T (°C) | 55 | |
| Stirring speed extraction (rpm) | 500 | |
| Extraction t (s) | 600 | |
| Extraction T (°C) | 55 | |
| Desorption T (°C) | 200 | |
| Desorption time (s) | 120 | |
| Conditioning t of the fiber after injection (s) | 420 | |
| Analyte | Characteristic Ions (m/z) |
|---|---|
| TCM | 83, 47, 85 |
| BDCM | |
| DBCM | 129, 79, 127 |
| TBM | 173, 79, 81 |
| 2-MIB | 107, 95, 135 |
| GM | 112, 111, 125 |
| DIM | 141, 127, 134 |
| IB | 95, 110, 121 |
| DHN | 112, 111, 125 |
| Analyte/IS | Linear Range (µg/L) | Equation | r2 |
|---|---|---|---|
| TCM/DIM | 0.8–50 | y = (1.95 ± 0.01) x − (1.8 ± 0.4) | 0.999 |
| BDCM/DIM | 0.05–20 | y = (3.76 ± 0.08) x − (2.3 ± 0.9) | 0.999 |
| DBCM/DIM | 0.01–20 | y = (7.4 ± 0.2) x − (4.0 ± 2.0) | 0.999 |
| TBM/DIM | y = (12.2 ± 0.1) x − (5.0 ± 1.0) | 0.999 | |
| 2-MIB/IB | 0.005–0.05 | y = (0.0225 ± 0.0007) x + (0.06 ± 0.02) | 0.995 |
| GM/DHN | y = (0.256 ± 0.00)5 x − (0.4 ± 0.1) | 0.998 |
| Analyte | LOD (µg/L) | LOQ (µg/L) | Repeatability (%RSD) | Accuracy (%R) | ||
|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | |||
| TCM | 0.374 | 0.798 | 17 | 19 | 120 | 119 |
| BDCM | 0.022 | 0.050 | 12 | 10 | 100 | 100 |
| DBCM | 0.005 | 0.008 | 9 | 9 | 100 | 97 |
| TBM | 0.006 | 0.010 | 5 | 4 | 91 | 91 |
| 2-MIB | 0.001 | 0.005 | 11 | 7 | 93 | 99 |
| GM | 0.002 | 0.005 | 8 | 4 | 105 | 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardina, D.; Santamaria, A.; Alonso, M.L.; Bartolomé, L.; Alonso, R.M.; Maña, J.A.; Bilbao, E.; Lombraña, J.I.; Bartolome, M.; Hernando, L.M. HS-SPME-GC/MS Method for the Simultaneous Determination of Trihalomethanes, Geosmin, and 2-Methylisoborneol in Water Samples. Chemosensors 2023, 11, 84. https://doi.org/10.3390/chemosensors11020084
Pardina D, Santamaria A, Alonso ML, Bartolomé L, Alonso RM, Maña JA, Bilbao E, Lombraña JI, Bartolome M, Hernando LM. HS-SPME-GC/MS Method for the Simultaneous Determination of Trihalomethanes, Geosmin, and 2-Methylisoborneol in Water Samples. Chemosensors. 2023; 11(2):84. https://doi.org/10.3390/chemosensors11020084
Chicago/Turabian StylePardina, Diego, Asier Santamaria, María Luz Alonso, Luis Bartolomé, Rosa M. Alonso, Jon Ander Maña, Elisabeth Bilbao, Jose Ignacio Lombraña, Mikel Bartolome, and Luis M. Hernando. 2023. "HS-SPME-GC/MS Method for the Simultaneous Determination of Trihalomethanes, Geosmin, and 2-Methylisoborneol in Water Samples" Chemosensors 11, no. 2: 84. https://doi.org/10.3390/chemosensors11020084
APA StylePardina, D., Santamaria, A., Alonso, M. L., Bartolomé, L., Alonso, R. M., Maña, J. A., Bilbao, E., Lombraña, J. I., Bartolome, M., & Hernando, L. M. (2023). HS-SPME-GC/MS Method for the Simultaneous Determination of Trihalomethanes, Geosmin, and 2-Methylisoborneol in Water Samples. Chemosensors, 11(2), 84. https://doi.org/10.3390/chemosensors11020084










