Mass-Mediated Phase Modulation of Thin Molybdenum Nitride Crystals on a Liquid Cu-Mo Alloy
Abstract
1. Introduction
2. Materials and Methods
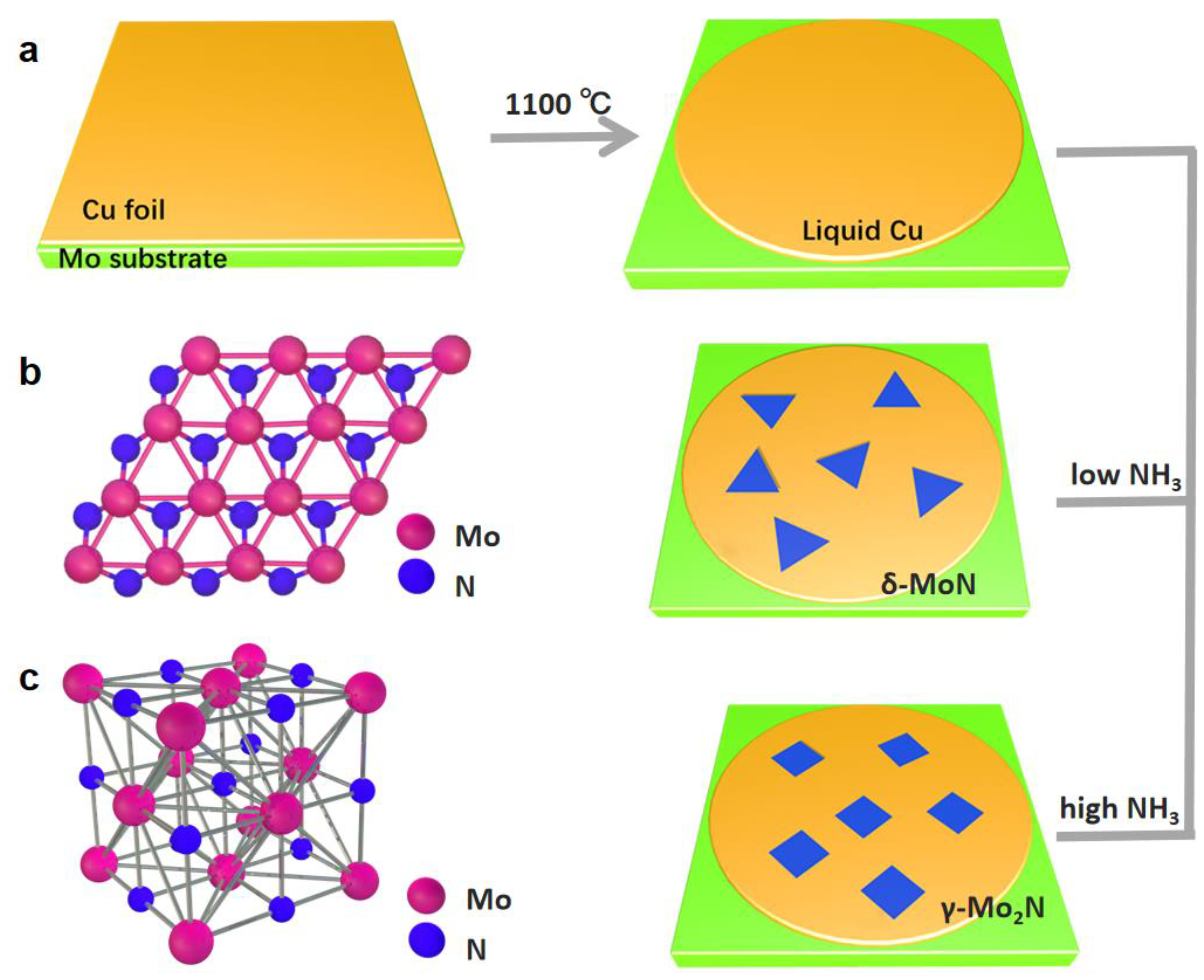
2.1. Selective Preparation of Thin MoxNy Crystals via CVD
2.2. Transfer of Thin MoxNy Crystals
2.3. Quality and Structure Characterization of MoxNy Crystals
3. Results
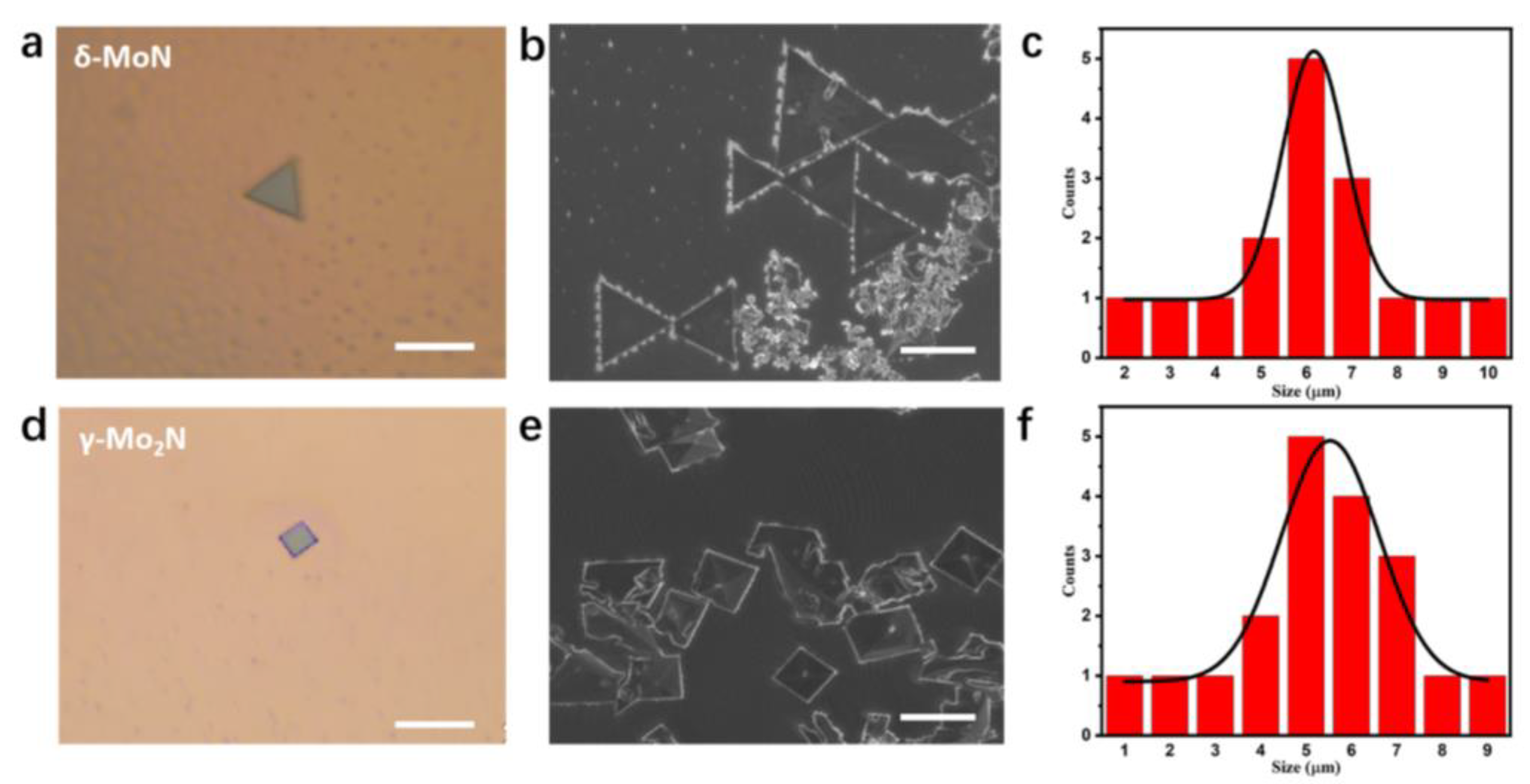
3.1. Phase Modulation of MoxNy Crystals
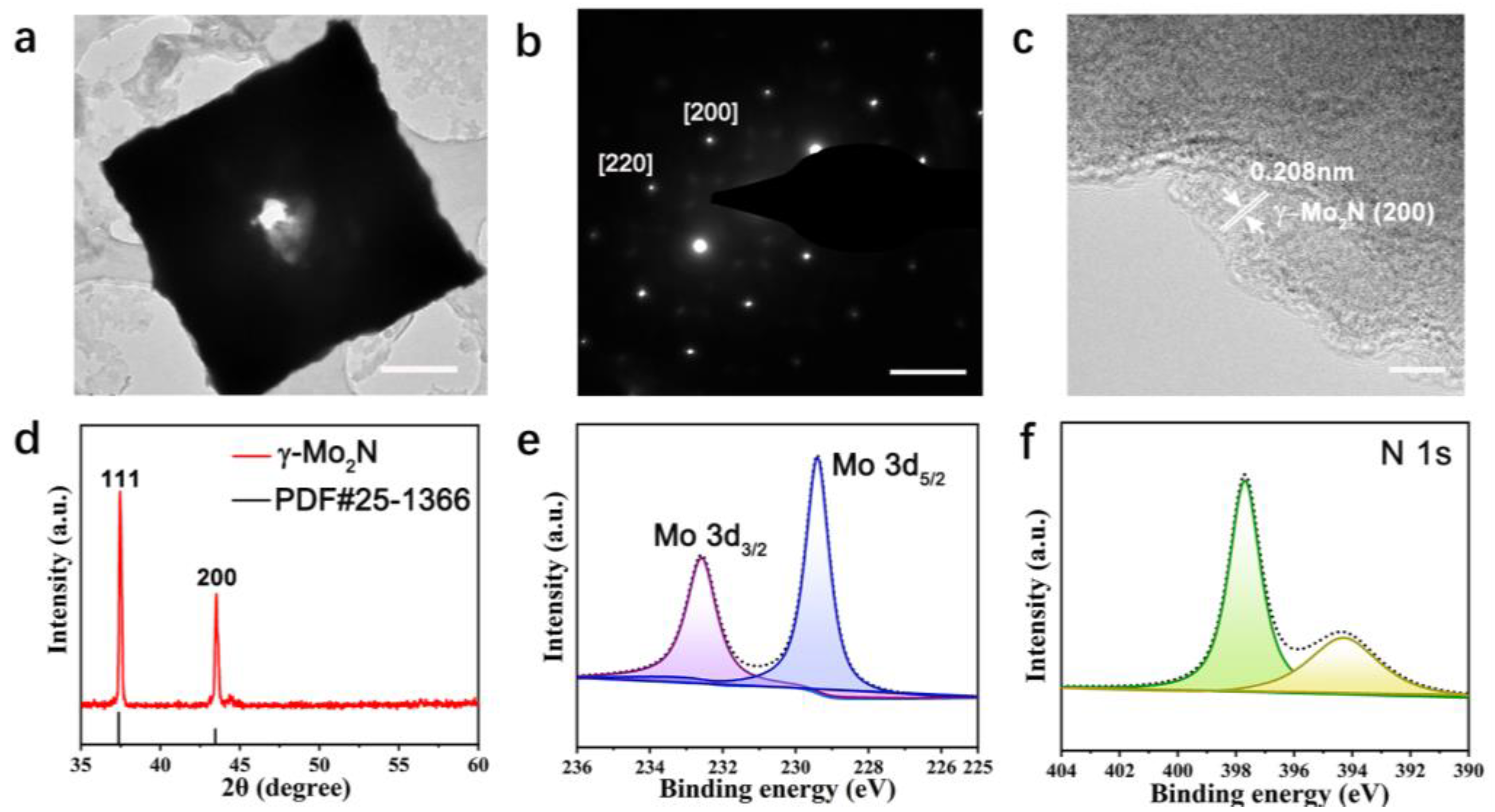
3.2. Quality and Structure Characterization of γ-Mo2N Crystals
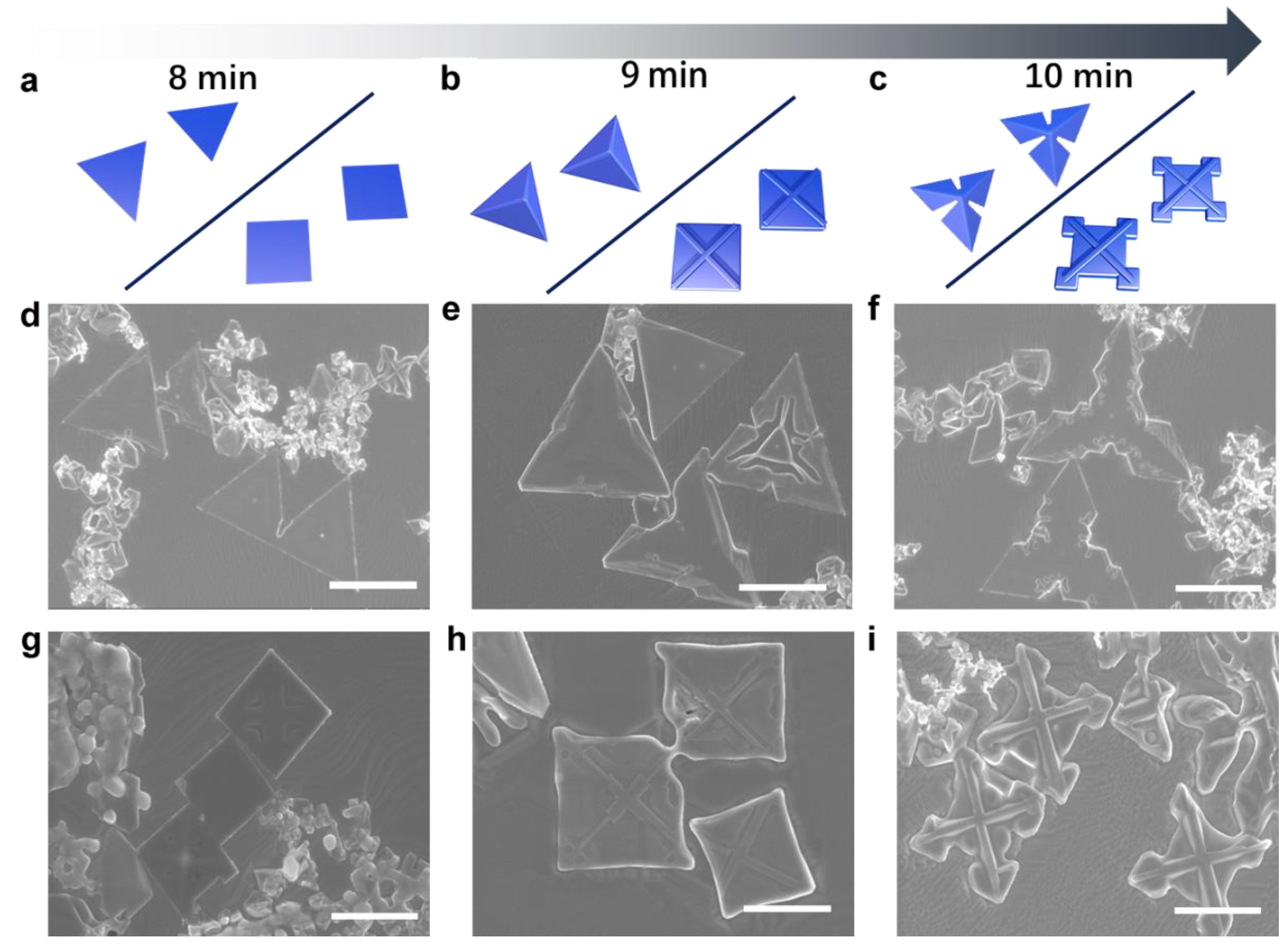
3.3. Morphology Evolution of δ-MoN and γ-Mo2N Crystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahid Mohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Zhang, C.; McKeon, L.; Kremer, M.P.; Park, S.H.; Ronan, O.; Seral-Ascaso, A.; Barwich, S.; Coileáin, C.Ó.; McEvoy, N.; Nerl, H.C.; et al. Additive-free MXene inks and direct printing of micro-supercapacitors. Nat. Commun. 2019, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.X.; Li, L.; Zhang, Y.; Zhang, X.T.; Geng, D.C.; Hu, W.P. Recent advances in growth of transition metal carbides and nitrides (MXenes) crystals. Adv. Funct. Mater. 2022, 32, 2111357. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, E.L.; Wang, Y.S.; Gao, C.S.; Wang, C.Y.; Li, L.; Geng, D.C.; Chen, H.P.; Chen, W.; Hu, W.P. Ultralow-power Vertical Transistors for Multilevel Decoding Modes. Adv. Mater. 2022, in press. [Google Scholar] [CrossRef]
- Jaf, Z.N.; Miran, H.A.; Jiang, Z.T.; Altarawneh, M. Molybdenum nitrides from structures to industrial applications. Rev. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, Y.H. Recent Progress in MXene Layers Materials for Supercapacitors: High-performance Electrodes. SmartMater. 2022; in press. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.T.; Zhao, S.J.; Wu, Z.G. High-entropy carbide ceramics: A perspective review. Tungsten 2021, 3, 131–142. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Ding, K.; Huang, C.; Li, Q.; Chu, P.K.; Huo, K. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
- Choi, D.; Kumta, P.N. Synthesis and characterization of nanostructured niobium and molybdenum nitrides by a two-step transition metal halide approach. J. Am. Ceram. Soc. 2011, 94, 2371–2378. [Google Scholar] [CrossRef]
- Liu, J.; Tang, S.S.; Lu, Y.K.; Cai, G.M.; Liang, S.Q.; Wang, W.J.; Chen, X.L. Synthesis of Mo2N nanolayer coated MoO2 hollow nanostructures as high-performance anode materials for lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2691–2697. [Google Scholar] [CrossRef]
- McKay, D.; Hargreaves, J.S.J.; Rico, J.L.; Rivera, J.L.; Sun, X.L. The influence of phase and morphology of molybdenum nitrides on ammonia synthesis activity and reduction characteristics. J. Solid State Chem. 2008, 181, 325–333. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Lamey, D.; Kiwi-Minsker, L.; Keane, M.A. Molybdenum nitrides: A study of synthesis variables and catalytic performance in acetylene hydrogenation. J. Mater. Sci. 2018, 53, 6707–6718. [Google Scholar] [CrossRef]
- Kreider, M.E.; Stevens, M.B.; Liu, Y.; Patel, A.M.; Statt, M.J.; Gibbons, B.M.; Gallo, A.; Ben-Naim, M.; Mehta, A.; Davis, R.C.; et al. Nitride or oxynitride? Elucidating the composition–activity relationships in molybdenum nitride electrocatalysts for the oxygen reduction reaction. Chem. Mater. 2020, 32, 2946–2960. [Google Scholar] [CrossRef]
- Tagliazucca, V.; Schlichte, K.; Schüth, F.; Weidenthaler, C. Molybdenum-based catalysts for the decomposition of ammonia: In situ X-ray diffraction studies, microstructure, and catalytic properties. J. Catal. 2013, 305, 277–289. [Google Scholar] [CrossRef]
- Klimashin, F.F.; Koutná, N.; Euchner, H.; Holec, D.; Mayrhofer, P.H. The impact of nitrogen content and vacancies on structure and mechanical properties of Mo–N thin films. J. Appl. Phys. 2016, 120, 185301. [Google Scholar] [CrossRef]
- Dongil, A.B. Recent progress on transition metal nitrides nanoparticles as heterogeneous catalysts. Nanomaterials 2019, 9, 1111. [Google Scholar] [CrossRef]
- Baek, S.H.; Yun, K.; Kang, D.C.; An, H.; Park, M.B.; Shin, C.H.; Min, H.K. Characteristics of high surface area molybdenum nitride and its activity for the catalytic decomposition of ammonia. Catalysts 2021, 11, 192. [Google Scholar] [CrossRef]
- Hao, Z.X.; Wei, Z.B.; Wang, L.J.; Li, X.H.; Li, C.; Min, E.Z.; Xin, Q. Selective hydrogenation of ethyne on γ-Mo2N. Appl. Catal. A-Gen. 2000, 192, 81–84. [Google Scholar] [CrossRef]
- Jaf, Z.N.; Altarawneh, M.; Miran, H.A.; Jiang, Z.T.; Dlugogorski, B.Z. Mechanisms governing selective hydrogenation of acetylene over γ-Mo2N surfaces. Catal. Sci. Technol. 2017, 7, 943–960. [Google Scholar] [CrossRef]
- Wyvratt, B.M.; Gaudet, J.R.; Pardue, D.B.; Marton, A.; Rudić, S.; Mader, E.A.; Cundari, T.R.; Mayer, J.M.; Thompson, L.T. Reactivity of hydrogen on and in nanostructured molybdenum nitride: Crotonaldehyde hydrogenation. ACS Catal. 2016, 6, 5797–5806. [Google Scholar] [CrossRef]
- Jujjuri, S.; Cárdenas-Lizana, F.; Keane, M.A. Synthesis of group VI carbides and nitrides: Application in catalytic hydrodechlorination. J. Mater. Sci. 2014, 49, 5406–5417. [Google Scholar] [CrossRef]
- Jaf, Z.N.; Altarawneh, M.; Miran, H.A.; Jiang, Z.T.; Dlugogorski, B.Z. Hydrodesulfurization of thiophene over γ-Mo2N catalyst. Mol. Catal. 2018, 459, 21–30. [Google Scholar] [CrossRef]
- Jaf, Z.N.; Altarawneh, M.K.; Miran, H.A.; Almatarneh, M.H.; Jiang, Z.T.; Dlugogorski, B.Z. Catalytic hydrogenation of p-chloronitrobenzene to p-chloroaniline mediated by γ-Mo2N. ACS Omega 2018, 3, 14380–14391. [Google Scholar] [CrossRef]
- Altarawneh, M.K.; Jaf, Z.N.; Oskierski, H.; Jiang, Z.T.; Gore, J.; Dlugogorski, B.Z. Conversion of NO into N2 over γ-Mo2N. J. Phys. Chem. C 2016, 120, 22270–22280. [Google Scholar] [CrossRef]
- Zaman, S.F.; Pasupulety, N.; Al-Zahrani, A.A.; Daous, M.A.; Al-Shahrani, S.S.; Driss, H.; Petrov, L.A.; Smith, K.J. Carbon monoxide hydrogenation on potassium promoted Mo2N catalysts. Appl. Catal. A-Gen. 2017, 532, 133–145. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J. Heterogeneous catalysis with metal nitrides. Coord. Chem. Rev. 2013, 257, 2015–2031. [Google Scholar] [CrossRef]
- Ganin, A.Y.; Kienle, L.; Vajenine, G.V. Synthesis and characterisation of hexagonal molybdenum nitrides. J. Solid State Chem. 2006, 179, 2339–2348. [Google Scholar] [CrossRef]
- Marchand, R.; Tessier, F.; DiSalvo, F.J. New routes to transition metal nitrides: And characterization of new phases. J. Mater. Chem. A 1999, 9, 297–304. [Google Scholar] [CrossRef]
- Geng, D.C.; Zhao, X.X.; Chen, Z.X.; Sun, W.W.; Fu, W.; Chen, J.Y.; Liu, W.; Zhou, W.; Loh, K.P. Direct synthesis of large-area 2D Mo2C on in situ grown graphene. Adv. Mater. 2017, 29, 1700072. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.C.; Zhao, X.X.; Li, L.J.; Song, P.; Tian, B.B.; Liu, W.; Chen, J.Y.; Shi, D.; Lin, M.; Zhou, W.; et al. Controlled growth of ultrathin Mo2C superconducting crystals on liquid Cu surface. 2D Mater. 2016, 4, 011012. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.B.; Liu, Z.B.; Chen, L.; Guo, J.K.; Kang, N.; Ma, X.L.; Cheng, H.M.; Ren, W.C. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.L.; Liu, Z.; Wang, L.; Zhou, T.; Ma, W.; Xu, C.; Feng, S.; Chen, L.; Chen, M.L.; Sun, D.M.; et al. Chemical vapor deposition of layered two-dimensional MoSi2N4 materials. Science 2020, 369, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Liu, D.; Wu, B.; Wang, J.M.; Zhang, L.Z.; Chen, C.; Su, Y.Y.; Yin, H.Y.; Zhang, J.N.; Gogotsi, Y.; et al. Ultrafast growth of thin hexagonal and pyramidal molybdenum nitride crystals and films. ACS Mater. Lett. 2019, 1, 383–388. [Google Scholar] [CrossRef]
- Ma, Y.C.; Lu, S.; Han, G.R.; Liu, Y.; Chen, Z.P. Chemical vapor deposition of two-dimensional molybdenum nitride/graphene van der Waals heterostructure with enhanced electrocatalytic hydrogen evolution performance. Appl. Surf. Sci. 2022, 589, 152934. [Google Scholar] [CrossRef]
- Geng, D.C.; Wu, B.; Guo, Y.L.; Luo, B.R.; Xue, Y.Z.; Chen, J.Y.; Yu, G.; Liu, Y.Q. Fractal etching of graphene. J. Am. Chem. Soc. 2013, 135, 6431–6434. [Google Scholar] [CrossRef]
- Li, M.H.; Li, L.; Fan, Y.X.; Jiao, F.; Geng, D.C.; Hu, W.P. From top to down—Recent advances in etching of 2D materials. Adv. Mater. Interfaces 2022, 9, 2201334. [Google Scholar] [CrossRef]
- Ahmed, A.; Sharma, S.; Adak, B.; Hossain, M.M.; LaChance, A.M.; Mukhopadhyay, S.; Sun, L. Two-dimensional MXenes: New frontier of wearable and flexible electronics. InfoMat 2022, 4, e12295. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Qiao, R.; Liang, J.; Liu, C.; Zheng, B.; Liu, L.; Gao, P.; Jiao, Q.; Yu, D.; et al. Visualizing grain boundaries in monolayer MoSe2 using mild H2O vapor etching. Nano Res. 2018, 11, 4082–4089. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, Q.; Fan, Y.; Li, L.; Geng, D.; Hu, W. Mass-Mediated Phase Modulation of Thin Molybdenum Nitride Crystals on a Liquid Cu-Mo Alloy. Chemosensors 2023, 11, 82. https://doi.org/10.3390/chemosensors11020082
Li M, Zhang Q, Fan Y, Li L, Geng D, Hu W. Mass-Mediated Phase Modulation of Thin Molybdenum Nitride Crystals on a Liquid Cu-Mo Alloy. Chemosensors. 2023; 11(2):82. https://doi.org/10.3390/chemosensors11020082
Chicago/Turabian StyleLi, Minghui, Qing Zhang, Yixuan Fan, Lin Li, Dechao Geng, and Wenping Hu. 2023. "Mass-Mediated Phase Modulation of Thin Molybdenum Nitride Crystals on a Liquid Cu-Mo Alloy" Chemosensors 11, no. 2: 82. https://doi.org/10.3390/chemosensors11020082
APA StyleLi, M., Zhang, Q., Fan, Y., Li, L., Geng, D., & Hu, W. (2023). Mass-Mediated Phase Modulation of Thin Molybdenum Nitride Crystals on a Liquid Cu-Mo Alloy. Chemosensors, 11(2), 82. https://doi.org/10.3390/chemosensors11020082





