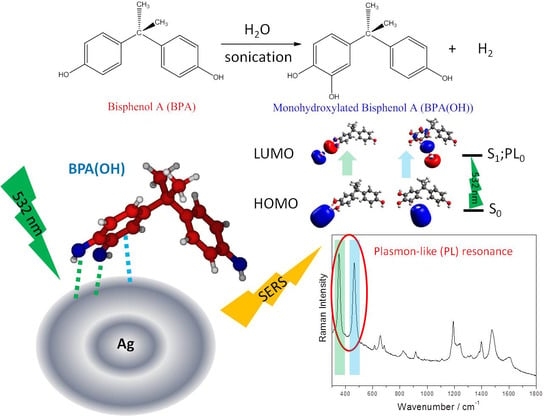
Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum
Abstract
1. Introduction
2. Materials and Methods
2.1. Colloidal AgNPs Experiments
2.2. Electrochemical Experiments
2.3. Instrumentation
2.4. Computational Details. Theoretical Resonance Raman Spectra
3. Results and Discussion
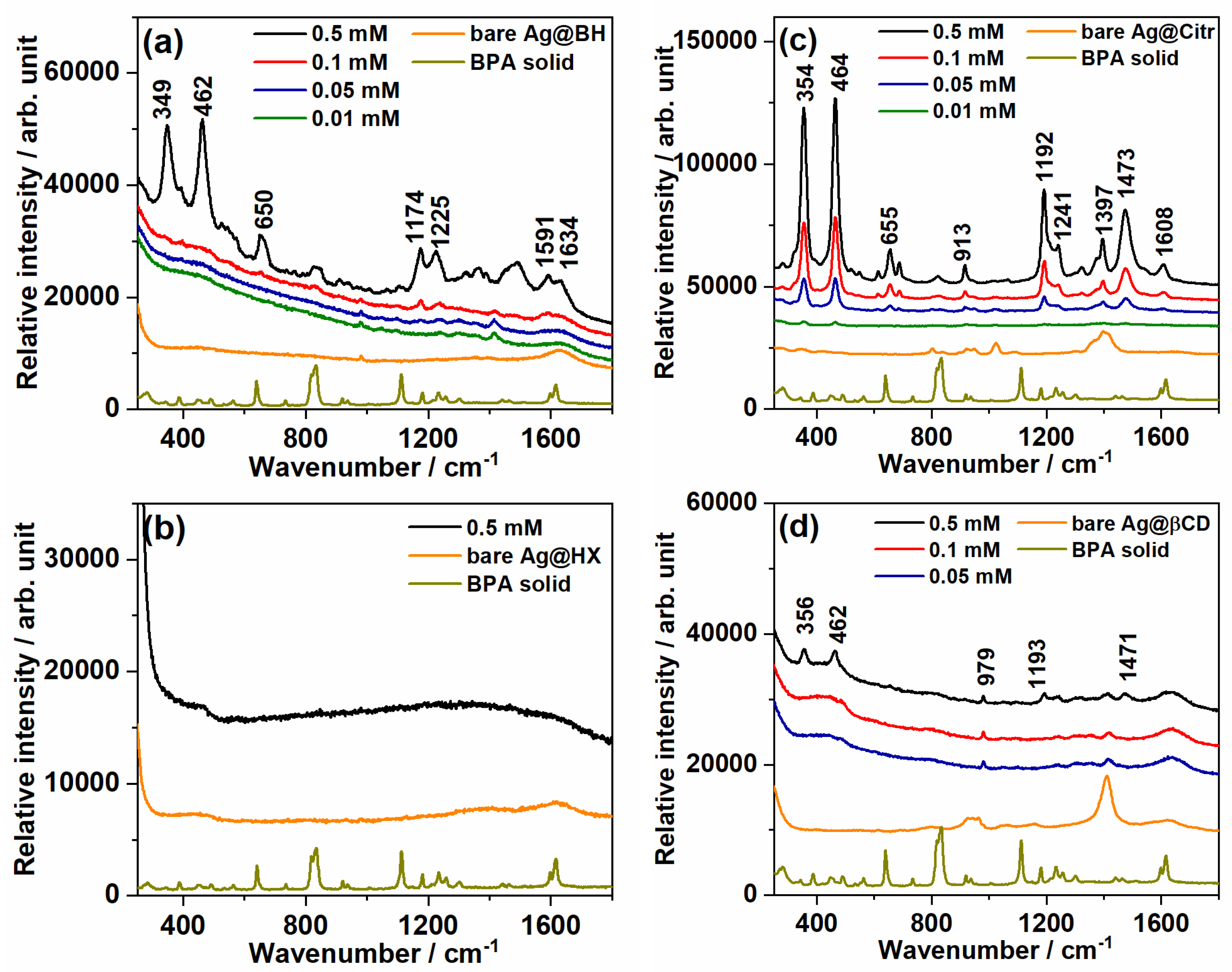
3.1. Standard AgNPs as a Chemical Sensor of BPA in a Sonicated Aqueous Solution
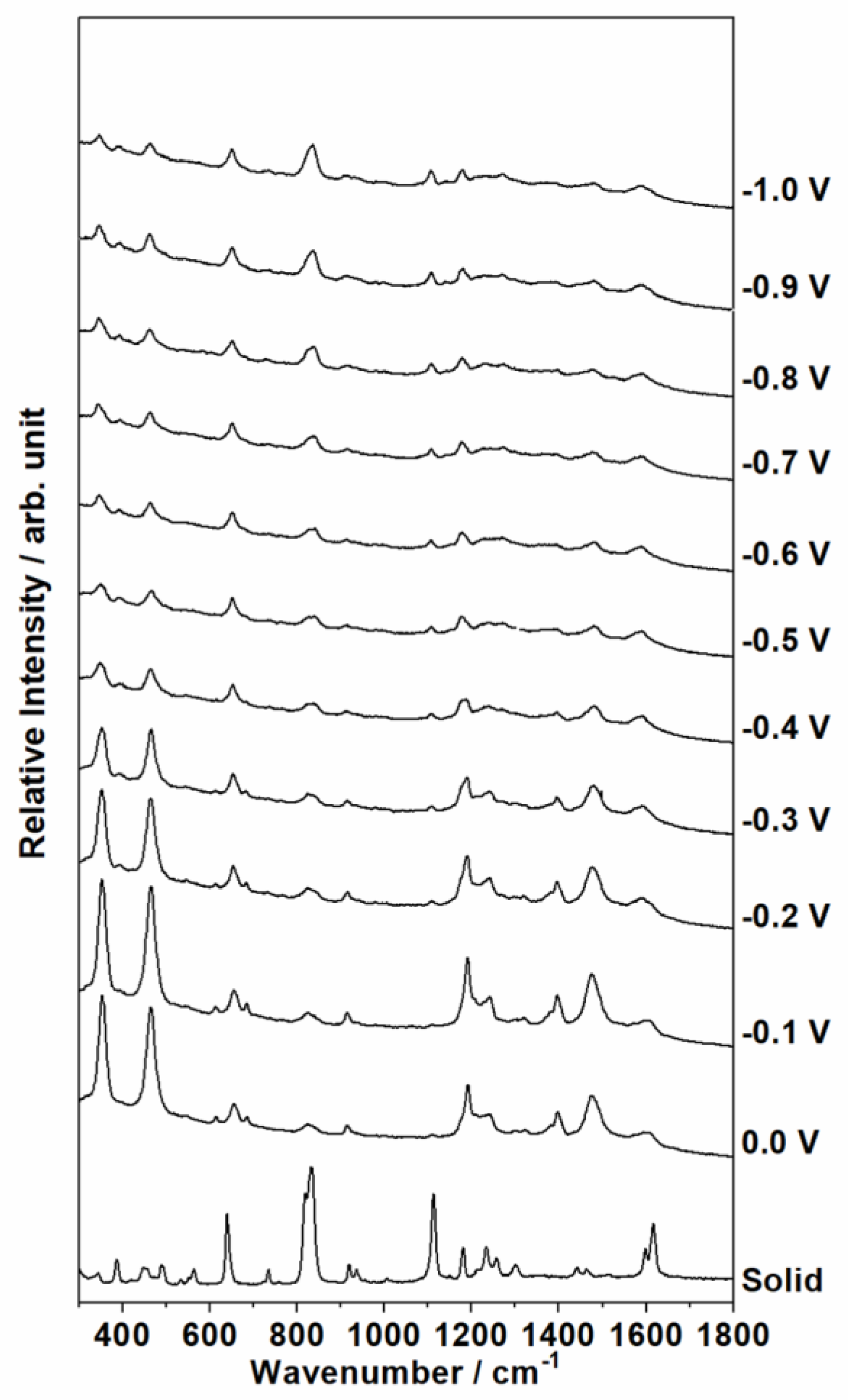
3.2. SERS Spectra of a Sonicated Aqueous Solution of BPA on Roughed Silver Electrode
3.3. DFT Calculations on Silver Coordination and Complexation Energy of the Ag2-BPA and Ag2-BPA(OH) Complexes
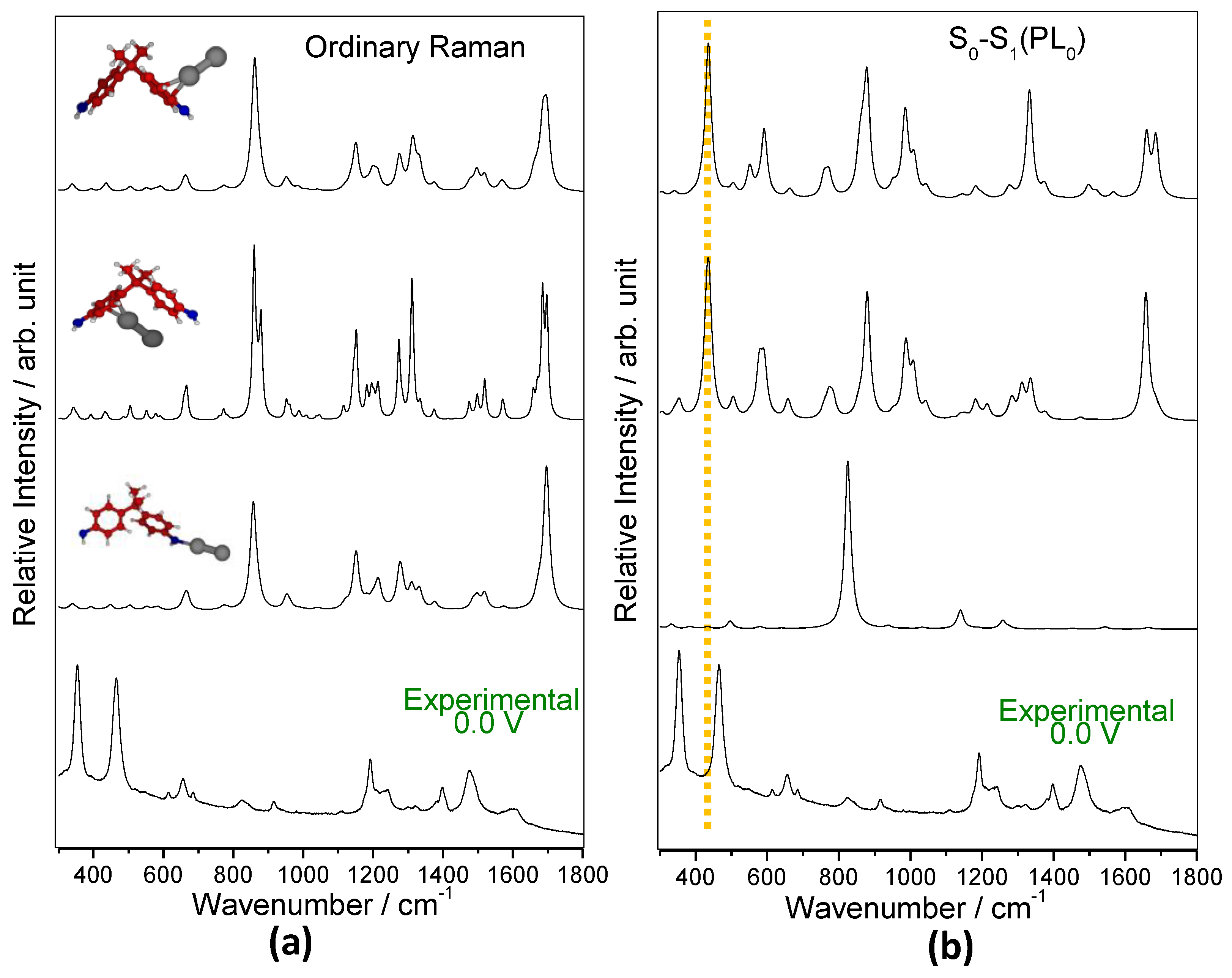
3.4. Insights into the Nature of SERS Enhancement by Analyzing TD-DFT Resonance Raman Spectra of Ag2-BPA and Ag2-BPA(OH) Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasiljevic, T.; Harner, T. Bisphenol A and Its Analogues in Outdoor and Indoor Air: Properties, Sources and Global Levels. Sci. Total Environ. 2021, 789, 148013. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Chakraborty, P. A Review on Sources and Health Impacts of Bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Foth, H.; Gebel, T.; Kramer, P.J.; Lilienblum, W.; Schweinfurth, H.; Völkel, W.; Wollin, K.M.; Gundert-Remy, U. Critical Evaluation of Key Evidence on the Human Health Hazards of Exposure to Bisphenol A. Crit. Rev. Toxicol. 2011, 41, 263–291. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; FAO. Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A. Final Report, Including Report of Stakeholder Meeting on Bisphenol A, 1–5 November 2010, Ottawa, Canada; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/44624 (accessed on 15 November 2022).
- Ragavan, K.V.; Rastogi, N.K.; Thakur, M.S. Sensors and Biosensors for Analysis of Bisphenol-A. TrAC Trends Analyt. Chem. 2013, 52, 248–260. [Google Scholar] [CrossRef]
- Sheng, W.; Duan, W.; Shi, Y.; Chang, Q.; Zhang, Y.; Lu, Y.; Wang, S. Sensitive Detection of Bisphenol A in Drinking Water and River Water Using an Upconversion Nanoparticles-Based Fluorescence Immunoassay in Combination with Magnetic Separation. Anal. Methods 2018, 10, 5313–5320. [Google Scholar] [CrossRef]
- Feng, Y.; Ning, B.; Su, P.; Wang, H.; Wang, C.; Chen, F.; Gao, Z. An Immunoassay for Bisphenol A Based on Direct Hapten Conjugation to the Polystyrene Surface of Microtiter Plates. Talanta 2009, 80, 803–808. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Rubio, S.; Pérez-Bendito, D. Analytical Methods for the Determination of Bisphenol A in Food. J. Chromatogr. A 2009, 1216, 449–469. [Google Scholar] [CrossRef]
- Sun, F.; Kang, L.; Xiang, X.; Li, H.; Luo, X.; Luo, R.; Lu, C.; Peng, X. Recent Advances and Progress in the Detection of Bisphenol A. Anal. Bioanal. Chem. 2016, 408, 6913–6927. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Martín-Bueno, J.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Methods of Bisphenol A Detection by Gas Chromatography and Mass Spectrometry (GC-Ms) in Human Breast Milk and Foodstuff. In Emerging Contaminants in the Environment: Challenges and Sustainable Practices; Sarma, H., Dominguez, D.C., Lee, W.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 18; pp. 465–493. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, D.; Huang, C.; Sun, Y.; Lee, Y.I. Sensitive Detection of Bisphenol A in Complex Samples by In-Column Molecularly Imprinted Solid-Phase Extraction Coupled with Capillary Electrophoresis. Microchem. J. 2015, 121, 1–5. [Google Scholar] [CrossRef]
- Yahaya, N.; Huang, Z.; Yan, B.; Chen, D.D.Y. Capillary Electrophoresis–Mass Spectrometry Analysis of Bisphenol A and Its Analogues in Bottled Tea Beverages with Dynamic PH Focusing. Food Chem. 2022, 372, 131220. [Google Scholar] [CrossRef]
- Shareef, A.; Angove, M.J.; Wells, J.D.; Johnson, B.B. Aqueous Solubilities of Estrone, 17β-Estradiol, 17α-Ethynylestradiol, and Bisphenol A. J. Chem. Eng. Data 2006, 51, 879–881. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Pilot, R. SERS Detection of Food Contaminants by Means of Portable Raman Instruments. J. Raman Spectrosc. 2018, 49, 954–981. [Google Scholar] [CrossRef]
- Furini, L.N.; Constantino, C.J.L.; Sanchez-Cortes, S.; Otero, J.C.; López-Tocón, I. Adsorption of Carbendazim Pesticide on Plasmonic Nanoparticles Studied by Surface-Enhanced Raman Scattering. J. Colloid. Interface Sci. 2016, 465, 183–189. [Google Scholar] [CrossRef]
- López-Tocón, I.; Otero, J.C.; Arenas, J.F.; García-Ramos, J.V.; Sánchez-Cortés, S. Trace Detection of Triphenylene by Surface Enhanced Raman Spectroscopy Using Functionalized Silver Nanoparticles with Bis-Acridinium Lucigenine. Langmuir 2010, 26, 6977–6981. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-Enhanced Raman Spectroscopy: A Brief Perspective. In Surface-Enhanced Raman Scattering. Topics in Applied Physics; Kneipp, K., Moskovits, M., Kneipp, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 103, pp. 1–17. [Google Scholar] [CrossRef]
- López-Tocón, I.; Otero, J.C.; Arenas, J.F.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Multicomponent Direct Detection of Polycyclic Aromatic Hydrocarbons by Surface-Enhanced Raman Spectroscopy Using Silver Nanoparticles Functionalized with the Viologen Host Lucigenin. Anal. Chem. 2011, 83, 2518–2525. [Google Scholar] [CrossRef]
- de Bleye, C.; Dumont, E.; Hubert, C.; Sacré, P.Y.; Netchacovitch, L.; Chavez, P.F.; Hubert, P.; Ziemons, E. A Simple Approach for Ultrasensitive Detection of Bisphenols by Multiplexed Surface-Enhanced Raman Scattering. Anal. Chim. Acta 2015, 888, 118–125. [Google Scholar] [CrossRef]
- Roschi, E.; Gellini, C.; Ricci, M.; Sanchez-Cortes, S.; Focardi, C.; Neri, B.; Otero, J.C.; López-Tocón, I.; Smulevich, G.; Becucci, M. Surface-Enhanced Raman Spectroscopy for Bisphenols Detection: Toward a Better Understanding of the Analyte–Nanosystem Interactions. Nanomaterials 2021, 11, 881. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zeng, Y.; Shen, A.G.; Hu, J.M. A Highly Sensitive SERS Probe for Bisphenol A Detection Based on Functionalized Au@Ag Nanoparticles. Anal. Methods 2018, 10, 5622–5628. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, S.K.; Kim, M.J.; Lee, S.W. Simple and Rapid Detection of Bisphenol A Using a Gold Nanoparticle-Based Colorimetric Aptasensor. Food Chem. 2019, 287, 205–213. [Google Scholar] [CrossRef]
- Liu, S.; Fu, Y.; Xiong, C.; Liu, Z.; Zheng, L.; Yan, F. Detection of Bisphenol A Using DNA-Functionalized Graphene Field Effect Transistors Integrated in Microfluidic Systems. ACS Appl. Mater. Interfaces 2018, 10, 23522–23528. [Google Scholar] [CrossRef]
- Guerrini, L.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Sensing Polycyclic Aromatic Hydrocarbons with Dithiocarbamate-Functionalized Ag Nanoparticles by Surface-Enhanced Raman Scattering. Anal. Chem. 2009, 81, 953–960. [Google Scholar] [CrossRef]
- Inoue, M.; Masuda, Y.; Okada, F.; Sakurai, A.; Takahashi, I.; Sakakibara, M. Degradation of Bisphenol A Using Sonochemical Reactions. Water Res. 2008, 42, 1379–1386. [Google Scholar] [CrossRef]
- Gültekin, I.; Ince, N.H. Ultrasonic Destruction of Bisphenol-A: The Operating Parameters. Ultrason. Sonochem. 2008, 15, 524–529. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.; Needham, L.L.; Calafat, A.M. In-Vitro Oxidation of Bisphenol A: Is Bisphenol A Catechol a Suitable Biomarker for Human Exposure to Bisphenol A? Anal. Bioanal. Chem. 2011, 399, 1071–1079. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, R. Ultrasonic Irradiation-Induced Degradation of Low-Concentration Bisphenol A in Aqueous Solution. J. Hazard. Mater. 2009, 163, 855–860. [Google Scholar] [CrossRef]
- López-Tocón, I.; Valdivia, S.; Soto, J.; Otero, J.C.; Muniz-Miranda, F.; Menziani, M.C.; Muniz-Miranda, M. A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles. Nanomaterials 2019, 9, 1211. [Google Scholar] [CrossRef]
- López-Tocón, I.; Imbarack, E.; Soto, J.; Sanchez-Cortes, S.; Leyton, P.; Otero, J.C. Intramolecular and Metal-to-Molecule Charge Transfer Electronic Resonances in the Surface-Enhanced Raman Scattering of 1,4-Bis((E)-2-(Pyridin-4-Yl)Vinyl)Naphthalene. Molecules 2019, 24, 4622. [Google Scholar] [CrossRef]
- Avila, F.; Ruano, C.; Lopez-Tocon, I.; Arenas, J.F.; Soto, J.; Otero, J.C. How the Electrode Potential Controls the Selection Rules of the Charge Transfer Mechanism of SERS. Chem. Commun. 2011, 47, 4213–4215. [Google Scholar] [CrossRef]
- Román-Pérez, J.; López-Tocón, I.; Castro, J.L.; Arenas, J.F.; Soto, J.; Otero, J.C. The Electronic Structure of Metal–Molecule Hybrids in Charged Interfaces: Surface-Enhanced Raman Selection Rules Derived from Plasmon-like Resonances. Phys. Chem. Chem. Phys. 2014, 17, 2326–2329. [Google Scholar] [CrossRef]
- Arenas, J.F.; López Tocón, I.; Otero, J.C.; Marcos, J.I. Charge Transfer Processes in Surface-Enhanced Raman Scattering. Franck−Condon Active Vibrations of Pyridine. J. Phys. Chem. 1996, 100, 9254–9261. [Google Scholar] [CrossRef]
- Yao, G.; Zhai, Z.; Zhong, J.; Huang, Q. DFT and SERS Study of 15N Full-Labeled Adenine Adsorption on Silver and Gold Surfaces. J. Phys. Chem. C 2017, 121, 9869–9878. [Google Scholar] [CrossRef]
- Yao, G.; Huang, Q. DFT and SERS Study of l-Cysteine Adsorption on the Surface of Gold Nanoparticles. J. Phys. Chem. C 2018, 122, 15241–15251. [Google Scholar] [CrossRef]
- de Souza, M.L.; Otero, J.C.; López-Tocón, I. Comparative Performance of Citrate, Borohydride, Hydroxylamine and β-Cyclodextrin Silver Sols for Detecting Ibuprofen and Caffeine Pollutants by Means of Surface-Enhanced Raman Spectroscopy. Nanomaterials 2020, 10, 2339. [Google Scholar] [CrossRef] [PubMed]
- Cañamares, M.V.; Garcia-Ramos, J.V.; Gómez-Varga, J.D.; Domingo, C.; Sanchez-Cortes, S. Comparative Study of the Morphology, Aggregation, Adherence to Glass, and Surface-Enhanced Raman Scattering Activity of Silver Nanoparticles Prepared by Chemical Reduction of Ag+ Using Citrate and Hydroxylamine. Langmuir 2005, 21, 8546–8553. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Pande, S.; Ghosh, S.K.; Praharaj, S.; Panigrahi, S.; Basu, S.; Jana, S.; Pal, A.; Tsukuda, T.; Pal, T. Synthesis of Normal and Inverted Gold−Silver Core−Shell Architectures in β-Cyclodextrin and Their Applications in SERS. J. Phys. Chem. C 2007, 111, 10806–10813. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Soto, J.; Imbarack, E.; López-Tocón, I.; Sánchez-Cortés, S.; Otero, J.C.; Leyton, P. Application of Surface-Enhanced Resonance Raman Scattering (SERS) to the Study of Organic Functional Materials: Electronic Structure and Charge Transfer Properties of 9,10-Bis((E)-2-(Pyridin-4-Yl)Vinyl)Anthracene. RSC Adv. 2019, 9, 14511–14519. [Google Scholar] [CrossRef]
- Valdivia, S.; Avila, F.J.; Otero, J.C.; López-Tocón, I. Voltage Selection of Physisorbed or Chemisorbed 4-Cyanobenzoate on a Nanostructured Silver Electrode and the Dual Electronic Structure of Charged Metal–Molecule Hybrids. Appl. Surf. Sci. 2022, 579, 152071. [Google Scholar] [CrossRef]
- Santoro, F.; Cerezo, J. FCclasses 3.0, A Code for Vibronic Calculations. 2021. Available online: http://www.iccom.cnr.it/en/fcclasses (accessed on 25 April 2022).
- Santoro, F.; Improta, R.; Lami, A.; Bloino, J.; Barone, V. Effective method tocompute Frank_condon integrals for optical spectra of large molecules in solution. J. Chem. Phys. 2007, 126, 084509. [Google Scholar] [CrossRef] [PubMed]
- Dirac, P.A.M. The quantum theory of dispersion. Proc. Math. Phys. Eng. 1927, 114, 710–728. [Google Scholar] [CrossRef]
- Albretch, A.C. On the theory of Raman intensities. J. Chem. Phys. 1961, 34, 1476–1484. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Schaftenaar, G.; Noordik, J.H. Molden: A Pre- and Post-Processing Program for Molecular and Electronic Structures. J. Comput. Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A Review of Dietary and Non-Dietary Exposure to Bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- EFSA. Bisphenol A: EFSA Draft Opinion Proposes Lowering the Tolerable Daily Intake; EFSA: Parma, Italy, 2021; Available online: https://www.efsa.europa.eu/en/news/bisphenol-efsa-draft-opinion-proposes-lowering-tolerable-daily-intake (accessed on 8 January 2023).
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]

| Coordination | |||
|---|---|---|---|
| Oxygen Atoms | Internal Face | External Face | |
| Ag2-BPA |  |  |  |
| (Kcal/mol) | −6.40 | −7.27 | −6.71 |
| Ag2-BPA(OH) |  |  |  |
| (Kcal/mol) | −8.41 | −7.08 | −7.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Souza, M.L.; Valdivia, S.; Otero, J.C.; López-Tocón, I. Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum. Chemosensors 2023, 11, 78. https://doi.org/10.3390/chemosensors11020078
De Souza ML, Valdivia S, Otero JC, López-Tocón I. Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum. Chemosensors. 2023; 11(2):78. https://doi.org/10.3390/chemosensors11020078
Chicago/Turabian StyleDe Souza, Michele Lemos, Samuel Valdivia, Juan Carlos Otero, and Isabel López-Tocón. 2023. "Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum" Chemosensors 11, no. 2: 78. https://doi.org/10.3390/chemosensors11020078
APA StyleDe Souza, M. L., Valdivia, S., Otero, J. C., & López-Tocón, I. (2023). Sensing Bisphenol A by Means of Surface-Enhanced Raman Spectroscopy and DFT Calculations to Elucidate the Enhancement Mechanism That Dominates the Spectrum. Chemosensors, 11(2), 78. https://doi.org/10.3390/chemosensors11020078