Voltammetric Electrochemical Behavior of Carbon Paste Electrode Containing Intrinsic Silver for Determination of Cysteine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Solution
2.2. Preparation of Modified Electrode
2.3. Apparatus and Measurements
3. Results and Discussion
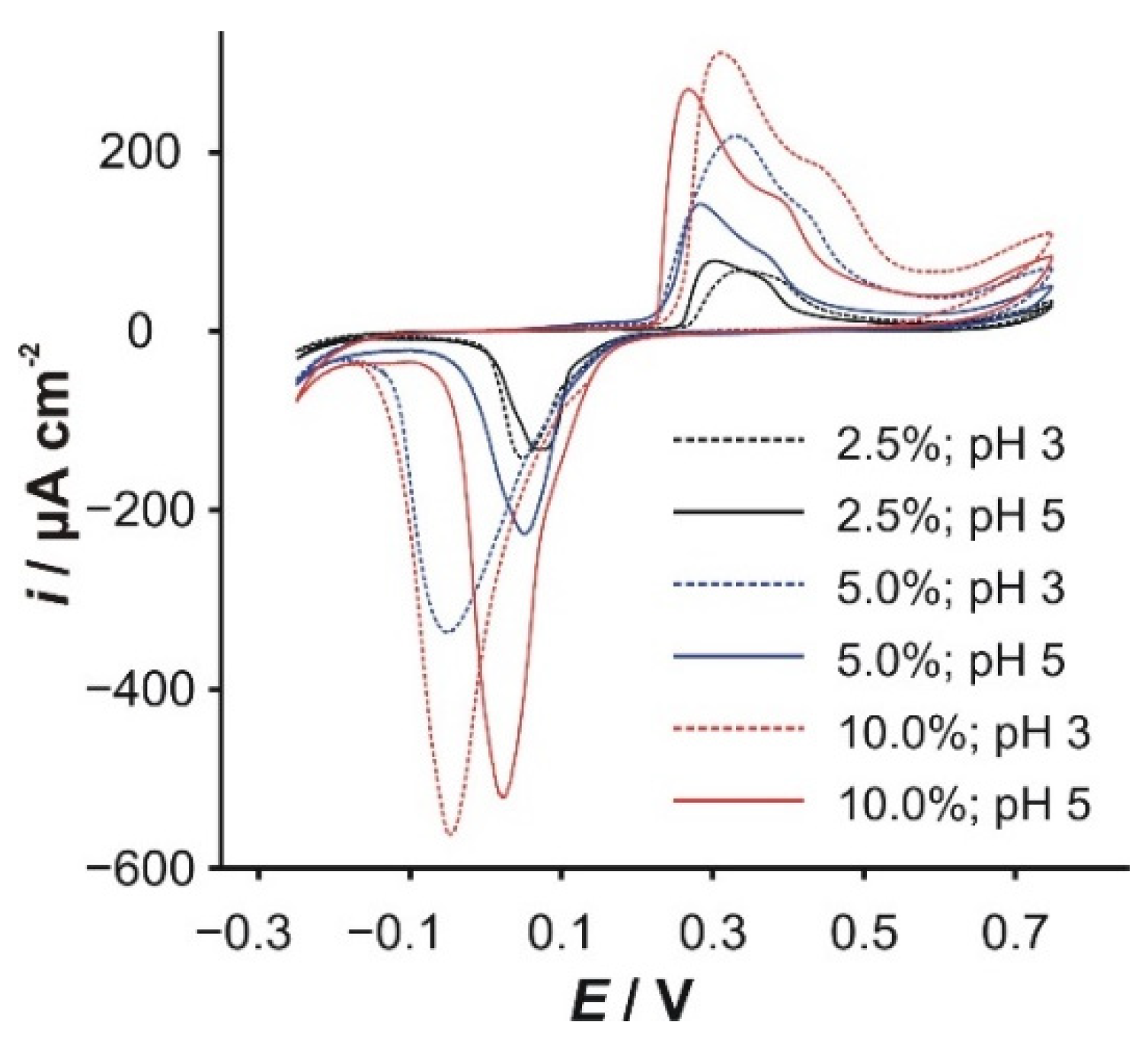
3.1. Composition of the Modifier
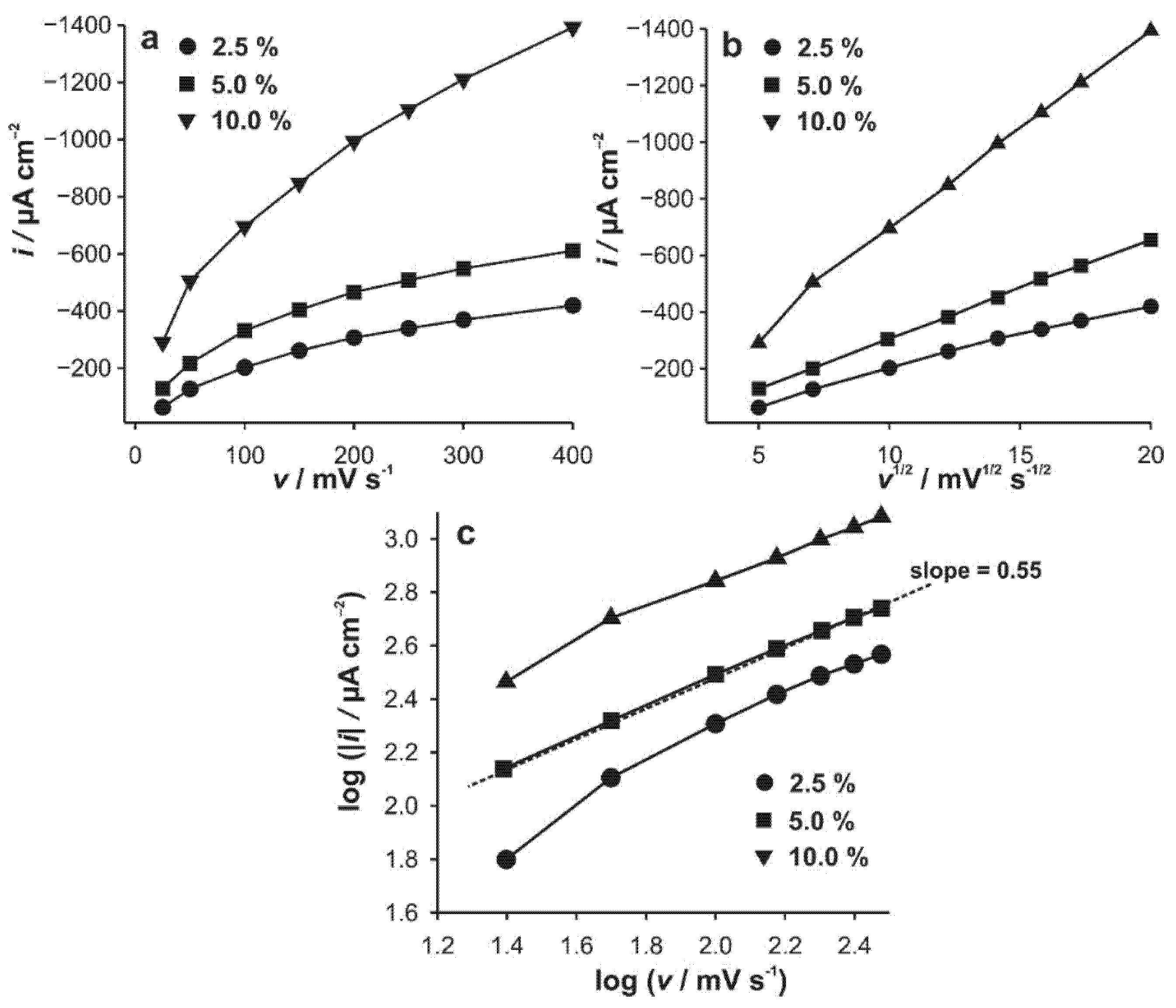
3.2. Cyclic Voltammetry
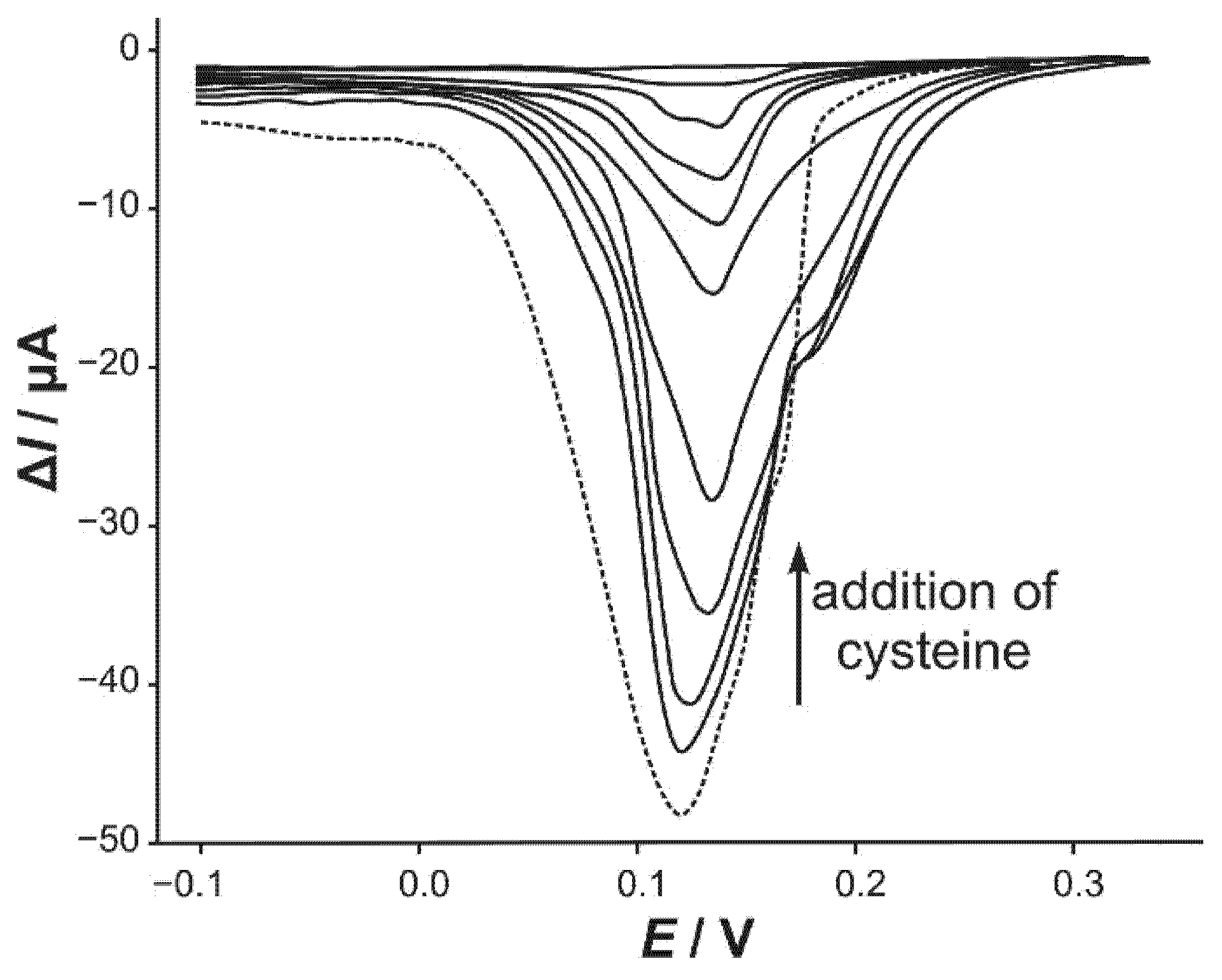
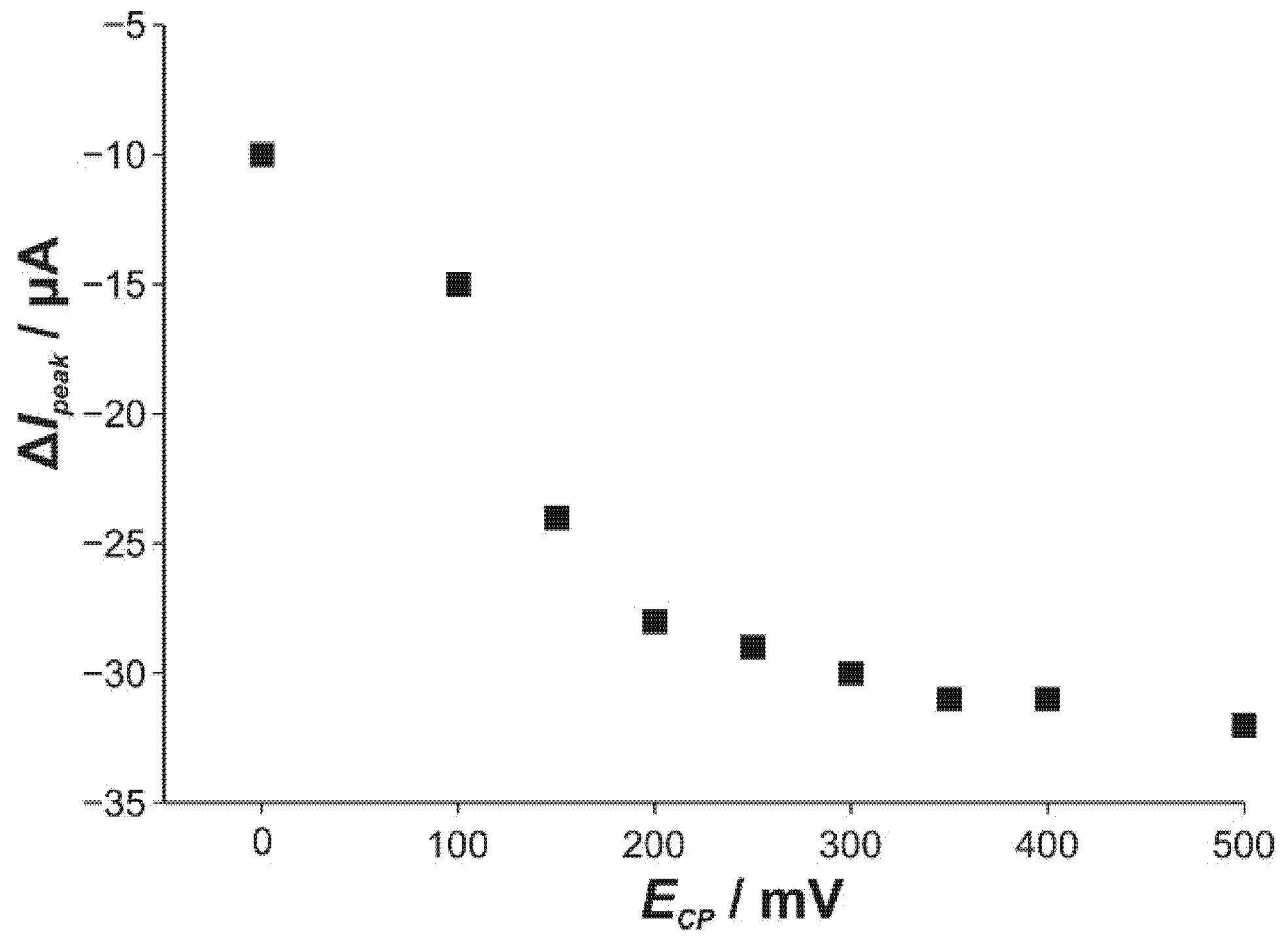
3.3. Differential Pulse Voltammetry (DPV)
3.4. Effects of Interfering Compounds
3.5. Detection of Cysteine in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, G.; Tang, Y.; Lin, W. A multi-signal fluorescent probe for discrimination of cysteine/homocysteine, and glutathione and the application in living cells and zebrafish. New J. Chem. 2018, 42, 12615–12620. [Google Scholar] [CrossRef]
- Ravindran, A.; Dhas, S.P.; Chandrasekaran, N.; Mukherjee, A. Differential interaction of silver nanoparticles with cysteine. J. Exp. Nanosci. 2013, 8, 589–595. [Google Scholar] [CrossRef]
- Chen, L.C.; Ho, K.C. Multimode optoelectrochemical detection of cysteine based on an electrochromic Prussian blue electrode. Sens. Actuators B Chem. 2008, 130, 418–424. [Google Scholar] [CrossRef]
- Santos, E.; Avalle, L.; Pötting, K.; Vélez, P.; Jones, H. Experimental and theoretical studies of l-cysteine adsorbed at Ag(111) electrodes. Electrochim. Acta 2008, 53, 6807–6817. [Google Scholar] [CrossRef]
- Koswattage, K.R.; Ishii, O. Photoemission investigation of interaction between L-cysteine and silver surface. Surf. Interface Anal. 2020, 52, 513–517. [Google Scholar] [CrossRef]
- Bell, R.A.; Kramer, J.R. Structural chemistry and geochemistry of silver–sulfur compounds: Critical review. Environ. Toxicol. Chem. 1999, 18, 9–22. [Google Scholar] [CrossRef]
- Toh, H.S.; Batchelor-Mcauley, C.; Tschulik, K.; Compton, R.G. Chemical interactions between silver nanoparticles and thiols: A comparison of mercaptohexanol against cysteine. Sci. China Chem. 2014, 57, 1199–1210. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Miao, L.; Kan, M.; Kong, L.; Zhang, H. Oxidation and detection of L-cysteine using a modified Au/Nafion/glass carbon electrode. Sci. China Chem. 2011, 54, 521–525. [Google Scholar] [CrossRef]
- Pei, L.Z.; Yang, L.J.; Wang, J.F.; Fan, C.G.; Hu, J.L. Synthesis and Electrochemical Properties of Ag2S and Ag2S/Cu2S Crystals. e-J. Surf. Sci. Nanotech. 2010, 8, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Vladislavić, N.; Škugor Rončević, I.; Buljac, M.; Brinić, S.; Krivić, D.; Buzuk, M. Electroanalytical Determination of Cysteine Using the Electrodes Based on Ternary Silver-Copper Sulfides. Sensors 2018, 18, 3753. [Google Scholar] [CrossRef] [Green Version]
- Aydogmus, Z.; Sarakbi, A.; Kauffmann, J.M. Determination of Thiols and Free Thiol Content in a Protein with Liquid Chromatography Coupled to Amperometric Detection at a Silver Based Carbon Paste Electrode. Electroanalysis 2016, 28, 2703–2708. [Google Scholar] [CrossRef]
- Moulaee, K.; Neri, G. Electrochemical Amino Acid Sensing: A Review on Challenges and Achievements. Biosensors 2021, 11, 502. [Google Scholar] [CrossRef]
- González-García, O.; Ariño, C.; Díaz-Cruz, J. Comparison of voltammetric detection assisted by multivariate curve resolution with amperometric detection in liquid chromatographic analysis of cysteine-containing compounds J. Chromatogr. A 2005, 1062, 95–101. [Google Scholar] [CrossRef]
- Alwael, H.; Connolly, D.; Barron, L.; Paull, B. Development of a rapid and sensitive method for determination of cysteine/cystine ratio in chemically defined media. J. Chromatogr. A 2010, 1217, 3863–3870. [Google Scholar] [CrossRef]
- Hou, X.Y.; Chen, S.; Tang, J.; Long, Y.F. Visual determination of trace Cysteine based on promoted corrosion of triangular silver nanoplates by sodium thiosulfate. Spectrochim. Acta A Mol. Biomol. 2014, 125, 285–289. [Google Scholar] [CrossRef]
- Liu, S.; Dai, G. Preparation and Electrochemical Behaviour of Silver Pentacyanonitrosylferrate Film Modified Glassy Carbon Electrode and Its Electrocatalytic Oxidation to L-cysteine. J. Chin. Chem. Soc. 2011, 58, 617–622. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Beitollahi, H.; Hosseinzadeh, R. Electrocatalytic Oxidation and Highly Selective Voltammetric Determination of L-Cysteine at the Surface of a 1-[4-(Ferrocenyl ethynyl)phenyl]-1-ethanone Modified Carbon Paste Electrode. Anal. Sci. 2006, 22, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- White, P.C.; Lawrence, N.S.; Davis, J.; Compton, R.G. Electrochemical Determination of Thiols: A Perspective. Electroanalysis 2002, 14, 89–98. [Google Scholar] [CrossRef]
- Yao, J.; Liu, C.; Liu, L.; Chen, M.; Yang, M. An Electrochemical Sensor for Sensitive Determination of L-cysteine and Its Electrochemical Kinetics on AgNPs/GQDs/GCE Composite Modified Electrode. J. Electrochem. Soc. 2018, 165, 551–558. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Kolbadinezhad, M. Differential Pulse Voltammetry Determination of L-Cysteine with Ferrocene-Modified Carbon Paste Electrode. Bull. Chem. Soc. Jpn. 2005, 78, 818–826. [Google Scholar] [CrossRef]
- Baldrianova, L.; Agrafiotou, P.; Svancara, I.; Vytras, K.; Sotiropoulos, S. The determination of cysteine at Bi-powder carbon paste electrodes by cathodic stripping voltammetry. Electrochem. Commun. 2008, 10, 918–921. [Google Scholar] [CrossRef]
- Zhou, M.; Ding, J.; Guo, L.; Shang, Q. Electrochemical Behavior of L-Cysteine and Its Detection at Ordered Mesoporous Carbon-Modified Glassy Carbon Electrode. Anal. Chem. 2007, 79, 5328–5335. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Zarei, E. Preparation of poly N,N-dimethylaniline/ferrocyanide film modified carbon paste electrode: Application to electrocatalytic oxidation of L-cysteine. J. Electroanal. Chem. 2010, 638, 241–245. [Google Scholar] [CrossRef]
- Mardini Farias, P.A.; Rebello Wagener, A.; Altamirano Junqueira, A.; Castro, A.A. Determination of Cysteine in the Presence of Copper in Diluted Alkaline Electrolyte by Adsorptive Stripping Voltammetry at the Mercury Film Electrode. Anal. Lett. 2007, 40, 2032–2044. [Google Scholar] [CrossRef]
- Yusoff, N.; Rameshkumar, P.; Noor, A.M.; Huang, N.M. Amperometric determination of L-cysteine using a glassy carbonelectrode modified with palladium nanoparticles grown on reduced graphene oxide in a Nafion matrix. Microchim. Acta 2018, 185, 246. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kondo, T.; Shitanda, I.; Hoshi, Y.; Itagaki, M.; Tojo, T.; Yuasa, M. Sensitive electrochemical detection of L-Cysteine at a screen-printed diamond electrode. Carbon 2021, 173, 395–402. [Google Scholar] [CrossRef]
- Zhai, X.; Li, S.; Chen, X.; Hua, Y.; Wang, H. Coating silver metal-organic frameworks onto nitrogen-doped porous carbons for the electrochemical sensing of cysteine. Microchim. Acta 2020, 187, 493. [Google Scholar] [CrossRef]
- Jerome, R.; Keerthivasan, P.V.; Murugan, N.; Devi, N.R.; Sundramoorthy, A.K. Preparation of Stable CuO/Boron Nitride Nanocomposite Modified Electrode for Selective Electrochemical Detection of L-Cysteine. Chem. Sel. 2020, 5, 9111–9118. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Alizadeh, Z. A Certain Electrochemical Nanosensor Based on Functionalized Multi-Walled Carbon Nanotube for Determination of Cysteine in the Presence of Paracetamol. J. Nanostruct. 2020, 10, 258–267. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadoss, G.; Thangamuthu, R.; Kumar, M.R.; Kumar, R.M.; Jayavel, R. CeO2-based heterostructure nanocomposite for electrochemical determination of L-cysteine biomolecule. Inorg. Chem. Commun. 2020, 113, 107793. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadoss, G.; Thangamuthu, R.; Kumar, M.R.; Kumar, R.M. Facile synthesis of CeO2-SnO2 nanocomposite for electrochemical determination of L-cysteine. J. Alloys Compd. 2019, 792, 1150–1161. [Google Scholar] [CrossRef]
- Atacan, K. CuFe2O4/reduced graphene oxide nanocomposite decorated with gold nanoparticles as a new electrochemical sensor material for L-cysteine detection. J. Alloys Compd. 2019, 791, 391–401. [Google Scholar] [CrossRef]
- Beitollahi, H.; Ganjali, M.R.; Norouzi, P.; Movlaee, K.; Hosseinzadeh, R.; Tajik, S. A novel electrochemical sensor based on graphene nanosheets and ethyl 2-(4-ferrocenyl-[1,2,3]triazol-1-yl) acetate for electrocatalytic oxidation of cysteine and tyrosine. Meas. J. Int. Meas. Confed. 2020, 152, 107302. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Wu, D.; Zhang, J.; Liu, X.; Gao, S.; Kong, Y. Electrochemical Chiral Recognition of Tryptophan Isomers Based on Nonionic Surfactant-Assisted Molecular Imprinting Sol-Gel Silica. ACS Appl. Mater. Interfaces 2019, 11, 2840–2848. [Google Scholar] [CrossRef]
- Kurniawan, A.; Kurniawan, F.; Gunawan, F.; Chou, S.H.; Wang, M.J. Disposable electrochemical sensor based on copper-electrodeposited screen-printed gold electrode and its application in sensing L-Cysteine. Electrochim. Acta 2019, 293, 318–327. [Google Scholar] [CrossRef]
- Zhou, H.; Ran, G.; Masson, J.F.; Wang, C.; Zhao, Y.; Song, Q. Rational Design of Magnetic Micronanoelectrodes for Recognition and Ultrasensitive Quantification of Cysteine Enantiomers. Anal. Chem. 2018, 90, 3374–3381. [Google Scholar] [CrossRef]
- Premlatha, S.; Selvarani, K.; Bapu, G.N.K.R. Facile Electrodeposition of Hierarchical Co-Gd2O3 Nanocomposites for Highly Selective and Sensitive Electrochemical Sensing of L-Cysteine. Chem. Sel. 2018, 3, 2665–2674. [Google Scholar] [CrossRef]
- Pei, L.Z.; Wei, T.; Lin, N.; Zhang, H.; Fan, C.G. Bismuth Tellurate Nanospheres and Electrochemical Behaviors of L-Cysteine at the Nanospheres Modified Electrode. Russ. J. Electrochem. 2018, 54, 84–91. [Google Scholar] [CrossRef]
- Buljac, M.; Krivić, D.; Škugor Rončević, I.; Vladislavić, N.; Vukadin, J.; Buzuk, M. Voltammetric behaviour and amperometric sensing of hydrogen peroxide on a carbon paste electrode modified with ternary silver-copper sulfides containing intrinsic silver. Monatsh. Chem. 2020, 151, 511–524. [Google Scholar] [CrossRef]
- Brinić, S.; Buzuk, M.; Bralić, M.; Buljac, M.; Jozić, D. Cu (II) Ion-Selective Electrode Based on Mixed Silver-Copper Sulfide: Phase Structure and Electrochemical Properties. Int. J. Electrochem. Sci. 2012, 7, 5217–5230. [Google Scholar]
- Andersson, L.O. Study of Some SilverThiol Complexes and Polymers: Stoichiometry and Optical Effects. J. Polym. Sci. Part A1 Polym. Chem. 1972, 10, 1963–1973. [Google Scholar] [CrossRef]
- Report No. ISO 11843-2; Capability of Detection. International Organization for Standardization (ISO): Genève, Switzerland, 2000.
- Buglak, A.A.; Ramazanov, R.R.; Kononov, A.I. Silver cluster–amino acid interactions: Quantum-chemical study. Amino Acids 2019, 51, 855–864. [Google Scholar] [CrossRef]
- Arenas, J.F.; Castro, J.L.; Otero, J.C.; Marcos, J.I. Study of Interaction Between Aspartic Acid and Silver by Surface-Enhanced Raman Scattering on H2O and D2O Sols. Biopolymers (Biospectroscopy) 2001, 62, 241–248. [Google Scholar] [CrossRef]
- Han, C.; Xu, K.; Liu, Q.; Liu, X.; Li, J. Colorimetric sensing of cysteine using label-free silver nanoparticles. Sens. Actuators B Chem. 2014, 202, 574–582. [Google Scholar] [CrossRef]
- Borase, H.P.; Patil, C.D.; Salunkhe, R.B.; Suryawanshi, R.K.; Patil, S.V. Bio-Functionalized Silver Nanoparticles: A Novel Colorimetric Probe for Cysteine Detection. Appl. Biochem. Biotechnol. 2015, 175, 3479–3493. [Google Scholar] [CrossRef]




| Supporting Electrode/Sensitive Layer | pH | Working Potential E/V | LOD/µM | Linear Range µM | Sensitivity | Method | Ref. |
|---|---|---|---|---|---|---|---|
| GCE/AgNPs/GQDs | 7 | 0.52 V vs. SCE | 0.01 | 0.1–5; | NA | CV, | [19] |
| 8–200 | DPV | ||||||
| silver-copper sulfide | 5 | 0.142 V vs. Ag/AgCl | 0.036 | 1–100 | 0.11 µA µM−1 | AMP | [10] |
| 7 | 0.04 V vs. Ag/AgCl | 0.024 | 0.10 µA µM−1 | ||||
| GCE/rGO-Nafion@Pd | 7 | 0.6 V vs. SCE | 0.15 | 0.5–10 | 1.30 μA μM−1 cm−2 | AMP | [25] |
| screen-printed diamond electrode | 7 | 0.663 V vs. Ag/AgCl | 0.872 | 1–194 | 0.226 μA μM−1 cm−2 | CV | [26] |
| N-PC/Ag-MOF | 7 | 0.02 V vs. Ag/AgCl | 0.05 | 0.1–1300 | NA | AMP | [27] |
| CuO/boron nitride | 7.4 | 0.45 V vs. Ag/AgCl | 0.58 | 1–10 | NA | AMP | [28] |
| Nanocomposite | |||||||
| functionalized MWCNT | 7 | 0.2 V vs. SCE | 0.16 nM | 0.7 nM–200µM | NA | DPV | [29] |
| CeO2-CuO nanocomposite | 7 | 0.8 V vs. SCE | 0.016 | 10–5000 | 21.6 μA cm−2 mM −1 | AMP | [30] |
| CeO2-SnO2 nanocomposite | 7 | 0.8 V vs. SCE | 16 | 10–2000 | 186.34 μA cm−2 mM −1 | AMP | [31] |
| CuFe2O4/rGO–Au composite | 6.5 | 0.4 V vs. Ag/AgCl | 0.383 | 50–200 | 100.01 μA cm−2 mM −1 | CV | [32] |
| ethyl 2-(4 ferrocenyl[1,2,3]triazol-1-yl) acetate/graphene | 7 | 0.33 V vs. Ag/AgCl | 0.9 | 4.0–2300.0 | 0.0078 µA µM−1 | SWV | [33] |
| Au nanoparticles/ | 7 | 0.8 V vs. SCE | 1.873 | 15–500 | NA | DPV | [34] |
| anthraquinone-2-carboxylic acid | |||||||
| electrodeposited copper/SPE | 7.4 | 0.5 vs. Ag pseudo-ref | 0.21 | 1–400 | 0.028 µA µM−1 | AMP | [35] |
| 400–1800 | 0.014 µA µM−1 | ||||||
| Cu2+ | 5 | 0.1 V vs. Ag/AgCl | 83.0 pM | 0.010–500 | 102 μA μM−1 cm−2 | SWV | [36] |
| modified | |||||||
| Fe3O4@polydopamine | |||||||
| Co-Gd2O3 nanocomposite | 7 | 0.4 V vs. Ag/AgCl | 0.23 | 1–100 | NA | AMP | [37] |
| bismuth tellurate nanospheres | 7 | 0.14 V vs. SCE | 0.046 | 0.1–2000 | NA | CV | [38] |
| CPE/ternary silver-copper sulfides | 5 | 0.055 V vs. Ag/AgCl | 0.032 | 0.1–2.5 | 11.78 μA μM−1 | DPV | This work |
| 0.08 | 5.6–28 |
| Composition of Mediator | Mole Ratio of Ions | Moles of Ions |
|---|---|---|
| metallic silver, Ag1.2Cu0.8S and CuAgS | (Cu2+; Ag+): S2− | Cu2+; Ag+; S2− |
| 1:0.5 | 0.02; 0.04; 0.02 |
| Sample | Expected/μM | Found/μM | Average/μM | RSD/% | Recovery/% |
|---|---|---|---|---|---|
| NOW Foods tablets | 1 | 0.96; 0.95; 1.02 | 0.98 | 3.9 | 97.7 |
| Fluimukan Akut | 6 | 6.13; 6.23; 5.94 | 6.10 | 2.4 | 101.7 |
| Propomucil | 24 | 23.91; 24.32; 24.15 | 24.13 | 0.9 | 100.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buljac, M.; Krivić, D.; Rončević, I.Š.; Vladislavić, N.; Buzuk, M. Voltammetric Electrochemical Behavior of Carbon Paste Electrode Containing Intrinsic Silver for Determination of Cysteine. Chemosensors 2022, 10, 240. https://doi.org/10.3390/chemosensors10070240
Buljac M, Krivić D, Rončević IŠ, Vladislavić N, Buzuk M. Voltammetric Electrochemical Behavior of Carbon Paste Electrode Containing Intrinsic Silver for Determination of Cysteine. Chemosensors. 2022; 10(7):240. https://doi.org/10.3390/chemosensors10070240
Chicago/Turabian StyleBuljac, Maša, Denis Krivić, Ivana Škugor Rončević, Nives Vladislavić, and Marijo Buzuk. 2022. "Voltammetric Electrochemical Behavior of Carbon Paste Electrode Containing Intrinsic Silver for Determination of Cysteine" Chemosensors 10, no. 7: 240. https://doi.org/10.3390/chemosensors10070240
APA StyleBuljac, M., Krivić, D., Rončević, I. Š., Vladislavić, N., & Buzuk, M. (2022). Voltammetric Electrochemical Behavior of Carbon Paste Electrode Containing Intrinsic Silver for Determination of Cysteine. Chemosensors, 10(7), 240. https://doi.org/10.3390/chemosensors10070240






