Recent Advances of Fluorescence Probes for Imaging of Ferroptosis Process
Abstract
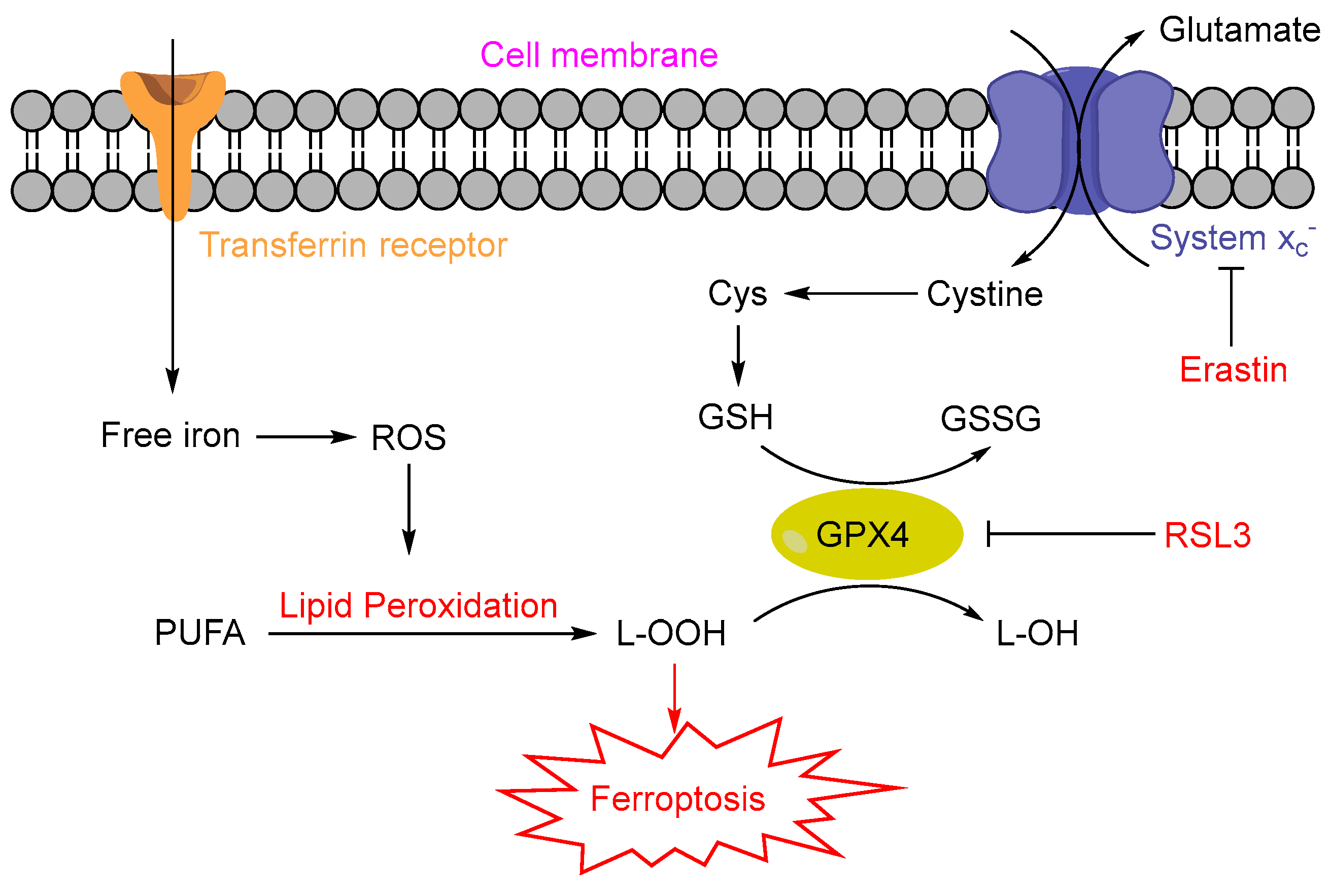
:1. Introduction
2. Fluorescence Probes for Iron and Its Related Bioactive Species
2.1. Probes for Fe2+ and Heme
2.2. Probes for Fe3+
3. Fluorescence Probes for ROS
3.1. Probes for •OH
3.2. Probes for H2O2
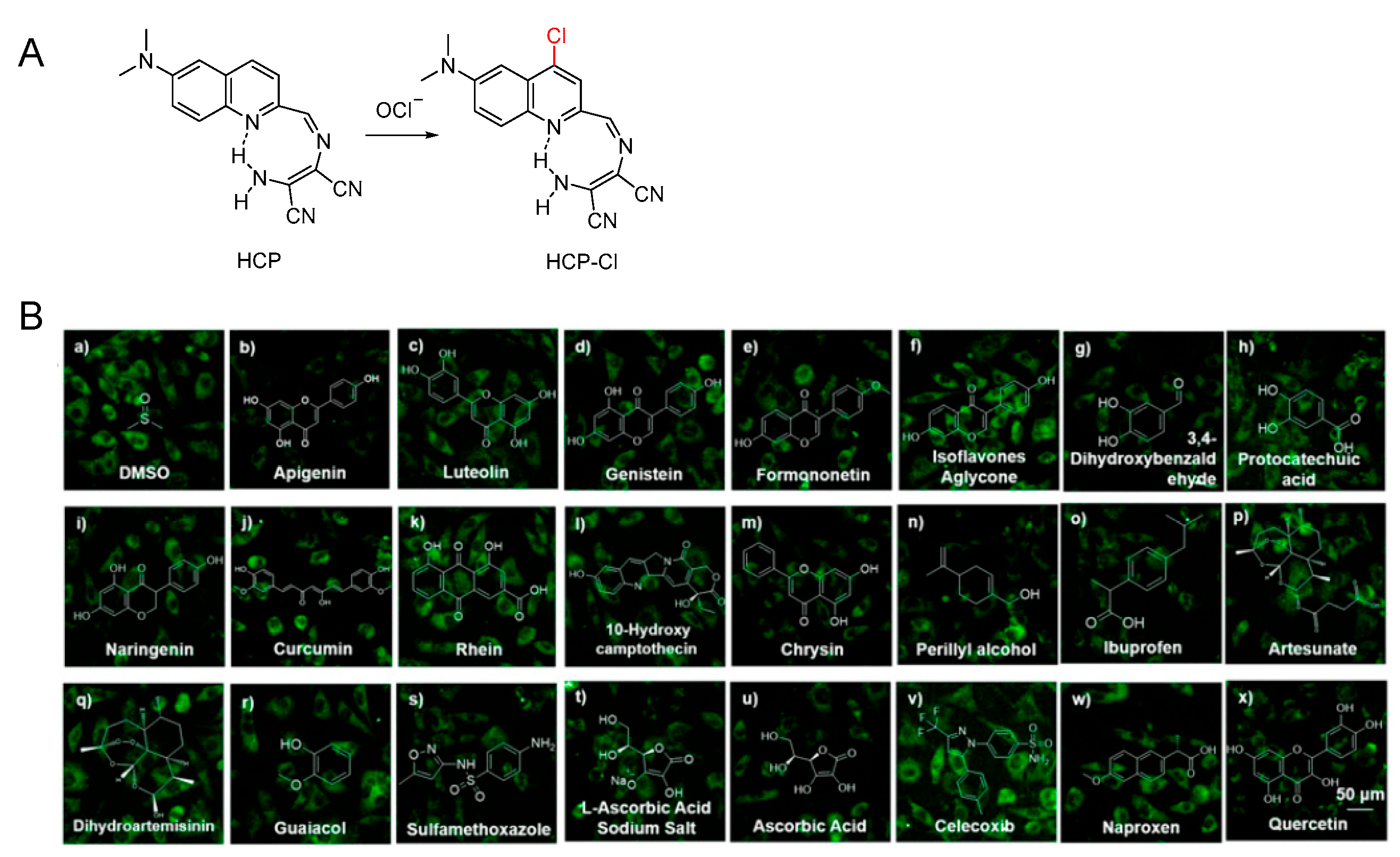
3.3. Probe for OCl−
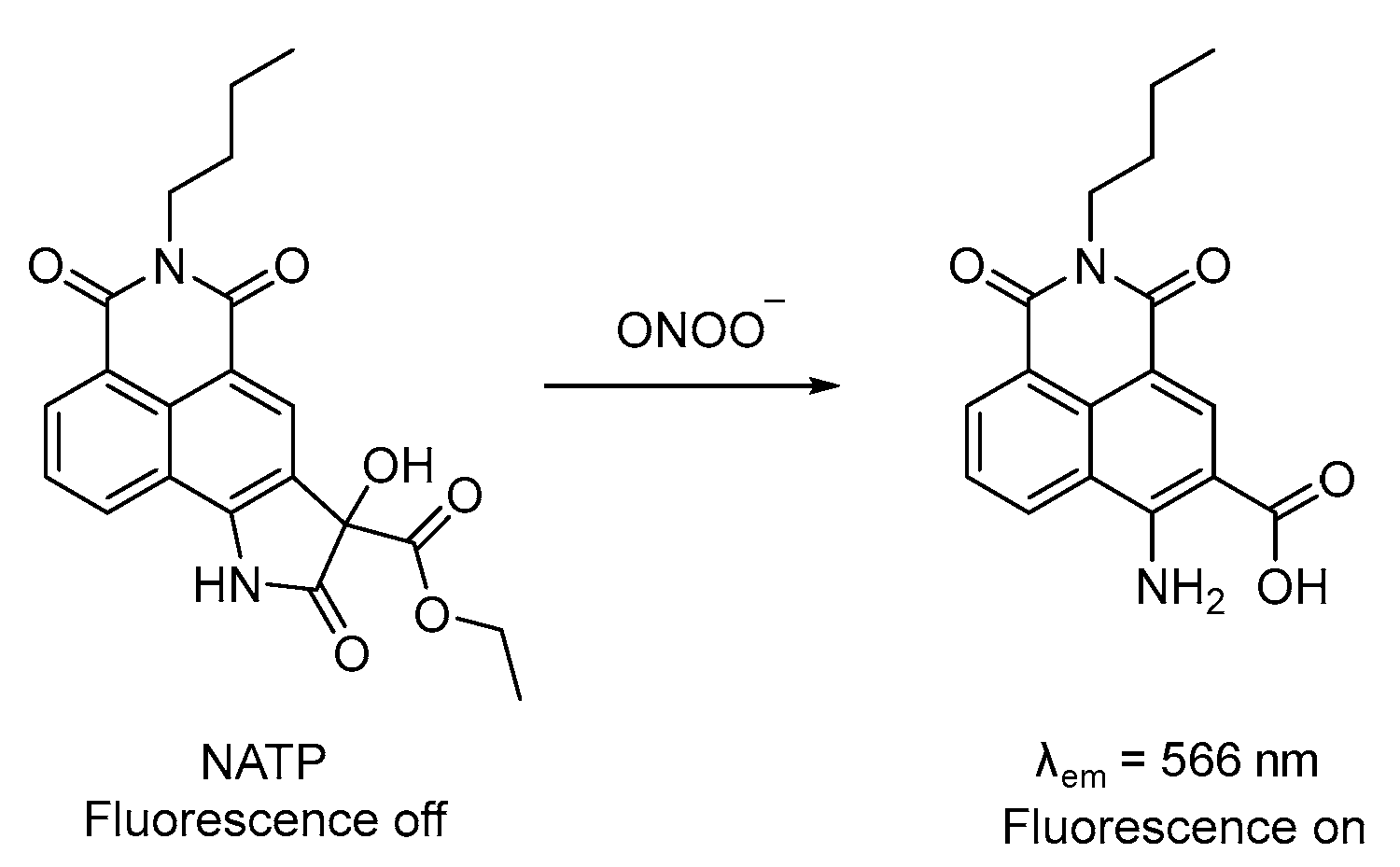
3.4. Probe for ONOO−
4. Fluorescence Probes for Biothiols
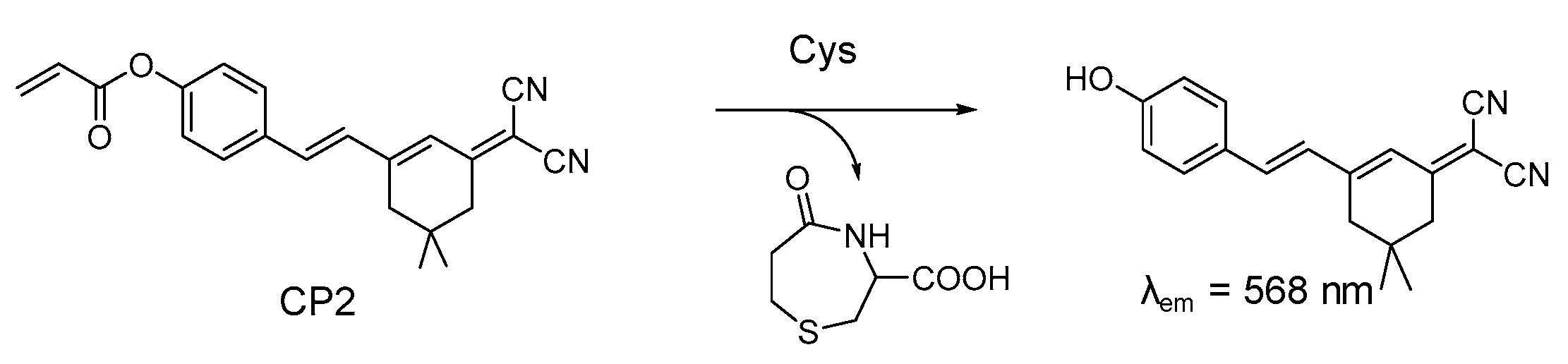
4.1. Probe for Cys
4.2. Probe for GSH
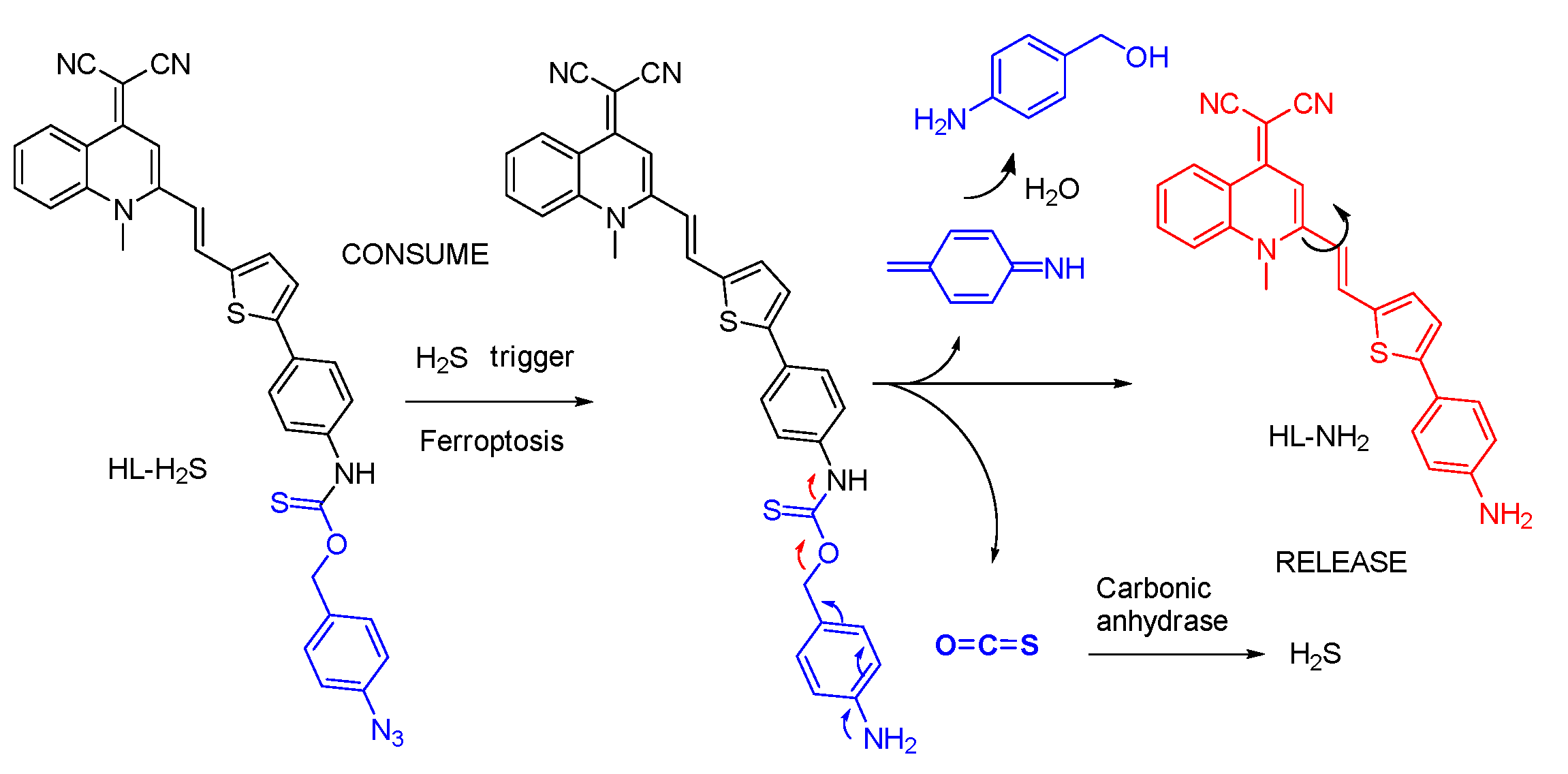
4.3. Probe for H2S
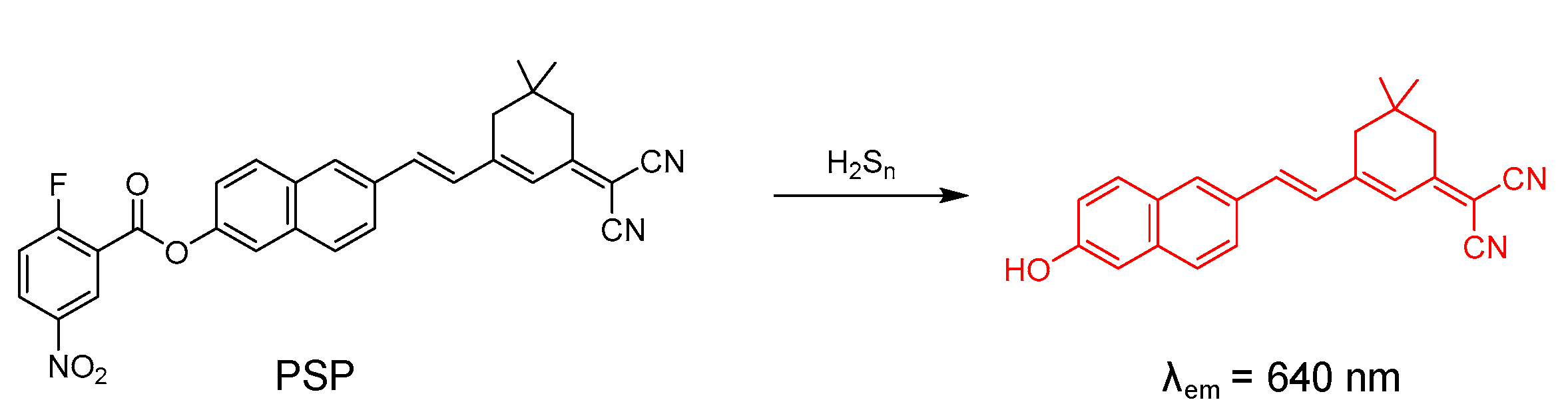
4.4. Probe for H2Sn
5. Fluorescence Probes for Cell Microenvironmental Factors
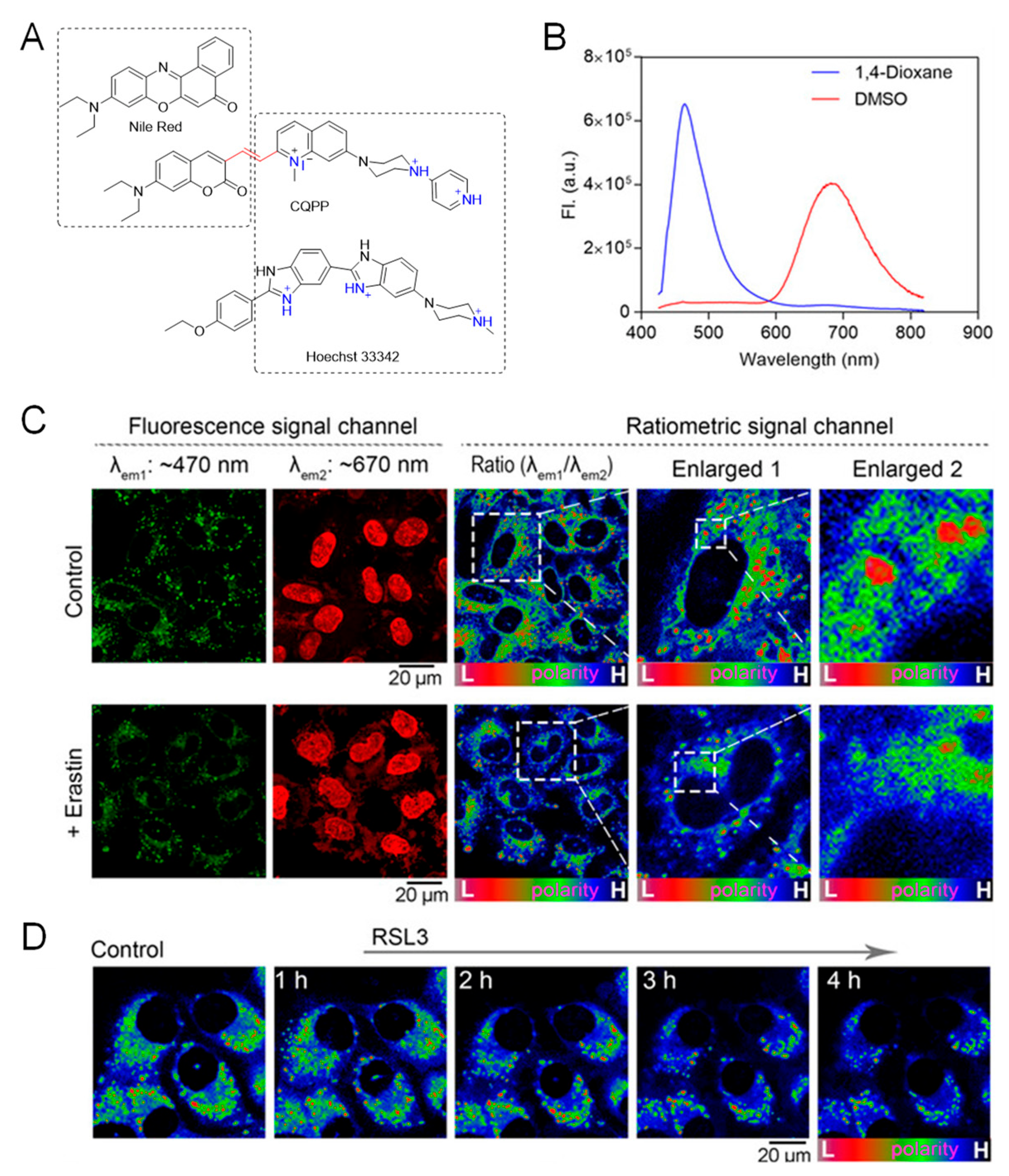
5.1. Probe for Polarity
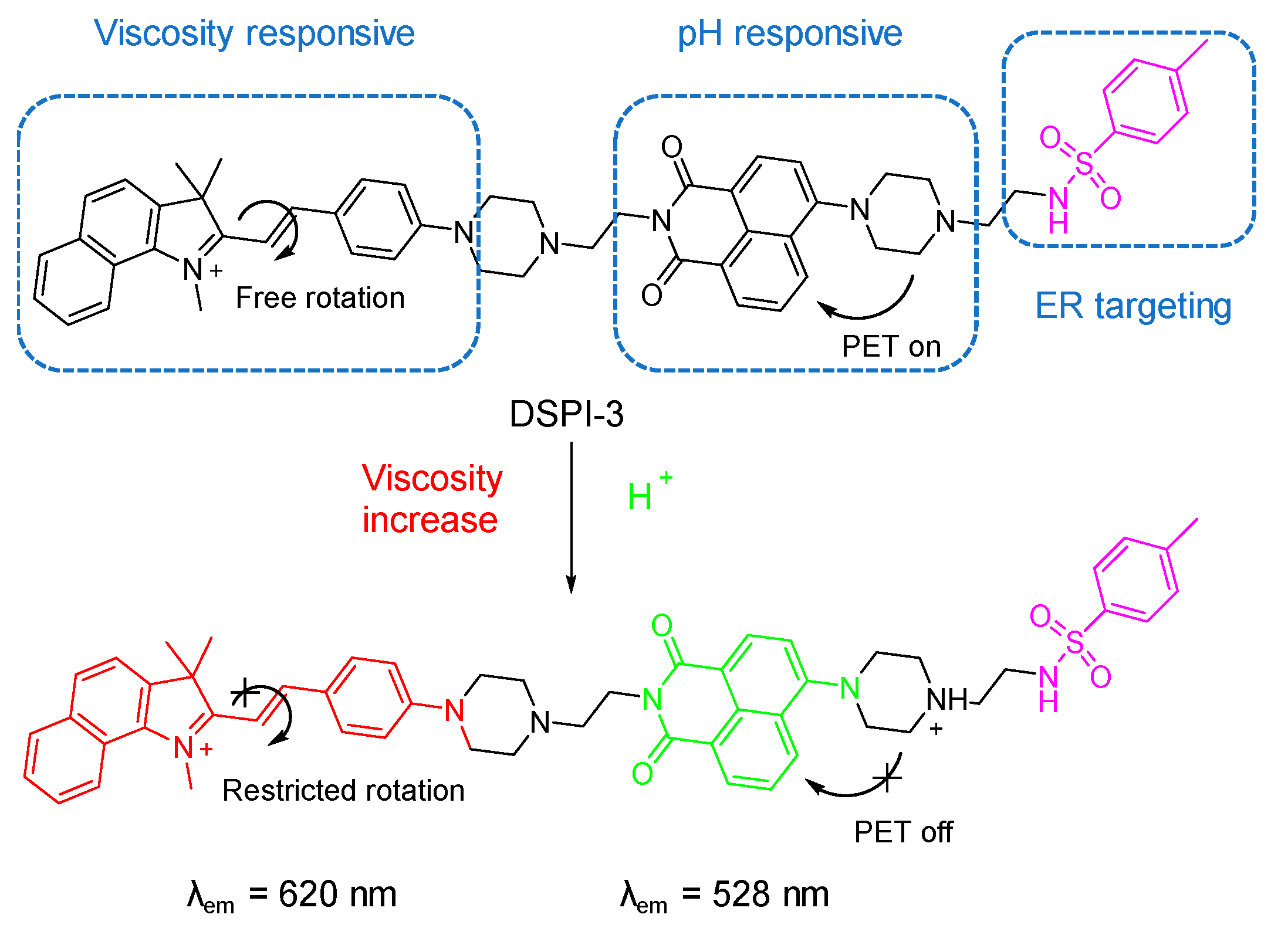
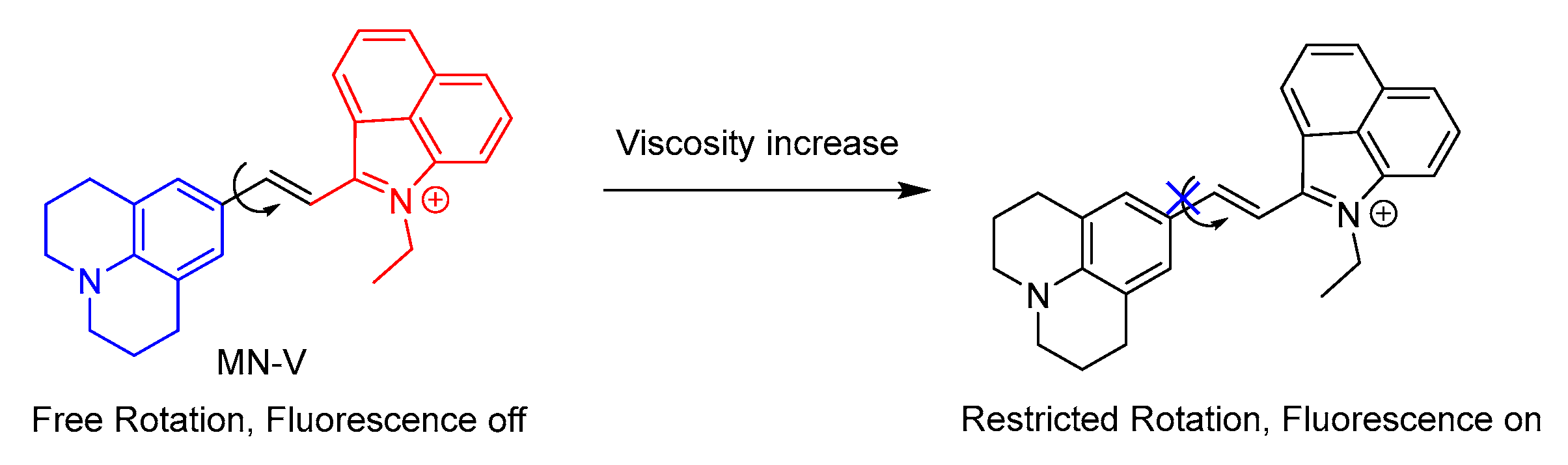
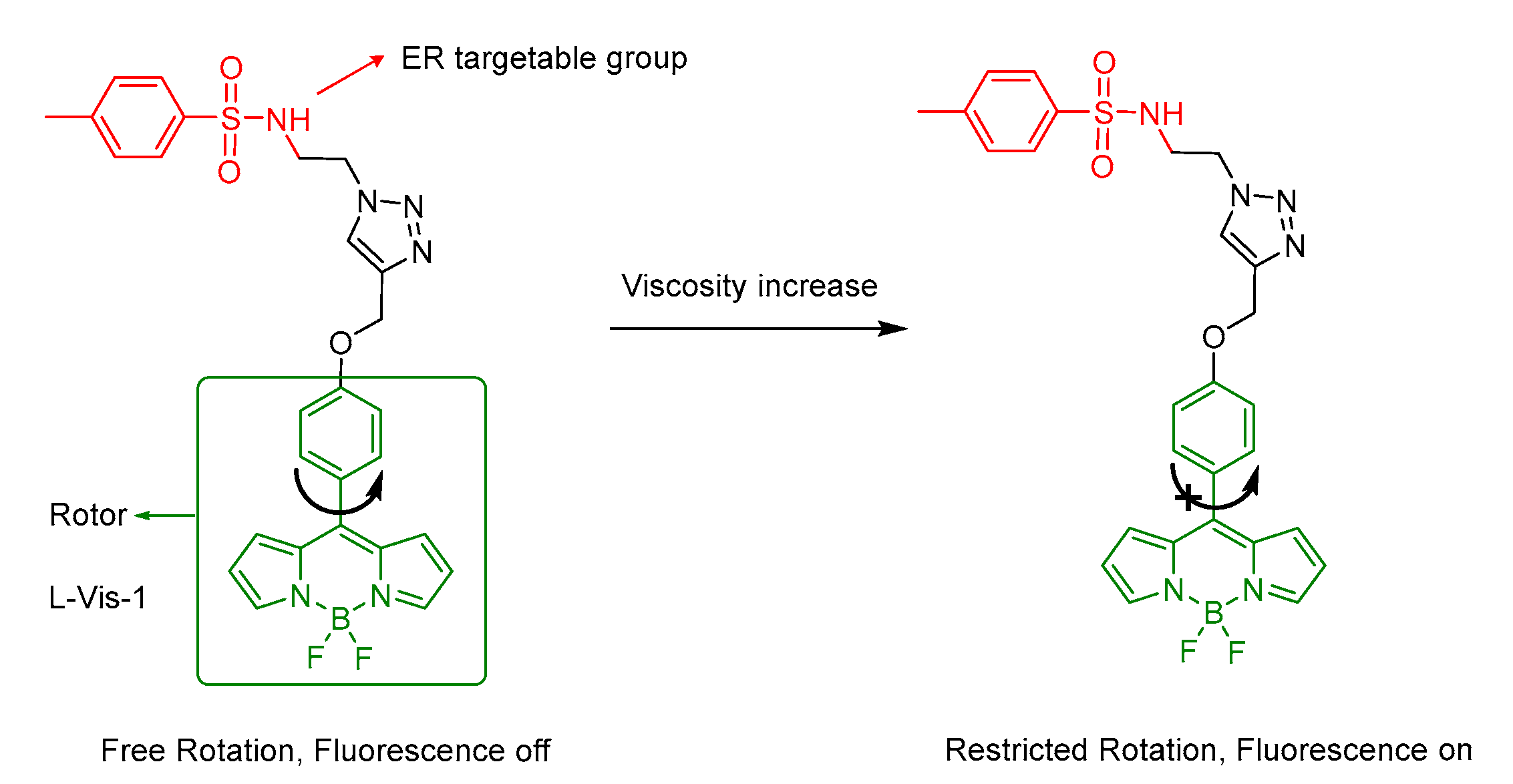
5.2. Probes for Viscosity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron−Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Stockwell, B.R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Graham, E.T.; Naowarojna, N.; Shi, Z.; Wang, Y.; Xie, G.; Zhou, L.; Salmon, W.; Jia, J.M.; Wang, X.; et al. PALP: A Rapid Imaging Technique for Stratifying Ferroptosis Sensitivity in Normal and Tumor Tissues in Situ. Cell Chem. Biol. 2022, 29, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Distéfano, A.M.; Martin, M.V.; Córdoba, J.P.; Bellido, A.M.; D’Ippólito, S.; Colman, S.L.; Soto, D.; Roldán, J.A.; Bartoli, C.G.; Zabaleta, E.J.; et al. Heat Stress Induces Ferroptosis−like Cell Death in Plants. J. Cell Biol. 2017, 216, 463–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 2019, 31, 1904197. [Google Scholar] [CrossRef] [PubMed]
- Hadian, K.; Stockwell, B.R. A Roadmap to Creating Ferroptosis−Based Medicines. Nat. Chem. Biol. 2021, 17, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gao, Y.; Ouyang, Z.; Fan, Y.; Yu, H.; Zhan, M.; Wang, H.; Shi, X.; Shen, M. Apoptosis−Enhanced Ferroptosis Therapy of Pancreatic Carcinoma through PAMAM Dendrimer−Iron(III) Complex−Based Plasmid Delivery. Sci. China Chem. 2022, 65, 778–788. [Google Scholar] [CrossRef]
- Zhang, N.; Shu, G.; Qiao, E.; Xu, X.; Shen, L.; Lu, C.; Chen, W.; Fang, S.; Yang, Y.; Song, J.; et al. DNA−Functionalized Liposomes In Vivo Fusion for NIR−II/MRI Guided Pretargeted Ferroptosis Therapy of Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2022, 14, 20603–20615. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Li, J.; Wang, G.; Sang, W.; Xu, M.; Li, W.; Yan, J.; Li, B.; Zhang, Z.; Zhao, Q.; et al. Phototheranostic Metal−Phenolic Networks with Antiexosomal PD−L1 Enhanced Ferroptosis for Synergistic Immunotherapy. J. Am. Chem. Soc. 2022, 144, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, J.K.; Lee, S.; Kang, G.W.; Lee, Y.R.; Park, S.Y.; Song, I.S.; Yun, J.W.; Lee, J.; Choi, Y.K.; Park, K.G. Macropinocytosis Is an Alternative Pathway of Cysteine Acquisition and Mitigates Sorafenib−Induced Ferroptosis in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liang, H.; Yang, R.; Yang, Y.; Dong, J.; Di, Y.; Sun, M. Glutathione Depletion-Induced Activation of Dimersomes for Potentiating the Ferroptosis and Immunotherapy of “Cold” Tumor. Angew. Chem. Int. Ed. 2022, 61, e202202843. [Google Scholar]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Murfin, L.C.; Wu, L.; Lewis, S.E.; James, T.D. Fluorescent Small Organic Probes for Biosensing. Chem. Sci. 2021, 12, 3406–3426. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, H. Design Principles of Spectroscopic Probes for Biological Applications. Chem. Sci. 2016, 7, 6309–6315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design Strategies for Water−Soluble Small Molecular Chromogenic and Fluorogenic Probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent Progress in the Development of Fluorescent, Luminescent and Colorimetric Probes for Detection of Reactive Oxygen and Nitrogen Species. Chem. Soc. Rev. 2016, 45, 2976–3016. [Google Scholar] [CrossRef]
- Han, H.H.; Tian, H.; Zang, Y.; Sedgwick, A.C.; Li, J.; Sessler, J.L.; He, X.P.; James, T.D. Small−Molecule Fluorescence−Based Probes for Interrogating Major Organ Diseases. Chem. Soc. Rev. 2021, 50, 9391–9429. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liew, S.S.; Wei, X.; Pu, K. Hemicyanine−Based Near−Infrared Activatable Probes for Imaging and Diagnosis of Diseases. Angew. Chem. Int. Ed. 2021, 60, 26454–26475. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; He, X.P.; Kim, H.J.; Yoon, J.; Tian, H. Fluorogenic Probes for Disease−Relevant Enzymes. Chem. Soc. Rev. 2019, 48, 683–722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Zhang, W.; Tang, B.; Li, P. Recent Advances in Small Molecule Fluorescent Probes for Simultaneous Imaging of Two Bioactive Molecules in Live Cells and in Vivo. Front. Chem. Sci. Eng. 2022, 16, 4–33. [Google Scholar] [CrossRef]
- Wu, X.; Shi, W.; Li, X.; Ma, H. Recognition Moieties of Small Molecular Fluorescent Probes for Bioimaging of Enzymes. Acc. Chem. Res. 2019, 52, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, W.; Li, X.; Ma, H. Recent Advances in Fluorescent Probes for Lipid Droplets. Chem. Commun. 2022, 58, 1495–1509. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torii, S.; Shintoku, R.; Kubota, C.; Yaegashi, M.; Torii, R.; Sasaki, M.; Suzuki, T.; Mori, M.; Yoshimoto, Y.; Takeuchi, T.; et al. An Essential Role for Functional Lysosomes in Ferroptosis of Cancer Cells. Biochem. J. 2016, 473, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, O.; da Silva, B.M.; Porto, G.; Lopes, C. Iron Homeostasis in Breast Cancer. Cancer Lett. 2014, 347, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress−Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Foret, M.K.; Lincoln, R.; Do Carmo, S.; Cuello, A.C.; Cosa, G. Connecting the “Dots”: From Free Radical Lipid Autoxidation to Cell Pathology and Disease. Chem. Rev. 2020, 120, 12757–12787. [Google Scholar] [CrossRef]
- Latunde−Dada, G.O. Ferroptosis: Role of Lipid Peroxidation, Iron and Ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varnes, A.W.; Dodson, R.B.; Wehry, E.L. Interactions of Transition−Metal Ions with Photoexcited States of Flavines. Fluorescence Quenching Studies. J. Am. Chem. Soc. 1972, 94, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Kemlo, J.A.; Shepherd, T.M. Quenching of Excited Singlet States by Metal Ions. Chem. Phys. Lett. 1977, 47, 158–162. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The Stability of Transition−Metal Complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
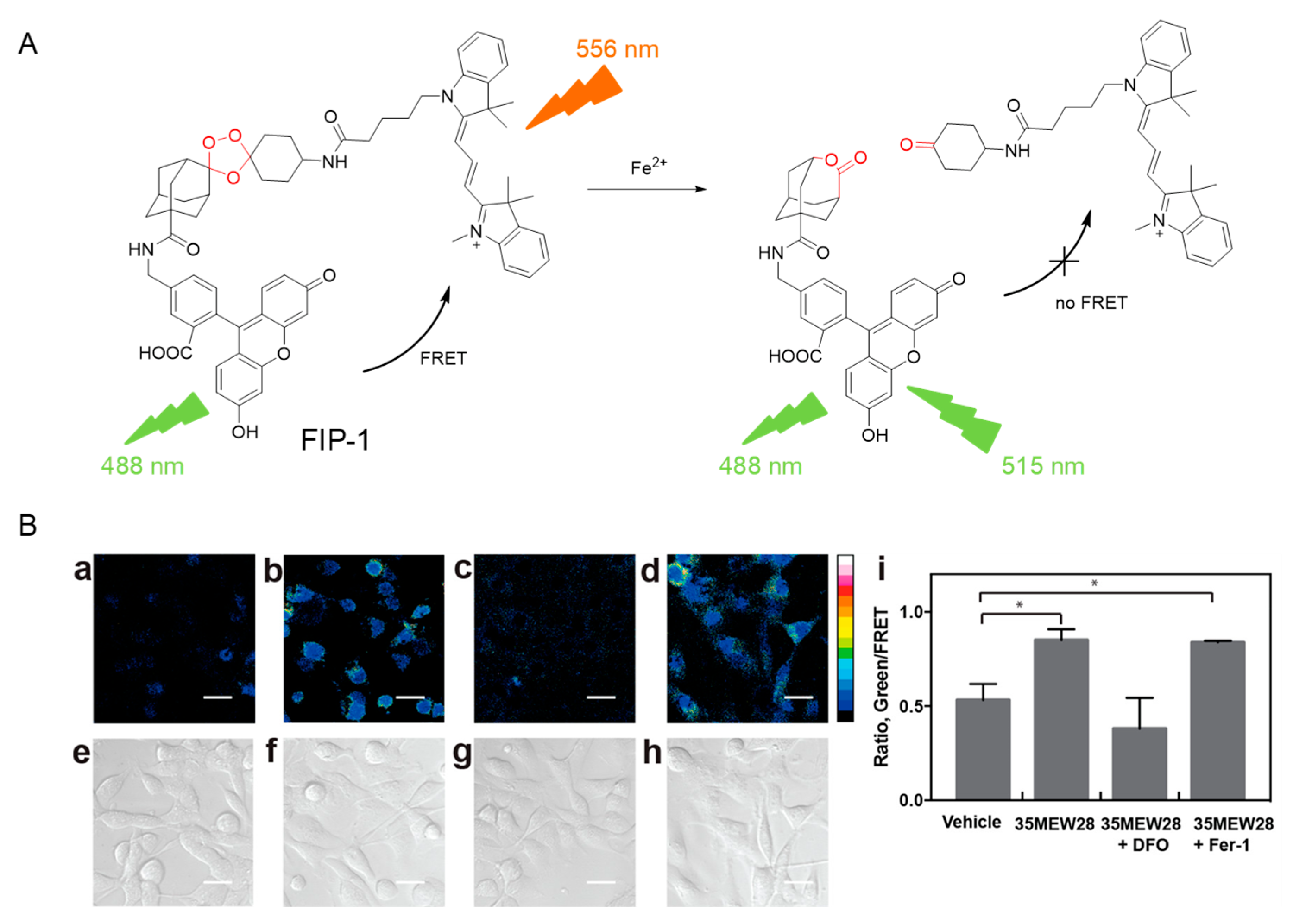
- Aron, A.T.; Loehr, M.O.; Bogena, J.; Chang, C.J. An Endoperoxide Reactivity−Based FRET Probe for Ratiometric Fluorescence Imaging of Labile Iron Pools in Living Cells. J. Am. Chem. Soc. 2016, 138, 14338–14346. [Google Scholar] [CrossRef]
- Hirayama, T.; Okuda, K.; Nagasawa, H. A Highly Selective Turn−on Fluorescent Probe for Iron(II) to Visualize Labile Iron in Living Cells. Chem. Sci. 2013, 4, 1250–1256. [Google Scholar] [CrossRef]
- Aron, A.T.; Reeves, A.G.; Chang, C.J. Activity−Based Sensing Fluorescent Probes for Iron in Biological Systems. Curr. Opin. Chem. Biol. 2018, 43, 113–118. [Google Scholar] [CrossRef] [Green Version]
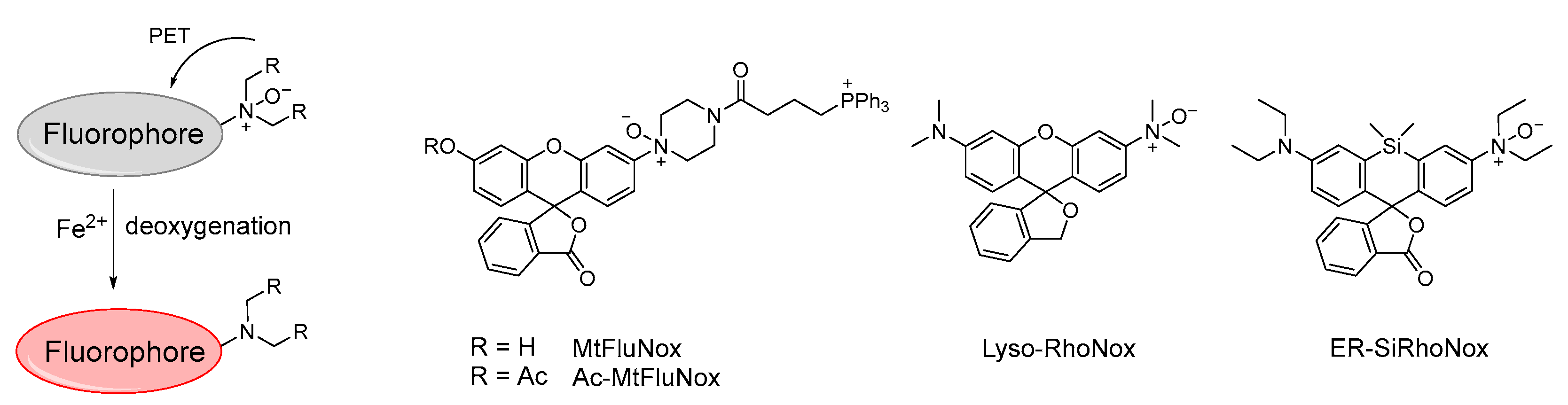
- Hirayama, T.; Kadota, S.; Niwa, M.; Nagasawa, H. A Mitochondria−Targeted Fluorescent Probe for Selective Detection of Mitochondrial Labile Fe(II). Metallomics 2018, 10, 794–801. [Google Scholar] [CrossRef]
- Niwa, M.; Hirayama, T.; Okuda, K.; Nagasawa, H. A New Class of High−Contrast Fe(II) Selective Fluorescent Probes Based on Spirocyclized Scaffolds for Visualization of Intracellular Labile Iron Delivered by Transferrin. Org. Biomol. Chem. 2014, 12, 6590–6597. [Google Scholar] [CrossRef]
- Hirayama, T.; Tsuboi, H.; Niwa, M.; Miki, A.; Kadota, S.; Ikeshita, Y.; Okuda, K.; Nagasawa, H. A Universal Fluorogenic Switch for Fe(II) Ion Based on N−Oxide Chemistry Permits the Visualization of Intracellular Redox Equilibrium Shift towards Labile Iron in Hypoxic Tumor Cells. Chem. Sci. 2017, 8, 4858–4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirayama, T.; Miki, A.; Nagasawa, H. Organelle−Specific Analysis of Labile Fe(II) during Ferroptosis by Using a Cocktail of Various Colour Organelle−Targeted Fluorescent Probes. Metallomics 2019, 11, 111–117. [Google Scholar] [CrossRef]
- Kawai, K.; Hirayama, T.; Imai, H.; Murakami, T.; Inden, M.; Hozumi, I.; Nagasawa, H. Molecular Imaging of Labile Heme in Living Cells Using a Small Molecule Fluorescent Probe. J. Am. Chem. Soc. 2022, 144, 3793–3803. [Google Scholar] [CrossRef]
- Groves, J.T.; Watanabe, Y. Reactive Iron Porphyrin Derivatives Related to the Catalytic Cycles of Cytochrome P−450 and Peroxidase. Studies of the Mechanism of Oxygen Activation. J. Am. Chem. Soc. 1988, 110, 8443–8452. [Google Scholar] [CrossRef]
- Xing, W.; Xu, H.; Ma, H.; Abedi, S.A.A.; Wang, S.; Zhang, X.; Liu, X.; Xu, H.; Wang, W.; Lou, K. A PET−Based Fluorescent Probe for Monitoring Labile Fe(II) Pools in Macrophage Activations and Ferroptosis. Chem. Commun. 2022, 58, 2979–2982. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H.; Bu, X.; Fu, Y.; Wang, Z.; Liu, Q. Recent Advances in Dual−Emission Ratiometric Fluorescence Probes for Chemo/Biosensing and Bioimaging of Biomarkers. Coord. Chem. Rev. 2019, 383, 82–103. [Google Scholar] [CrossRef]
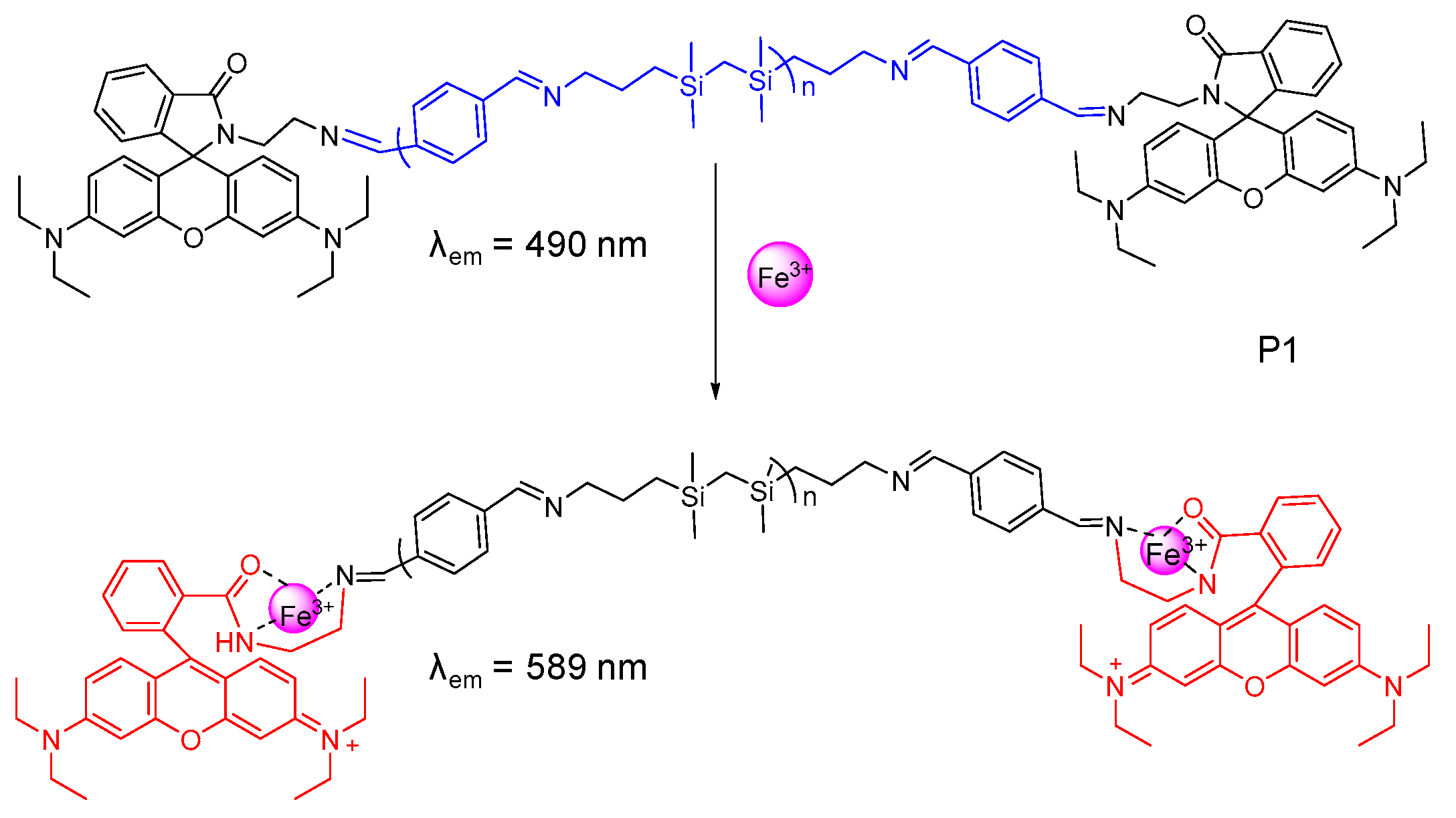
- Zuo, Y.; Yang, T.; Wang, X.; Zhang, Y.; Tian, M.; Gou, Z.; Lin, W. Visualizing the Cell Ferroptosis via a Novel Polysiloxane−Based Fluorescent Schiff Base. Sens. Actuators Chem. 2019, 298, 126843. [Google Scholar] [CrossRef]
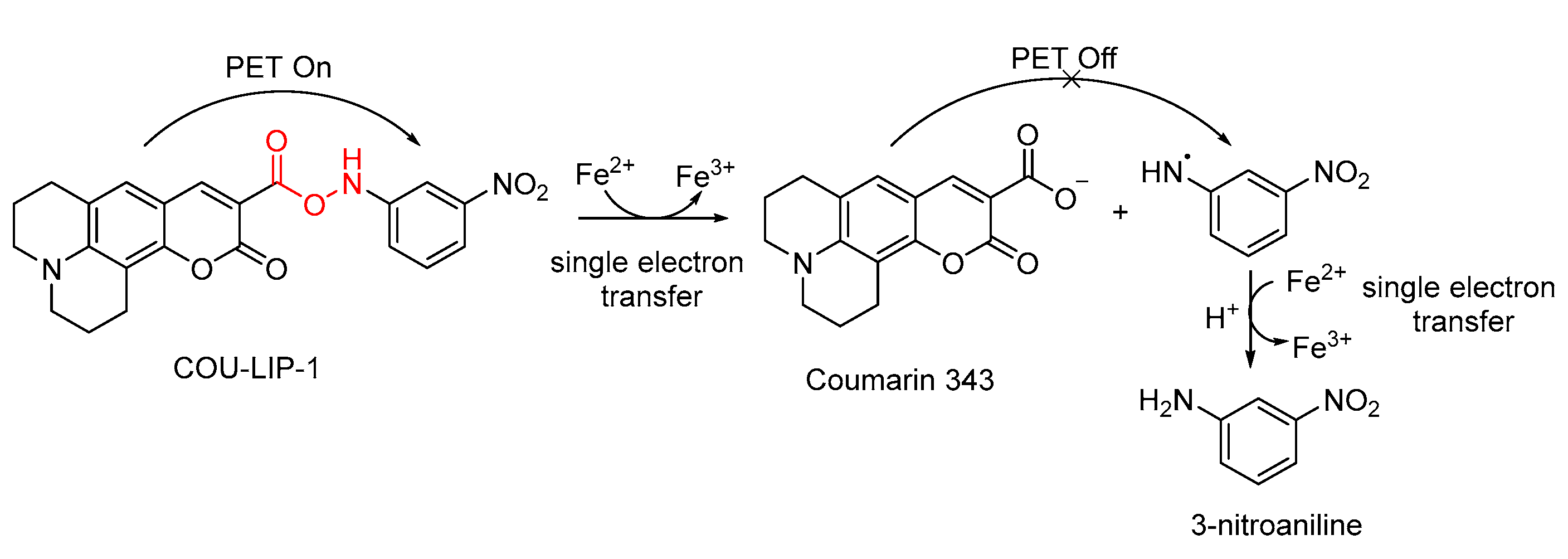
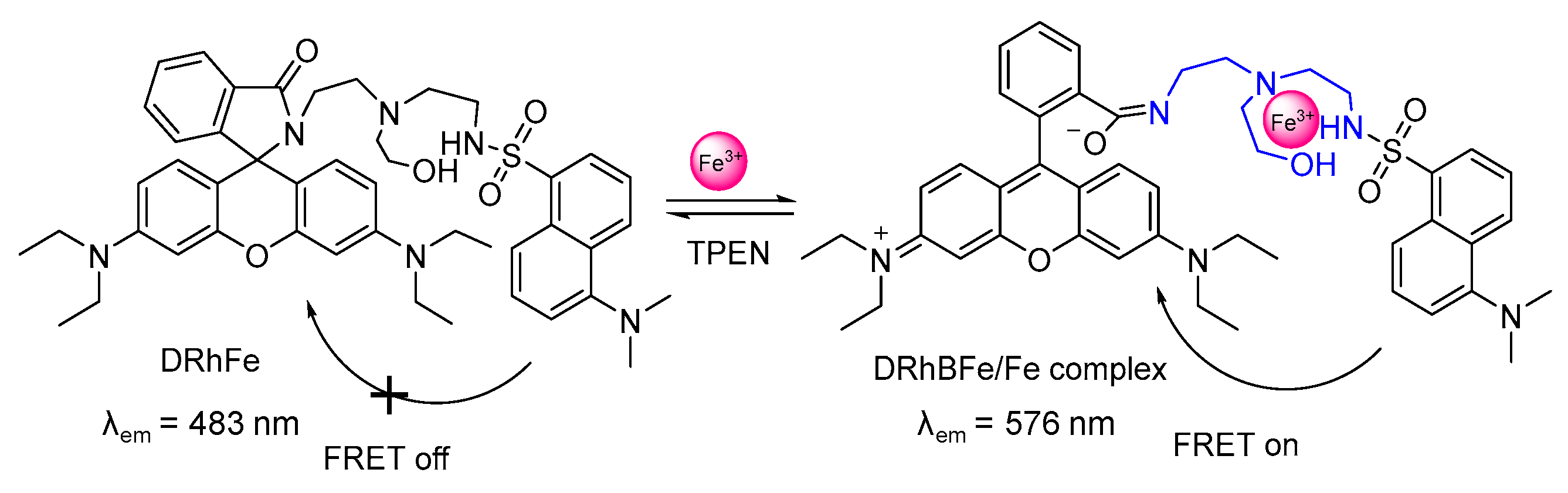
- Gao, J.; He, Y.; Chen, Y.; Song, D.; Zhang, Y.; Qi, F.; Guo, Z.; He, W. Reversible FRET Fluorescent Probe for Ratiometric Tracking of Endogenous Fe3+in Ferroptosis. Inorg. Chem. 2020, 59, 10920–10927. [Google Scholar] [CrossRef] [PubMed]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
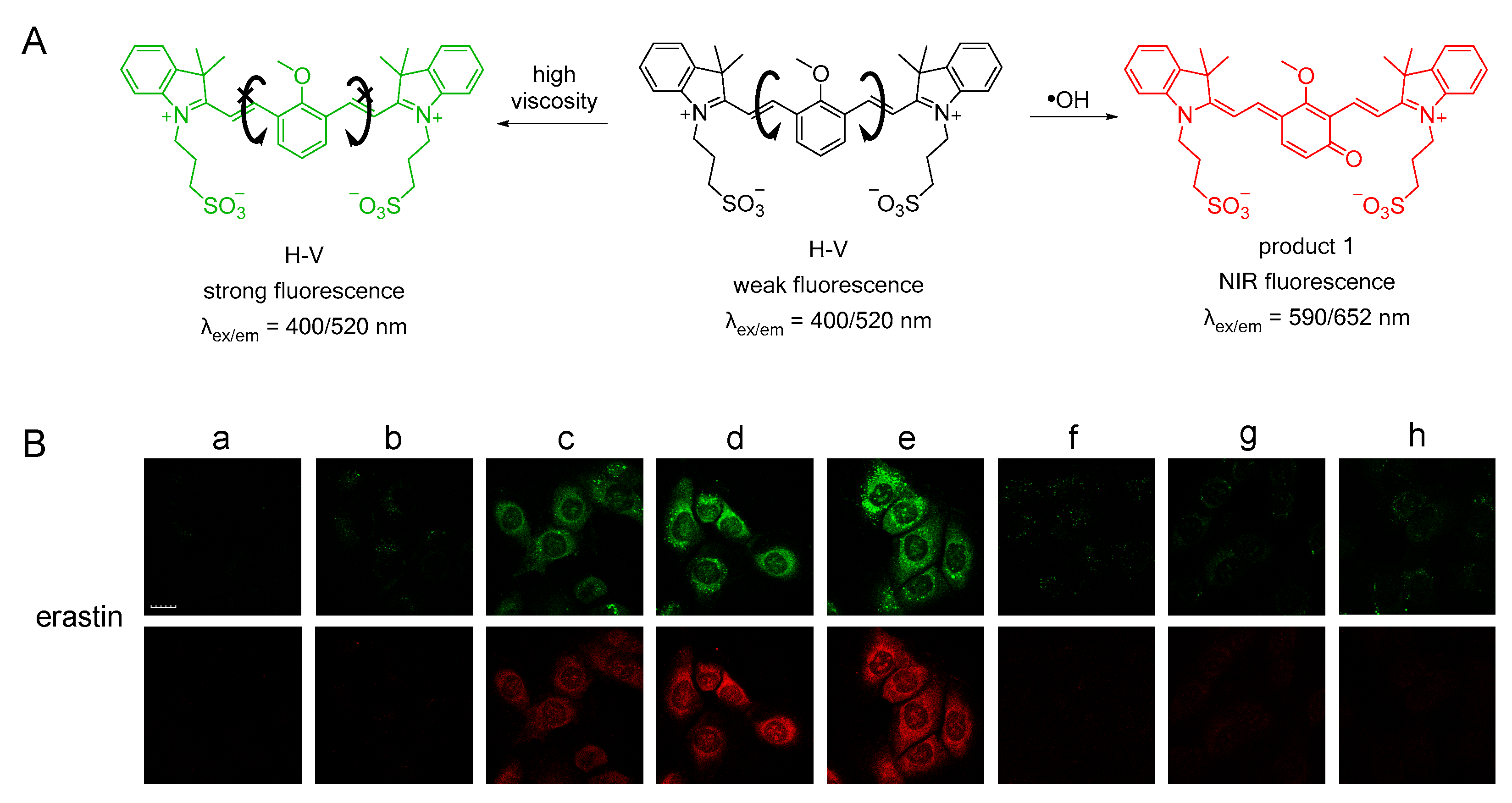
- Li, H.; Shi, W.; Li, X.; Hu, Y.; Fang, Y.; Ma, H. Ferroptosis Accompanied by •OH Generation and Cytoplasmic Viscosity Increase Revealed via Dual−Functional Fluorescence Probe. J. Am. Chem. Soc. 2019, 141, 18301–18307. [Google Scholar] [CrossRef]
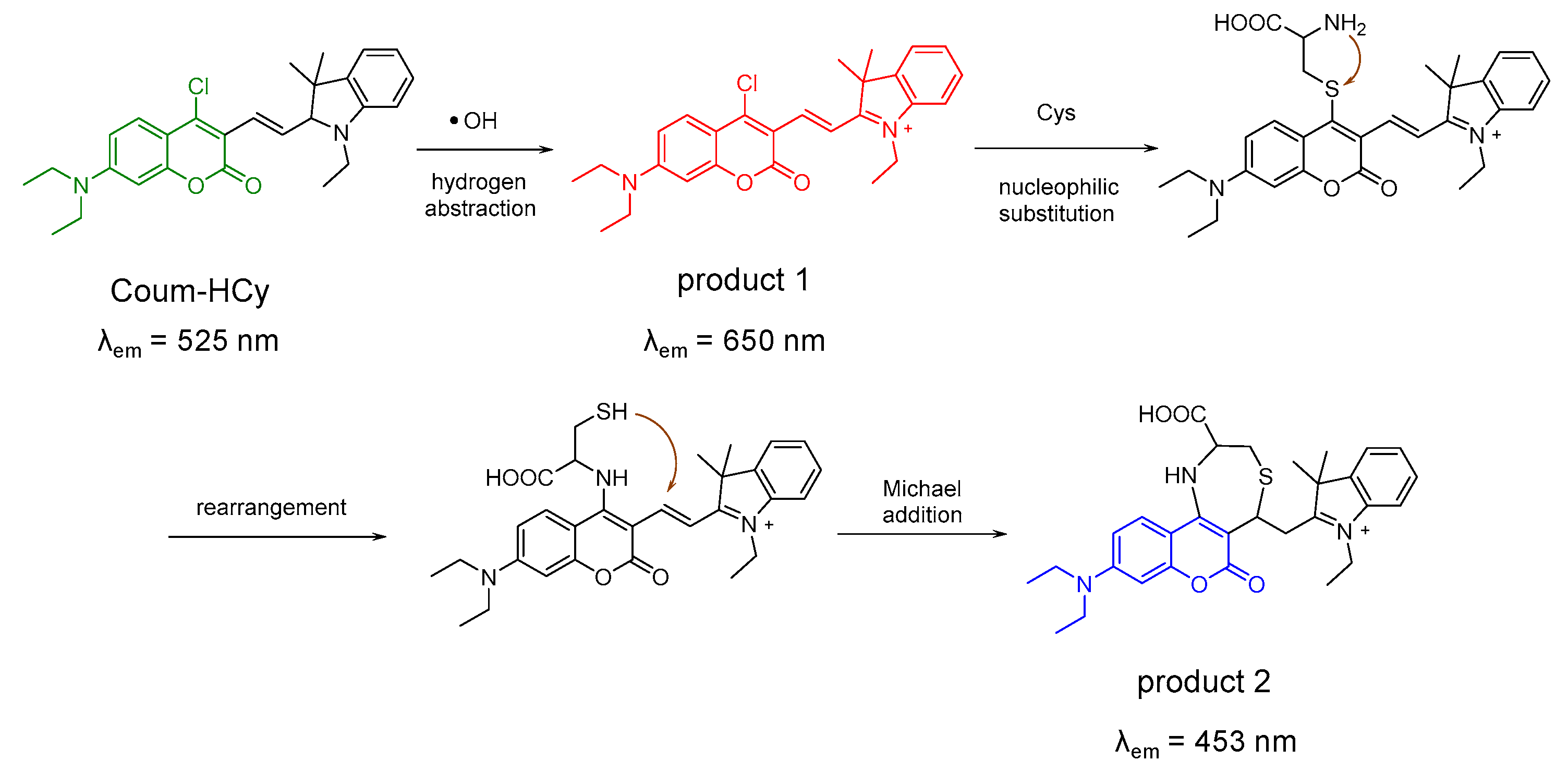
- Chen, Y.; Hu, Z.; Yang, M.; Gao, J.; Luo, J.; Li, H.; Yuan, Z. Monitoring the Different Changing Behaviors of •OH and Cysteine in Two Ferroptosis Pathways by a Dual−Functional Fluorescence Probe. Sens. Actuators Chem. 2022, 362, 131742. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, Z.R.; Ren, M.; Kong, X.; Liu, K.; Deng, B.; Lin, W. A Fast−Responsive Turn on Fluorescent Probe for Detecting Endogenous Hydroxyl Radicals Based on a Hybrid Carbazole−Cyanine Platform. Sens. Actuators Chem. 2016, 236, 60–66. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Song, J. Ratiometric Fluorescent Detection of Intracellular Hydroxyl Radicals Based on a Hybrid Coumarin−Cyanine Platform. Chem. Commun. 2010, 46, 7930–7932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Wu, X.; Shi, W.; Ma, H. N −Acetylcysteine and Cysteine in Their Ability to Replenish Intracellular Cysteine by a Specific Fluorescent Probe. Chem. Commun. 2016, 52, 9410–9413. [Google Scholar] [CrossRef]
- Li, H.; Ma, H. New Progress in Spectroscopic Probes for Reactive Oxygen Species. J. Anal. Test. 2018, 2, 2–19. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small−Molecule Fluorescent Probes for Imaging and Detection of Reactive Oxygen, Nitrogen, and Sulfur Species in Biological Systems. Anal. Chem. 2018, 90, 533–555. [Google Scholar] [CrossRef]
- Xu, Q.; Lee, K.A.; Lee, S.; Lee, K.M.; Lee, W.J.; Yoon, J. A Highly Specific Fluorescent Probe for Hypochlorous Acid and Its Application in Imaging Microbe−Induced Hocl Production. J. Am. Chem. Soc. 2013, 135, 9944–9949. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Lee, H.; Choi, Y.; Kim, Y. A Boronate−Based Fluorescent Probe for the Selective Detection of Cellular Peroxynitrite. Chem. Commun. 2014, 50, 9353–9356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.; Shen, J.; Li, Q.; Wang, R.; Xu, Y.; Qian, X. A Ratiometric Fluorescent Probe for Fast and Sensitive Detection of Peroxynitrite: A Boronate Ester as the Receptor to Initiate a Cascade Reaction. RSC Adv. 2014, 4, 51589–51592. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Li, X.; Li, X.; Ma, H. Design, Synthesis and Application of a Dual−Functional Fluorescent Probe for Reactive Oxygen Species and Viscosity. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2021, 246, 119059. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Imaging of Hydrogen Peroxide (H2O2) during the Ferroptosis Process in Living Cancer Cells with a Practical Fluorescence Probe. Talanta 2020, 212, 120804. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited−State Intramolecular Proton−Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.R.; Ghanavatkar, C.W.; Sekar, N. Towards NIR−Active Hydroxybenzazole (HBX)−Based ESIPT Motifs: A Recent Research Trend. ChemistrySelect 2020, 5, 2103–2113. [Google Scholar] [CrossRef]
- Zhang, H.C.; Tian, D.H.; Zheng, Y.L.; Dai, F.; Zhou, B. Designing an ESIPT−Based Fluorescent Probe for Imaging of Hydrogen Peroxide during the Ferroptosis Process. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2021, 248, 119264. [Google Scholar] [CrossRef]
- Ren, M.; Dong, D.; Xu, Q.; Yin, J.; Wang, S.; Kong, F. A Biotin−Guided Two−Photon Fluorescent Probe for Detection of Hydrogen Peroxide in Cancer Cells Ferroptosis Process. Talanta 2021, 234, 122684. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yuan, J.; Liu, Y.; Qin, Y.; Wang, X.; Gu, J.; Chen, G.; Zhang, B.; Liu, H.K.; Zhao, J.; et al. Epileptic Brain Fluorescent Imaging Reveals Apigenin Can Relieve the Myeloperoxidase−Mediated Oxidative Stress and Inhibit Ferroptosis. Proc. Natl. Acad. Sci. USA 2020, 117, 10155–10164. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Liu, G.; Zhao, Y.; Liu, J.; Li, Y.; Zhang, J.; Jiao, X.; Wang, X.; Tang, B. Two−Photon Fluorescence Imaging of the Cerebral Peroxynitrite Stress in Alzheimer’s Disease. Chem. Commun. 2022, 58, 6300–6303. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Yang, Y.; Gong, Z.; Zhu, H.; Qian, Y. In Vivo Tracking Cystine/Glutamate Antiporter−Mediated Cysteine/Cystine Pool under Ferroptosis. Anal. Chim. Acta 2020, 1125, 66–75. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, J.; Bajić, A.; Zhang, C.; Song, X.; Carroll, S.L.; Cai, Z.L.; Tang, M.; Xue, M.; Cheng, N.; et al. Quantitative Real−Time Imaging of Glutathione. Nat. Commun. 2017, 8, 16087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, T.; Qiang, T.; Ren, L.; Cheng, F.; Wang, B.; Li, M.; Hu, W.; James, T.D. Near−Infrared Fluorescent Probe for Hydrogen Sulfide: High−Fidelity Ferroptosis Evaluation in Vivo during Stroke. Chem. Sci. 2022, 13, 2992–3001. [Google Scholar] [CrossRef]
- Di, X.; Ge, C.; Liu, Y.; Shao, C.; Zhu, H.L.; Liu, H.K.; Qian, Y. Monitoring Hydrogen Polysulfide during Ferroptosis with a Two−Photon Fluorescent Probe. Talanta 2021, 232, 122467. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.P.; Abney, J.R.; Verkman, A.S. Determinants of the Translational Mobility of a Small Solute in Cell Cytoplasm. J. Cell Biol. 1993, 120, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Wang, S.; Ren, W.X.; Hou, J.T.; Won, M.; An, J.; Chen, X.; Shu, J.; Kim, J.S. Fluorescence Imaging of Pathophysiological Microenvironments. Chem. Soc. Rev. 2021, 50, 8887–8902. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.N.; Liu, L.Y.; Mao, D.; Xu, S.; Tan, C.P.; Cao, Q.; Mao, Z.W.; Liu, B. A Polarity−Sensitive Ratiometric Fluorescence Probe for Monitoring Changes in Lipid Droplets and Nucleus during Ferroptosis. Angew. Chem. Int. Ed. 2021, 60, 15095–15100. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Liu, J.; Yang, X.; Zhu, W.; Ye, Y. A Bifunctional Fluorescent Probe for Imaging Lipid Droplets Polarity/SO2 during Ferroptosis. Sens. Actuators Chem. 2022, 365, 131937. [Google Scholar] [CrossRef]
- Kuimova, M.K. Mapping Viscosity in Cells Using Molecular Rotors. Phys. Chem. Chem. Phys. 2012, 14, 12671–12686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Heo, J.; Woo, H.C.; Lee, J.A.; Seo, Y.H.; Lee, C.L.; Kim, S.; Kwon, O.P. Fluorescent Molecular Rotors for Viscosity Sensors. Chem. Eur. J. 2018, 24, 13706–13718. [Google Scholar] [CrossRef]
- Abdel−Mottaleb, M.S.A.; Loutfy, R.O.; Lapouyade, R. Non−Radiative Deactivation Channels of Molecular Rotors. J. Photochem. Photobiol. A Chem. 1989, 48, 87–93. [Google Scholar] [CrossRef]
- Dong, B.; Song, W.; Lu, Y.; Sun, Y.; Lin, W. Revealing the Viscosity Changes in Lipid Droplets during Ferroptosis by the Real−Time and in Situ Near−Infrared Imaging. ACS Sens. 2021, 6, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, W.; Yue, L.; Lin, W. Revealing the Effects of Endoplasmic Reticulum Stress on Ferroptosis by Two−Channel Real−Time Imaging of PH and Viscosity. Anal. Chem. 2022, 94, 6557–6565. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, Y.; Wu, G.; Wang, Y.; Duan, S.; Han, J.; Cheng, W.; Li, C.; Tian, X.; Zhang, X. Dual−Responsive Fluorescent Probe for Imaging NAD(P)H and Mitochondrial Viscosity and Its Application in Cancer Cell Ferroptosis. Sens. Actuators Chem. 2022, 350, 130862. [Google Scholar] [CrossRef]
- Yin, J.; Xu, Q.; Mo, X.; Dai, L.; Ren, M.; Wang, S.; Kong, F. Construction of a Novel Mitochondria−Targeted near−Infrared (NIR) Probe for Detection of Viscosity Changes in Cancer Cells Ferroptosis Process. Dyes Pigments 2022, 200, 110184. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, L.; Xie, L.; Zheng, Y.; Man, H.; Xiao, Y. Forthrightly Monitoring Ferroptosis Induced by Endoplasmic Reticulum Stresses through Fluorescence Lifetime Imaging of Microviscosity Increases with a Specific Rotor. Chin. Chem. Lett. 2021, 33, 2537–2540. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; An, Y.; Gao, J.; Yang, M.; Luo, J.; Li, X.; Lv, J.; Li, X.; Yuan, Z.; Ma, H. Recent Advances of Fluorescence Probes for Imaging of Ferroptosis Process. Chemosensors 2022, 10, 233. https://doi.org/10.3390/chemosensors10060233
Li H, An Y, Gao J, Yang M, Luo J, Li X, Lv J, Li X, Yuan Z, Ma H. Recent Advances of Fluorescence Probes for Imaging of Ferroptosis Process. Chemosensors. 2022; 10(6):233. https://doi.org/10.3390/chemosensors10060233
Chicago/Turabian StyleLi, Hongyu, Yan An, Jie Gao, Mingyan Yang, Junjun Luo, Xinmin Li, Jiajia Lv, Xiaohua Li, Zeli Yuan, and Huimin Ma. 2022. "Recent Advances of Fluorescence Probes for Imaging of Ferroptosis Process" Chemosensors 10, no. 6: 233. https://doi.org/10.3390/chemosensors10060233
APA StyleLi, H., An, Y., Gao, J., Yang, M., Luo, J., Li, X., Lv, J., Li, X., Yuan, Z., & Ma, H. (2022). Recent Advances of Fluorescence Probes for Imaging of Ferroptosis Process. Chemosensors, 10(6), 233. https://doi.org/10.3390/chemosensors10060233






