3.1. Quantitative
In Goodman and Gilman’s
The Pharmacological Basis of Therapeutics (
GGPBT) [
22], there were 115 authors, of which 32 contributed to more than one chapter. The highest degree was MD for half of authors (47.0%), PhD for two-fifths (45.2%), and the remainder (7.8%) were PharmDs. The preponderance (90.4%) of authors were based in the US.
One-fifth (20.9%) of authors were female—or 19.0% if including multiple chapter contributions. Female authorship in GGPBT was equivalent to that of Katzung’s Basic and Clinical Pharmacology (KatBCP, 17.0%, p = 0.52), but less than Remington’s Science and Practice of Pharmacy (RemSPP, 37.0%, χ2(1) = 8.84, p ≤ 0.003) and Koda-Kimble and Young’s Applied Therapeutics (KKYAT, 53.0%, χ2(1) = 8.84, p < 0.001).
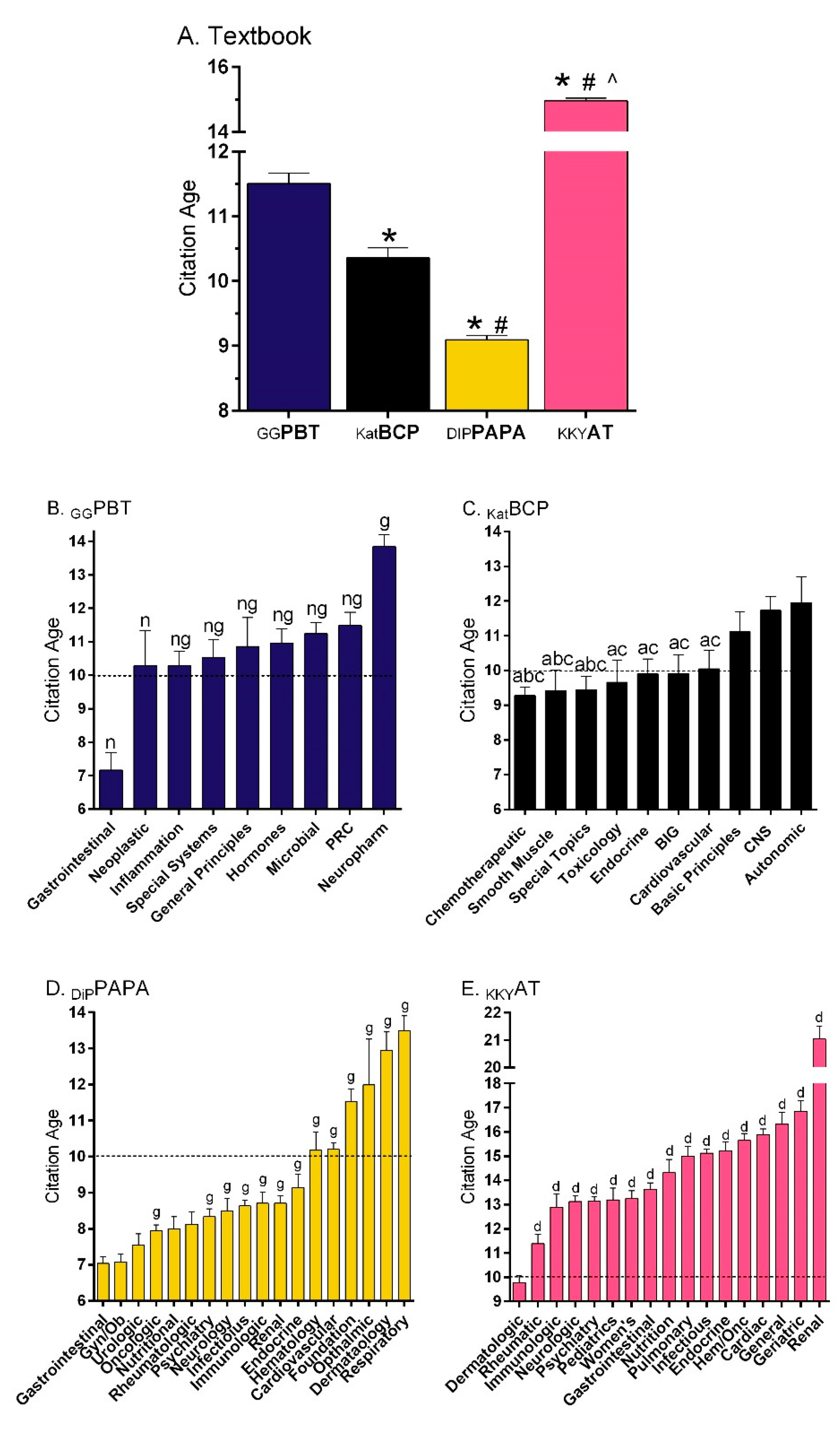
Figure 1A shows that the references in
GGPBT [
22] were 12.3% older (11.5 years) than those in
KATBCP [
23] (10.4,
t(5321) = 4.42,
p < 0.0005). The DiPiro’s
Pharmacotherapy: A Pathophysiological Approach (
DiPPAPA) [
11] citations (9.1 years) were also more recent than
GGPBT (
t(16,951) = 15.59,
p < 0.0001). Similarly, additional analysis determined that more references were at least a decade old for
GGPBT (49.1%) compared to
KatBCP (44.7%, χ
2(1) = 9.20,
p ≤ 0.002) or
DiPPAPA (34.0%, χ
2(1) = 278.21,
p < 0.0001).
KatBCP also differed from
DiPPAPA on this measure (χ
2(1) = 78.44,
p < 0.0001). However,
GGPBT had less citations that were ten or more years old relative to
KKYAT [
24] (64.4%, χ
2(1) = 283.08,
p < 0.0001).
Figure 1B depicts reference age by section of
GGPBT. The Drugs Affecting Gastrointestinal Function section was significantly newer than all other sections. Conversely, the Neuropharmacology references were 6.7 years older than those for Gastrointestinal, and also significantly less recent than all other sections. There were some broad similarities between
GGPBT and
KatBCP (
Figure 1C), with oncology citations being more recent, endocrinology intermediate, and neuroscience the oldest. The gastrointestinal, as well as gynecological, citations were the most recent relative to many other sections in
DiPPAPA (
Figure 1D). The citations in
KKYAT [
24] were significantly older (14.96 ± 0.08) than those of the other three textbooks [
10,
22,
23]. Citations in the dermatologic disorders section (9.77 ± 0.30) were significantly more recent than all other sections, particularly renal disorders (21.04 ± 0.47,
t (201.03) = 16.36,
p < 0.0005,
Figure 1E) and the eye disorders chapter (27.59 ± 1.05, data in
Supplementary Materials).
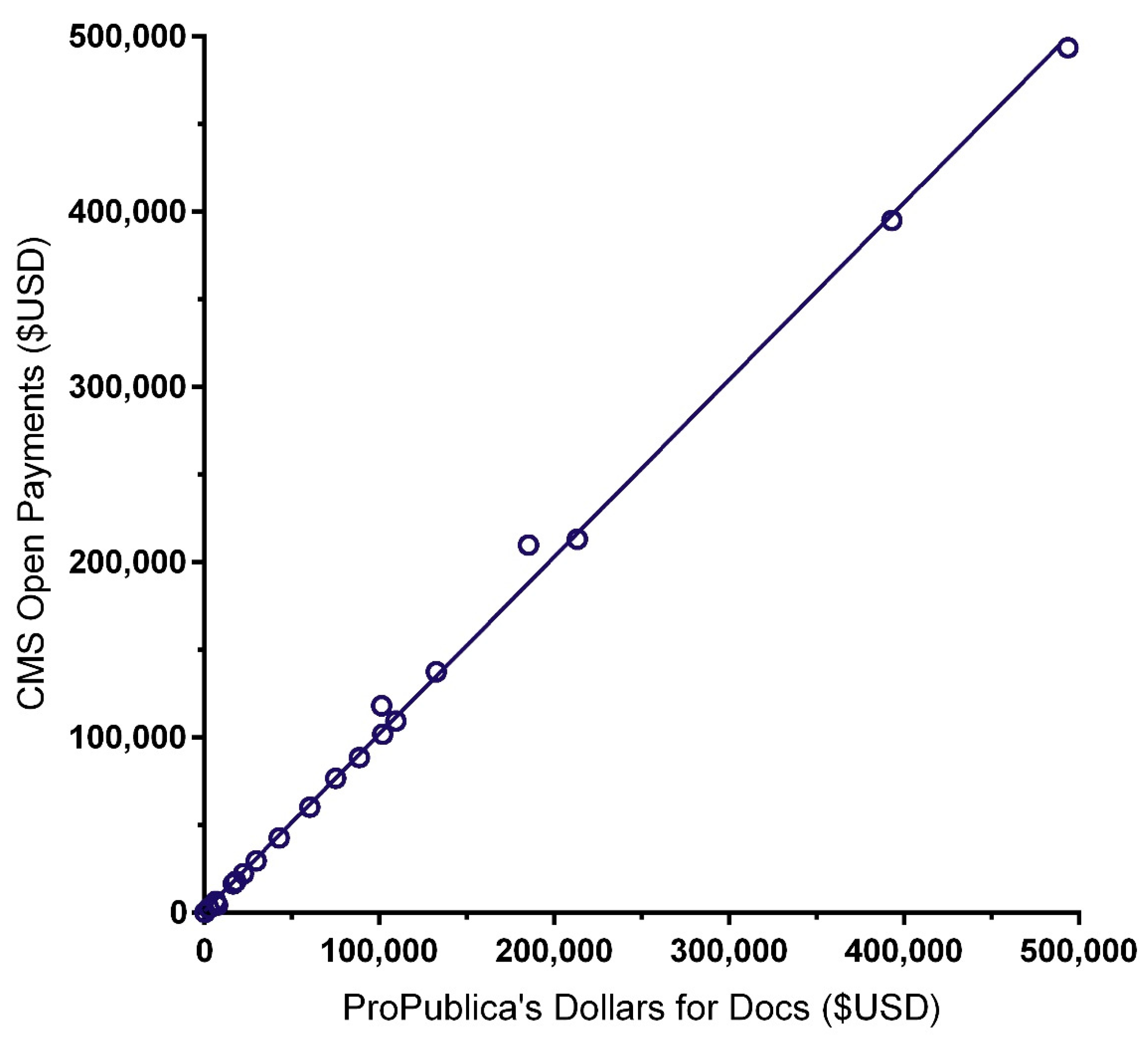
The similarity of the CoI databases ProPublica’s Dollars for Docs (
PPDD) and Medicaid Service’s Open Payments (
CMSOP) was examined with two complementary analyses. The correlation between the total received from 2013 to 2016 (i.e., the most recent available in both databases) was high (
r (24) = 0.999,
p < 0.0005;
Figure 2). However, the mean amount received was eighteen-hundred dollars higher for
CMSOP (USD 82,923 ± 24,404) than
PPDD (USD 81,105 ± 24,122)—a non-significant difference (
t (25) = 1.59,
p = 0.13).
The total undisclosed remuneration received by twenty-seven GGPBT authors (57.4% of eligible US-based physician-authors, 96.3% male, Minimum = USD 23, Maximum = USD 743,718) from 2013 to 2017 was USD 2.97 million of which almost three-quarters (71.4%) went to the top five authors. The most compensated author received USD 493,536 (66.0% of their total) for royalty or licenses and USD 250,108 (34.0%) for ownership or investment interest. One of the dermatological pharmacology authors received USD 493,536 for ivermectin, a head lice treatment. The psychosis and mania chapter author received USD 238,413 in payments related to two atypical antipsychotics. The oncologist author of “Hormones and Related Agents in the Therapy of Cancer” received USD 228,493 for five breast cancer agents.
Additional analyses were completed on
DiPPAPA [
11] which provides CoI information. Approximately one out of every twelve contributors (20/233 or 8.6%) self-reported a potential CoI. Among the 35 eligible (MD or DO with a US affiliation) authors, 74.3% had a
PPDD entry (Min = USD 14, Max = USD 729,695, total = USD 1.8 million). However, some discrepancies were identified. A status epilepticus author whose disclosures were self-reported as “none” received USD half-a-million for anti-epilepsy drugs according to
PPDD. Two authors who did not report disclosures to
DiPPAPA accepted USD 70–350 thousand in potentially relevant remuneration from 2013 to 2016 (
Table 1).
3.2. Narrative Reviews
Open-ended evaluations were provided on sixteen chapters, including representation of seven of the nine sections of Goodman and Gilman’s
The Pharmacological Basis of Therapeutics (
GGPBT).
Table 2 provides strengths and limitations.
3.2.1. General Principles
Chapter 2, “Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination”, is a comprehensive and thorough explanation and discussion of the basic concepts of pharmacokinetics. The characteristics and processes of absorption, distribution, metabolism, and elimination are reviewed respectively, including an in-depth discussion of the types of transport across membranes and the influence of pH. The first-pass effect, as well as the benefits and limitations of various routes of drug administration, are also explored. Potentially beneficial additions to the discussion are further examples of specific drugs and their routes of administration, as well as the factors that influence onset of action. There is a brief discussion of rectal administration that could be improved by expanding upon the reasoning behind its incomplete and irregular absorption. Distribution is briefly explored, including a discussion of the differences of distribution among various types of tissues as well as an explanation of protein and tissue binding. While the bone portion of this section focuses mostly on tetracycline antibiotics, an introduction to the pathophysiologic changes in bone that make distribution difficult overall would be helpful, space permitting. The next section on metabolism is a simplistic but comprehensive explanation of phase one and two reactions, as well as first and zero-order kinetics. The excretion section focuses primarily on renal excretion but also includes that by other routes. Learning is aided by highly beneficial figures that depict renal drug handling and a helpful visual of the afferent and efferent arterioles. The final section discusses clinical pharmacokinetics and the “four most important parameters governing drug disposition.” While this section is extensive and includes strong drug examples to explain clinical pharmacokinetics, it could be confusing to the reader that components of “clinical pharmacokinetics” are separated from ADME. It may be beneficial to integrate this section into earlier content. Some examples include moving the discussion of extent and rate of absorption under absorption, clearance under excretion, and volume of distribution under distribution. There is a helpful explanation of maintenance and loading doses, therapeutic window, and dosing intervals along with drug examples with each. However, the explanation of therapeutic drug monitoring is somewhat limited and potentially too generalized. Additional examples of therapeutic drug monitoring (for example, a comparison of therapeutic drug monitoring used for vancomycin, aminoglycosides, and warfarin) would be beneficial to the reader in order to understand the parameters that influence different timing and results of monitoring.
Chapter 3, “Pharmacodynamics: The Molecular Mechanisms of Drug Action” includes a comprehensive and highly detailed discussion of three primary areas of pharmacodynamics including basic concepts, mechanisms of drug action, and signaling pathways. The first section provides a detailed explanation of different receptor types as well as a short discussion of the increased development of biologic agents. The definitions of agonists, antagonists, and their subtypes are provided. A useful addition to this General Principles section would be drug examples of agonists, antagonists, and their subtypes in order to illustrate these concepts further. Specificity of drug receptors and tachyphylaxis are explored, followed by a detailed explanation of affinity, efficacy, and potency. Graphs and mathematical models are used to elaborate upon these concepts. Individual and population pharmacodynamics as well as factors that affect the variability of drug dosing are included. There is also a useful section that expands on the individual patient characteristics that contribute to variability of dosing. There is a section on drug interactions, but it does not mention the effect of CYP enzymes on drug interactions. While this is typically categorized in pharmacokinetics, it may be beneficial to mention their substantial effects on drug levels here. The following section explores the mechanisms of drug action, and expands upon the effects of drugs on ligands, extracellular responses, intracellular pathways, and ions. The mechanisms of anti-infective drugs are also reviewed, which can potentially be eliminated in the overall discussion, or converted to an example instead. There is a helpful review of the structure and function of specific receptor types including second messenger systems and other signaling pathways. Tachyphylaxis and desensitization are discussed again here and could be more beneficial if the discussions on this topic were condensed to one area of the chapter. The chapter ends with an extensively detailed exploration of diseases associated with transcription and translation along with the pharmacotherapies that treat them. Considering that this is an introductory chapter on pharmacodynamics, it may be more useful for this information to be included at a later point in the book. It may be advantageous to consider narrowing this topic to only basic pharmacodynamic concepts. A thorough evaluation of the Pharmacogenomics chapter may be found in
Appendix A.
3.2.2. Neuropharmacology
Chapter 15, “Drug Therapy of Depression and Anxiety Disorders”, includes several strengths. Figure 15.1 illustrates detailed information about the variety of antidepressant mechanisms or action. It provides a list of long term cellular regulatory changes in addition to increasing neurotransmitter “dwell time” in the synapse. The development of ketamine and other approaches as novel antidepressants are well described. Information about pharmacokinetics and CYP action is helpful in a practical way to predict, and avoid, drug interaction. This chapter provides a general summary of the basic treatments for anxiety and depression.
As a weakness, chapter 15 does not place very much emphasis on the dopamine or histamine systems/actions in antidepressant effects or depressive etiology. Information about side effects, therapeutic laboratory values, wash-out periods, serotonin syndrome and discontinuation effects are dispersed throughout the text and are not highlighted or emphasized as key pieces of information in designing a treatment strategy. At the beginning of the chapter, depression is immediately categorized into bipolar I and II. It might be more accurate to introduce mood disorders more generally, with an emphasis on depressive symptoms/diagnoses. The authors suggest that there has been limited progress in developing animal models sensitive to antidepressants and anxiolytics. Anxiety is described as “a normal human emotion that serves an adaptive function,” however depression is not.
Another concern is that there are places where information is presented without research citations, such as “Anxious patients appear to be particularly prone to severe discontinuation reactions with certain medications such as venlafaxine and paroxetine; therefore, slow-tapering is required.” The chapter does not include information about options for drug-treatment-resistant symptoms or suggestions for combination approaches [
21]. As this is a pharmacology text, that is reasonable. However, it might be helpful to have at least a mention of electroconvulsive therapy or transcranial magnetic stimulation, so readers are better informed of other options if drug treatment has been unsuccessful. More specialized textbooks [
21] provide, at least, a nod that the nomenclature for the “Selective” Serotonin Reuptake Uptake Inhibitors is an inaccurate oversimplification [
26]. Although recognizing space constraints, greater incorporation of evidence-based psychotherapies like cognitive behavioral therapy, alone and in combination with pharmacotherapies [
27,
28], is needed.
Chapter 20, “Opioids, analgesia, and pain management”, is well-organized and includes an emphasis on history, receptor signaling, the pathophysiology of pain, tolerance, withdrawal, and medical chemistry for this important drug class. On rare occasions, there is some unusual content and minor areas for reconsideration. Mentioning that opioids do not bind to sigma receptors, twice, may be less useful for new members of the field. The space devoted to specific opioids does not show any simple relationship with their current use patterns [
29]. Meperidine and normeperidine, despite concerns with CNS excitation and seizures, are mentioned four times more commonly than the ubiquitous hydrocodone. Tramadol is described, twice, as a “weak opiate agonist”. Katzung’s
Basic and Clinical Pharmacology (
KatBCP) [
23] and others have a more nuanced description of the mechanism of action of this agent and the importance of desmethyltramadol [
30]. The terms “addict” or “addicts” are used eight times, vs. zero for the less stigmatizing “opioid use disorder” [
31].
Chapter 23, “Ethanol”, begins with a brief description about the historical use of alcohol in human civilization as well as epidemiologic overview of problems associated with it; this is quite helpful. There is also a practical overview about alcohol content of different beverages, as well as information about estimating the blood ethanol concentration in end-expiratory alveolar air. Excellent sections with pharmacological properties of methanol and ethanol and its effects are arranged by system. The addition of a Shakespearean quote added emphasis and served as a nice anecdote to illustrate the overview. This is also a good overview of the postulated neurological pathways that are thought to be involved in tolerance and dependence. Descriptions of teratogenicity, genetics, and drug interactions are adequate. Information about treatment of alcohol withdrawal is lacking. No information about choice of drug therapy for withdrawal, or for symptoms triggered therapy protocols (the Clinical Institute Withdrawal Assessment or CIWA protocol for example) for assessment and treatment, is provided. This is a surprising omission as alcohol withdrawal is very prevalent and is associated with considerably increased mortality and morbidity as compared to opioid withdrawal.
3.2.3. Modulation of Pulmonary, Renal, and Cardiovascular Function
Chapter 25 has the challenging task of introducing normal renal structure and function as a basis for the mechanistic understanding of drugs that affect the renal excretory function. The chapter does an incredible job in covering clinically relevant renal concepts. However, it is somewhat arbitrary in what is introduced in the chapter’s introduction as “normal” and what is mentioned later, because it is necessary to mention to understand the mechanics of diuretics and other drugs. Some figures and tables are very helpful (e.g., Table 25-1), while others are overloaded and do not add much value (e.g., Figure 25-2). Overall, it is a good chapter, despite the fact that every pathophysiologist will miss some mechanistic understanding of normal (e.g., Mg2+ reabsorption) or abnormal processes (e.g., reduction of urinary Ca2+ excretion by thiazide diuretics).
3.2.4. Hormones and Hormone Antagonists
Chapter 48, “Agents Affecting Mineral Ion Homeostasis and Bone Turnover” provides a thorough review of the pharmacological agents used in treating and preventing mineral ion imbalances and bone metabolism. The tables and figures are highly useful and summarize the main points of discussion, particularly the drug summary table at the end of the chapter, which provides a quick reference for understanding the pharmacological agents, their uses, and their clinical effects. This chapter will be useful for both the medical student and seasoned healthcare professional in that, in addition to discussing all of the major pharmacological players involved in treating ion and bone diseases, it makes great attempts to review and contextualize these treatment approaches by providing significant background information. For example, this chapter contains a review of the basic hormonal control mechanisms involved in ion homeostasis, target organs/systems, the relevant bone cell physiology, and a summary of the biology of disorders of mineral homeostasis and bone. The chapter focuses on general concepts that have a high relevance in the clinical setting and are useful for directly understanding the underlying pharmacology. This approach, while on the whole effective, does sometimes come at the expense of missing some detailed but relevant information. For example, the chapter really does not discuss osteoclast function in depth or bone resorption/remodeling effectively, which would aid in the reader’s understanding of drugs like bisphosphonates. This chapter also contains a decent summary discussing the integrated approach to prevention and treatment of osteoporosis. This section does a more than adequate job of generally summarizing the field with respect to the treatment strategies used for managing and preventing osteoporosis.
3.2.5. Gastrointestinal (GI) Pharmacology
Chapters 49 through 51 focus on GI disorders. There are several positive aspects to these chapters. First, the physiology and pathogenesis overview of each chapter and pharmacology of each medication class is brief yet manages to remain quite detailed. The use of figures throughout allows information to be more easily digested. Also, as a new edition, this resource incorporates information on recently approved agents, including medications like eluxadoline (Viberzi®) and vedolizumab (Entyvio®), which naturally would be omitted by older resources. Finally, information on each drug and drug class appears complete and accurate. Even adverse events recently identified by newer studies, like dementia and chronic kidney disease for proton pump inhibitors (PPIs), are included.
On the other hand, there are several drawbacks as well. This section groups a large number of topics into a small number of chapters, which could make readings challenging to assign to students. For instance, to discuss a topic like Helicobacter pylori infection, one must first learn about PPIs early in Chapter 49, then navigate through about two-thirds of the chapter before finally coming across a brief discussion on managing the disorder.
The organization of Chapter 50, which covers motility disorders, emesis, and biliary and pancreatic disease, is particularly hard to follow. The chapter begins by introducing antimotility agents used in small populations and only available through limited access programs, if at all. Meanwhile, very common motility disorders like diarrhea and constipation are buried throughout the lengthy chapter. Also, the complications of cirrhosis, an important GI disorder, are mixed in only in short blurbs and in a confusing manner throughout the chapter. A more complete discussion is vital and its absence is a concerning flaw of this section.
Also, while it may be fitting to limit or omit information on medical interventions, such as fundoplication surgery, relative to a therapeutics textbook, there is limited coverage on nonpharmacologic interventions, which are some of the most common and important recommendations pharmacists can make to patients. Additionally, some aspects of these chapters assume a baseline understanding, which may not be beneficial for students who do not possess this prior knowledge. For instance, terms like distal proctitis are left undefined, yet are used freely when describing the therapeutic role of certain agents. Finally, compared to other resources, this section uses fewer tables, making differences between medications or diseases more difficult to establish.
Overall, chapters 49 to 51 allow individuals to build a strong foundation in the pharmacological treatment of GI disorders by providing a great deal of information in a small number of chapters. However, this section is not perfect. Improvement is needed with regard to organization, coverage of cirrhosis, information on nonpharmacologic interventions, and the use of tables to present data.
3.2.6. Chemotherapy of Infectious Diseases
Chapters 52, 56, 57 and 58 focus on infectious diseases. Chapter 52 is a general chapter focusing on the general principles of antibacterial therapy. There are a few positive aspects to this chapter including the step by step description of which antibiotic type (prophylactic, definitive, suppressive, etc.) should be utilized in the infectious disease (ID) process and detailed explanations of each type. The overview of the pharmacokinetics of antibiotics is very detailed and the use of figures throughout the descriptions provides visual explanations to better understand the information. The explanation on medication resistance is very thorough and ensures that detailed information for the resistance of each medication class does not have to be discussed in each respective chapter.
Chapter 52, on the general principles of antimicrobial therapy, was very informative but does have some disadvantages which make it somewhat difficult to follow. The pharmacokinetics section, though detailed and useful, covers information beyond the need of healthcare providers. The descriptions of Emax and Ka, including equations, are explained well but are not necessary to fully describe the antibiotic classes. This information is not useful when determining what antibiotic to utilize, nor does it explain how dosing is affected based on these values. The explanation of time-dependent and concentration-dependent antibiotics, and antibiotics with a post-antibiotic effect are explained very well; however, a comprehensive table detailing what medications fall into each category is not provided. Most other textbooks include a description of what an infection entails and appropriate selection of antibiotics; however, this chapter jumps directly into the pharmacokinetic information.
Chapters 56–58 discuss various antibacterial agents with descriptions of structure activity relationships, mechanism of actions, antibacterial spectrum, pharmacokinetics and indications for each medication within each respective class. The organization of the chapters has an excellent flow and is consistent from class to class. In chapter 56, the discussion on medications used for urinary tract infections is not consistent with the other medication classes; however, the information provided is very thorough and does incorporate the same information provided by the other class of medications, just in a different manner. Even though there are copious amounts of information provided on each respective class and each medication within the class, the format and explanations are done in a very lay method allowing non-healthcare providers to understand the material as well. The inclusion of historical facts about certain medication classes improves the reading quality and provides background information on either the class or the antibacterial spectrum of the class. In chapter 57, the inclusion of the carbenicillin class is necessary to explain the class as a whole. Other texts will exclude these medications and only discuss piperacillin/tazobactam; however, including the medications not available in the United States, such as ticarcillin and mezlocillin, increases the credibility of piperacillin. Most texts have difficulty explaining the cephalosporin generations; however, chapter 57 does an impeccable job detailing out medications in each generation and relating it back to the antibacterial spectrum. The summary tables at the end of chapters 57 and 58 are all-inclusive and do a superb job at summarizing the chapter.
These chapters are very thorough and useful to a variety of healthcare providers; however, there are a few limitations within these three chapters, which provide information that is not needed or lacks information in certain areas. In chapter 56, when discussing sulfonamides, several medications are referenced which are not commonly used, including one which is not approved for use in the United States. This excess information makes it more difficult to find the relevant information for medications more commonly utilized. One major limitation within these three chapters is the lack of tables throughout the chapter to simplify indications and dosing. All indications are separated into respective paragraphs which makes it more difficult to follow, providing tables after each class highlighting the indication and doses would allow readers to connect all medications within the class. It is understandable that not all new medications could be included due to the publication process being lengthy; however, four medications released within the last year are not included, two of which make a huge impact in practice. Delafloxacin (Baxdela®) and the combination of meropenem and vabobactam (Vabomere®) were introduced within the last year, but they have not warranted major changes; however, the combination of ceftazidime and avibactam (Avycaz®) and the combination of ceftolozane and tazobactam (Zerbaxa®), medications introduced specifically to fight off resistant pathogens such a carbapenemase-resistant enterobacteriaceae, are essential for healthcare providers to know about due to increased resistance to other medications in recent years.
Overall, these chapters allow healthcare providers to obtain the necessary pharmaceutics information needed to make decisions in patient care; however, improvements in formatting and the inclusion of newer medications would benefit the text as a whole.
3.2.7. Pharmacotherapy of Neoplastic Disease
Chapter 65, “General Principles in the Pharmacotherapy of Cancer”, is an excellent and reasonably comprehensive background to cancer pharmacology. Strengths of this chapter include: (a) distinguishing slower-growing cancers that have a smaller proportion of actively cycling tumor cells and are thus less responsive to drugs that target the cell cycle; (b) stressing the importance of combinatorial therapy, particularly combinations of molecularly targeted drugs and immunotherapy with more generalized cytotoxic chemotherapy; (c) a discussion of the various challenges of molecular testing to determine those patients for whom specific targeted therapies would be most efficacious. With respect to the last issue, the chapter not only discusses the issue of tumor heterogeneity but also properly cites inherited genetic variation (and not just tumor-specific variation) as a factor affecting treatment response. One criticism is, in the section on resistance, it is stated that the resistant cells pre-exist the treatment which selects for these cells. While this no doubt occurs in the (vast) majority of cases (and the evidence for this for kinase inhibitors is discussed in Chapter 67), it should be noted that resistant cells can occur during treatment as a result of random mutations, unrelated to treatment, that may occur, particularly in tumors exhibiting a hypermutable condition (e.g., MSI+). Also, the much more controversial issue of adaptive mutation, which is starting to attract serious attention from a subset of researchers, could have at least been mentioned in passing. For example, there has been a report of the possible role played by adaptive mutation in the development of resistance to the androgen receptor antagonist bicalutamide in prostate cancer cells [
32].
Chapter 66 goes into great detail about several important classes of cytotoxic drugs, mostly those that block cell division and/or promote apoptosis, but also the differentiating agent all-trans retinoic acid (ATRA). Alkylating agents and platinum analogs come in for a particularly extensive review, and one that does justice not only to mechanisms of action and therapeutic efficacy, but also the (often serious) side effects of these agents. The chapter also makes an important distinction between bifunctional agents and monofunctional methylating agents, which is helpful.
Chapter 67, “Pathway-Targeted Therapies: Monoclonal Antibodies, Protein Kinases Inhibitors, and Various Small Molecules”, delves into a detailed examination of pathway-targeted therapies, centered on small molecule inhibitors and monoclonal antibodies, two approaches that are contrasted with respect to range of action and to their side effects (the small molecules tend to have both a greater range of desirable activities as well as negative side effects). Similar to the examination of cytotoxic therapies in the preceding chapter and to the more general introduction of chapter 65, attention is paid to issues of mechanism of action, matching specific therapies to the appropriate type of cancer, including the genetic variation of the cancer, preexisting inherited genetic variation of the patient that affects response, side effects, and the development of resistance. Also, combinatorial therapy is touched upon, an approach particularly useful with the monoclonal antibodies. The sections on angiogenesis inhibitors and immunotherapy (particularly the immune checkpoint inhibitors) was quite good, and this reviewer positively notes the discussion of combinatorial immune checkpoint therapy targeting both cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed death-1 (PD-1). There was some discussion about how to overcome resistance; for example, resistance to the mitogen-activated protein kinase kinase (MEK) inhibitor trametinib can be overcome by combinatorial therapy with the BRAF inhibitor dabrafenib, and there is also discussion about dealing with imatinib resistance. This is all significant; if anything, more on this topic can be included; the same can be said about the ameliorating side effects of these agents. There was some decent discussion on this latter topic; particularly useful was the note about colony-stimulating factors used to deal with hematopoietic toxicity of many anti-cancer therapies. Certainly, an expanded analysis of such topics is always welcomed. The section on histone deacetylase inhibitors was adequate, but there could have been a discussion about butyrate—a fermentation product of dietary fiber—which is a histone deacetylase inhibitor, and one that may significantly mediate the anti-cancer properties of dietary fiber for the colon. These topics could have been broached, which would have also allowed for a discussion of how naturally occurring agents in food may exert preventive action against cancer. Although the book is focusing on pharmacological therapy, the overlap between pharmacology and “medicinal food,” as well as that between prevention and treatment, could have been productively addressed for the sake of completeness. Chemoprevention is a legitimate topic in the field of cancer, both with respect to natural products as well as, perhaps, other pharmacological agents. One issue that could have been included as additional detailed discussion is the approach of inducing apoptosis through hyper-activation (rather than inhibition) of signaling pathways the cancer cell is “addicted to.” There also could have been some discussion of cutting-edge small molecules in clinical trial that target signaling pathways, such as ICG-001-like compounds that inhibit CBP-mediated Wnt signaling. Further, by connecting issues such as targeting signaling pathways, hyperactivating signaling pathways, and molecularly targeted drugs, one could cite the finding that some histone deacetylase inhibitors can hyperactivate Wnt signaling, inducing apoptosis of colorectal cancer cells in culture, pointing to a possible novel therapeutic approach. That underscores one weakness of these chapters—while they tend to give an excellent overview of existing therapies, there is not much about possible novel future approaches.
Chapter 68 offers sound coverage on the role of hormone modulation for cancer therapeutics. Two main approaches were addressed. First, there was discussion about glucocorticoids, which can not only be used for anti-cancer treatment, but also to ameliorate side effects from other forms of cancer therapy. Second, hormone-based therapy for breast and prostate cancer was addressed. Interestingly, while anti-estrogen therapy is now important in breast cancer treatment, in the past, high doses of estrogen were used for certain breast cancers to induce apoptosis. The chapter notes that it is necessary to address the physiological type of breast cancer before commencing therapy, as some forms of the disease are hormone-therapy resistant. The chapter also goes into detail about anti-androgen therapy for prostate cancer, and this is well done, although an analysis of quality-of-life issues would have strengthened the discussion of pharmacology-based treatment options for this disease.
3.2.8. Special Systems Pharmacology
The dermatological and ocular pharmacology chapters have been greatly expanded compared to the 12th edition of GGPBT. Five new agents (bentoquatam, coal tar, anthralin, brimonidine, and propranolol) were added to the “miscellaneous agents” section and one agent was removed (podophyllin) in the dermatological chapter compared to last edition. Both chapters have high quality colorful figures of pathways and tables with details such as structural class and efficacy for each agent. Additionally, there is a drug summary table at the end of the chapter with therapeutic uses, clinical pharmacology, and tips for each agent to compliment the text.
The ocular chapter provides excellent explanation of the medications’ mechanisms of action and describes which agent is preferred or which agent provides fewer side effects compared to an agent within the same class. For example, brimonidine is less likely to cause ocular allergy and for that reason, it is more commonly used. Although this information is presented throughout the chapter, it is not accessible in one place. An algorithm highlighting what is first-line and second-line treatment for glaucoma in the ocular chapter would have been beneficial to the reader.
When compared to Koda-Kimble 10
th edition [
33], the dermatological and ocular chapters in
GGPBT lack cases. It would have been helpful to have included case questions within the chapter, so the reader can easily see how the pharmacological information is applicable to patients in practice. In comparison to
KatBCP [
23], the
GGPBT dermatology chapter reviews more medications. However, the medication section headlines are not as clearly defined and are presented in a less organized format. Within the sunscreen portion in the dermatological chapter, it might have been desirable to have more details about each agent. The chapters use only the generic name of medications. Since pharmacy students are expected to know both brand and generic names for the NAPLEX and in practice, it would have been appropriate to use both within the tables. In contrast, use of brand names would be less useful for medical students. Additional information from a student perspective may be found in
Appendix B.