Nano-Sized and Mechanically Activated Composites: Perspectives for Enhanced Mass Burning Rate in Aluminized Solid Fuels for Hybrid Rocket Propulsion
Abstract
1. Introduction
2. Background
2.1. Fundamentals of Solid Fuel Combustion Process
2.2. Regression Rate Performance and Metal Additives
2.3. Aluminized Solid Fuel Formulations: Aggregation and Agglomeration
3. Investigated Materials
3.1. Aluminum and Al-Based Powders
3.2. Tested Formulations
4. Experimental Methods
4.1. Aluminum and Aluminum-Based Additives Pre-Burning Characterization
4.2. Burning Behavior Investigation
4.2.1. Lab-Scale Two-Dimensional (2D) Radial Micro-Burner
4.2.2. Time-Resolved Regression Rate
4.2.3. Lab-Scale Micro-Slab Motor
5. Experimental Results and Discussion
5.1. Additives Pre-Burning Characterization
5.2. Burning Behavior
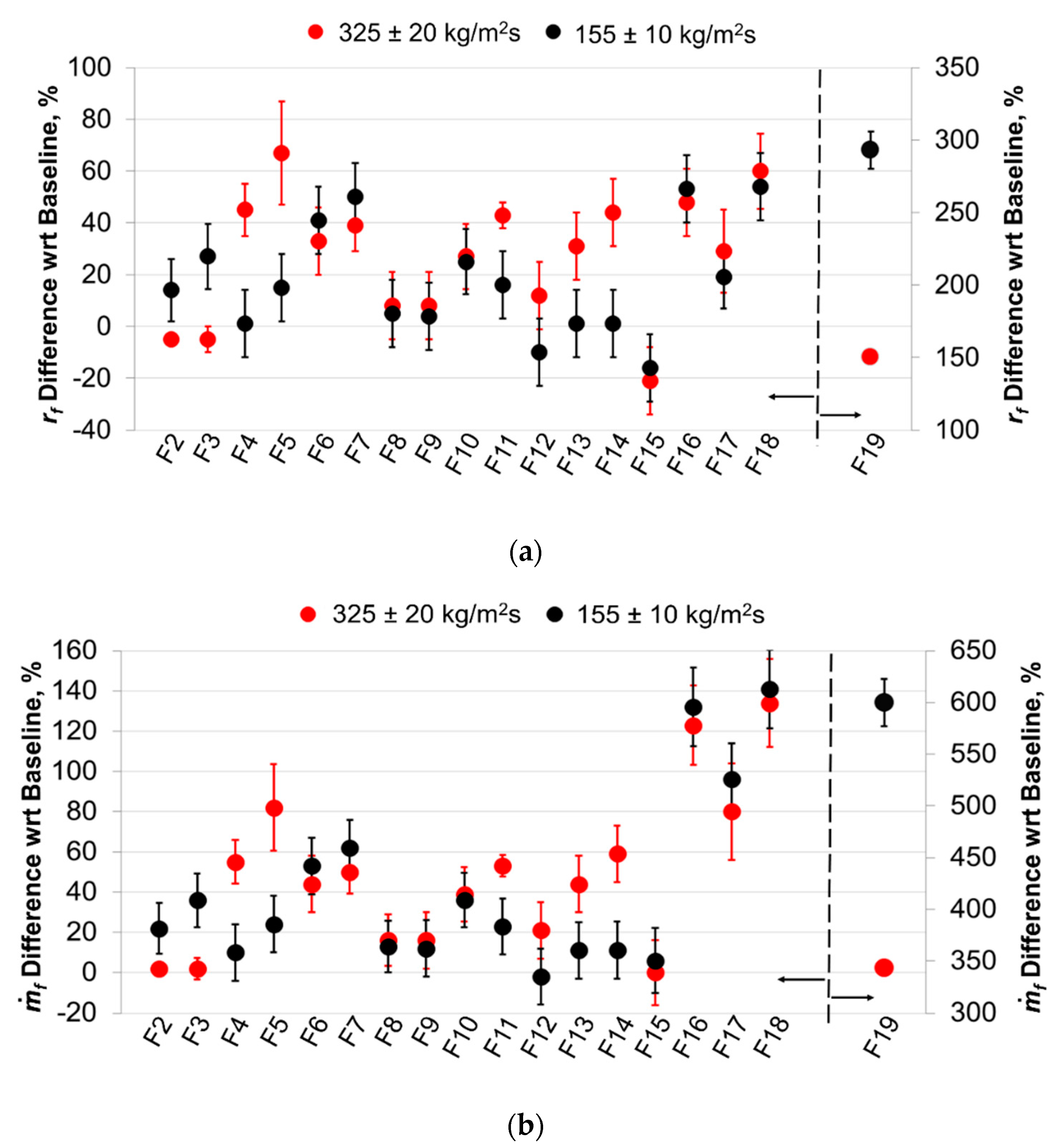
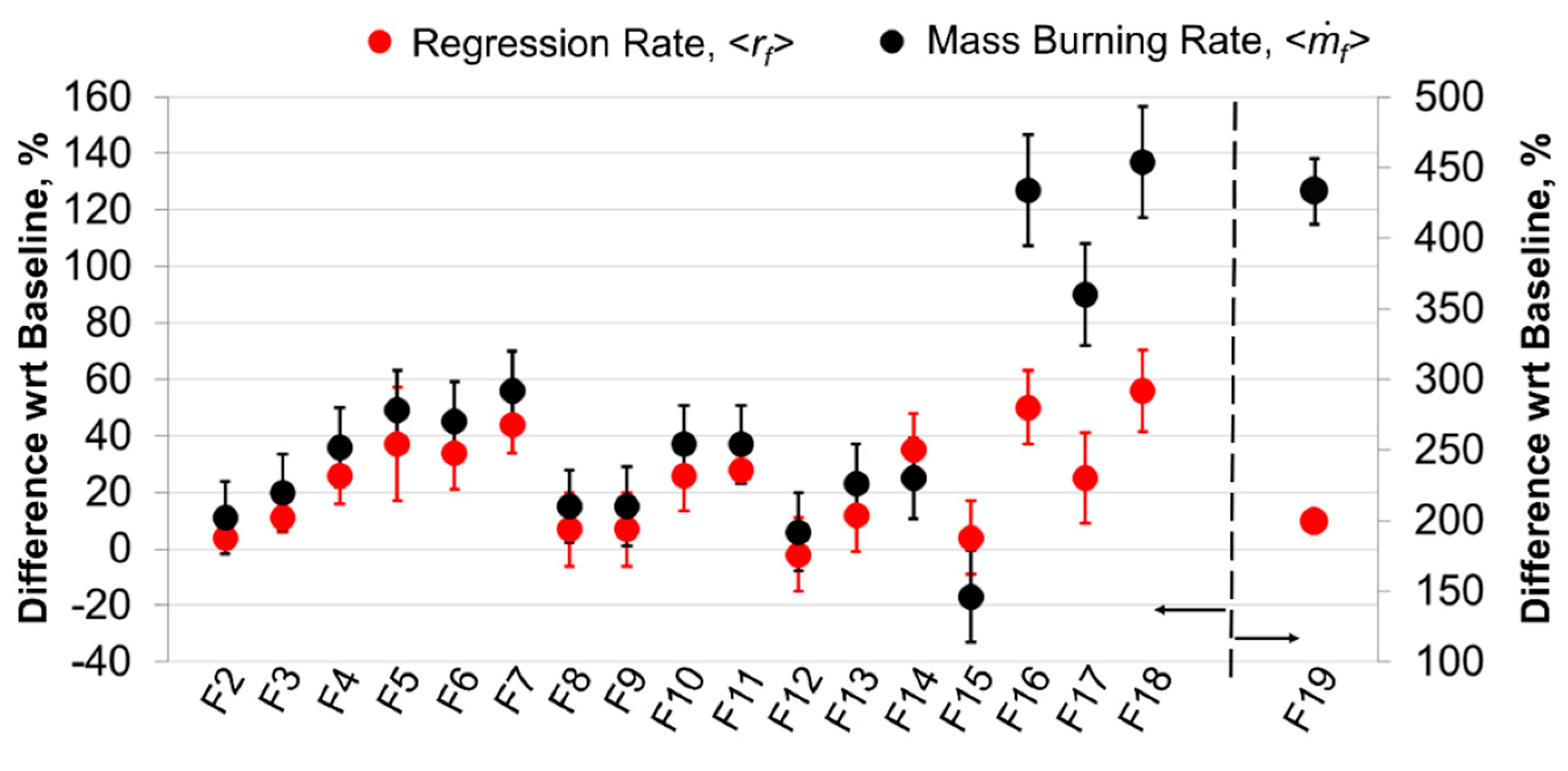
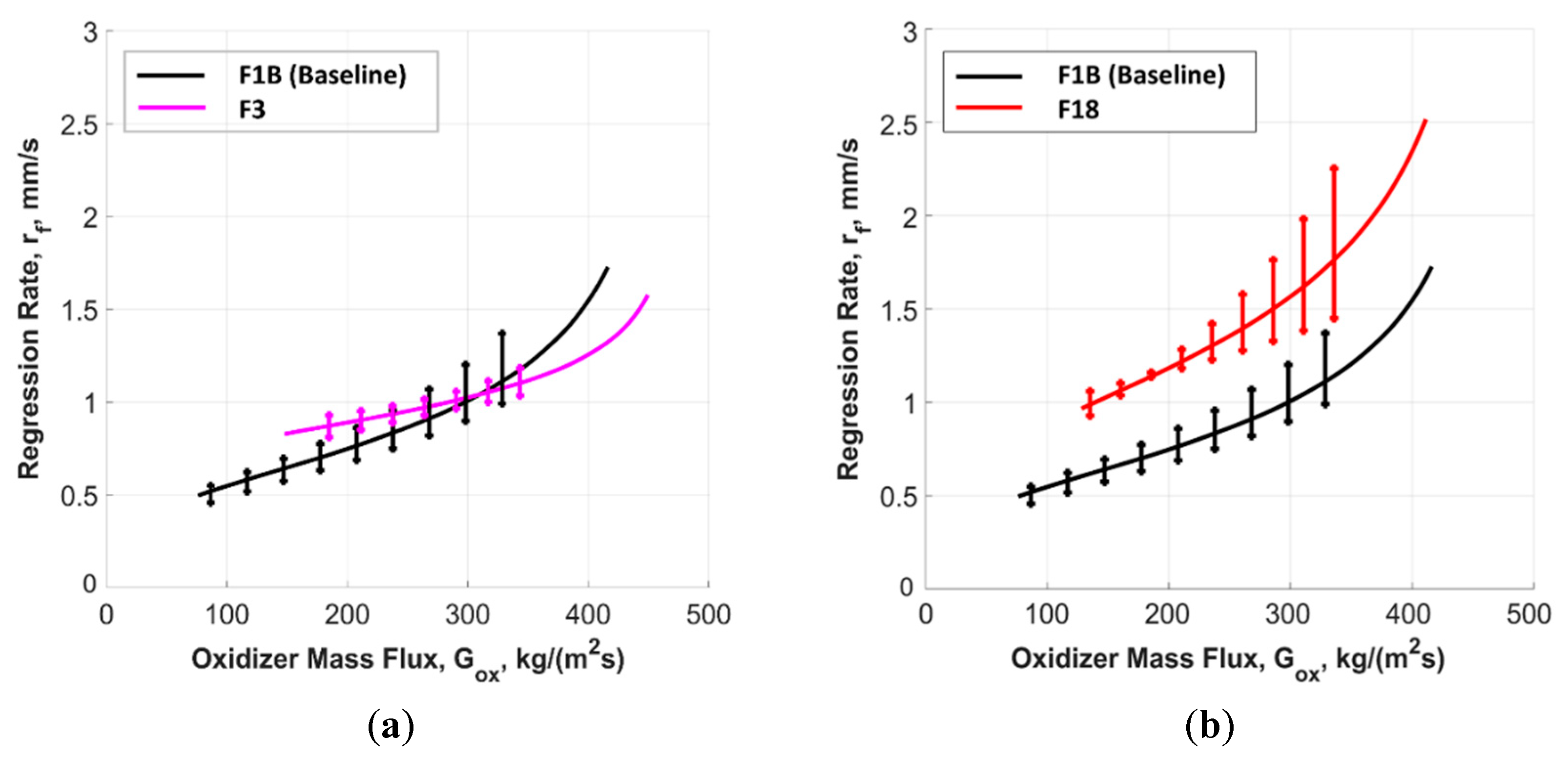
5.2.1. Time-Resolved Regression Rate
5.2.2. Combustion Surface Visualization
5.2.3. Concluding Remarks
6. Conclusions and Future Developments
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Roman and Greek Symbols | |
| space-averaged value | |
| time-averaged value | |
| α(T) | Al → Al2O3 conversion factor, % |
| Δm0 | mass change before the first intense oxidation onset temperature, % |
| Δm(T) | mass change at the temperature T, % |
| μAl | micron-sized Al powder |
| ρAl | Al density, kg/m3 |
| ρf | fuel density, kg/m3 |
| aD | pre-exponential coefficient in diameter change power law fitting, mm/snD |
| ar | pre-exponential coefficient in regression rate power law approximation, mm/s/(kg/(m2∙s))nr |
| as | particle size derived from the specific surface area, nm |
| CAl | active Al content, wt.% |
| D(0.1) | particle diameter below which 10% of the particles lay, μm |
| D(0.5) | particle diameter below which 50% of the particles lay, μm |
| D(0.9) | particle diameter below which 90% of the particles lay, μm |
| D32 | surface-based mean particle diameter, μm |
| D43 | volume-based mean particle diameter, μm |
| i-th sampled port diameter, mm | |
| G | total mass flux (G = Gox + Gf), kg/(m2∙s) |
| Gf | fuel mass flux, kg/(m2∙s) |
| Gox | oxidizer mass flux, kg/(m2∙s) |
| fuel grain length, m | |
| fuel mass burning rate, kg/s | |
| oxidizer mass flow rate, kg/s (except where otherwise stated) | |
| nAl | nano-sized Al powder |
| nD | exponent in diameter change power law fitting |
| nr | exponent in regression rate power law approximation |
| pc | combustion chamber pressure, MPa |
| R2 | data fitting parameter |
| rf | solid fuel regression rate, mm/s (m/s in Equation (4)) |
| T | temperature, K |
| t | time, s |
| tend | end time, s |
| Tfl | calculated flame temperature, K |
| ti | time of the i-th diameter sampling, s |
| tign | ignition time, s |
| Ton,i | i-th intense oxidation onset temperature, K |
| Acronyms | |
| ALEX | aluminum exploded (nAl produced by electrical explosion of wires, typically air-passivated) |
| AP | ammonium perchlorate |
| CB | carbon black |
| CCP | condensed combustion product |
| DTG | differential of the thermogravimetry trace |
| GOX | gaseous oxygen |
| HE | high energy (mechanical activation) |
| HRE | hybrid rocket engine |
| HTPB | hydroxyl-terminated polybutadiene |
| LE | low energy (mechanical activation) |
| MA | mechanical activation |
| PDL | pressure deflagration limit, MPa |
| PTFE | polytetrafluoroethylene |
| SEM | scanning electron microscopy |
| SSA | specific surface area, m2/g |
| SOP | small oxide particles |
| TED | transmission energy dispersion |
| TEM | transmission electron microscopy |
| TOT | thickness over time |
| TG | thermogravimetry |
| TMD | theoretical maximum density, kg/m3 |
| VFHFP | vinylidene fluoride hexafluoropropylene copolymer |
| wrt | with respect to |
References
- Marxman, G.A.; Gilbert, M. Turbulent boundary layer combustion in the hybrid rocket. In 9th International Symposium on Combustion; Academic Press, Inc.: New York, NY, USA, 1963; pp. 371–383. [Google Scholar]
- Marxman, G.A. Boundary layer combustion in propulsion. In 11th Symposium (International) on Combustion; The Combustion Institute: Pittsburg, PA, USA, 1964; pp. 269–289. [Google Scholar]
- Marxman, G.A.; Wooldridge, C.E. Research on the Combustion Mechanism of Hybrid Rockets. In Advances in Tactical Rocket Propulsion; Penner, S.S., Ed.; AGARD Conference Proceedings No. 1; Technivision Services: London, UK, 1968; pp. 421–477. [Google Scholar]
- Chiaverini, M.J. Review of solid fuel regression rate behavior in classical and non-classical hybrid rocket motors. In Fundamentals of Hybrid Rocket Combustion and Propulsion; Chiaverini, M.J., Kuo, K.K., Eds.; AIAA Progress in Astronautics and Aeronautics: Reston, VA, USA, 2007; Volume 218, pp. 37–125. [Google Scholar]
- Marquardt, T.; Majdalani, J. Review of Classical Diffusion-Limited Regression Rate Models in Hybrid Rockets. Aerospace 2019, 6, 75. [Google Scholar] [CrossRef]
- Pastrone, D. Approaches to low fuel regression rates in hybrid rocket engines. Int. J. Aerosp. Eng. 2012, 2012, 649753. [Google Scholar] [CrossRef]
- Karabeyoglu, M.A.; Altman, D.; Cantwell, B.J. Combustion of liquefying hybrid propellants: Part 1, general theory. J. Propul. Power 2002, 18, 610–620. [Google Scholar] [CrossRef]
- Karabeyoglu, M.A.; Cantwell, B.J. Combustion of liquefying hybrid propellants: Part 2, stability of liquid films. J. Propul. Power 2002, 18, 621–630. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Moon, H.; Sung, H.; Kim, J.; Cho, J. Effect of paraffin-LDPE blended fuel in hybrid rocket motor. In Proceedings of the 46th AIAA. ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Nashville, TN, USA, 25–28 July 2010. AIAA Paper No. 2010–7031. [Google Scholar]
- Boiocchi, M.; Paravan, C.; Dossi, S.; Maggi, F.; Colombo, G.; Galfetti, L. Paraffin-Based Fuels and Energetic Additives for Hybrid Rocket Propulsion. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015. AIAA Paper No. 2015–4042. [Google Scholar]
- Paravan, C.; Galfetti, L.; Maggi, F. A Critical Analysis of Paraffin-based Fuel Formulations for Hybrid Rocket Propulsion. In Proceedings of the 53rd AIAA/SAE/ASEE Joint Propulsion Conference, Atlanta, GA, USA, 10–12 July 2017. AIAA Paper No. 2017–4830. [Google Scholar]
- Paravan, C.; Bisin, R.; Piscaglia, F.; Galfetti, L. Combustion Processes in Hybrid Propulsion. Int. J. Energ. Mater. Chem. Propuls. 2019, 18, 255–286. [Google Scholar]
- Veale, K.; Adali, S.; Pitot, J.; Brooks, M. A Review of the Performance and Structural Considerations of Paraffin Wax Hybrid Rocket Fuels with Additives. Acta Astronaut. 2017, 141, 196–208. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, S.; Zhang, W.; Shen, R.; DeLuca, L.T.; Ye, Y. The Effects of Mechanical Modification Additives on the Regression Rate of Paraffin-Based Fuels for Hybrid Rocket. In Proceedings of the 7th European Conference for Aeronautics and Space Sciences, Milan, Italy, 1–4 July 2017. [Google Scholar]
- Risha, G.A.; Evans, B.J.; Boyer, E.; Kuo, K.K. Metals, energetic additives and special binders used in solid fuels for hybrid rockets. In Fundamentals of Hybrid Rocket Combustion and Propulsion; Chiaverini, M.J., Kuo, K.K., Eds.; AIAA Progress in Astronautics and Aeronautics: Reston, VA, USA, 2007; Volume 218, pp. 413–456. [Google Scholar]
- Paravan, C. Ballistics of Innovative Solid Fuel Formulations for Hybrid Rocket Engines. Ph.D. Thesis, Politecnico di Milano, Aerospace Science and Technology Department, Milan, Italy, 2012. [Google Scholar]
- De Luca, L.T.; Galfetti, L.; Maggi, F.; Colombo, G.; Paravan, C.; Reina, A.; Dossi, S.; Fassina, M.; Sossi, A. Characterization and combustion of aluminum nanopowders in energetic systems. In Metal Nanopowders Production, Characterization, and Energetic Applications; Gromov, A.A., Teipel, U., Eds.; Wiley VCH: Weinheim, Germany, 2014; pp. 299–408. [Google Scholar]
- Evans, B.; Favorito, N.A.; Boyer, E.; Risha, G.A.; Wehrman, R.B.; Kuo, K.K. Characterization of Nano-sized Energetic Particle Enhancement of Solid Fuel Burning Rates in an X-Ray Transparent Hybrid Rocket Engine. In Proceedings of the 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit (AIAA), Fort Lauderdale, FL, USA, 11–14 July 2004. AIAA Paper No. 2004–3821. [Google Scholar]
- Galfetti, L.; Merotto, L.; Boiocchi, M.; Maggi, F.; DeLuca, L.T. Ballistic and Rheological Characterization of Paraffin-based Fuels for Hybrid Rocket Propulsion. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit (AIAA), San Diego, CA, USA, 31 July–3 August 2011. AIAA Paper No. 2011–5680. [Google Scholar]
- Strand, L.D.; Ray, R.L.; Anderson, F.; Cohen, N.S. Hybrid rocket fuel combustion and regression rate study. In Proceedings of the 28th Joint Propulsion Conference and Exhibit, Nashville (AIAA), Nashville, TN, USA, 6–8 July 1992. AIAA Paper No. 1992–3302. [Google Scholar]
- Grosse, A.V.; Conway, J.B. Combustion of metals in oxygen. Ind. Eng. Chem. 1958, 50, 663–672. [Google Scholar] [CrossRef]
- DeLuca, L.T.; Maggi, F.; Dossi, S.; Fassina, M.; Paravan, C.; Sossi, A. Prospects of Aluminum Modifications as Energetic Fuels in Chemical Rocket Propulsion. In Chemical Rocket Propulsion: A Comprehensive Survey of Energetic Materials; DeLuca, L.T., Shimada, T., Sinditskii, V.P., Calabro, M., Eds.; Springer: Berlin, Germany, 2017; pp. 191–233. [Google Scholar]
- Dossi, S. Mechanically Activated Al Fuels for High Performance Solid Rocket Propellants. Ph.D. Thesis, Politecnico di Milano, Aerospace Science and Technology Department, Milan, Italy, 2014. [Google Scholar]
- Dossi, S.; Paravan, C.; Maggi, F.; Galfetti, L. Enhancing micrometric aluminum reactivity by mechanical activation. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015. AIAA Paper No. 2015–4221. [Google Scholar]
- Paravan, C.; Dossi, S.; Maggi, F.; Galfetti, L.; Marra, G. Pre-burning characterization of nano-sized aluminum in condensed energetic systems. In Energetic Nanomaterials: Synthesis, Production, and Characterization; Gromov, A.A., Zarko, V.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 341–368. [Google Scholar]
- Juergens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Structure and morphology of aluminum oxide films formed by thermal oxidation of aluminum. Thin Solid Films 2002, 418, 89–101. [Google Scholar] [CrossRef]
- Trunov, M.A.; Schoenitz, M.; Zhu, X.; Dreizin, E.L. Effect of polymorphic phase transformations on Al2O3 film on oxidation kinetics of aluminum powders. Combust. Flame 2005, 140, 310–318. [Google Scholar] [CrossRef]
- Paravan, C.; Verga, A.; Maggi, F.; Galfetti, L. Accelerated ageing of aluminum powders: Metal content, composition, and non-isothermal oxidation reactivity. Acta Astronaut. 2019, 158, 397–406. [Google Scholar] [CrossRef]
- Schoenitz, M.; Patel, B.; Agboh, O.; Dreizin, E.L. Oxidation of aluminum powders at high heating rates. Thermochim. Acta 2010, 507, 115–122. [Google Scholar] [CrossRef]
- Trunov, M.; Schoenitz, M.; Dreizin, E.L. Ignition of aluminum powders under different experimental conditions. Propellants Explos. Pyrotech. 2005, 30, 36–43. [Google Scholar] [CrossRef]
- Sundaram, S.D.; Puri, P.; Yang, V. A general theory of ignition and combustion of nano- and micron-sized aluminum particles. Combust. Flame 2016, 169, 94–109. [Google Scholar] [CrossRef]
- Gen, M.Y.; Frolov, Y.V.; Storozhev, V.B. On combustion of particles of subdispersed aluminum. Combust. Explos. Shock Waves 1978, 14, 153–155. [Google Scholar] [CrossRef]
- Ivanov, G.V.; Tepper, F. Activated aluminum as stored energy source for propellants. In Challenges in Propellants and Combustion 100 Years after Nobel; Kuo, K.K., Ed.; Begell House: New York, NY, USA, 1997; pp. 636–645. [Google Scholar]
- Ilyin, A.P.; Gromov, A.A.; An, V.; Faubert, F.; de Izarra, C.; Espagnacq, A.; Brunet, L. Characterization of aluminum powders I. Parameters of reactivity of aluminum powders. Propellants Explos. Pyrotech. 2002, 27, 361–364. [Google Scholar] [CrossRef]
- Gromov, A.A.; Ilyin, A.P.; Förther-Barth, U.; Teipel, U. Characterization of aluminum powders: II. Aluminum nanopowders passivated by non-inert coatings. Propellants Explos. Pyrotech. 2006, 31, 401–409. [Google Scholar] [CrossRef]
- Hahma, A.; Gany, A.; Palovuori, K. Combustion of activated aluminum. Combust. Flame 2006, 145, 464–480. [Google Scholar] [CrossRef]
- Yogodnikov, D.A.; Andreev, E.A.; Vorob’ev, V.S.; Glotov, O.G. Ignition, combustion, and agglomeration of encapsulated aluminum particles in a composite solid propellant. I. Theoretical study of the ignition and combustion of aluminum with fluorine-containing coatings. Combust. Explos. Shock Waves 2006, 42, 153–155. [Google Scholar] [CrossRef]
- Dreizin, E.; Schoenitz, M. Reactive and Metastable Nanomaterials Prepared by Mechanical Milling. In Metal Nanopowders Production, Characterization, and Energetic Applications; Gromov, A.A., Teipel, U., Eds.; Wiley VCH: Weinheim, Germany, 2014; pp. 227–278. [Google Scholar]
- Maggi, F.; Dossi, S.; Paravan, C.; DeLuca, L.T.; Liljedhal, M. Activated aluminum powders for space propulsion. Powder Technol. 2015, 270, 46–52. [Google Scholar] [CrossRef]
- Sossi, A.; Duranti, E.; Paravan, C.; DeLuca, L.T.; Vorozhtsov, A.B.; Gromov, A.A.; Pautova, Y.I.; Lerner, M.I.; Rodkevich, N.G. Non-isothermal oxidation of aluminum nanopowder coated by hydrocarbons and fluorohydrocarbons. Appl. Surf. Sci. 2013, 271, 337–343. [Google Scholar] [CrossRef]
- Paravan, C.; Reina, A.; Sossi, A.; Manzoni, M.; Massini, G.; Rambaldi, G.; Duranti, E.; Adami, A.; Seletti, E.; DeLuca, L.T. Time-resolved Regression Rate of Innovative Solid Fuel Formulations. In Advances in Propulsion Physics; DeLuca, L.T., Bonnal, C., Haidn, O., Frolov, S., Eds.; Torus Press: Moscow, Russia, 2013; Volume 4, pp. 75–98. [Google Scholar]
- DeLuca, L.T.; Galfetti, L.; Maggi, F.; Colombo, G.; Merotto, L.; Boiocchi, M.; Paravan, C.; Reina, A.; Tadini, P.; Fanton, L. Characterization of HTPB-based solid fuel formulations: Performance, mechanical properties, and pollution. Acta Astronaut. 2013, 92, 150–162. [Google Scholar] [CrossRef]
- Zare, A.; Harriman, T.A.; Lucca, D.A.; Roncalli, S.; Kosowski, B.; Paravan, C.; DeLuca, L.T. Mapping of aluminum particle dispersion in solid rocket fuel formulations. In Chemical Rocket Propulsion: A Comprehensive Survey of Energetic Materials; DeLuca, L.T., Shimada, T., Sinditskii, V.P., Calabro, M., Eds.; Springer: Berlin, Germany, 2017; pp. 673–688. [Google Scholar]
- Teipel, U.; Förter-Barth, U. Rheology of nano-aluminum suspensions. Propellants Explos. Pyrotech. 2001, 26, 268–272. [Google Scholar] [CrossRef]
- Sippel, T.; Son, S.F.; Groven, L.J. Altering the reactivity of aluminum with selective inclusion of polytetrafluoroethylene through mechanical activation. Propellants Explos. Pyrotech. 2013, 38, 286–295. [Google Scholar] [CrossRef]
- Sippel, T.R.; Son, S.F.; Groven, L.J.; Zhang, S.; Dreizin, E.L. Exploring mechanisms for agglomerate reduction in composite solid propellants with polyethylene inclusion modified aluminum. Combust. Flame 2015, 162, 846–854. [Google Scholar] [CrossRef]
- Terry, B.C.; Son, S.F.; Groven, L.J. Altering combustion of silicon/polytetrafluoroethylene with two-step mechanical activation. Combust. Flame 2015, 162, 1350–1357. [Google Scholar] [CrossRef]
- Dossi, S.; Paravan, C.; Maggi, F.; Di Lorenzo, M.; Ardalic, J.; Galfetti, L. Novel activated metal powders for improved hybrid fuels and green solid propellants. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016. AIAA Paper No. 2016–4596. [Google Scholar]
- Chiaverini, M.J.; Serin, N.; Johnson, D.; Lu, Y.C.; Kuo, K.K.; Risha, G.A. Regression Rate Behavior of Hybrid Rocket Solid Fuels. J. Propul. Power 2000, 16, 125–132. [Google Scholar] [CrossRef]
- Risha, G.A.; Ulas, A.; Boyer, E.; Kumar, S.; Kuo, K.K. Combustion of HTPB-Based Solid Fuels for Containing Nano-Sized Energetic Powder in a Hybrid Rocket Motor. In Proceedings of the 37th Joint Propulsion Conference and Exhibit, Salt Lake City, UT, USA, 8–11 July 2001. AIAA Paper No. 2001–3535. [Google Scholar]
- Risha, G.A.; Boyer, E.; Wehrman, R.B.; Kuo, K.K. Performance Comparison of HTPB-Based Solid Fuels Containing Nano-Sized Energetic Powder in a Cylindrical Hybrid Rocket Motor. In Proceedings of the 38th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Indianapolis, IN, USA, 7–10 July 2002. AIAA Paper No. 2002–3576. [Google Scholar]
- Risha, G.A.; Evans, B.; Boyer, E.; Wehrman, R.B.; Kuo, K.K. Nano-Sized Aluminum and Boron-Based Solid Fuel Characterization in a Hybrid Rocket Engine. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Huntsville, AL, USA, 20–23 July 2003. AIAA Paper No. 2003–4593. [Google Scholar]
- Fanton, L.; Paravan, C.; DeLuca, L.T. Testing and modeling fuel regression rate in a miniature hybrid burner. Int. J. Aerosp. Eng. 2012, 2012, 673838. [Google Scholar] [CrossRef]
- Sossi, A.; Duranti, E.; Manzoni, M.; Paravan, C.; DeLuca, L.T.; Vorozhtsov, A.B.; Lerner, M.I.; Rodkevich, N.G.; Gromov, A.A.; Savin, N.L. Combustion of HTPB-based solid fuels loaded with coated nanoaluminum. Combust. Sci. Technol. 2013, 185, 17–36. [Google Scholar] [CrossRef]
- Carmicino, C.; Sorge, A.R. Experimental Investigation into the Effect of Solid-Fuel Additives on Hybrid Rocket Performance. J. Propul. Power 2015, 31, 699–713. [Google Scholar] [CrossRef]
- Thomas, J.; Petersen, E.L.; DeSain, J.D.; Brady, B.B. Enhancement of Regression Rates in Hybrid Rockets with HTPB Fuel Grains by Metallic Additives. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015. AIAA Paper No. 2015–4041. [Google Scholar]
- 3M Material Safety Datasheet. Available online: http://www.machichemicals.com/pdf/3M_FC-2175.pdf (accessed on 19 July 2019).
- George, P.; Krishnan, S.; Varkey, P.M.; Ravindran, M.; Ramachandran, L. Fuel regression rate enhancement studies in HTPB/GOX hybrid rocket motors. In Proceedings of the 34th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Cleveland, OH, USA, 13–15 July 1998. AIAA Paper No. 1998–3188. [Google Scholar]
- Frederick, R.A.; Whitehead, J.J.; Knox, L.R.; Moser, M.D. Regression rates study of mixed hybrid propellants. J. Propul. Power 2007, 23, 175–180. [Google Scholar] [CrossRef]
- Lengellé, G. Solid-Fuel Pyrolysis Phenomena and Regression Rate, Part 1: Mechanisms. In Fundamentals of Hybrid Rocket Combustion and Propulsion; Chiaverini, M.J., Kuo, K.K., Eds.; AIAA Progress in Astronautics and Aeronautics: Reston, VA, USA, 2007; Volume 218, pp. 127–166. [Google Scholar]
- Strand, L.D.; Ray, R.L.; Cohen, N.S. Hybrid rocket combustion study. In Proceedings of the 29th Joint Propulsion Conference and Exhibit (AIAA), Monterey, CA, USA, 28–30 July 1993. AIAA Paper No. 1993–2412. [Google Scholar]
- Strand, L.D.; Jones, M.D.; Ray, R.L.; Cohen, N.S. Characterization of hybrid rocket internal heat flux and HTPB fuel pyrolysis. In Proceedings of the 30th Joint Propulsion Conference and Exhibit (AIAA), Indianapolis, IN, USA, 27–29 June 1994. AIAA Paper No. 1994–2876. [Google Scholar]
- Paravan, C.; Manzoni, M.; Rambaldi, G.; DeLuca, L.T. Analysis of quasi-steady and transient burning of hybrid fuels in a laboratory-scale burner by an optical technique. Int. J. Energ. Mater. Chem. Propuls. 2013, 12, 385–410. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Zhang, W.; Shen, R.; Yu, H.; Ye, Y.; DeLuca, L.T. Innovative Methods to Enhance the Combustion Properties of Solid Fuels for Hybrid Rocket Propulsion. Aerospace 2019, 6, 47. [Google Scholar] [CrossRef]
- Bisin, R.; Paravan, C.; Alberti, S.; Galfetti, L. An Innovative Strategy for Paraffin-based Fuels Reinforcement: Part I, Mechanical and Pre-Burning Characterization. In Proceedings of the 8th European Conference for Aeronautics and Space Sciences (EUCASS 2019), Madrid, Spain, 1–4 July 2019; pp. 1–14. [Google Scholar]
- Bisin, R.; Paravan, C.; Verga, A.; Galfetti, L. An Innovative Strategy for Paraffin-based Fuels Reinforcement: Part II, Ballistic Characterization. In Proceedings of the 8th European Conference for Aeronautics and Space Sciences (EUCASS 2019), Madrid, Spain, 1–4 July 2019; pp. 1–14. [Google Scholar]
- Grosse, M. Effect of a Diaphragm on Effect of a Diaphragm on Performance and Fuel Regression of a Laboratory Scale Hybrid Rocket Motor Using Nitrous Oxide and Paraffin. In Proceedings of the 45th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Denver, CO, USA, 2–5 August 2009. AIAA Paper No. 2009–5113. [Google Scholar]
- Yuasa, S.; Shiraishi, N.; Hirata, K. Controlling Parameters for Fuel Regression Rate of Swirling-oxidizer-flow-type Hybrid Rocket Engine. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. AIAA Paper No. 2012–4106. [Google Scholar]
- Yuasa, S.; Yamamoto, K.; Hachiya, H.; Kitagawa, K.; Oowada, Y. Development of a small sounding hybrid rocket with a swirling-oxidizer-type engine. In Proceedings of the 37th Joint Propulsion Conference and Exhibit (AIAA), Salt Lake City, UT, USA, 8–11 July 2001. AIAA Paper No. 2001–3537. [Google Scholar]
- Ozawa, K.; Kitagawa, K.; Aso, S.; Shimada, T. Hybrid Rocket Firing Experiments at Various Axial–Tangential Oxidizer-Flow-Rate Ratios. J. Propul. Power 2019, 35, 94–108. [Google Scholar] [CrossRef]
- Paravan, C.; Glowacki, J.; Carlotti, S.; Maggi, F.; Galfetti, L. Vortex Combustion in a Lab-scale Hybrid Rocket Motor. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016. AIAA Paper No. 2016–4562. [Google Scholar]
- Connell, T.L., Jr.; Yetter, R.A.; Risha, G.A.; Huba, Z.J.; Epsheytn, A.; Fisher, B.T. Enhancement of Solid Fuel Combustion in a Hybrid Rocket Motor Using Amorphous Ti–Al–B Nanopowder Additives. J. Propul. Power 2019, 35, 662–664. [Google Scholar] [CrossRef]
- Connell, T.L., Jr.; Risha, G.A.; Yetter, R.A.; Roberts, C.W.; Young, G. Boron and Polytetrafluoroethylene as a Fuel Composition for Hybrid Rocket Applications. J. Propul. Power 2015, 31, 373–385. [Google Scholar] [CrossRef]
- Morothiya, G.; Ramakrishna, P.A. Utilization of Mechanical Activated Aluminum in Hybrid Rockets. J. Propul. Power 2018, 34, 1206–1213. [Google Scholar] [CrossRef]
- Price, E.W.; Sigman, R.K. Combustion of Aluminized Solid Propellants. In Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics; Yang, V., Brill, T.B., Ren, W.Z., Eds.; AIAA Progress in Astronautics and Aeronautics: Reston, VA, USA, 2000; Volume 185, pp. 66–687. [Google Scholar]
- Babuk, V.A.; Vassiliev, V.A.; Sviridov, V.V. Formation of Condensed Combustion Products at the Burning Surface of Solid Rocket Propellant. In Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics; Yang, V., Brill, T.B., Ren, W.Z., Eds.; AIAA Progress in Astronautics and Aeronautics: Reston, VA, USA, 2000; Volume 185, pp. 749–776. [Google Scholar]
- DeLuca, L.T.; Galfetti, L.; Colombo, G.; Maggi, F.; Bandera, A.; Babuk, V.A.; Sindistskii, V.P. Microstructure Effects in Aluminized Solid Rocket Propellants. J. Propul. Power 2010, 26, 724–733. [Google Scholar] [CrossRef]
- Gany, A. Micro and Nano Scale Phenomena of Aluminum Agglomeration during Solid Propellant Combustion. Eurasian Chem. Technol. J. 2016, 18, 161–170. [Google Scholar] [CrossRef]
- Caveny, L.; Gany, A. Breakup of Al/Al2O3 Agglomerates in Accelerating Flowfields. AIAA J. 1979, 17, 1368–1371. [Google Scholar] [CrossRef]
- Rashkovskii, S.A. Statistical Simulation of Aluminum Agglomeration during Combustion of Heterogeneous Condensed Mixtures. Combust. Explos. Shock Waves 2005, 41, 174–184. [Google Scholar] [CrossRef]
- Jackson, T.; Najjar, F.; Buckmaster, J. New Aluminum Agglomeration Models and Their Use in Solid Propellant Rocket Simulations. J. Propul. Power 2005, 21, 925–936. [Google Scholar] [CrossRef]
- Maggi, F.; DeLuca, L.T.; Bandera, A. A Pocket Model for Aluminum Agglomeration Based on Propellant Microstructure. AIAA J. 2015, 53, 3395–4303. [Google Scholar] [CrossRef]
- Plaud, M.; Gallier, S. A Numerical Mesoscale Model for Aluminum Agglomeration in Solid Propellants. In Proceedings of the 7th European Conference for Aeronautics and Space Sciences (EUCASS 2017), Milan, Italy, 1–4 July 2017. [Google Scholar]
- DeLuca, L.T.; Paravan, C.; Reina, A.; Marchesi, E.; Maggi, F.; Bandera, A.; Colombo, G.; Kosowski, B. Aggregation and Incipient Agglomeration in Metallized Solid Propellants and Solid Fuels for Rocket Propulsion. In Proceedings of the 46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Nashville, TN, USA, 25–28 July 2010. AIAA Paper No. 2010–6752. [Google Scholar]
- Purdue University. Heats of Formation and Chemical Compositions. Available online: https://engineering.purdue.edu/~propulsi/propulsion/comb/propellants.html (accessed on 19 April 2018).
- Koch, E.C.; Hahma, A.; Klapotke, T.M.; Radies, H. Metal-Fluorocarbon Pyrolants: XI. Radiometric Performance of Pyrolants Based on Magnesium, Perfluorinated Tetrazolates, and Viton-A. Propellants Explos. Pyrotech. 2010, 35, 248–253. [Google Scholar] [CrossRef]
- Koch, E.C. (Ed.) Metal-Fluorocarbon Based Energetic Materials; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2012. [Google Scholar]
- Biswas, S.W.; Vijaian, K. Friction and wear of PTFE–A review. Wear 1992, 158, 193–211. [Google Scholar] [CrossRef]
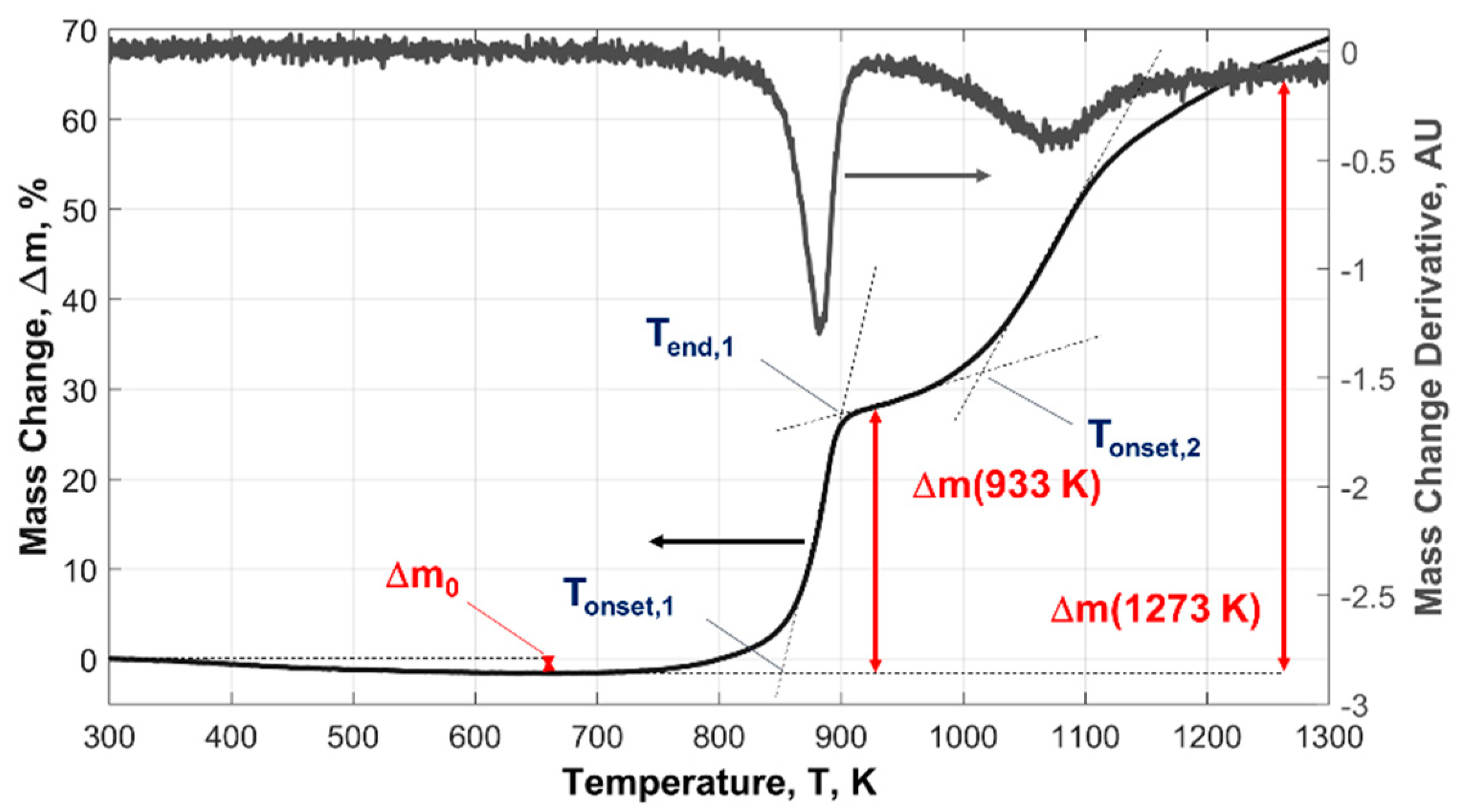
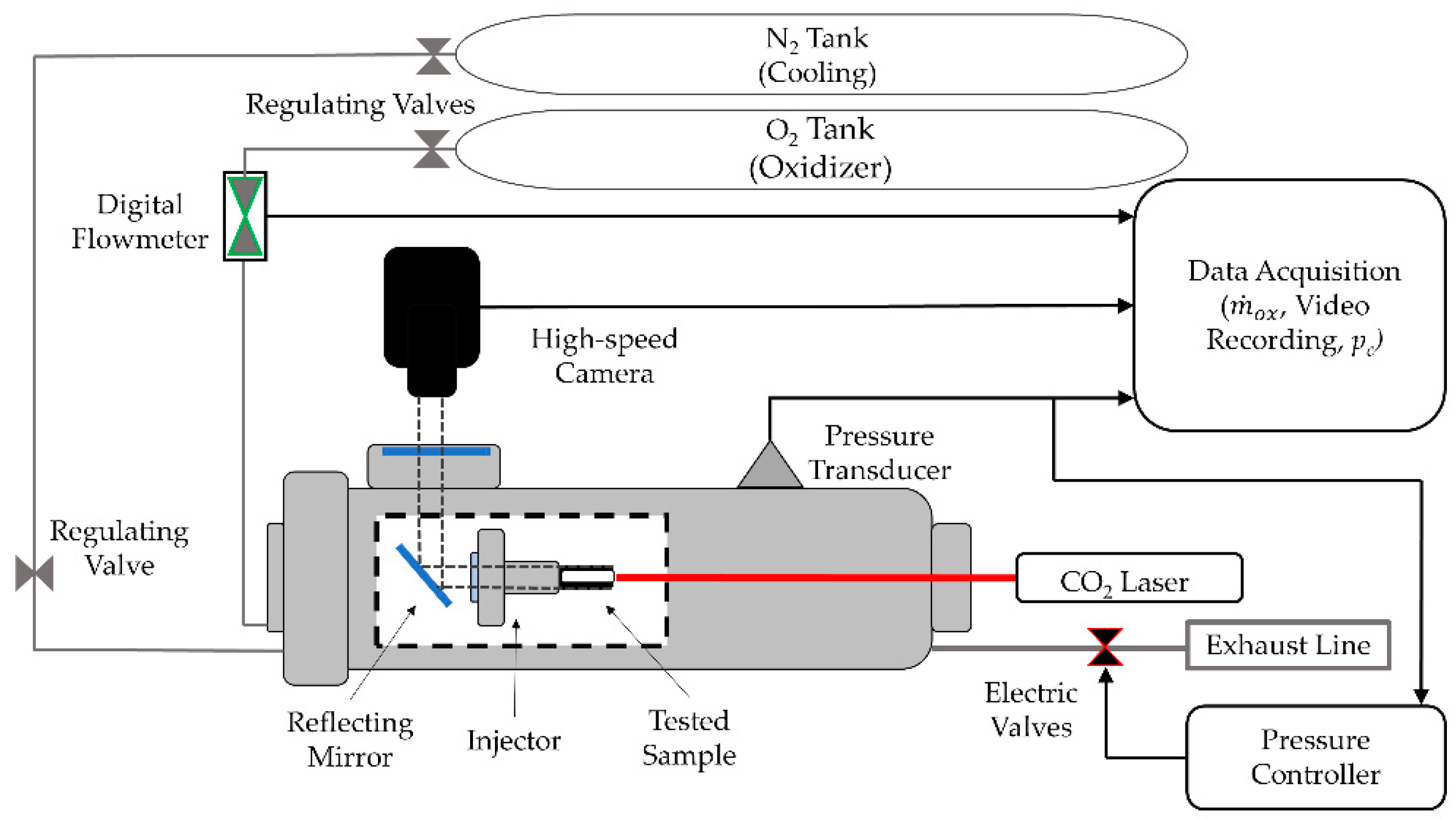
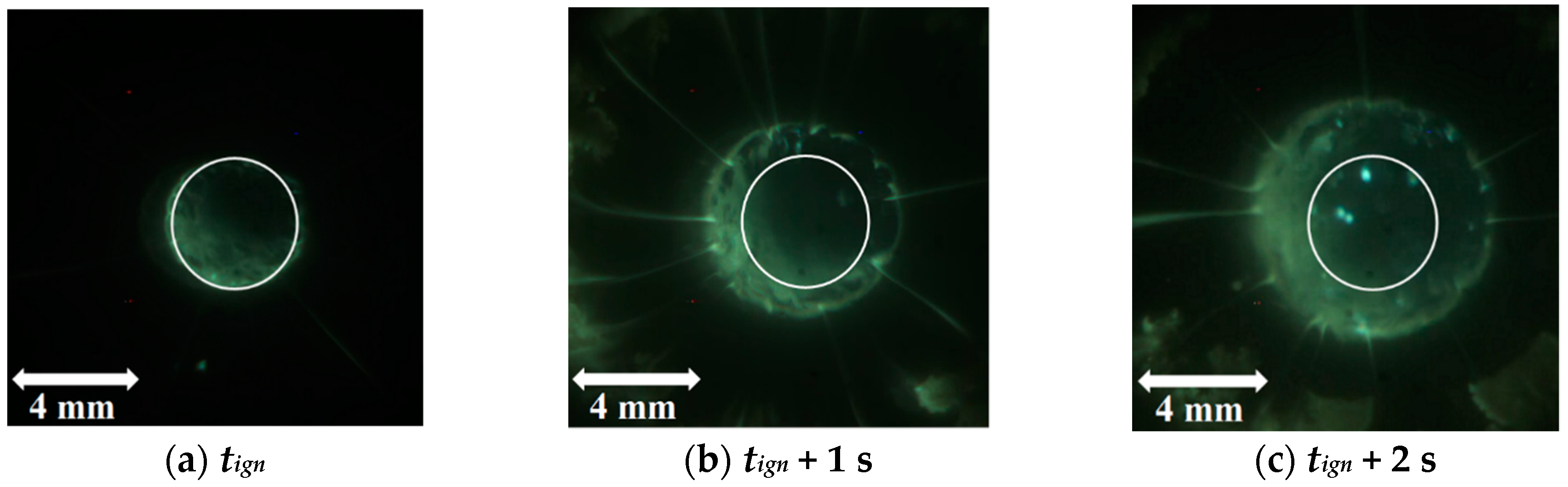
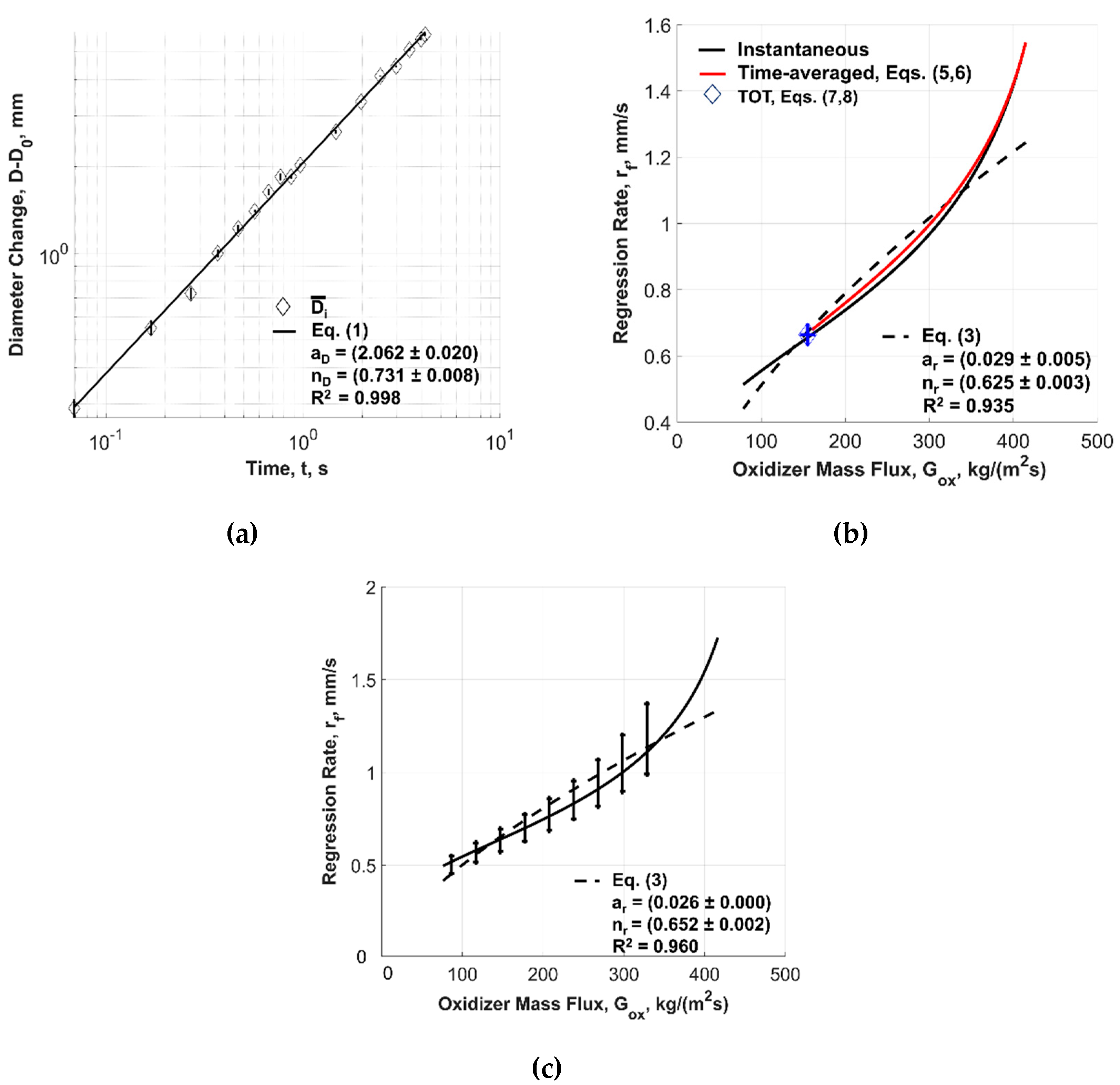

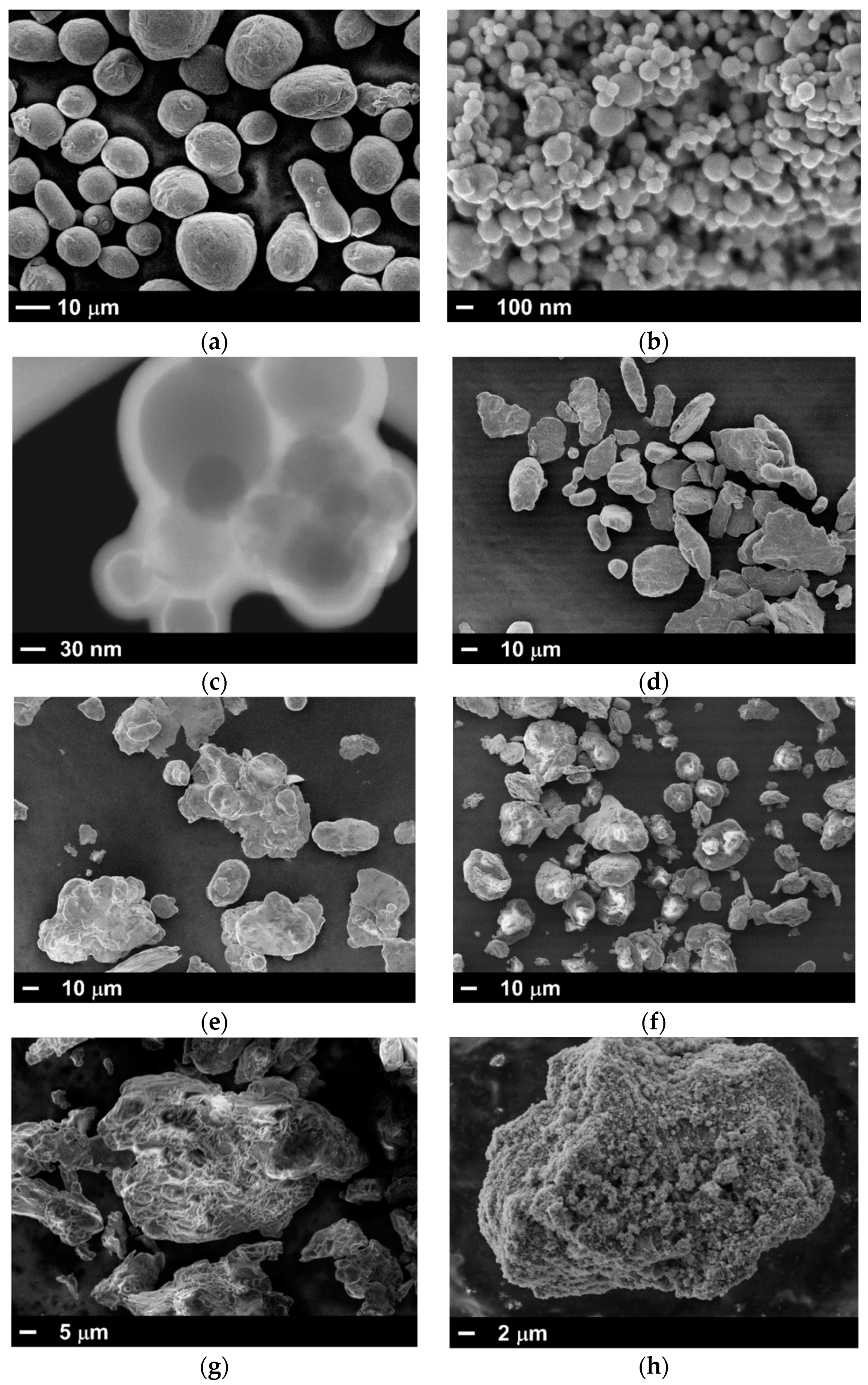
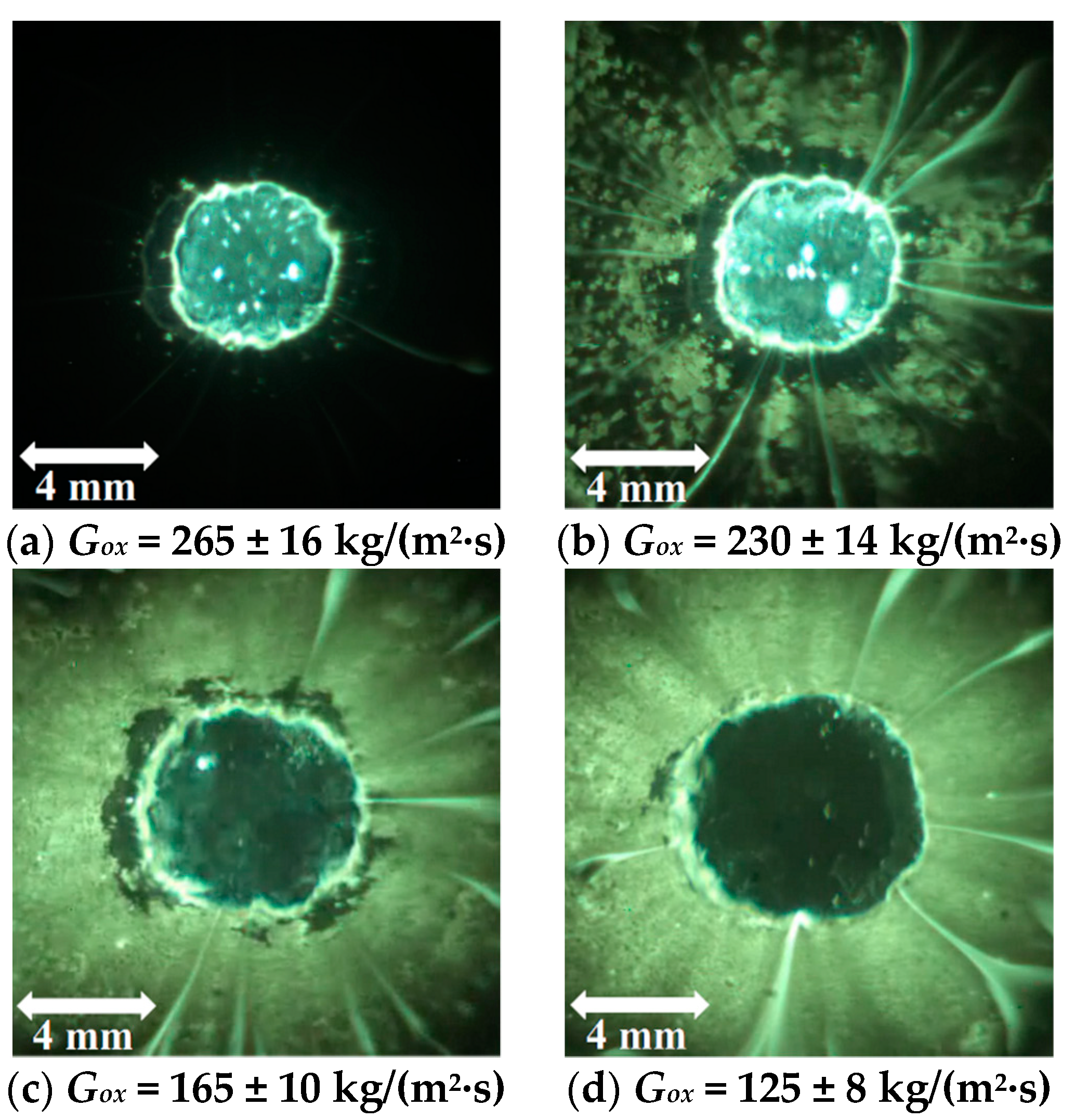
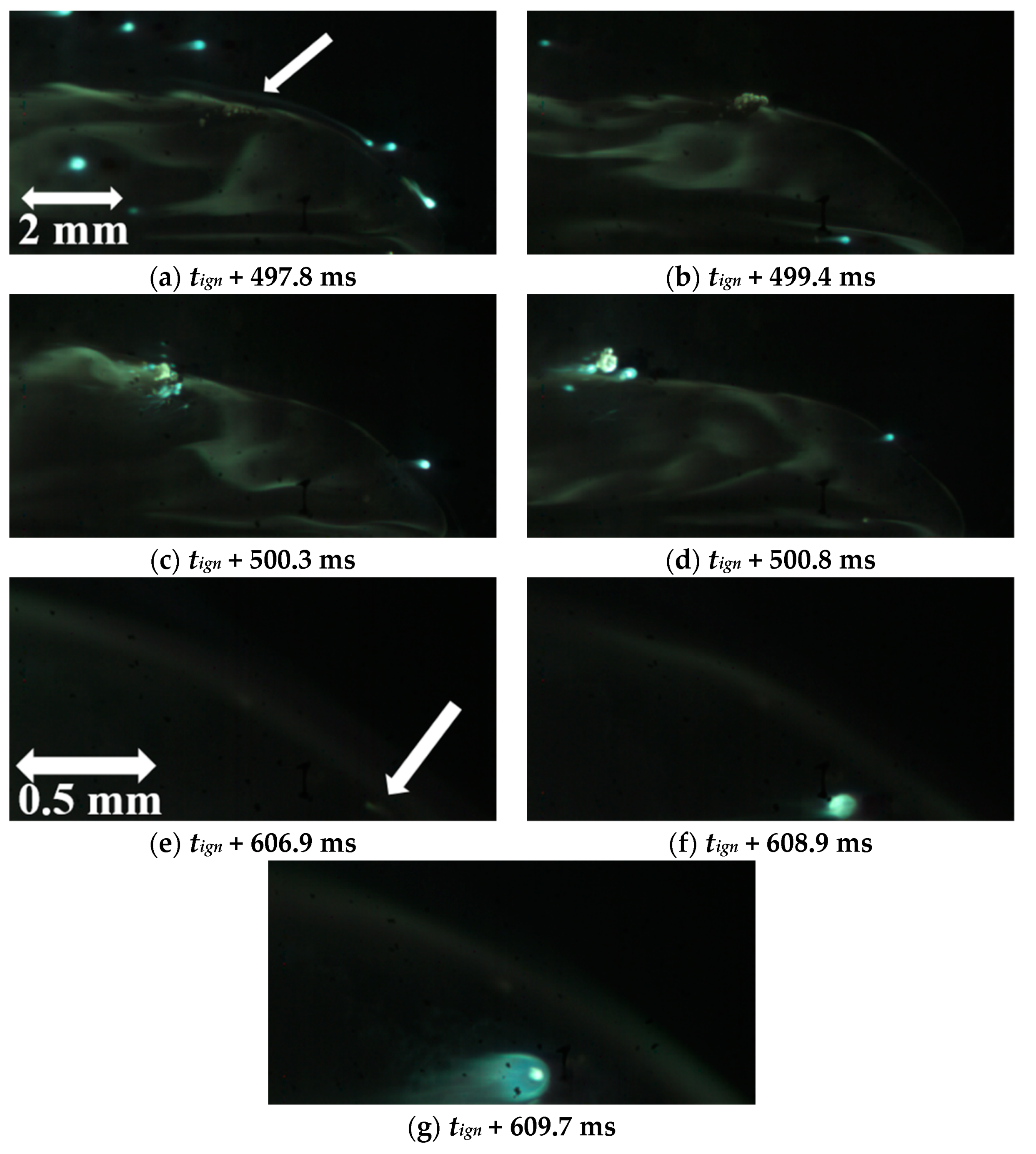
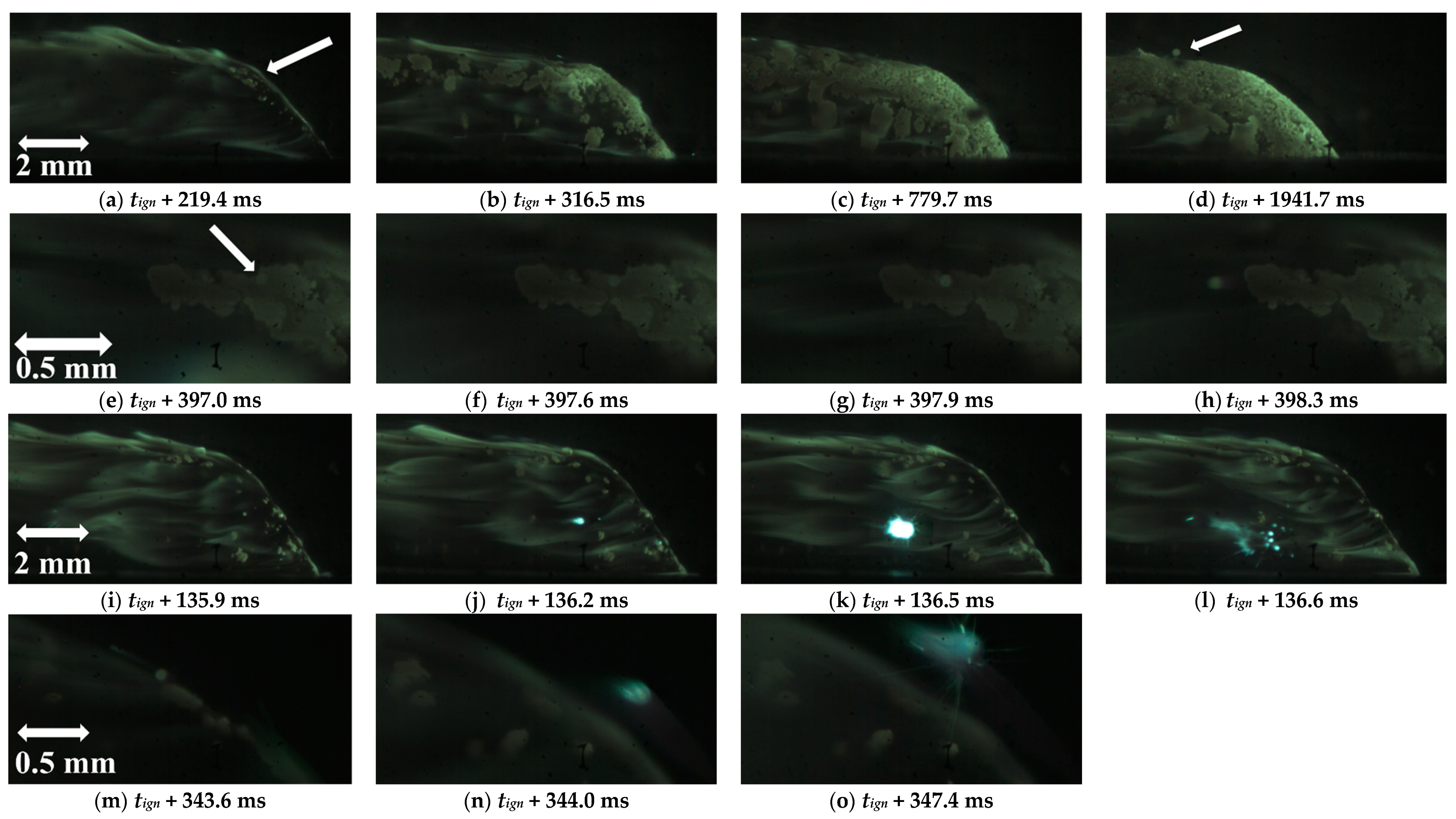
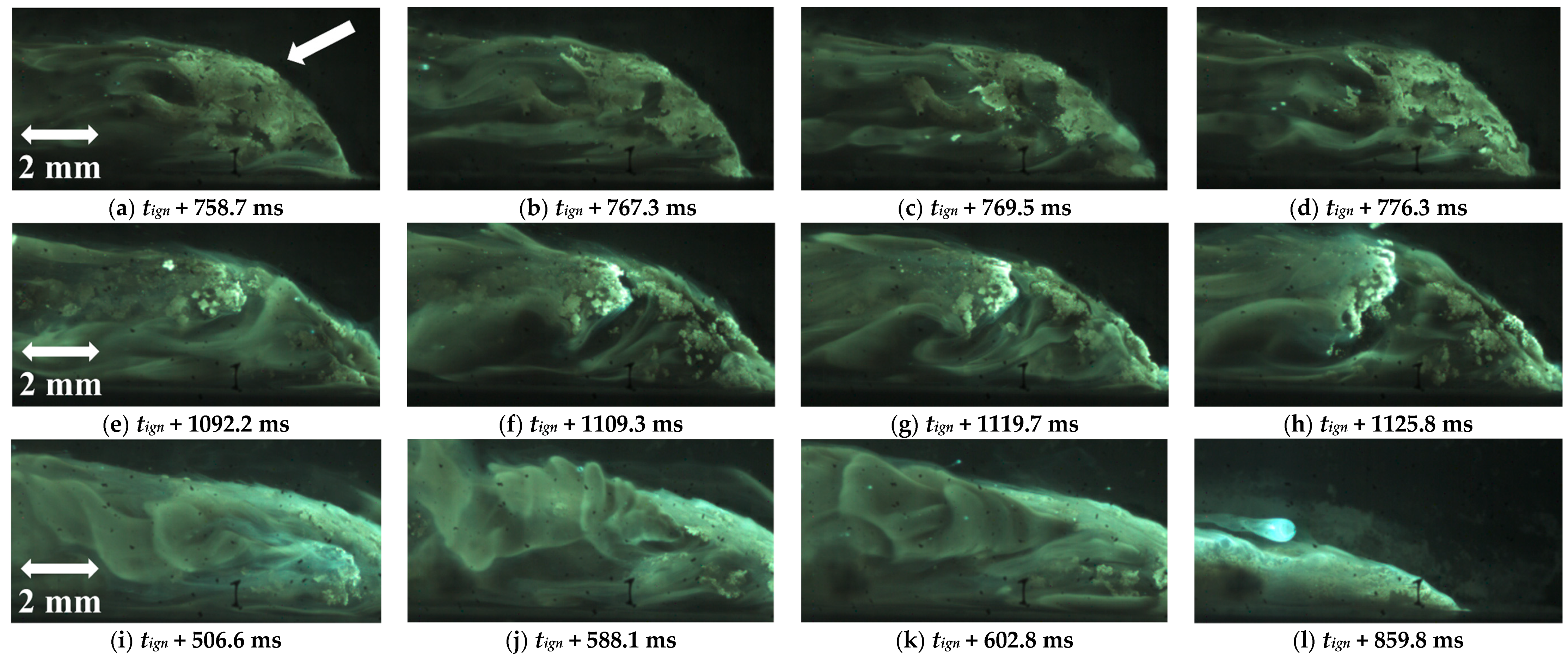
| Powder Id. | Powder Description and Notes | Particle Surface Composition | Producer and References |
|---|---|---|---|
| μAl15 | Air-passivated μAl with nominal size of 15 μm | Al2O3 | AMG Alpoco (UK) [25] |
| μAl7.5 | Air-passivated μAl with nominal size of 7.5 μm | ||
| ALEX-100 | Air-passivated nAl with nominal size of 100 nm | Al2O3 | Advanced Powder Technology LLC (Tomsk, Russia) [40,54] |
| ALEX-50 | Air-passivated nAl with nominal size of 50 nm | ||
| ALEX-100B | ALEX-100 coated with 0.2 wt.% pyrocatechol (C6H6O2) | C6H6O2 | |
| L-ALEX-100 | Stearic acid coated ALEX-100 | C17H35COOH | |
| VF-ALEX-100F | Fluorotelomer alcohol-derived ester-coated ALEX-100 (coating solvent removed by filtration) | Vinylidene fluoride–hexafluoropropylene (70:30) copolymer (VFHFP) + ester | |
| VF-ALEX-50E | Fluorotelomer alcohol-derived ester-coated ALEX-50 (coating solvent removed by evaporation) | ||
| AP15-ALEX-100B | AP (15 wt.%) + ALEX-100B (85 wt.%), ALEX-100B clustered and coated by AP (solvent removal by evaporation) | Al2O3 + C6H6O2 + NH4ClO4 | SPLab |
| LE-μAl15 | Low-energy MA, μAl15 | Al2O3 + (C2F4)n | SPLab (MA procedure) [23,24] |
| LE-μAl15-T10 | Low-energy MA, μA15 (90 wt.%) + PTFE (10 wt.%) | ||
| LE-μAl15-T30 | Low-energy MA, μAl15 (70 wt.%) + PTFE (30 wt.%) | ||
| LE-μAl7.5-T30 | Low-energy MA, μAl7.5 (70 wt.%) + PTFE (30 wt.%) | ||
| HE-μAl15-T45 | High-energy MA, μAl15 (55 wt.%) + PTFE (45 wt.%) | ||
| HE-ALEX-50-T45 | High-energy MA, ALEX-50 (55 wt.%) + PTFE (45 wt.%) |
| Oxidizer | Density, kg/m3 | 10 wt.% | 15 wt.% | 30 wt.% | 45 wt.% |
|---|---|---|---|---|---|
| AP | 1950 | 1754 K | 2327 K | 2656 K | 3524 K |
| VFHFP | 1800 | 933.6 K | 1304 K | 1752 K | 1975 K |
| PTFE | 2200 | 1013 K | 1440 K | 1853 K | 2095 K |
| Solid Fuel ID | HTPB, wt.% | Energetic Additive ID, wt.% | TMD, kg/m3 | Notes |
|---|---|---|---|---|
| F1 | 100 | - | 920 | Relative grading baseline |
| F2 | 90.0 | μAl15, 10.0 | 980 | - |
| F3 | 90.0 | μAl7.5, 10.0 | 980 | - |
| F4 | 88.0 | ALEX-100, 10.0 | 992 | Formulation includes 2 wt.% CB, additive dispersion by ultrasound irradiation during mixing |
| F5 | 88.0 | L-ALEX-100, 10.0 | 992 | |
| F6 | 88.0 | VF-ALEX-100F, 10.0 | 992 | |
| F7 | 88.0 | VF-ALEX-50E, 10.0 | 992 | |
| F8 | 88.7 | ALEX-100B, 11.3 | 906 | Formulation Al content is equimolar to F2, no additive dispersion by ultrasound irradiation during mixing |
| F9 | 87.4 | AP 1.9% + ALEX-100B 10.7% a | 999 | |
| F10 | 87.4 | AP15-ALEX-100B, 12.6 | 999 | |
| F11 | 89.9 | LE-μAl15, 10.1 | 980 | |
| F12 | 89.0 | LE-μAl15-T10, 11.0 | 986 | |
| F13 | 85.5 | LE-μAl15-T30, 14.5 | 986 | |
| F14 | 84.9 | LE-μAl7.5-T30, 15.1 | 1006 | |
| F15 | 65.0 | PTFE, 35.0 | 1150 | Evaluation of HTPB + PTFE ballistics |
| F16 | 45.5 | PTFE, 24.5 | 1389 | Evaluation of the effects of HTPB + PTFE on nAl combustion (no mechanical activation) |
| ALEX-50, 30.0 | ||||
| F17 | 45.5 | HE-μAl15-T45, 54.5 | 1389 | HE activation effects evaluation with respect to LE, increased PTFE content |
| F18 | 45.5 | HE-ALEX-50-T45, 54.5 | 1389 | |
| F19 | 30.0 | HE-ALEX-50-T45, 70.0 | 1630 |
| Powder ID | D43, μm | D(0.1), μm | D(0.5), μm | D(0.9), μm | SSA, m2/g | CAl, wt.% |
|---|---|---|---|---|---|---|
| μAl15 | 19.8 | 10.0 | 17.9 | 32.3 | <0.1 | 99.5 ± 0.4 |
| μAl7.5 | 6.6 | 2.99 | 6.08 | 11.0 | <0.1 | 95.3 ± 0.2 |
| ALEX-100 | 0.138 | 0.105 | 0.135 | 0.176 | 11.8 ± 0.4 | 89.0 ± 5.0 |
| ALEX-50 | - | - | - | - | 15.5 ± 0.1 | 86.3 ± 4.1 |
| ALEX-100B | 0.141 | 0.104 | 0.138 | 0.182 | 11.3 ± 0.1 | 88.0 ± 1.5 |
| L-ALEX-100 | - | - | - | - | 9.1 ± 0.3 | 70.3 ± 4.3 a |
| VF-ALEX-100F | - | - | - | - | 6.9 ± 0.2 | 78.3 ± 4.4 |
| VF-ALEX-50E | - | - | - | - | 10.9 ± 0.3 | 78.4 ± 4.4 |
| AP15-ALEX-100B | 6.73 | 0.42 | 1.66 | 16.0 | - | 76.0 ± 1.3 |
| LE-μAl15 | 123 | 15.9 | 90.8 | 284 | <1.0 | 98.2 ± 0.1 |
| LE-μAl15-T10 | 28.4 | 12.0 | 25.1 | 49.5 | <1.0 | 90.5 ± 0.1 |
| LE-μAl15-T30 | 31.2 | 5.46 | 21.7 | 59.4 | <1.0 | 68.6 ± 0.6 |
| LE-μAl7.5-T30 | 13.5 | 2.67 | 8.13 | 29.2 | <1.0 | 65.8 ± 0.4 |
| HE-μAl15-T45 | 52.2 | 4.05 | 22.6 | 141 | 1.5 ± 0.1 | 53.7 ± 1.2 |
| HE-ALEX-50-T45 | 85.2 | 3.41 | 41.7 | 237 | 5.9 ± 0.1 | 47.3 ± 1.7 |
| AP | 10.2 | 0.68 | 5.74 | 26.4 | - | - |
| PTFE | 7.24 | 0.36 | 0.88 | 8.28 | - | - |
| Powder ID | Δm0, wt.% | Ton,1, K | α (933 K), % | Ton,2, K | α (1273 K), % |
|---|---|---|---|---|---|
| μAl15 | −0.1 | 845 | 1.1 | 1173 | 8.0 |
| μAl7.5 | −0.2 | 843 | 2.2 | 1203 | 13.7 |
| ALEX-100 | −1.0 | 850 | 40.4 | 983.2 | 83.3 |
| ALEX-50 | −1.0 | 850 | 52.1 | 1000 | 90.9 |
| ALEX-100B | −1.3 | - | 36.3 | - | 79.3 a |
| L-ALEX-100 | −3.1 | 858 | 45.1 b | 985.0 | 103.5 b |
| VF-ALEX-100F | −5.8 | 888 | 33.9 | 1088 | 76.8 |
| VF-ALEX-50E | −5.1 | 883 | 39.4 | 1091 | 88.5 |
| AP15-ALEX-100B | −12.3 | 858 | 39.8 | 993.0 | 84.0 |
| LE-μAl15 | −0.1 | 831 | 3.9 | 1198 | 14.5 |
| LE-μAl15-T10 | −7.1 | 899 | 2.5 | 1216 | 18.1 |
| LE-μAl15-T30 | −24.1 | 902 | 0.8 | 1175 | 9.8 |
| LE-μAl7.5-T30 | −26.7 | 902 | 1.7 | 1174 | 41.0 |
| HE-μAl15-T45 | −36.5 | 1148 | 0.0 | - | 27.4 |
| HE-ALEX-50-T45 | −34.2 | 893 | 43.0 | 988 | 61.0 a |
| Solid Fuel | rf(Gox) Power Law Approximation (Equation (3)) | ||
|---|---|---|---|
| ID | ar | nr | R2 |
| F1A | 0.018 ± 0.001 | 0.680 ± 0.003 | 0.88 |
| F1B | 0.026 ± 0.000 | 0.652 ± 0.002 | 0.96 |
| F2 | 0.059 ± 0.001 | 0.477 ± 0.001 | 0.95 |
| F3 | 0.064 ± 0.001 | 0.494 ± 0.001 | 0.90 |
| F4 | 0.005 ± 0.001 | 0.959 ± 0.022 | 0.85 |
| F5 | 0.007 ± 0.001 | 0.905 ± 0.012 | 0.96 |
| F6 | 0.052 ± 0.003 | 0.535 ± 0.010 | 0.91 |
| F7 | 0.041 ± 0.003 | 0.595 ± 0.014 | 0.85 |
| F8 | 0.017 ± 0.000 | 0.630 ± 0.002 | 0.95 |
| F9 | 0.016 ± 0.000 | 0.643 ± 0.002 | 0.92 |
| F10 | 0.019 ± 0.000 | 0.747 ± 0.002 | 0.91 |
| F11 | 0.006 ± 0.000 | 0.902 ± 0.003 | 0.93 |
| F12 | 0.006 ± 0.000 | 0.908 ± 0.002 | 0.95 |
| F13 | 0.006 ± 0.000 | 0.897 ± 0.002 | 0.95 |
| F14 | 0.006 ± 0.000 | 0.954 ± 0.002 | 0.96 |
| F15 | 0.065 ± 0.000 | 0.491 ± 0.001 | 0.94 |
| F16 | 0.011 ± 0.000 | 0.869 ± 0.002 | 0.94 |
| F17 | 0.012 ± 0.002 | 0.815 ± 0.003 | 0.88 |
| F18 | 0.021 ± 0.002 | 0.769 ± 0.002 | 0.95 |
| F19 | 1.969 ± 0.002 | 0.053 ± 0.000 | 0.88 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paravan, C. Nano-Sized and Mechanically Activated Composites: Perspectives for Enhanced Mass Burning Rate in Aluminized Solid Fuels for Hybrid Rocket Propulsion. Aerospace 2019, 6, 127. https://doi.org/10.3390/aerospace6120127
Paravan C. Nano-Sized and Mechanically Activated Composites: Perspectives for Enhanced Mass Burning Rate in Aluminized Solid Fuels for Hybrid Rocket Propulsion. Aerospace. 2019; 6(12):127. https://doi.org/10.3390/aerospace6120127
Chicago/Turabian StyleParavan, Christian. 2019. "Nano-Sized and Mechanically Activated Composites: Perspectives for Enhanced Mass Burning Rate in Aluminized Solid Fuels for Hybrid Rocket Propulsion" Aerospace 6, no. 12: 127. https://doi.org/10.3390/aerospace6120127
APA StyleParavan, C. (2019). Nano-Sized and Mechanically Activated Composites: Perspectives for Enhanced Mass Burning Rate in Aluminized Solid Fuels for Hybrid Rocket Propulsion. Aerospace, 6(12), 127. https://doi.org/10.3390/aerospace6120127




