A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector
Abstract
1. Introduction
2. Natural Oil-Based Epoxies
3. Isosorbide Based Epoxy Resins
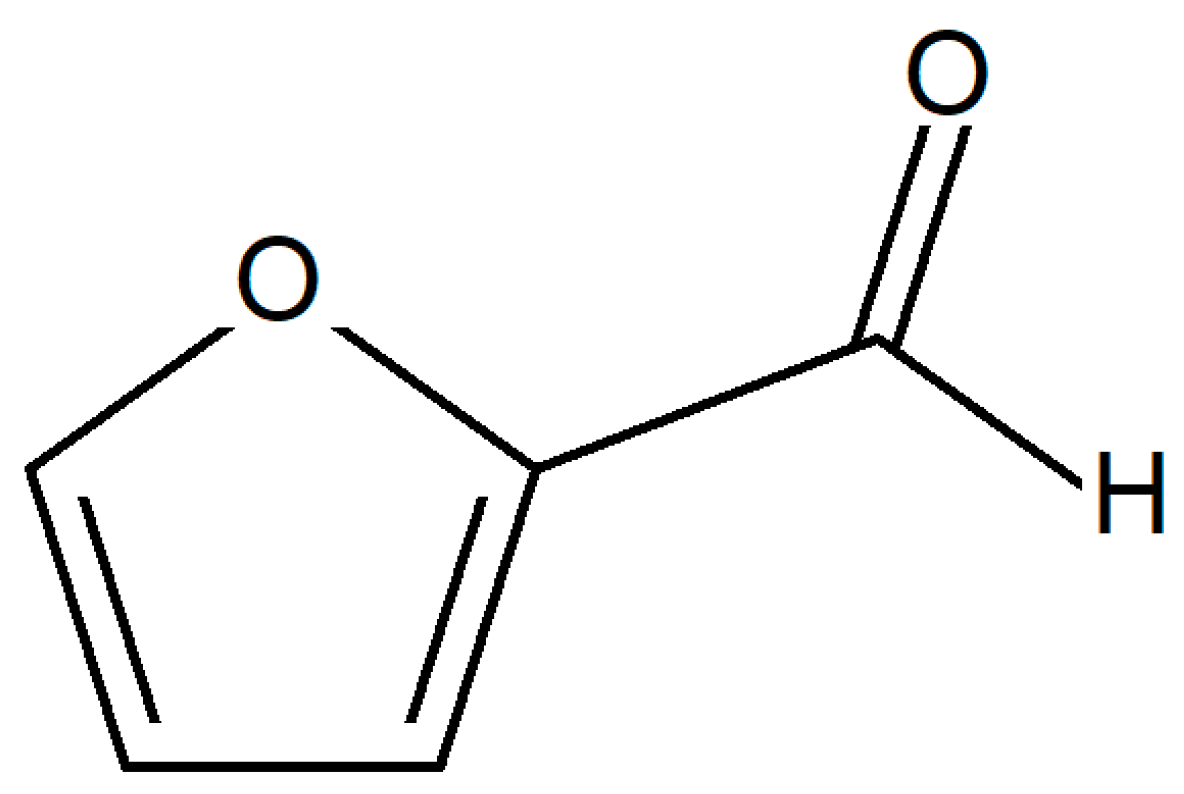
4. Furan Based Epoxy Resins
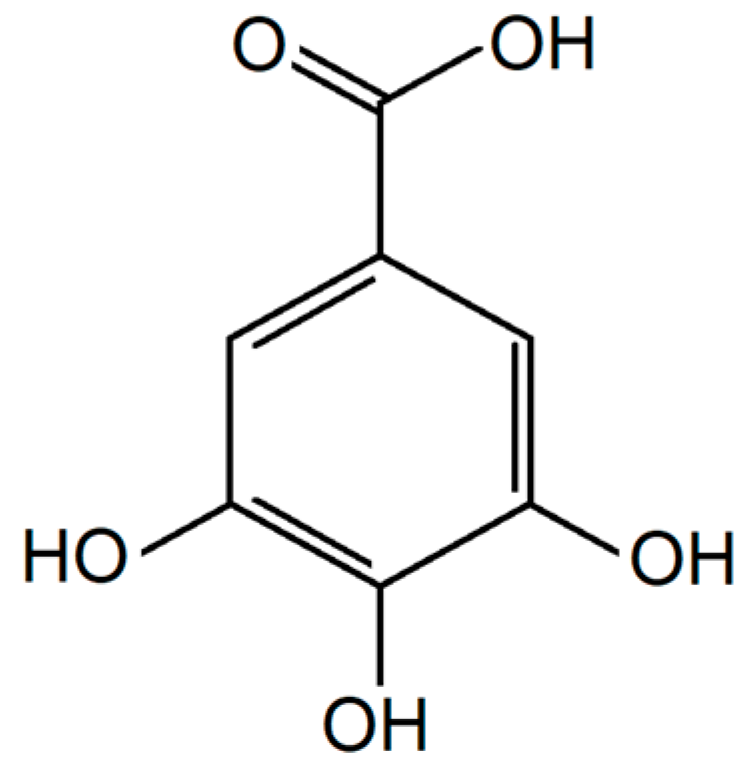
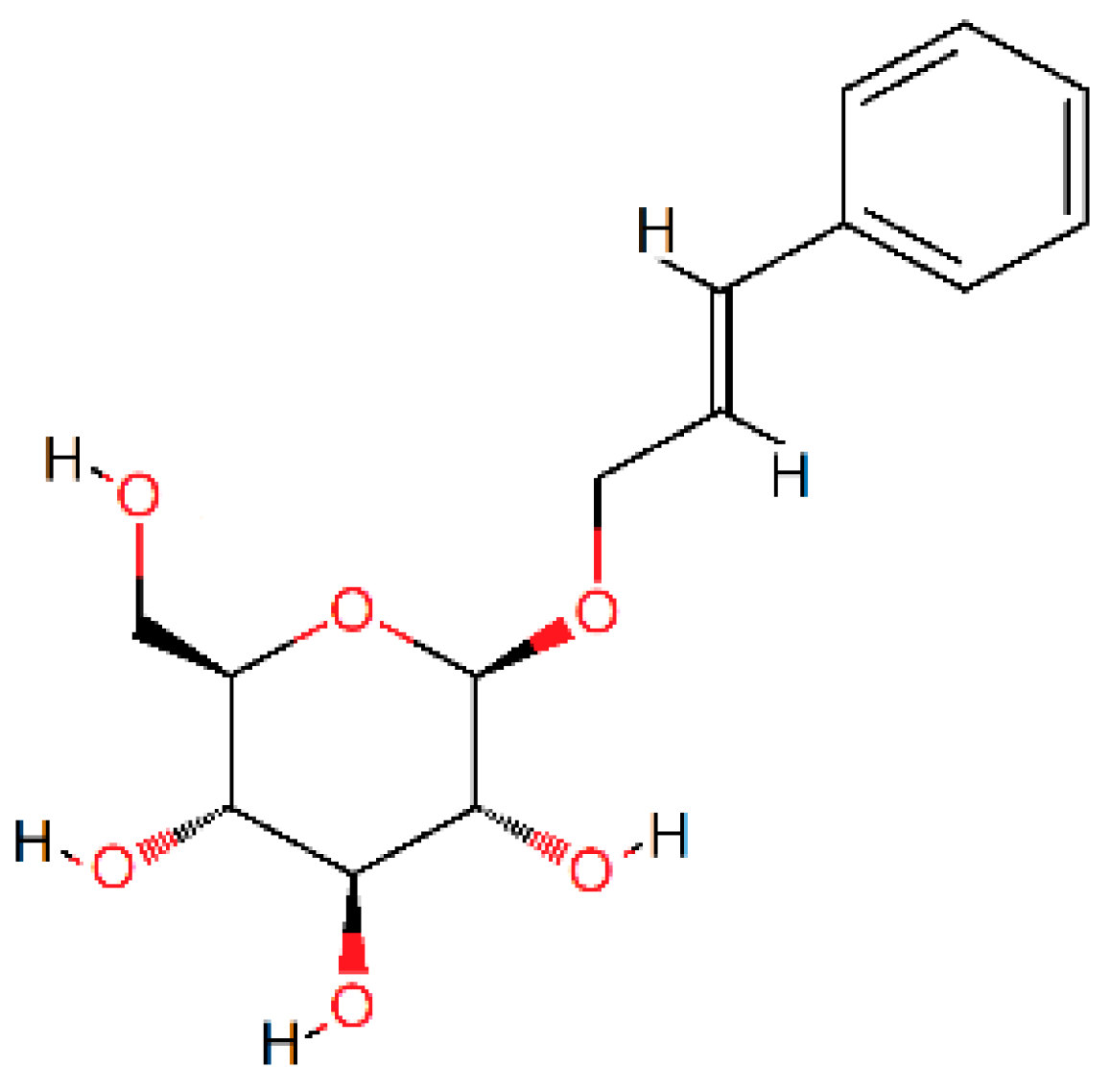
5. Phenolic and Polyphenolic Epoxies
6. Epoxidized Natural Rubber
7. Epoxy Lignin Derivatives
8. Rosin Based Resin
9. Summary and Discussion
Funding
Conflicts of Interest
Appendix A
| Nature | Epoxy System | Sample Description | Tg (°C) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Potential Applications Suggested by the Authors | References |
|---|---|---|---|---|---|---|---|---|---|
| Natural oil-based epoxy | Epoxidized soybean oil (ESO) | Commercial ESO (from 30 to 10 wt %) mixed with Shell Epon 9500 epoxy resin | 61.9–72.3 respectively | 60–51 respectively | 3193–2807 respectively | 99–119 respectively | 2910–3234 respectively | Composites (enhancement of mechanical properties needed) | Zhu et al. [17] |
| Epoxidized allyl soyate (from 30 to 10 wt %) mixed with Shell Epon 9500 | 65.0–75.1 respectively | 54–53 respectively | 2952–2972 respectively | 103–127 respectively | 2979–3503 respectively | Zhu et al. [17] | |||
| ESO (from 0 to 100 wt %)-DGEBA blends | 108–57 respectively | - | - | - | - | Altuna et al. [18] | |||
| ESO (from 0 to 60 wt %)-DGEBA blends | - | - | - | 106–63 respectively | 3300–1600 respectively | Jin and Park [19] | |||
| Epoxidized linseed oil (ELO) | ELO-bio-based long chain diacid (Pripol 1009) | - | 1.65 | ≈7.3–7.9 | - | - | Composites, laminates, adhesives | Supanchaiyamat et al. [30] | |
| ELO-Adipic acid | 1.5 (DMA) | 8.8 | 22 | - | - | Ding et al. [31] | |||
| ELO-methyl nadic anhydride reinforced with slate fibres with differed silane treatments | - | 328.2–359.1 | 21,900–25,600 | 299.2–402.1 | 18,400–19,700 | Samper et al. [29] | |||
| Epoxidized canola oil (ECO) | ECO-phthalic anhydride (PA) | −24.1–16.1 depending on the curing temperature and PA proportions | - | - | - | - | For making lignocellulosic fibre- and particle-based biocomposites | Omonov and Curtis [37] | |
| Epoxidized castor oil | Epoxidized castor oil-DGEBA Curing catalyst: N-benzylpyrazinium hexafluoroantimonate (BPH) (99:1 wt ratio) | 197–38 (0 to 100 wt % epoxidized castor oil) | - | - | 82.5 | 3400 | - | Park et al. [38] | |
| Epoxidized castor oil-DGEBA Curing catalyst: N-benzylquinoxalinium hexafluoroantimonate (BQH) (99:1 wt ratio) | - | - | - | 122.8 | 2800 | - | Park et al. [39] | ||
| Epoxidized castor oil (0–50 wt % ) DGEBA Curing agent: TETA | 96.64–39.21 respectively | 70.18–18.26 respectively | 3343.11–900.59 respectively | 95.644–40.04 respectively | 3358.05–1200.79 respectively | - | Sudha et al. [41] | ||
| Epoxidized karanja oil (KO) | Epoxidized KO Curing agents: CA and TA | 112.70 (CA) 108.64 (TA) | 10.60 (CA) 4.50 (TA) | 2.65 (CA) 2.58 (TA) | - | - | Coatings and lamination | Kadam et al. [42] |
| Nature | Epoxy System | Sample Description | Tg (°C) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Potential Applications Suggested by the Authors | References |
|---|---|---|---|---|---|---|---|---|---|
| Isosorbide based epoxy | Diglicidyl eter of isosorbide (DGEI) | DGEI Curing agents: DETA and ISODA | 76 (DETA) 43 (ISODA) | 62 (DETA) 41 (ISODA) | 1798 (DETA) 1532 (ISODA) | - | 4027 (DETA) 1168 (ISODA) | Replacement of BPA (for food contact applications), Industry additives, can coatings, biomedical applications like bone cements and drug delivery systems, packaging, automotive industry. | Hong et al. |
| DGEI Curing agents: PHA, THPHA, TETA and IPHA | 108 (PHA) 95 (THPHA) 49 (TETA) 73 (IPHA) | - | - | 225.5 (PHA) 100.5 (THPHA) 228.3 (TETA) 158.8 (IPHA) | 17,400 (PHA) 15,100 (THPHA) 5500 (TETA) 14,600 (IPHA) | Łukaszczyk et al. [50] | |||
| Bisisosorbide diglicidyl eter | Bisisosorbide diglydicyl eter Curing agent: Jeffamine T403 | 48 but can be increased to 200 °C changing the curing agent | 68.8 | 2944 | - | - | Feng et al. [49] | ||
| Furan based epoxy | BOF and BOB | BOF/BOB—DGEBA Curing agents: PACM and EPIKURE W | 80 to 150 depending on the proportions and curing agent. | - | - | - | - | Adhesives, structural and engineering materials and composites | Hu et al. [58] |
| BOF Curing agents: DFDA, CH3-DGBA, PACM | 69 (DFDA) 62 (CH3-DGBA) 72 (PACM) | - | - | - | - | Hu et al. [59] | |||
| DGF | DGF Curing agents: MHHPA, D230 | 152 (MHHPA) 101.2 (D230) | 84 (MHHPA) 68 (D230) | 3000 (MHHPA) 2700 (D230) | 96 (MHHPA) 75 (D230) | 3100 (MHHPA) 2500 (D230) | Deng et al. [61] |
| Nature | Epoxy System | Sample Description | Tg (°C) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Potential Applications Suggested by the Authors | References |
|---|---|---|---|---|---|---|---|---|---|
| Phenolic and polyphenolic epoxy | GEHDGTE, GEFDGTE, GEC | GEGTE, GEC Curing agent: IPDA | 142 (GEGTE) 179 (GEC) | - | - | - | - | Electronic applications, composites | Benyahya et al. [65] |
| GEHDGTE, GEFDGTE, GEC Curing agent: Lignin derivative | 178 (GEC) 155 (GEFDGTE) 173 (GEFHDGTlE) | - | - | 63 (GEC) 56 (GEFDGTE) 40 (GEFHDGTE) | - | Basnet et al. [66] | |||
| GEGA | GEGA Curing agents: IPDA, DPG, BDMA | 158 (IPDA) 98 (DPG) 136 (BDMA) | 43.1 (IPDA) 70.6 (DPG) 31.2 (BDMA) | 3600 (IPDA) 3500 (DPG) 3200 (BDMA) | - | - | Tarzia et al. [74] | ||
| TA | GPE, SPE Curing agent: TA | 87.3 (GPE) 106.6 (SPE) | 36.7 (GPE) 60.6 (SPE) | 2400 (GPE) 1710 (SPE) | - | - | Shibata et al. [77] | ||
| Cardanol epoxy | Cardanol based resol-DGEBA Curing agent: Amine catalyst or an acid catalyst | - | 12 | 864 | - | - | Composites, binders and coatings | Maffezzoli et al. [80] | |
| BPA-Cardanol epoxy (80:20 and 50:50) | - | 31.7 (80:20) 23.5 (50:50) | 2045 (80:20) 1926 (50:50) | 80.8 (80:20) 71.45 (50:50) | - | Unnikrishnan et al. [75] | |||
| NC-514 Curing agents: IPDA, Jeffamine D400 | 50 (IPDA) 15 (Jeffamine D400) | - | - | - | - | Jaillet et al. [81] | |||
| NC-514-Sorbitol/Isosorbide epoxies Curing agents: IPDA, Jeffamine T403 | 83 (25:75 Epoxidized cardanol: Epoxidized isosorbide cured with IPDA) 60 (50 wt % Epoxidized cardanol/Epoxidized sorbitol cured with IPDA) 37 (50 wt % Epoxidized cardanol/Epoxidized sorbitol cured with Jeffamine T403) | - | - | - | - | Darroman et al. [82] | |||
| CNE Curing agent: CPA | 50–84 | - | - | - | Atta et al. [83] |
| Nature | Epoxy System | Sample Description | Tg (°C) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Potential Applications Suggested by the Authors | References |
|---|---|---|---|---|---|---|---|---|---|
| Epoxidized Natural rubber (ENR) | ENR | ENR-DGEBA Curing agent: Nadic methyl anhydride (K 68) | 112 (5 wt % ENR)–109 (20 wt % ENR) | - | - | - | - | - | Mathew et al. [93] |
| ENR Curing agent: DTDB | - | 12 | 1.67 | - | - | - | Imbernon et al. [94] | ||
| Epoxy lignin derivatives | Depolymerized lignin epoxy | DHL epoxy-DGEBA Curing agent: DDM | - | 138 (100% DHL)—187 (25% DHL) | 12,300 (100% DHL) 23,200 (25% DHL) | 47 (100% DHL)—258 (25% DHL) | 5000 (100% DHL)—13,200 (25% DHL) | Electronics, substitute for fossil resource-derived bisphenol A, polymer matrix for manufacture of bio-based fibre-reinforced plastics or composites | Ferdosian et al. [101] |
| Vanillin derivatives | Diglycidyl ethers of vanillyl alcohol, vanillic acid and methoxyhydroquinone Curing agent: IPDA | 97 (diglycidyl ether of vanillyl alcohol) 132 (Diglycidyl ether of methoxyhydroquinone) 152 (Diglycidyl ether of vanillic acid) | - | - | - | - | Fache et al. [104] |
| Nature | Epoxy System | Sample Description | Tg (°C) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Potential Applications Suggested by the Authors | References |
|---|---|---|---|---|---|---|---|---|---|
| Rosin based | Triglycidyl ester FPAE and glycidyl ethers FPEG1, FPEG2, and FPEG3 obtained from Rosin | E-44 FPAE1C FPEG1C FPEG2C FPEG3C | 140 167 81 79 75 | 56.25 48.54 68.75 58.18 42.41 | 290 471 495 300 270 | - | - | - | Deng et al. [111] |
| Rosin based- epoxy monomer and curing agent | Maleopimaric acid (MPA) and triglycidyl ester of maleopimaric acid | 164 | - | - | 70 | 2200 | - | Liu et al. [109] | |
| Rosin-based Epoxy Monomer | Two glycidyl amine type epoxies: diglycidyl dehydroabietylamine (DGDHAA) derived from DHAA (rosin) and diglycidyl benzylamine (DGBA) derived from benzylamine hexahydrophthalic anhydride HHPA | 47 43 54 | 2180 2350 2400 | 68 69 98 | 3250 3417 3540 | Li et al. [112] |
Appendix B
| Type of Measurement | Type of Bio-Based Resin System | Range of Values | Temperature of Measurements | Reference |
|---|---|---|---|---|
| Kinematic viscosity | ESO | 170.87 mm2·s–1— 20.41 mm2·s–1 | 40 °C at 100 °C | Erhan et al. [15]. |
| Dynamic viscosity | ELO with different amine catalysts | 400 mPa s—2000 mPa s depending on the catalyst | at 140°C | Supanchaiyamat et al. [30] |
| Dynamic viscosity | castor oil/DGEBA blends at various wt %—TETA as curing agent | 950 mPa s—1050 mPa s (initial) | 20 °C | Sudha et al. [41] |
| Dynamic viscosity | EHO | 845 mPa s | 25 °C | Manthey et al. [44] |
| Dynamic viscosity | Pollit/MMA (70/30) Pollit/TPGDA (70/30) Tribest MMSO | 500 mPa s 13,900 mPa s 5700 mPa s 1200 mPa s | 24 °C | Åkesson et al. [45] |
| Dynamic viscosity | Isosorbide-based DGEI/ISODA | <10,000 mPa s (initial) | 25 °C | Hong et al. [48] |
| Kinematic viscosity | IS-EPO | 60,120 mPa s | 20 °C | Łukaszczyk et al. [50] |
| Dynamic viscosity | neat IM | 156 mPa s | 25 °C | Sadler et al. [51] |
| Dynamic viscosity | AESO IM-AESO IM-MAESO IM | 4789 ± 69 mPa s 151 ± 1 mPa s 186 ± 7 mPa s 12 ± 1 mPa s | 30 °C | Liu et al. [52] |
| Dynamic viscosity | GEGA | 2000 mPa s | room temperature | Tarzia et al. [74] |
| Dynamic viscosity | BPA/cardanol epoxy 80:20 BPA/cardanol epoxy 50:50 | 10,485 mPa s 9868 mPa s | 25°C | Unnikrishnan et al. [75] |
| Dynamic viscosity | GPE SPE | 150 mPa s 5000 mPa s | 25 °C | Shibata et al. [77] |
| Dynamic viscosity | Resole–epoxy Resole–epoxy Resole–epoxy Resole–epoxy–acid catalyst | 470 mPa s 2800 mPa s 4200 mPa s 4000 mPa s | 25 °C | Maffezzoli et al. [80] |
| Dynamic viscosity | cardanol novolac epoxy (CNE) resin cardanol polyamine hardener (CPA) | 1150 mPa s 2800 mPa s | - | Atta et al. [83] |
| Dynamic viscosity | triglycidyl ester FPAE glycidyl ethers from rosin FPEG1 glycidyl ethers from rosin FPEG2 glycidyl ethers from rosin FPEG3 | >100,000 mPa s >100,000 mPa s 43,500 mPa s 7800 mPa s | 25 °C | Deng et al. [111] |
| Dynamic viscosity | RTM 6 | 32–38 mPa s (initial) 59–89 mPa s (after 2 h) | 120 °C | RTM 6-TDS [113] |
References
- Pascault, J.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers; Marcel Dekker: New York, NY, USA, 2002; ISBN 0824706706. [Google Scholar]
- Holbery, J.; Houston, D. Natural-fibre-reinforced polymer composites in automotive applications. J. Miner. Met. Mater. Soc. 2006, 58, 80–86. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Gude, M.R.; Sanchez, J.; Ureña, A. Nanoreinforced epoxy adhesives for aerospace industry. J. Adhes. 2009, 85, 180–199. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, L.; Lv, J. Effects of expandable graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- Sharmin, E.; Alam, M.S.; Philip, R.K.; Ahmad, S. Linseed amide diol/DGEBA epoxy blends for coating applications: Preparation, characterization, ageing studies and coating properties. Prog. Org. Coat. 2010, 67, 170–179. [Google Scholar] [CrossRef]
- Gibson, R.F. A review of recent research on mechanics of multifunctional composite materials and structures. Compos. Struct. 2010, 92, 2793–2810. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Shen, L.; Haufe, J.; Patel, M. Product Overview and Market Projection of Emerging Biobased Plastics (PROBIP 2009); Utrecht University: Utrecht, The Netherlands, 2009. [Google Scholar]
- Soutis, C. Fibre reinforced composites in aircraft construction. Prog. Aerosp. Sci. 2005, 41, 143–151. [Google Scholar] [CrossRef]
- Hamerton, I.; Mooring, L. The use of thermosets in aerospace applications. Thermosets Struct. Prop. Appl. 2012, 189–227. [Google Scholar] [CrossRef]
- Yang, Y.; Boom, R.; Irion, B.; van Heerden, D.J.; Kuiper, P.; de Wit, H. Recycling of composite materials. Chem. Eng. Process. Process Intensif. 2012, 51, 53–68. [Google Scholar] [CrossRef]
- Soutis, C. Carbon fiber reinforced plastics in aircraft construction. Mater. Sci. Eng. A 2005, 412, 171–176. [Google Scholar] [CrossRef]
- Kausar, A. Role of Thermosetting Polymer in Structural Composite. Am. J. Polym. Sci. Eng. 2017, 5, 1–12. [Google Scholar]
- Park, S.J.; Jin, F.L.; Lee, J.R. Thermal and mechanical properties of tetrafunctional epoxy resin toughened with epoxidized soybean oil. Mater. Sci. Eng. A 2004, 374, 109–114. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z. Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind. Crops Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zlatanić, A.; Lava, C.C.; Sinadinović-Fišer, S. Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids—Kinetics and side reactions. Eur. J. Lipid Sci. Technol. 2002, 104, 293–299. [Google Scholar] [CrossRef]
- Zhu, J.; Chandrashekhara, K.; Flanigan, V.; Kapila, S. Curing and mechanical characterization of a soy-based epoxy resin system. J. Appl. Polym. Sci. 2004, 91, 3513–3518. [Google Scholar] [CrossRef]
- Altuna, F.I.; Espósito, L.H.; Ruseckaite, R.A.; Stefani, P.M. Thermal and mechanical properties of anhydride-cured epoxy resins with different contents of biobased epoxidized soybean oil. J. Appl. Polym. Sci. 2011, 120, 789–798. [Google Scholar] [CrossRef]
- Jin, F.-L.; Park, S.-J. Impact-strength improvement of epoxy resins reinforced with a biodegradable polymer. Mater. Sci. Eng. A 2008, 478, 402–405. [Google Scholar] [CrossRef]
- Gupta, A.P.; Ahmad, S.; Dev, A. Modification of novel bio-based resin-epoxidized soybean oil by conventional epoxy resin. Polym. Eng. Sci. 2011, 51, 1087–1091. [Google Scholar] [CrossRef]
- Tan, S.G.; Chow, W.S. Curing characteristics and thermal properties of Epoxidized soybean oil based thermosetting resin. Am. Oil Chem. Soc. 2011, 88, 915–923. [Google Scholar] [CrossRef]
- Cavusoglu, J.; Çayli, G. Polymerization reactions of epoxidized soybean oil and maleate esters of oil-soluble resoles. J. Appl. Polym. Sci. 2015, 132, 1–6. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Takayama, T.; Uyama, H. Biodegradable Shape Memory Polymeric Material from Epoxidized Soybean Oil and Polycaprolactone. Polymers 2015, 7, 2165–2174. [Google Scholar] [CrossRef]
- Miyagawa, H.; Mohanty, A.K.; Misra, M.; Drzal, L.T. Thermo-physical and impact properties of epoxy containing epoxidized linseed oil, 1: Anhydride-cured epoxy. Macromol. Mater. Eng. 2004, 289, 629–635. [Google Scholar] [CrossRef]
- Miyagawa, H.; Mohanty, A.K.; Misra, M.; Drzal, L.T. Thermo-Physical and Impact Properties of Epoxy Containing Epoxidized Linseed Oil, 2. Macromol. Mater. Eng. 2004, 289, 636–641. [Google Scholar] [CrossRef]
- Kanno, S.; Kawamura, Y.; Mutsuga, M.; Tanamoto, K. Determination of Epoxidized Soybean Oil and Linseed Oil in Wrapping Film and Cap Sealing. J. Food Hyg. Soc. Jpn. (Shokuhin Eiseigaku Zasshi) 2006, 47, 89–94. [Google Scholar] [CrossRef]
- Sánchez, N.; Chirinos, J. Estabilizantes térmicos alternativos para el PVC. Rev. Iberoam. Polímeros 2014, 15, 178–197. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Samper, M.D.; Petrucci, R.; Sánchez-Nacher, L.; Balart, R.; Kenny, J.M. New environmentally friendly composite laminates with epoxidized linseed oil (ELO) and slate fiber fabrics. Compos. Part B Eng. 2015, 71, 203–209. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Shuttleworth, P.S.; Hunt, A.J.; Clark, J.H.; Matharu, A.S. Thermosetting resin based on epoxidised linseed oil and bio-derived crosslinker. Green Chem. 2012, 14, 1759–1765. [Google Scholar] [CrossRef]
- Ding, C.; Shuttleworth, P.S.; Makin, S.; Clark, J.H.; Matharu, A.S. New insights into the curing of epoxidized linseed oil with dicarboxylic acids. Green Chem. 2015, 17, 4000–4008. [Google Scholar] [CrossRef]
- Pin, J.M.; Sbirrazzuoli, N.; Mija, A. From epoxidized linseed oil to bioresin: An overall approach of epoxy/anhydride cross-linking. ChemSusChem 2015, 8, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Samper, M.D.; Fombuena, V.; Boronat, T.; García-Sanoguera, D.; Balart, R. Thermal and Mechanical Characterization of Epoxy Resins (ELO and ESO) Cured with Anhydrides. J. Am. Oil Chem. Soc. 2012, 89, 1521–1528. [Google Scholar] [CrossRef]
- Pérez, J.D.E.; Haagenson, D.M.; Pryor, S.W.; Ulven, C.A.; Wiesenborn, D.P. Production and Characterization of Epoxidized Canola Oil. Trans. ASABE 2009, 52, 1289–1297. [Google Scholar] [CrossRef]
- Campanella, A.; Fahimian, M.; Wool, R.P.; Raghavan, J. Synthesis and Rheology of Chemically Modified Canola Oil. J. Biobased Mater. Bioenergy 2009, 3, 91–99. [Google Scholar] [CrossRef]
- Mungroo, R.; Pradhan, N.C.; Goud, V.V.; Dalai, A.K. Epoxidation of Canola Oil with Hydrogen Peroxide Catalyzed by Acidic Ion Exchange Resin. J. Am. Oil Chem. Soc. 2008, 85, 887–896. [Google Scholar] [CrossRef]
- Omonov, T.S.; Curtis, J.M. Biobased epoxy resin from canola oil. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, M.-K.; Lee, J.-R.; Lee, D.-R. Studies on epoxy resins cured by cationic latent thermal catalysts: The effect of the catalysts on the thermal, rheological, and mechanical properties. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 187–195. [Google Scholar] [CrossRef]
- Park, S.-J.; Jin, F.-L.; Lee, J.-R. Effect of Biodegradable Epoxidized Castor Oil on Physicochemical and Mechanical Properties of Epoxy Resins. Macromol. Chem. Phys. 2004, 205, 2048–2054. [Google Scholar] [CrossRef]
- Park, S.-J.; Jin, F.-L.; Lee, J.-R. Synthesis and Thermal Properties of Epoxidized Vegetable Oil. Macromol. Rapid Commun. 2004, 25, 724–727. [Google Scholar] [CrossRef]
- Sudha, G.S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Biobased epoxy blends from epoxidized castor oil: Effect on mechanical, thermal, and morphological properties. Macromol. Res. 2017, 25, 420–430. [Google Scholar] [CrossRef]
- Kadam, A.; Pawar, M.; Yemul, O.; Thamke, V.; Kodam, K. Biodegradable biobased epoxy resin from karanja oil. Polymer 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Stemmelen, M.; Pessel, F.; Lapinte, V.; Caillol, S.; Habas, J.-P.; Robin, J.-J. A fully biobased epoxy resin from vegetable oils: From the synthesis of the precursors by thiol-ene reaction to the study of the final material. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2434–2444. [Google Scholar] [CrossRef]
- Manthey, N.W.; Cardona, F.; Francucci, G.; Aravinthan, T. Thermo-mechanical properties of epoxidized hemp oil-based bioresins and biocomposites. J. Reinf. Plast. Compos. 2013, 32, 1444–1456. [Google Scholar] [CrossRef]
- Åkesson, D.; Skrifvars, M.; Lv, S.; Shi, W.; Adekunle, K.; Seppälä, J.; Turunen, M. Preparation of nanocomposites from biobased thermoset resins by UV-curing. Prog. Org. Coat. 2010, 67, 281–286. [Google Scholar] [CrossRef]
- Flèche, G.; Huchette, M. Isosorbide. Preparation, Properties and Chemistry. Starch 1986, 38, 26–30. [Google Scholar] [CrossRef]
- Rose, M.; Palkovits, R. Isosorbide as a Renewable Platform chemical for Versatile Applications—Quo Vadis? ChemSusChem 2012, 5, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Radojčić, D.; Ionescu, M.; Petrović, Z.S.; Eastwood, E. Advanced materials from corn: Isosorbide-based epoxy resins. Polym. Chem. 2014, 5, 5360–5368. [Google Scholar] [CrossRef]
- Feng, X.; East, A.J.; Hammond, W.B.; Zhang, Y.; Jaffe, M. Overview of advances in sugar-based polymers. Polym. Adv. Technol. 2011, 22, 139–150. [Google Scholar] [CrossRef]
- Łukaszczyk, J.; Janicki, B.; Kaczmarek, M. Synthesis and properties of isosorbide based epoxy resin. Eur. Polym. J. 2011, 47, 1601–1606. [Google Scholar] [CrossRef]
- Sadler, J.M.; Nguyen, A.-P.T.; Toulan, F.R.; Szabo, J.P.; Palmese, G.R.; Scheck, C.; Lutgen, S.; La Scala, J.J. Isosorbide-methacrylate as a bio-based low viscosity resin for high performance thermosetting applications. J. Mater. Chem. A 2013, 1, 12579. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Biobased Thermosets Prepared from Rigid Isosorbide and Flexible Soybean Oil Derivatives. ACS Sustain. Chem. Eng. 2017. [Google Scholar] [CrossRef]
- Gandini, A. Furans as offspring of sugars and polysaccharides and progenitors of a family of remarkable polymers: a review of recent progress. Polym. Chem. 2010, 1, 245–251. [Google Scholar] [CrossRef]
- Spillman, P.J.; Pollnitz, A.P.; Liacopoulos, D.; Pardon, K.H.; Sefton, M.A. Formation and Degradation of Furfuryl Alcohol, 5-Methylfurfuryl Alcohol, Vanillyl Alcohol, and Their Ethyl Ethers in Barrel-Aged Wines. J. Agric. Food Chem. 1998, 46, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Lamminpää, K.; Ahola, J.; Tanskanen, J. Kinetics of Xylose Dehydration into Furfural in Formic Acid. Ind. Eng. Chem. Res. 2012, 51, 6297–6303. [Google Scholar] [CrossRef]
- Lamminpää, K. Formic Acid Catalysed Xylose Dehydration into Furfural. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2015. [Google Scholar]
- Cho, J.K.; Lee, J.-S.; Jeong, J.; Kim, B.; Kim, B.; Kim, S.; Shin, S.; Kim, H.-J.; Lee, S.-H. Synthesis of carbohydrate biomass-based furanic compounds bearing epoxide end group(s) and evaluation of their feasibility as adhesives. J. Adhes. Sci. Technol. 2013, 27, 2127–2138. [Google Scholar] [CrossRef]
- Hu, F.; La Scala, J.J.; Sadler, J.M.; Palmese, G.R. Synthesis and Characterization of Thermosetting Furan-Based Epoxy Systems. Macromolecules 2014, 47, 3332–3342. [Google Scholar] [CrossRef]
- Hu, F.; Yadav, S.K.; La Scala, J.J.; Sadler, J.M.; Palmese, G.R. Preparation and Characterization of Fully Furan-Based Renewable Thermosetting Epoxy-Amine Systems. Macromol. Chem. Phys. 2015, 216, 1441–1446. [Google Scholar] [CrossRef]
- Hu, F.; Yadav, S.K.; Sharifi, M.; La Scala, J.; Sadler, J.; McAninch, I.; Palmesea, G. Characterization of Furanyl Thermosetting Polymers with Superior Mechanical Properties and High-Temperature Char Yield. In Proceedings of the International SAMPE Technical Conference, Long Beach, CA, USA, 23–26 May 2016. [Google Scholar]
- Deng, J.; Liu, X.; Li, C.; Jiang, Y.; Zhu, J. Synthesis and properties of a bio-based epoxy resin from 2,5-furandicarboxylic acid (FDCA). RSC Adv. 2015, 5, 15930–15939. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Pizzi, A. Monomers, Polymers and Composites from Renewable Resources. Monomers Polym. Compos. Renew. Resour. 2008, 179–199. [Google Scholar] [CrossRef]
- Nouailhas, H.; Aouf, C.; Le Guerneve, C.; Caillol, S.; Boutevin, B.; Fulcrand, H. Synthesis and properties of biobased epoxy resins. Part 1. Glycidylation of flavonoids by epichlorohydrin. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2261–2270. [Google Scholar] [CrossRef]
- Benyahya, S.; Aouf, C.; Caillol, S.; Boutevin, B.; Pascault, J.P.; Fulcrand, H. Functionalized green tea tannins as phenolic prepolymers for bio-based epoxy resins. Ind. Crops Prod. 2014, 53, 296–307. [Google Scholar] [CrossRef]
- Basnet, S.; Otsuka, M.; Sasaki, C.; Asada, C.; Nakamura, Y. Functionalization of the active ingredients of Japanese green tea (Camellia sinensis) for the synthesis of bio-based epoxy resin. Ind. Crops Prod. 2015, 73, 63–72. [Google Scholar] [CrossRef]
- Haslam, E.; Cai, Y. Plant polyphenols (Vegetable tannins): Gallic acid metabolism. Nat. Prod. Rep. 1994, 11, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Samanidou, V.; Tsagiannidis, A.; Sarakatsianos, I. Simultaneous determination of polyphenols and major purine alkaloids in Greek Sideritis species, herbal extracts, green tea, black tea, and coffee by high-performance liquid chromatography-diode array detection. J. Sep. Sci. 2012, 35, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Schmitzer, V.; Slatnar, A.; Veberic, R.; Stampar, F.; Solar, A. Roasting Affects Phenolic Composition and Antioxidative Activity of Hazelnuts (Corylus avellana L.). J. Food Sci. 2011, 76. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Yonezawa, K. Epoxy Resin and Process for Preparing the Same. U.S. Patent No. 4,540,802, 10 September 1985. [Google Scholar]
- Aouf, C.; Lecomte, J.; Villeneuve, P.; Dubreucq, E.; Fulcrand, H. Chemo-enzymatic functionalization of gallic and vanillic acids: Synthesis of bio-based epoxy resins prepolymers. Green Chem. 2012, 14, 2328–2336. [Google Scholar] [CrossRef]
- Tarzia, A.; Montanaro, J.; Casiello, M.; Annese, C.; Nacci, A.; Maffezzoli, A. Synthesis, Curing, and Properties of an Epoxy Resin Derived from Gallic Acid. BioResources 2017, 13, 632–645. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P.; Thachil, E.T. Synthesis and characterization of cardanol-based epoxy systems. Des. Monomers Polym. 2008, 11, 593–607. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Na, H.; Wu, Y.; Zheng, W.; Zhu, J. How a bio-based epoxy monomer enhanced the properties of diglycidyl ether of bisphenol A (DGEBA)/graphene composites. J. Mater. Chem. A 2013, 1, 5081–5088. [Google Scholar] [CrossRef]
- Shibata, M.; Nakai, K. Preparation and properties of biocomposites composed of bio-based epoxy resin, tannic acid, and microfibrillated cellulose. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 425–433. [Google Scholar] [CrossRef]
- Kumar, P.P.; Paramashivappa, R.; Vithayathil, P.J.; Rao, P.V.S.; Rao, A.S. Process for isolation of cardanol from technical cashew (Anacardium occidentale L.) Nut shell liquid. J. Agric. Food Chem. 2002, 50, 4705–4708. [Google Scholar] [CrossRef]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Maffezzoli, A.; Calò, E.; Zurlo, S.; Mele, G.; Tarzia, A.; Stifani, C. Cardanol based matrix biocomposites reinforced with natural fibres. Compos. Sci. Technol. 2004, 64, 839–845. [Google Scholar] [CrossRef]
- Jaillet, F.; Darroman, E.; Ratsimihety, A.; Auvergne, R.; Boutevin, B.; Caillol, S. New biobased epoxy materials from cardanol. Eur. J. Lipid Sci. Technol. 2014, 116, 63–73. [Google Scholar] [CrossRef]
- Darroman, E.; Durand, N.; Boutevin, B.; Caillol, S. New cardanol/sucrose epoxy blends for biobased coatings. Prog. Org. Coat. 2015, 83, 47–54. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Hodan, H.A.; Hameed, R.S.A.; Ezzat, A.O. Preparation of green cardanol-based epoxy and hardener as primer coatings for petroleum and gas steel in marine environment. Prog. Org. Coat. 2017, 111, 283–293. [Google Scholar] [CrossRef]
- Mooibroek, H.; Cornish, K. Alternative sources of natural rubber. Appl. Microbiol. Biotechnol. 2000, 53, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.S.L.; Gelling, I.R.; Newell, R. Epoxidized Natural Rubber. Rubber Chem. Technol. 1985, 58, 67–85. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Nematzadeh, N.; Wahit, M.U.; Hassan, A.; Imran, M. Epoxidized natural rubber toughened polyamide 6/organically modified montmorillonite nanocomposites. J. Thermoplast. Compos. Mater. 2014, 27, 395–412. [Google Scholar] [CrossRef]
- Hashim, A.S.; Ong, S.K. Study on polypropylene/natural rubber blend with polystyrene-modified natural rubber as compatibilizer. Polym. Int. 2002, 51, 611–616. [Google Scholar] [CrossRef]
- Yoksan, R. Epoxidized Natural Rubber for Adhesive Applications. Kasetsart J. (Nat. Sci.) 2008, 42, 325–332. [Google Scholar]
- Grande, A.M.; Rahaman, A.; Landro, L.D.; Penco, M.; Spagnoli, G. Self Healing of Blends Based on Sodium Salt of Poly(Ethylene-co-Methacrylic Acid)/Poly(Ethylene-co-Vinyl Alcohol) and Epoxidized Natural Rubber Following High Energy Impact. In Proceedings of the 3rd International Conference on Self-Healing Materials, Bath, UK, 27–29 June 2011. [Google Scholar]
- Arroyo, M.; López-Manchado, M.A.; Valentín, J.L.; Carretero, J. Morphology/behaviour relationship of nanocomposites based on natural rubber/epoxidized natural rubber blends. Compos. Sci. Technol. 2007, 67, 1330–1339. [Google Scholar] [CrossRef]
- Greve, H.-H. Rubber, 2. Natural. ULLMANN’S Encycl. Ind. Chem. 2012, 31, 583–594. [Google Scholar]
- Hamzah, R.; Bakar, M.A.; Khairuddean, M.; Mohammed, I.A.; Adnan, R. A structural study of epoxidized natural rubber (ENR-50) and its cyclic dithiocarbonate derivative using NMR spectroscopy techniques. Molecules 2012, 17, 10974–10993. [Google Scholar] [CrossRef] [PubMed]
- Mathew, V.S.; George, S.C.; Parameswaranpillai, J.; Thomas, S. Epoxidized natural rubber/epoxy blends: Phase morphology and thermomechanical properties. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Imbernon, L.; Oikonomou, E.K.; Norvez, S.; Leibler, L. Chemically crosslinked yet reprocessable epoxidized natural rubber via thermo-activated disulfide rearrangements. Polym. Chem. 2015, 6, 4271–4278. [Google Scholar] [CrossRef]
- Pire, M.; Norvez, S.; Iliopoulos, I.; Rossignol, B. Le Leibler, L. Epoxidized natural rubber/dicarboxylic acid self-vulcanized blends. Polymer 2010, 51, 5903–5909. [Google Scholar] [CrossRef]
- Pire, M.; Norvez, S.; Iliopoulos, I.; Le Rossignol, B.; Leibler, L. Dicarboxylic acids may compete with standard vulcanisation processes for crosslinking epoxidised natural rubber. Compos. Interfaces 2014, 21, 45–50. [Google Scholar] [CrossRef]
- Imbernon, L.; Pire, M.; Oikonomou, E.K.; Norvez, S. Macromol. Chem. Phys. 7/2013. Macromol. Chem. Phys. 2013, 214, 745. [Google Scholar] [CrossRef]
- McCarthy, J.L.; Islam, A. Lignin Chemistry, Technology, and Utilization: A Brief History. In Lignin: Historical, Biological, and Materials Perspectives; American Chemical Society: Washington, DC, USA, 2000. [Google Scholar]
- Chung, H.; Washburn, N.R. Extraction and Types of Lignin; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780323355667. [Google Scholar]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Synthesis and characterization of hydrolysis lignin-based epoxy resins. Ind. Crops Prod. 2016, 91, 295–301. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C.C. Chemically modified lignin through epoxidation and its thermal properties. J-FOR 2012, 2, 11–15. [Google Scholar]
- Asada, C.; Basnet, S.; Otsuka, M.; Sasaki, C.; Nakamura, Y. Epoxy resin synthesis using low molecular weight lignin separated from various lignocellulosic materials. Int. J. Biol. Macromol. 2015, 74, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Fache, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New vanillin-derived diepoxy monomers for the synthesis of biobased thermosets. Eur. Polym. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; Zhu, J. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Zhang, J. Rosin-Based Chemicals and Polymers, 1st ed.; Smithers Rapra Technology Ltd.: Shrewsbury, UK, 2012; ISBN 978-1-84735-506-5. [Google Scholar]
- Liu, X.Q.; Huang, W.; Jiang, Y.H.; Zhu, J.; Zhang, C.Z. Preparation of a bio-based epoxy with comparable properties to those of petroleum-based counterparts. eXPRESS Polym. Lett. 2012, 6, 293–298. [Google Scholar] [CrossRef]
- Liu, X.; Xin, W.; Zhang, J. Rosin-based acid anhydrides as alternatives to petrochemical curing agents. Green Chem. 2009, 11, 1018–1025. [Google Scholar] [CrossRef]
- Deng, L.; Ha, C.; Sun, C.; Zhou, B.; Yu, J.; Shen, M.; Mo, J. Properties of Bio-based Epoxy Resins from Rosin with Different Flexible Chains. Ind. Eng. Chem. Res. 2013, 52, 13233–13240. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Zhu, J.; Zhang, C.; Guo, J. Synthesis, Characterization of a Rosin-based Epoxy Monomer and its Comparison with a Petroleum-based Counterpart. J. Macromol. Sci. Part A 2013, 50, 321–329. [Google Scholar] [CrossRef]
- HEXCEL HexFlow® RTM 6 Product Data Sheet. Available online: https://www.hexcel.com/user_area/content_media/raw/HexFlow_RTM6_DataSheet.pdf (accessed on 1 October 2018).
- Raquez, J.-M.; Deléglise, M.; Lacrampe, M.-F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Ganesan, A.; Muthusamy, S. Mechanical properties of high temperature cyanate ester/BMI blend composites. Polym. Compos. 2009, 30, 782–790. [Google Scholar] [CrossRef]
- Wang, J.; Liang, G.; Zhu, B. Modification of Cyanate Resin by Nanometer Silica. J. Reinf. Plast. Compos. 2007, 26, 419–429. [Google Scholar] [CrossRef]
- Yuan, L.; Gu, A.; Liang, G.; Zhang, Z. Microcapsule-modified bismaleimide (BMI) resins. Compos. Sci. Technol. 2008, 68, 2107–2113. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694–8701. [Google Scholar] [CrossRef]
- Dicker, M.P.M.; Duckworth, P.F.; Baker, A.B.; Francois, G.; Hazzard, M.K.; Weaver, P.M. Green composites: A review of material attributes and complementary applications. Compos. Part A Appl. Sci. Manuf. 2014, 56, 280–289. [Google Scholar] [CrossRef]
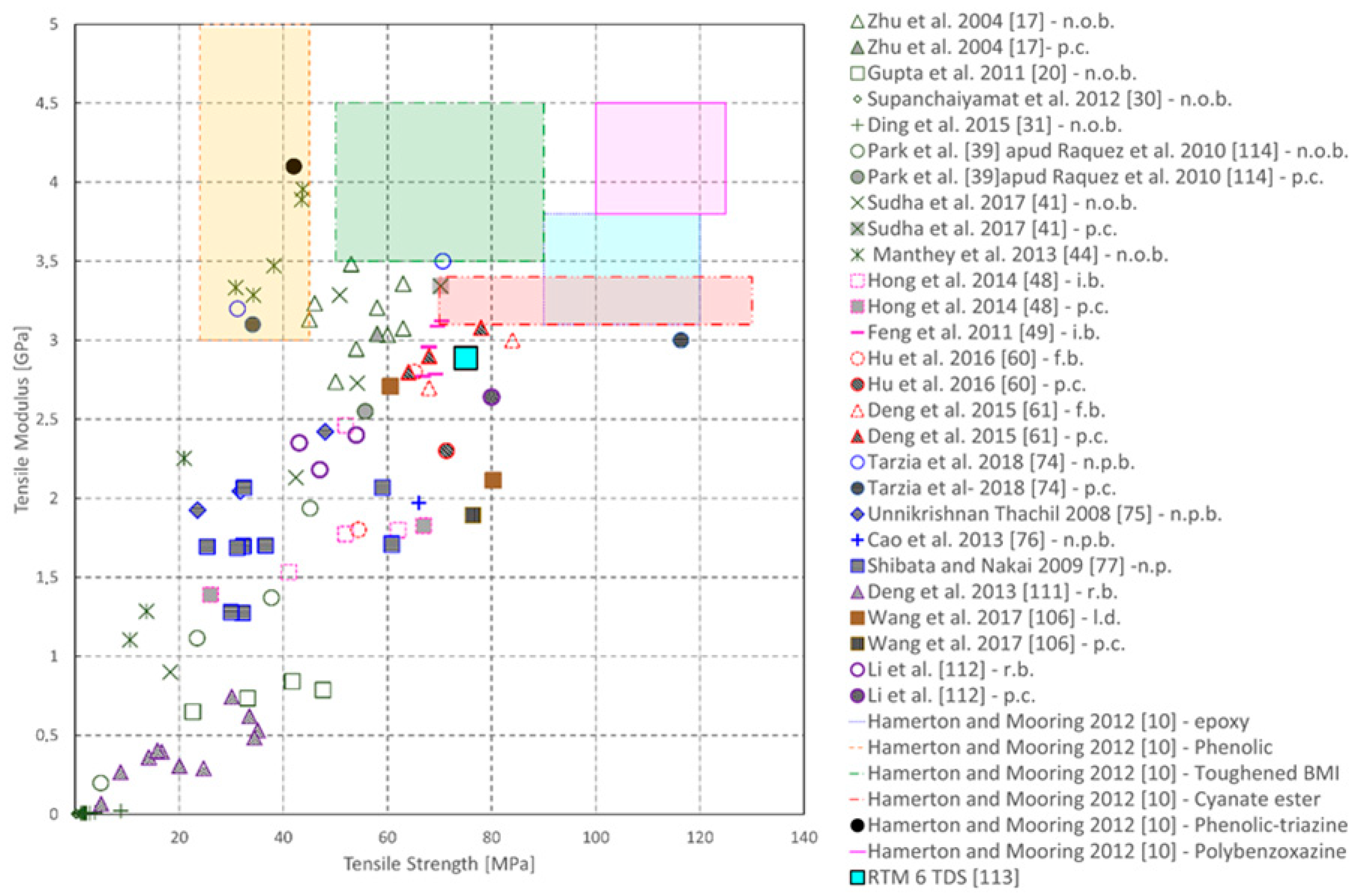
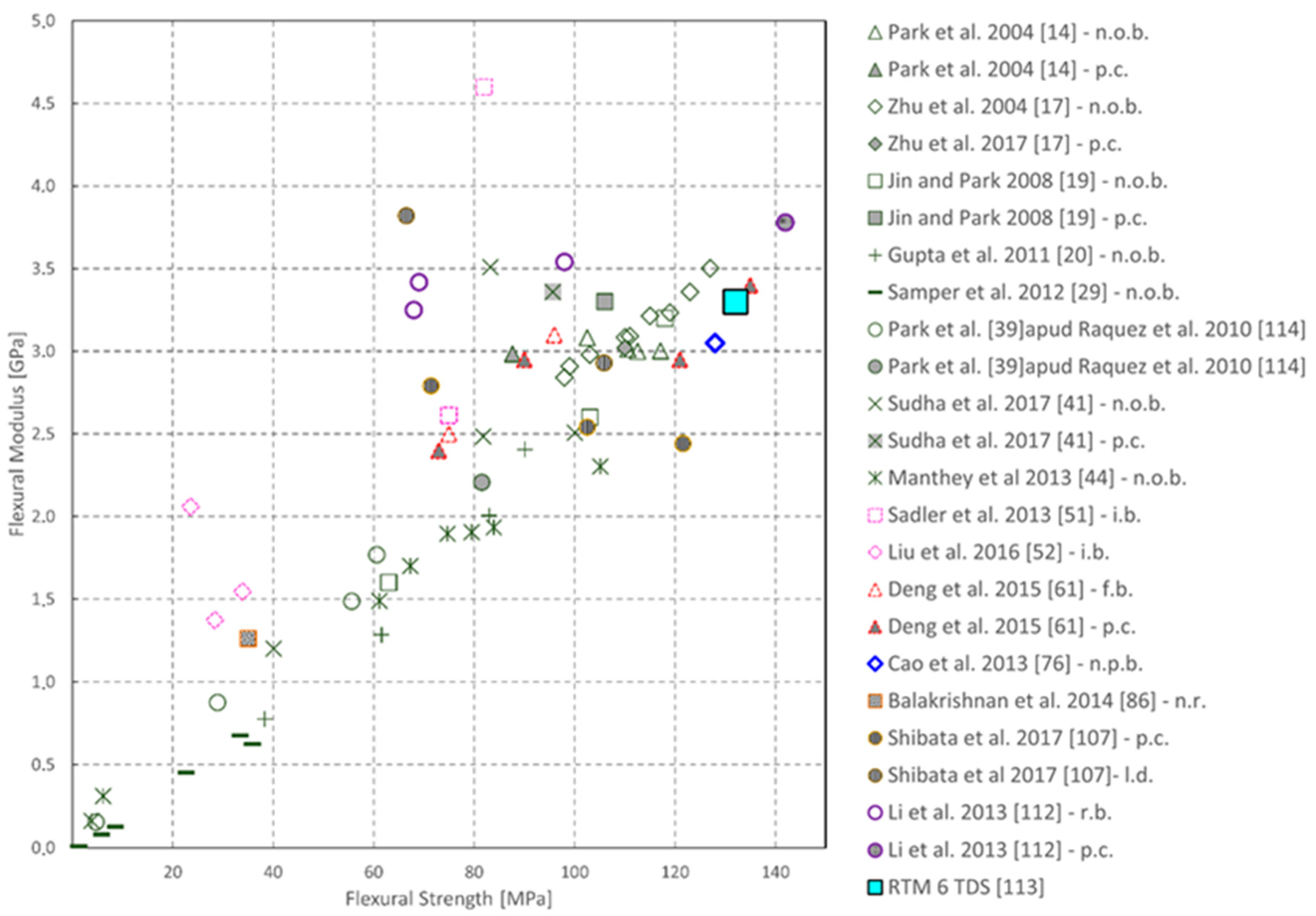
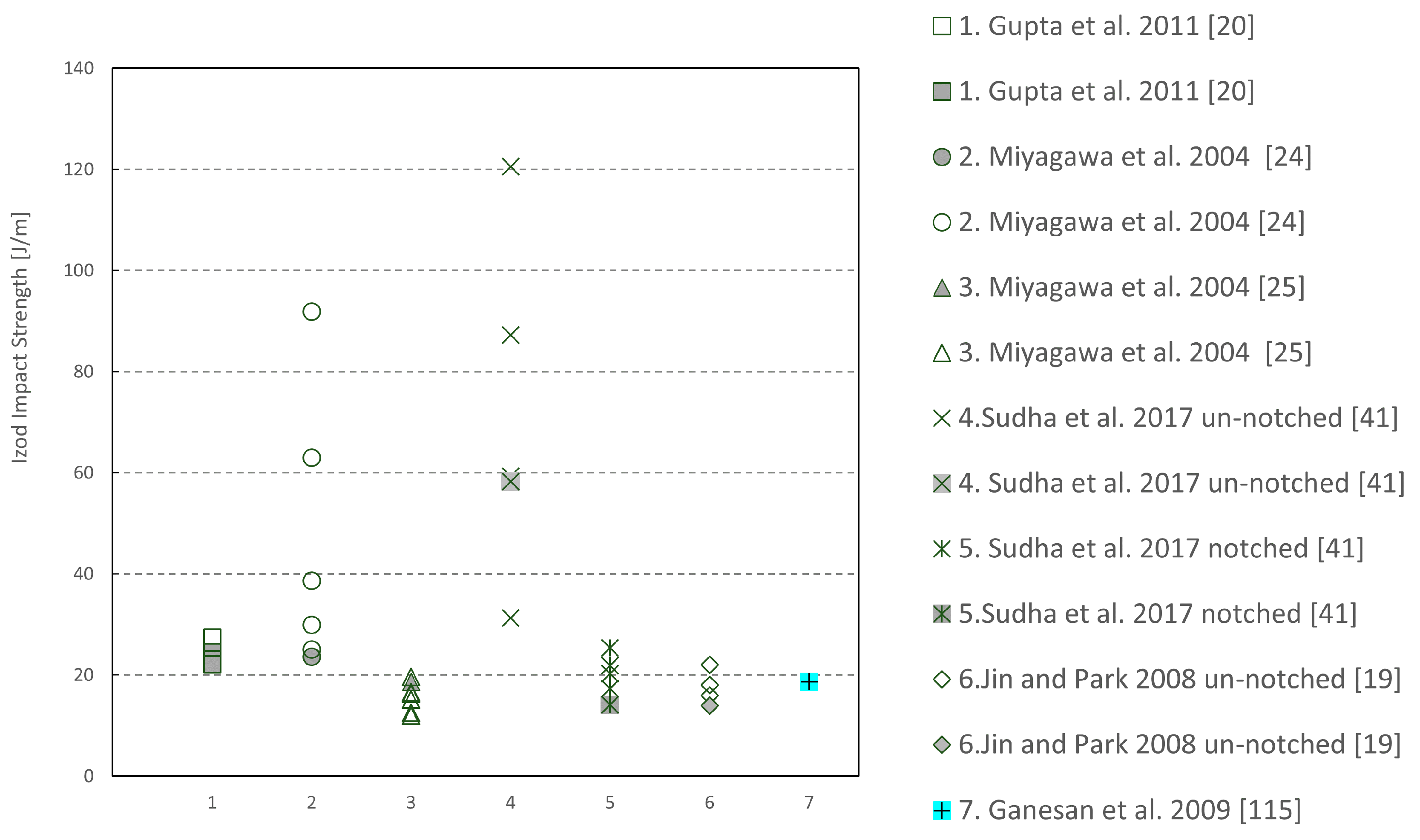
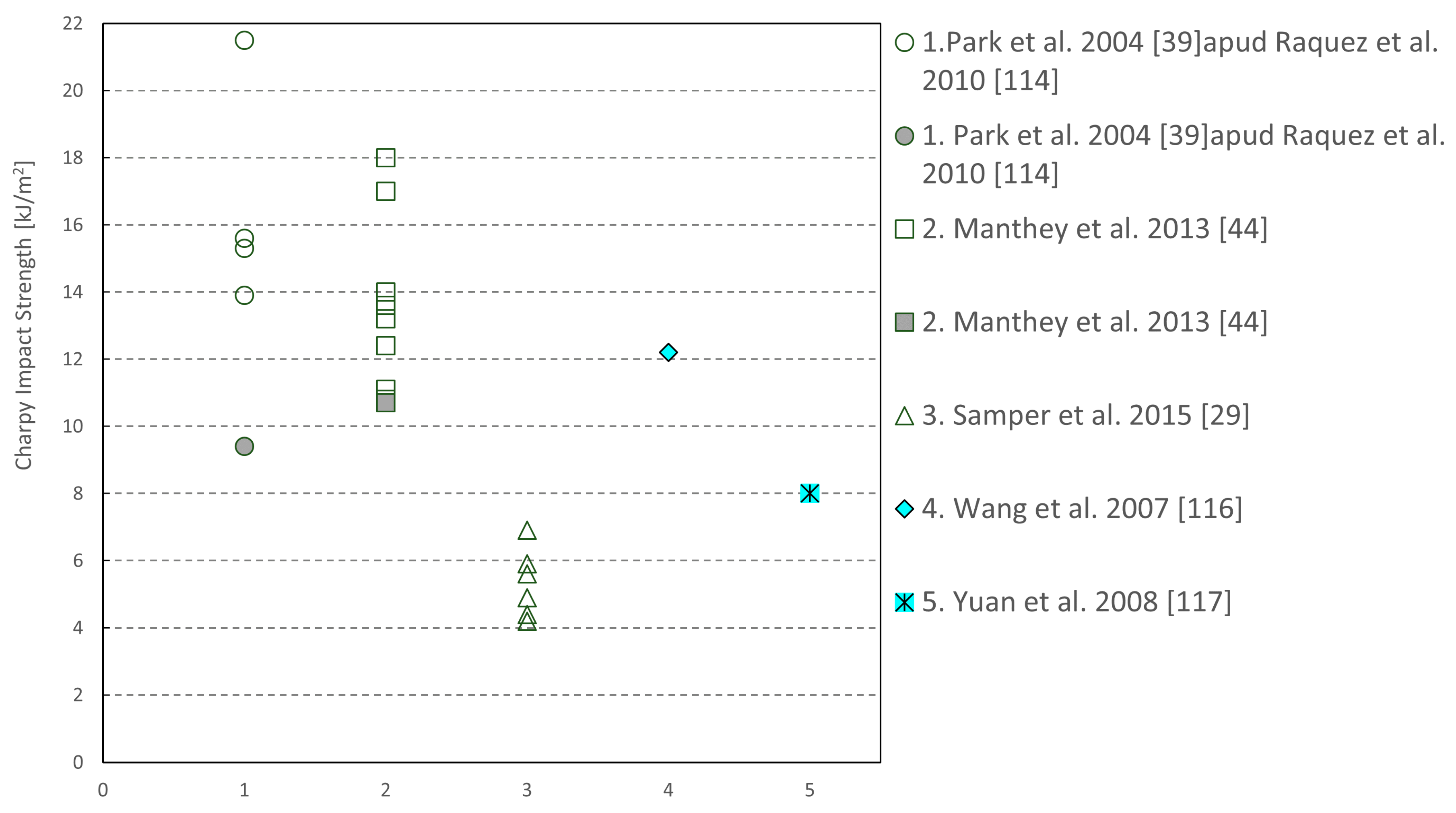
| Sample | Tg (°C) | Young’s Modulus (MPa) | Peak Strength (MPa) | Flexural Modulus (MPa) | Flexural Strength (MPa) |
|---|---|---|---|---|---|
| Epon epoxy | 74.8 | 3145 | 59 | 3021 | 110 |
| 10 wt % ESO | 72.3 | 2807 | 51 | 3234 | 119 |
| 20 wt % ESO | 67.0 | 2434 | 36 | 3090 | 111 |
| 30 wt % ESO | 61.9 | 3193 | 60 | 2910 | 99 |
| 10 wt % EAS | 75.1 | 2972 | 53 | 3503 | 127 |
| 20 wt % EAS | 69.2 | 2979 | 41 | 3359 | 123 |
| 30 wt % EAS | 65.0 | 2952 | 54 | 2979 | 103 |
| 10 wt % EMS | 68.0 | 2890 | 45 | 3214 | 115 |
| 20 wt % EMS | 63.3 | 2621 | 31 | 3083 | 110 |
| 30 wt % EMS | 55.3 | 3145 | 59 | 2841 | 98 |
| Sample | Elongation (%) | Stress at break (MPa) | Storage Modulus at 30 °C (MPa) |
|---|---|---|---|
| ESO–p-TBPMA–150 a | 34 | 4 | 40 |
| ESO–p-NPMA–150 | 128 | 1.5 | 10 |
| ESO–p-TBPMA–190 | 20 | 12 | 1088 |
| ESO–p-NPMA–190 | 48 | 13 | 180 |
| System (ECO 1:DGEBA) (wt %:wt %) | Tg (°C) | Storage Modulus at 30 °C (109 Pa) | Storage Modulus at Tα + 30 °C (109 Pa) | ρ (10−3 mol·cm−3) |
|---|---|---|---|---|
| 0:100 | 197 | 1.27 | 0.102 | 4.61 |
| 10:90 | 169 | 1.19 | 0.077 | 3.68 |
| 20:80 | 158 | 1.22 | 0.065 | 3.18 |
| 30:70 | 150 | 1.15 | 0.051 | 2.54 |
| 40:60 | 131 | 1.15 | 0.041 | 2.13 |
| 100:0 | 38 | - | 0.0079 | 0.57 |
| Sample (ECO 1:DGEBA) (wt %:wt %) | Tg (°C) | Tensile Strength (MPa) | Flexural Strength (MPa) | Crosslink Density, νe (×103 mol/m3) | Impact Strength Un-Notched (J/m) | Impact Strength Notched (J/m) |
|---|---|---|---|---|---|---|
| 0:100 | 96.64 | 70.18 ± 8 | 95.644 ± 3 | 2.81 | 58.23 ± 6 | 14.05 ± 2 |
| 10:90 | 91.37 | 50.79 ± 6 | 83.263 ± 28 | 2.43 | 87.20 ± 4 | 20.27 ± 2 |
| 20:80 | 71.37 | 54.22 ± 3 | 100.07 ± 18 | 2.33 | 120.53 ± 11 | 25.33 ± 2 |
| 30:70 | 47.44 | 42.41 ± 4 | 81.847 ± 26 | 1.15 | 59.31 ± 1 | 21.80 ± 1 |
| 50:50 | 39.21 | 18.26 ± 2 | 40.04 ± 7 | 0.66 | 31.25 ± 3 | 17.25 ± 1 |
| Sample | Tg (°C) | Tensile Strength (MPa) | Young’s Modulus (E) (MPa) | Shore Hardness (A) |
|---|---|---|---|---|
| Bioepoxy CA 1 | 112.70 | 10.60 | 2.65 | 56 |
| Bioepoxy TA 2 | 108.64 | 4.50 | 2.58 | 45 |
| Sample | Tg (°C) * | Tensile Strength (MPa) ** | Young Modulus (MPa) ** | Flexural Modulus (MPa) ** | Impact Strength (J/m) ** |
|---|---|---|---|---|---|
| DGEBA/DETA | 129 (134) | 26 (8.2%) | 1389 (4.7%) | 3061 (1.6%) | 60 (1.2%) |
| DGEBA/ISODA | 74 (79) | 67 (4%) | 1825 (4.5%) | 3364 | 94 (65%) |
| DGEI(mono)/DETA | 75 (76) | 62 (9%) | 1798 (1.2%) | 4027 | 72 (16.8%) |
| DGEI(mono)/ISODA | 32 (43) | 41 (21%) | 1532 (2.6%) | 1168 | 65 (23%) |
| DGEI(polymeric)/ISODA | 36 (43) | 52 (8.1% | 2461 (9.5%) | 3520 | 57 (14.7%) |
| DGEI(polymeric)/DETA | 48 (63) | 52 (18%) | 1774 (8%) | 2747 | 113 (33%) |
| Sample Composition | Tg (°C) | Flexural Strength (MPa) | Compression Strength (MPa) | Brinell Hardness (MPa) | Izod Impact Strength (kJ/m2) |
|---|---|---|---|---|---|
| Epidian 5/PHA | 171 | 158.4 | 290.8 | 198.0 | 7.2 |
| IS-EPO/PHA | 108 | 225.5 | 254.1 | 202.4 | 30.9 |
| Epidian 5/THPHA | 172 | 27.9 | 122.2 | 209.4 | 4.1 |
| IS-EPO/THPHA | 95 | 100.5 | 88.8 | 214.3 | 2.9 |
| Epidian 5/TETA | 116 | 170.8 | 234.2 | 212.1 | 9.5 |
| IS-EPO/TETA | 49 | 228.3 | 311.6 | 193.8 | 20.8 |
| Epidian 5/IPHA | 141 | 175.4 | 193.9 | 231.2 | 13.5 |
| IS-EPO/IPHA | 73 | 158.5 | 318.1 | 205.7 | 33.8 |
| Furanyl Monomer | Source | Status |
|---|---|---|
| Furfural (F) | Sugar cane bagasse or corn cobs (derived from pentoses) | Commercial |
| Furfuryl alcohol (FA) | from furfural. | Commercial |
| 5-Hydroxymethylfurfural (HMF) | Plant based sugars (derived from hexoses) | Commercial |
| 2-Furfurylmethacrylate (FM) | From HMF or furfural | Non-commercial |
| Bis-2,5-hydroxymethylfuran (BHMF) | From HMF or furfural | Non-commercial |
| 2,5-Furandicarboxylic acid (FDCA) | From HMF or furfural | Commercial |
| Weight Ratio Monomers (BOF:BOB:DGEBA) | Tg (°C) | |
|---|---|---|
| PACM | EPIKURE W | |
| 100:0:0 | 71 (80) 1 | 88 (94) |
| 70:0:30 | 96 (106) | 114 (120) |
| 50:0:50 | 111 (121) | 133 (139) |
| 30:0:70 | 131 (140) | 153 (160) |
| 0:100:0 | 55 (63) | 80 (90) |
| 0:70:30 | 84 (94) | 104 (103) |
| 0:50:50 | 103 (114) | 126 (136) |
| 0:30:70 | 124 (134) | 148 (159) |
| 0:0:100 | 167 (176) | 185 (198) |
| Sample | Tg (°C) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Strength (MPa) | Flexural Modulus (MPa) |
|---|---|---|---|---|---|
| DGF/MHHPA | 152 | 84 ± 4 | 3000 ± 50 | 96 ± 3 | 3100 ± 110 |
| DGT/MHPPA | 128.8 | 78 ± 2 | 3080 ± 80 | 90 ± 5 | 2950 ± 40 |
| DGF/D230 | 101.2 | 68 ± 3 | 2700 ± 110 | 75 ± 2 | 2500 ± 90 |
| DGT/D230 | 91.8 | 64 ± 2 | 2800 ± 60 | 73 ± 3 | 2400 ± 100 |
| DGEBA/MHHPA | 125 | 68 | 2900 | 135 | 3400 |
| DGEBA/D230 | 97 | NA | NA | 121 | 2950 |
| Sample | Tg (°C) | Storage Modulus (GPa) at 30 °C | Storage Modulus (GPa) at Tg + 30 °C |
|---|---|---|---|
| DGEBA | 209 | 2.81 | 0.019 |
| 75DGEBA/25GEC | 221 | 2.46 | 0.016 |
| 50DGEBA/50GEC | 202 | 2.40 | 0.014 |
| Sample | Tg (°C) | Storage Modulus (GPa) at 30 °C | Storage Modulus (GPa) at Tg + 30 °C |
|---|---|---|---|
| GEGTE-IPDA | 142 | 2.34 | 0.0593 |
| GEC-IPDA | 179 | 1.50 | 0.0364 |
| DER352-IPDA | 140 | 1.29 | 0.0136 |
| Sample | Tg (°C) | Flexural Strength (MPa) |
|---|---|---|
| GEC-Lignin | 178 | 63 |
| GEHDGTE-Lignin | 155 | 56 |
| GEFHDGTE-Lignin | 173 | 40 |
| BPA-Lignin | 150 | 29 |
| Sample | Tg (°C) | Tensile Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| GEGA/IPDA | 158 | 3.6 ± 0.3 | 43.1 ± 13.1 | 1.4 ± 0.3 |
| GEGA/DPG | 98 | 3.5 ± 0.2 | 70.6 ± 2.9 | 6.1 ± 0.6 |
| GEGA/BDMA | 136 | 3.2 ± 0.2 | 31.2 ± 2.3 | 1.1 ± 0.1 |
| DGEBA/IPDA | - | 3.1 ± 0.8 | 34.1 ± 2.0 | 1.7 ± 0.2 |
| DGEBA/DPG | - | 3.0 ± 0.2 | 116.4 ± 7.0 | 8.6 ± 0.3 |
| Sample | Epoxy/–OH | Tg (°C) [TMA] | Tensile Strength (MPa) |
|---|---|---|---|
| GPE-TA | 1.0 | 87.3 | 36.7 |
| SPE-TA | 1.0 | 106.6 | 60.6 |
| Sample | Tensile Strength (MPa) | Compressive Strength (MPa) | Elongation at Break (%) | Flexural Strength (MPa) | Impact Strength (Izod, J/m) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|---|
| Commercial DGEBA | 48.0 | 108 | 3.1 | 91.45 | 28.5 | 2420 |
| BPA/cardanol epoxy (80:20) a | 31.7 | 92.55 | 5.68 | 80.8 | 22.25 | 2045 |
| BPA/cardanol epoxy (50:50) | 23.5 | 78 | 8.42 | 71.45 | 20.4 | 1926 |
| Sample | Stress at Break (MPa) | Young’s Modulus (MPa) |
|---|---|---|
| ENR/DTDB | 12 ± 2 | 1.67 ± 0.2 |
| ENR/DA | 10 ± 1 | - |
| Sample (% by Weight) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Flexural Strength (MPa) | Flexural Modulus (GPa) |
|---|---|---|---|---|
| 100%DGEBA-DDM | 214 ± 4 | 17.5 ± 0.4 | 266 ± 5 | 13 ± 0.3 |
| 25%DHL-Epoxy-75%DGEBA-DDM a | 187 ± 5 | 23.2 ± 0.7 | 258 ± 4 | 13.2 ± 0.2 |
| 50%DHL-Epoxy-50%DGEBA-DDM | 187 ± 6 | 18.5 ± 0.6 | 214 ± 4 | 13 ± 0.3 |
| 75%DHL-Epoxy-25%DGEBA-DDM | 182 ± 3 | 23.1 ± 0.4 | 149 ± 3 | 10.6 ± 0.2 |
| 100%DHL-Epoxy-DDM | 138 ± 4 | 12.3 ± 0.3 | 47 ± 2 | 5 ± 0.1 |
| Sample | Tg (°C) |
|---|---|
| DGEBA/IPDA | 166 |
| Diglycidyl ether of vanillyl alcohol/IPDA | 97 |
| Diglycidyl ether of methoxyhydroquinone | 132 |
| Diglycidyl ether of vanillic acid | 152 |
| Sample | Flexural Modulus (MPa) | Flexural Strength (MPa) | Impact Strength (kJ/m2) | Strain at Break (%) |
|---|---|---|---|---|
| rosin-based | 2200 ± 30 | 70 ± 1 | 2.1 ± 0.2 | 1.9 ± 0.3 |
| DGEBA | 3000 ± 200 | 80 ± 3 [24] | 3.2 [25] | 2.6 [24] |
| Sample | Tg (°C) | Tensile Strength (MPa) | Tensile Modulus (GPa) | Breaking Elongation (%) |
|---|---|---|---|---|
| E-44 | 140 | 56.25 | 0.29 | 12.35 |
| FPAE1C | 167 | 48.54 | 0.471 | 13.37 |
| FPEG1C | 81 | 68.75 | 0.495 | 17.35 |
| FPEG2C | 79 | 58.18 | 0.300 | 20.54 |
| FPEG3C | 75 | 42.41 | 0.270 | 13.67 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramon, E.; Sguazzo, C.; Moreira, P.M.G.P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. https://doi.org/10.3390/aerospace5040110
Ramon E, Sguazzo C, Moreira PMGP. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace. 2018; 5(4):110. https://doi.org/10.3390/aerospace5040110
Chicago/Turabian StyleRamon, Eric, Carmen Sguazzo, and Pedro M. G. P. Moreira. 2018. "A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector" Aerospace 5, no. 4: 110. https://doi.org/10.3390/aerospace5040110
APA StyleRamon, E., Sguazzo, C., & Moreira, P. M. G. P. (2018). A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace, 5(4), 110. https://doi.org/10.3390/aerospace5040110





