Experimental Investigation of Hydrogen Peroxide and Nitrous Oxide in a 1-Newton Catalyst-Based Monopropellant Research Thruster †
Abstract
1. Introduction
2. Hydrogen Peroxide as Monopropellant
3. Nitrous Oxide as Monopropellant
Further, it was found by Pachatouridou et al. that Ir/Al2O3 performs better than Pt/Al2O3 and Pd/Al2O3 [45].Rh/Al2O3 > Pd/Al2O3 > Pt/Al2O3
4. Design of the Monopropellant Thruster “MoCa”
5. Experimental Test Setup
5.1. Facility and P&ID
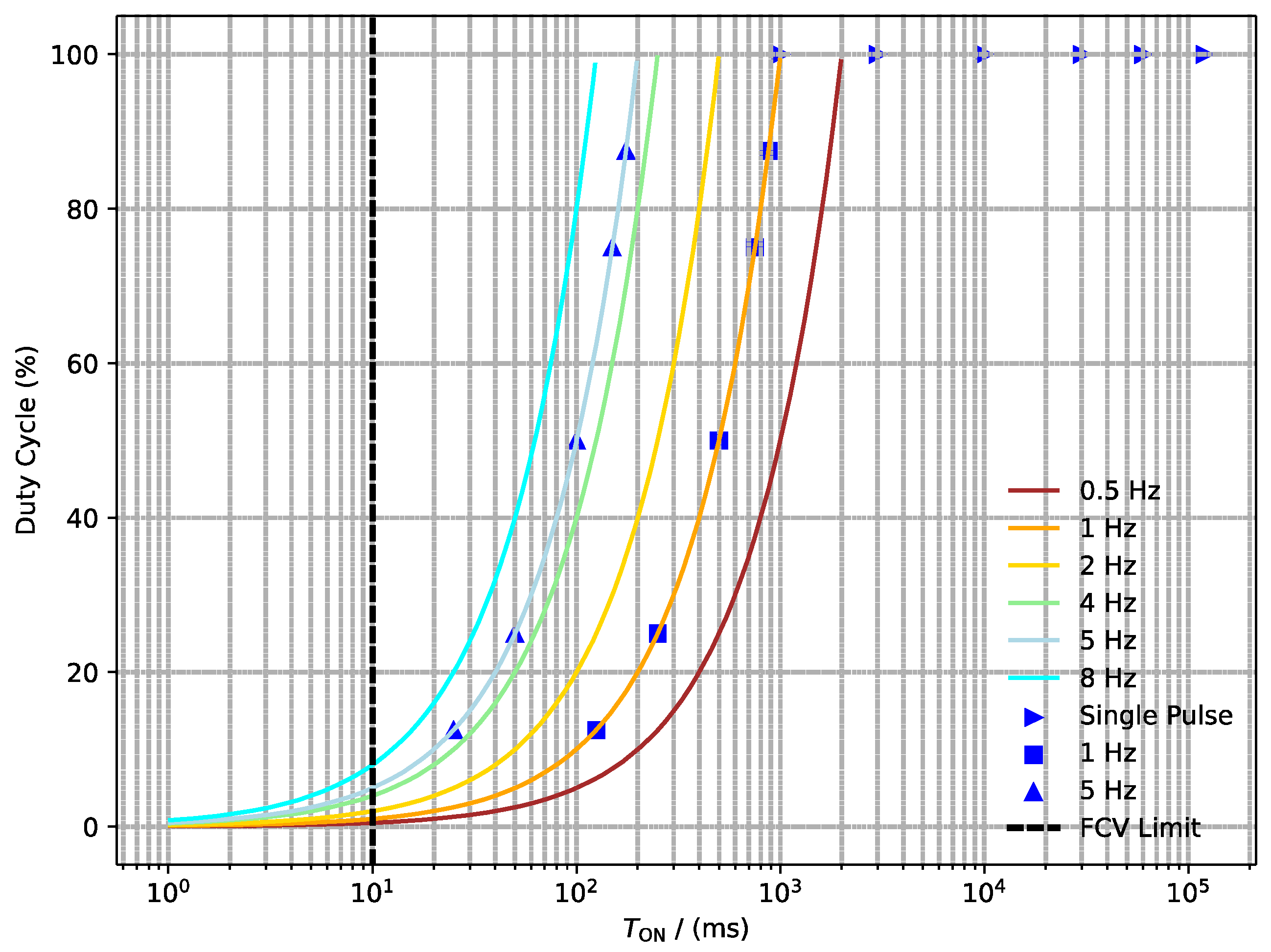
5.2. Design of Experiments
5.3. Estimation of Errors
6. Results and Discussion
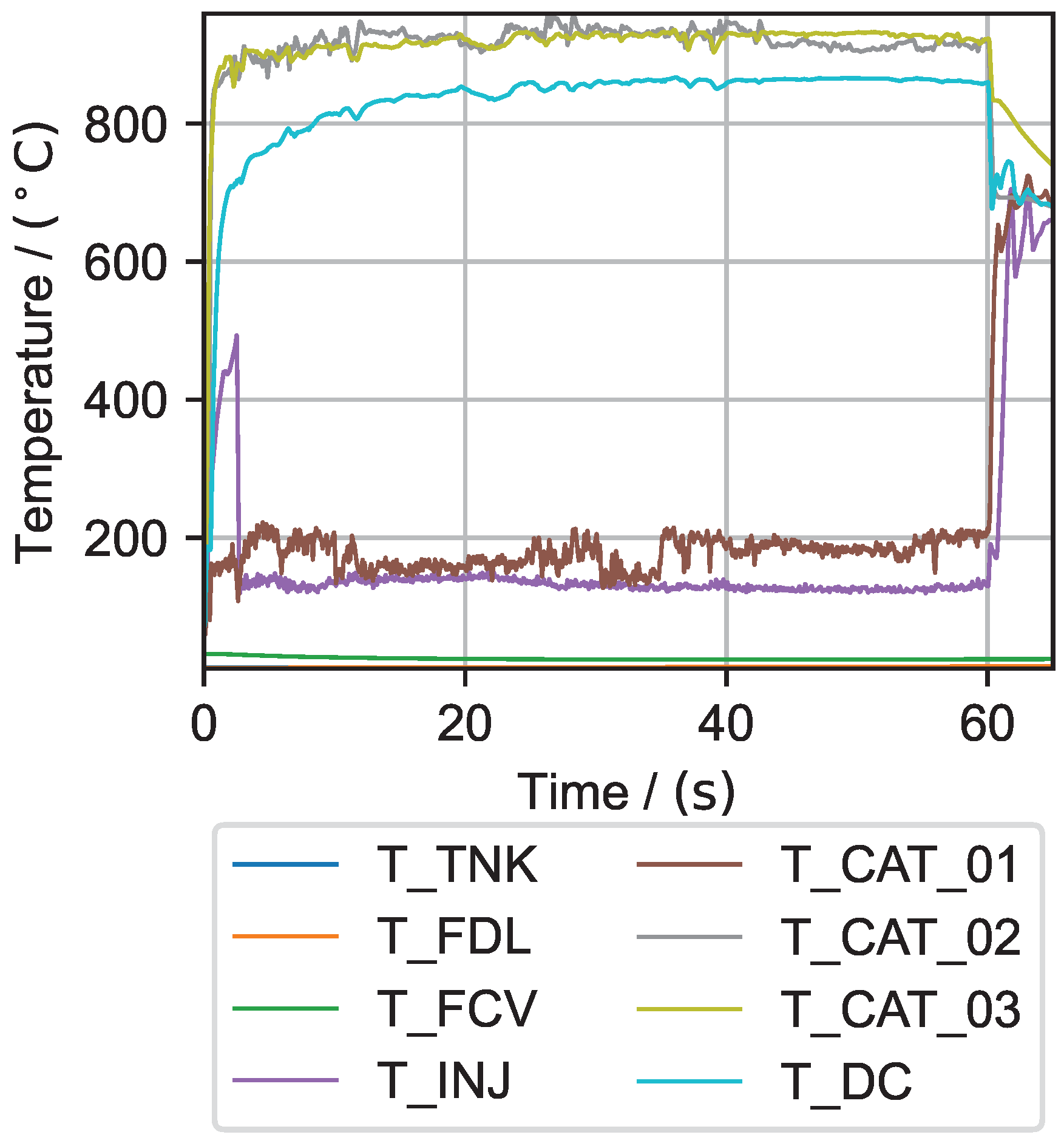
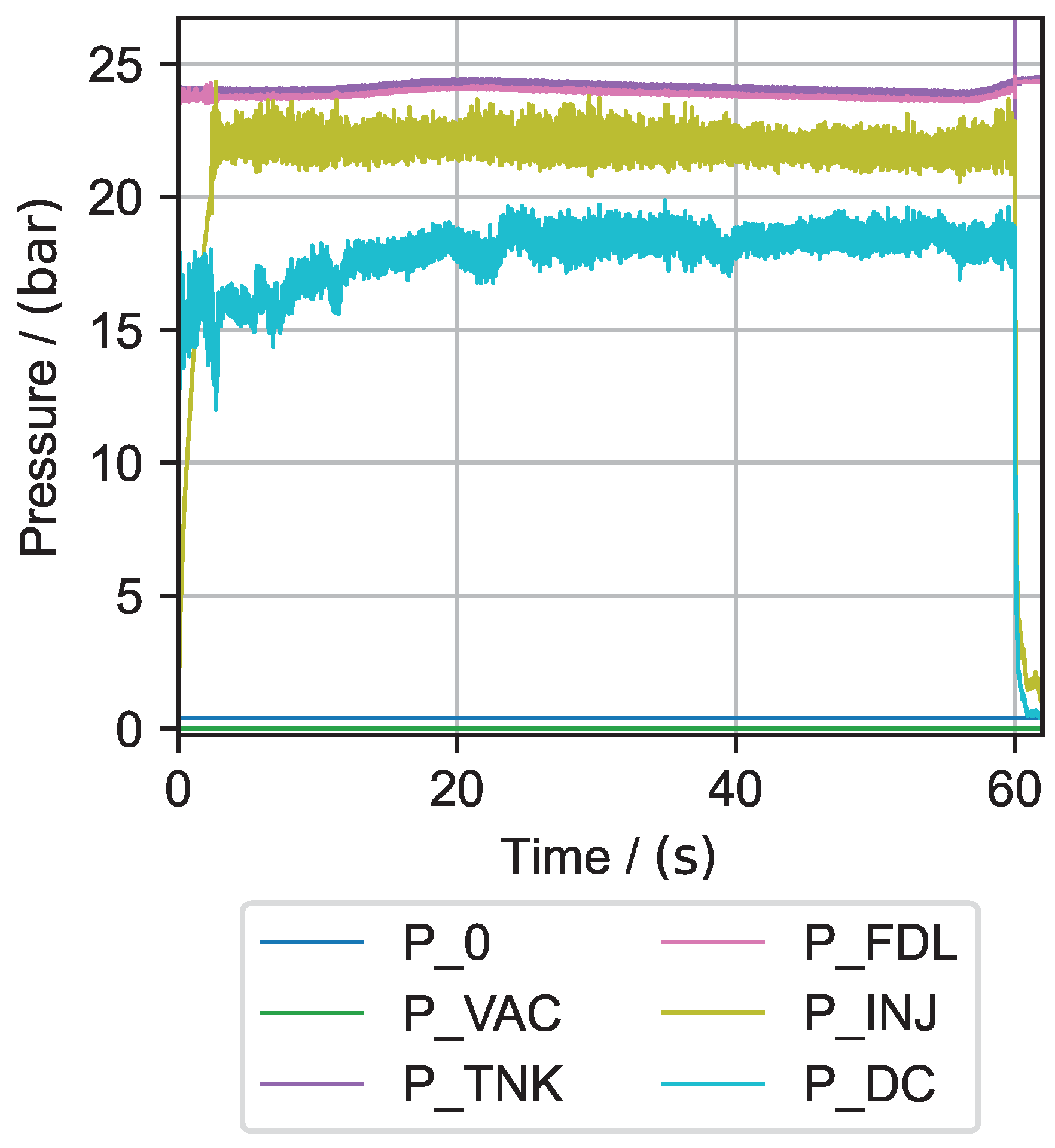
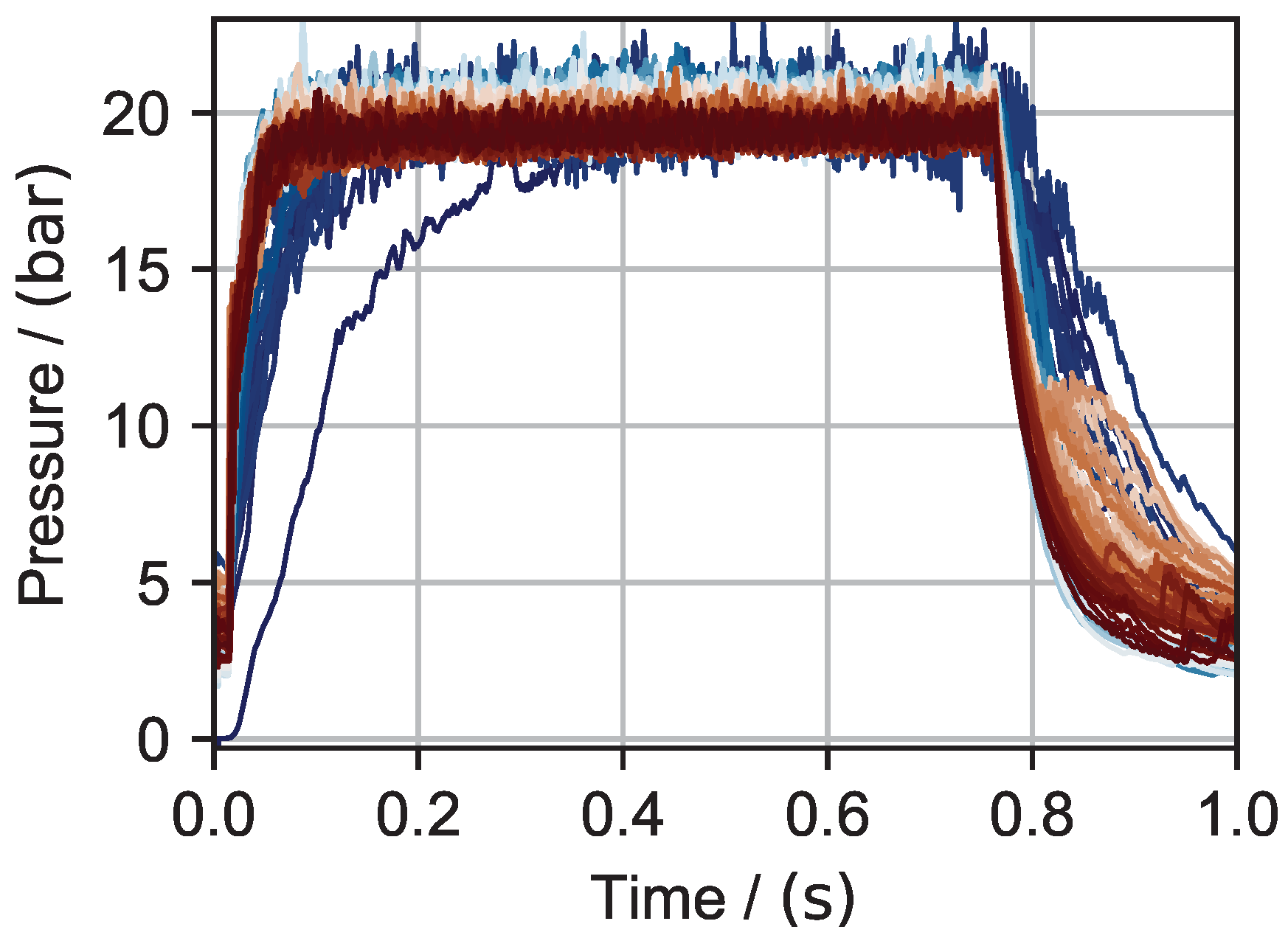
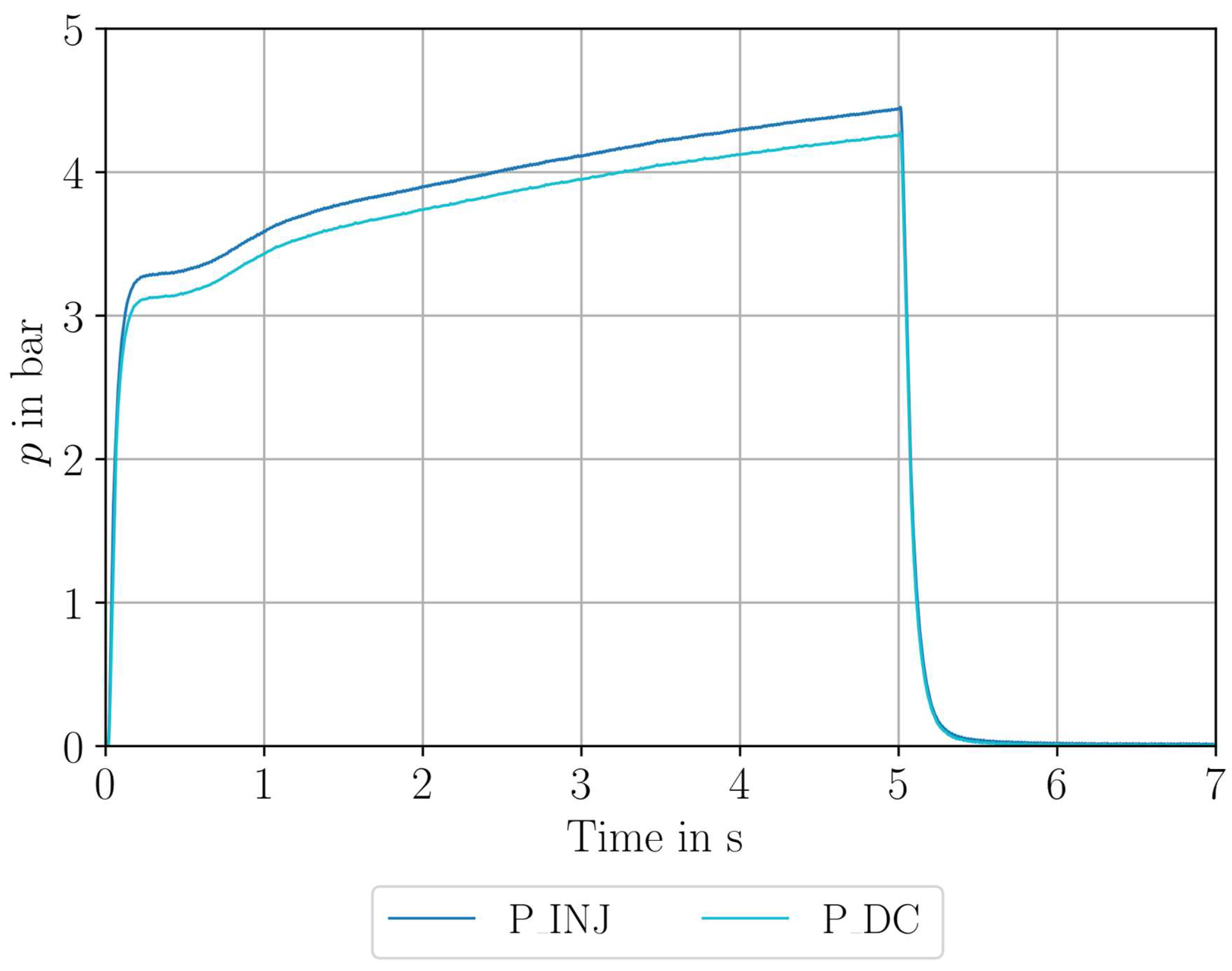
6.1. Hydrogen Peroxide Testing
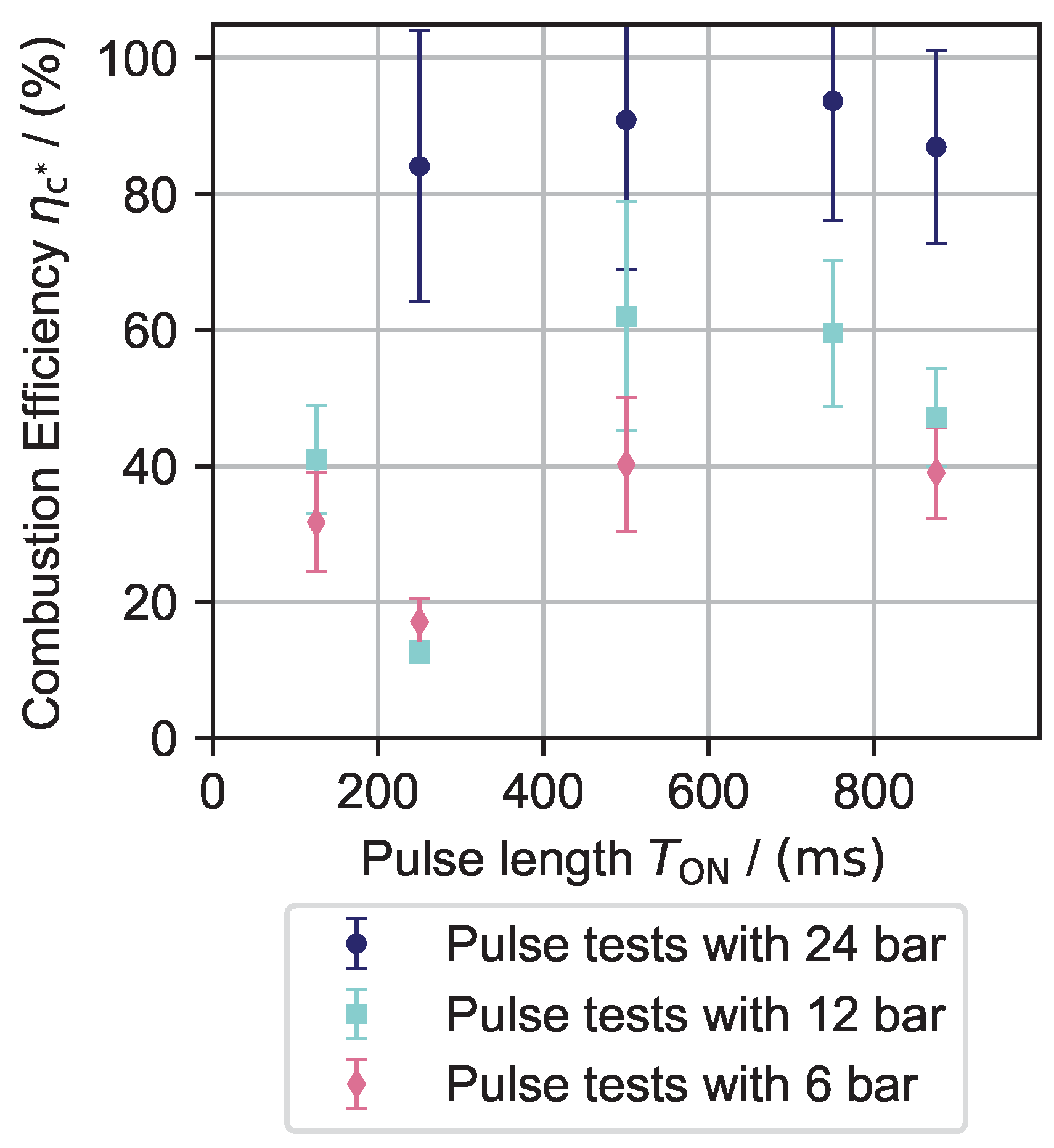
6.2. Nitrous Oxide Testing
- Higher pre-heating temperatures are needed to achieve decomposition (room temperature for H2O2 and >500 °C for N2O).
- Only efficiencies below 90% can be observed for N2O experiments; for H2O2, the observed efficiencies were mostly above 90%.
- The increase in efficiency during operation is much slower than with H2O2. This may be a result of the decreased reaction rate.
- Through the higher adiabatic decomposition temperature of N2O in comparison with H2O2, other thrust chamber materials should be considered.
- The storage of N2O is relatively uncomplicated. A wide range of materials is available, and, unlike H2O2, there is no need to worry about the decomposition of the propellant inside the tank.
- Catalyst bed loading should be optimized for the given thruster and the specific catalyst/propellant combination to achieve better performance.
- The position of the hottest point inside the catalyst bed is not stable and can be nonlinearly influenced, for example, by the mass flow, chamber pressure, pre-heating temperature, or the age of the catalyst.
- Pre-heating to temperatures above 500 °C is challenging in the given setup. The temperature must be equally distributed in the catalyst bed, and the overheating of the cartridge heater must be avoided. Moreover, an effective thermal standoff is needed to avoid temperature loss through heat conduction into the feedline system on the one hand, and to prevent the pulse valve from too-high temperatures on the other hand. Heating to such high temperatures required long pre-heating times; in this setup, it was over 30 min.
- A cold start is not possible or will only deliver cold gas performance.
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarritzu, A.; Pasini, A. Performance comparison of green propulsion systems for future Orbital Transfer Vehicles. Acta Astronaut. 2024, 217, 100–115. [Google Scholar] [CrossRef]
- Marshall, W.M.; Deans, M.C. Recommended figures of merit for green monopropellants. In Proceedings of the 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, San Jose, CA, USA, 15–17 July 2013; p. 3722. [Google Scholar]
- Hörger, T.; Krishti, D.; Koopmanns, R.J.; Lauck, F.; Merz, F.; Werling, L.; Kirchberger, C.; Steelant, J. Green Propellants for Satellite Propulsion: An Updated Literature Survey in the Frame of the ESA Project GreenRAIM. In Proceedings of the 13th ISICP-International Symposium on Special Topics in Chemical Propulsion, Gjovik, Norway, 30 May–2 June 2023. [Google Scholar]
- Nosseir, A.E.S.; Cervone, A.; Pasini, A. Review of state-of-the-art green monopropellants: For propulsion systems analysts and designers. Aerospace 2021, 8, 20. [Google Scholar] [CrossRef]
- Kirchberger, C.U.; Hörger, T.; Scholl, J.; Merz, F.; Teuffel, P.; Kurilov, M.; Lauck, F.; Werling, L.; Scholl, R.; Ricker, S.; et al. Green Propellants for Satellite Propulsion: Status of Research and Developments at DLR Lampoldshausen. In Proceedings of the AIAA SCITECH 2024 Forum, Orlando, FL, USA, 8–12 January 2024; p. 1789. [Google Scholar]
- Werling, L.; Freudenmann, D.; Ricker, S.C.; Wilhelm, M.; Lauck, F.; Strauss, F.; Manassis, K.; Kurilov, M.; Petrarolo, A.; Hörger, T.; et al. Research and Test Activities on Advanced Rocket Propellants at DLR’s Institute of Space Propulsion in Lampoldshausen. In Proceedings of the 9th European Conference for Aeronautics and Space Sciences (EUCASS), Lille, France, 27 June–1 July 2022. [Google Scholar]
- Lauck, F.; Negri, M.; Wilhelm, M.; Freudenmann, D.; Schlechtriem, S.; Wurdak, M.; Gotzig, U. Test bench preparation and hot firing tests of a 1N hydrogen peroxide monpropellant thruster. In Proceedings of the Space Propulsion Conference, Seville, Spain, 14–18 May 2018; Volume 14. [Google Scholar]
- Gotzig, U.; Wurdak, M.; Lauck, F. Development of a Flight Type 1N Hydrogen Peroxide Thruster. In Proceedings of the Space Propulsion Conference, Seville, Spain, 14–18 May 2018; pp. 14–18. [Google Scholar]
- Stollenwerk, M.; Schäfer, T.; Stadtmüller, J.; Döhring, T.; Freudenmann, D.; Röcke, N. Sputtered highly effective iridium catalysts: A new approach for green satellite propulsion. J. Mater. Sci. 2021, 56, 9974–9984. [Google Scholar] [CrossRef]
- Negri, M.; Lauck, F. Hot firing tests of a novel green hypergolic propellant in a thruster. J. Propuls. Power 2022, 38, 467–477. [Google Scholar] [CrossRef]
- Sarritzu, A.; Pasini, A.; Merz, F.; Werling, L.; Lauck, F. Experimental investigation of combustion performance of a green hypergolic bipropellant based on hydrogen peroxide. Acta Astronaut. 2024, 219, 278–290. [Google Scholar] [CrossRef]
- Ricker, S.; Lauck, F.; Teuffel, P.; Merz, F.; Freudenmann, D.; Kirchberger, C. HIM_30: Hot-Firing Tests and Characterisation of a Green Hypergolic Propellant Based on Ionic Liquids and Hydrogen Peroxide. In Proceedings of the Space Propulsion Conference Glasgow, Glasgow, UK, 20–23 May 2024. [Google Scholar]
- GESTIS-Stoffdatenbank Entry Distickstofftetroxid/Dinitrogen Tetraoxide. 2022. Available online: https://gestis.dguv.de/data?name=001950 (accessed on 9 September 2025).
- Kobald, M.; Fischer, U.; Tomilin, K.; Petrarolo, A.; Schmierer, C. Hybrid Experimental Rocket Stuttgart: A Low-Cost Technology Demonstrator. J. Spacecr. Rocket. 2018, 55, 484–500. [Google Scholar] [CrossRef]
- Werling, L.; Lauck, F.; Dobusch, J.; Gritzka, M.A.; Stratmann, V.; Merz, F.; Hörger, T.; Teuffel, P.J.; Braune, L. From Lampoldshausen to Space: DLR Spin-off InSpacePropulsion Technologies and the Development Status of Green Propellant Thrusters Based on H2O2 and N2O. In Proceedings of the Aerospace Europe Conference 2023–10th EUCASS–9th CEAS, Lausanne, Switzerland, 9–13 July 2023. [Google Scholar]
- Hennemann, L.; Andrade, J.C.; Costa, F.d.S. Experimental investigation of a monopropellant thruster using nitrous oxide. J. Aerosp. Technol. Manag. 2014, 6, 363–372. [Google Scholar] [CrossRef]
- Zakirov, V.; Sweeting, M.; Lawrence, T.; Sellers, J. Nitrous oxide as a rocket propellant. Acta Astronaut. 2001, 48, 353–362. [Google Scholar] [CrossRef]
- Scherson, Y.; Lohner, K.; Lariviere, B.; Cantwell, B.; Kenny, T. A monopropellant gas generator based on N2O decomposition for “green” propulsion and power generation. In Proceedings of the 45th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Denver, CO, USA, 2–5 August 2009; p. 4875. [Google Scholar]
- Haag, G.; Sweeting, M.; Richardson, G. Low cost propulsion development for small satellites at the surrey space centre. In Proceedings of the 13th AIAA/USU Conference on Small Satellites, Logan, UT, USA, 23–26 August 1999. [Google Scholar]
- Werling, L.; Hörger, T.; Manassis, K.; Grimmeisen, D.; Wilhelm, M.; Erdmann, C.; Ciezki, H.; Schlechtriem, S.; Richter, S.; Methling, T. Nitrous Oxide Fuels Blends: Research on premixed Monopropellants at the German Aerospace Center (DLR) since 2014. In Proceedings of the AIAA Propulsion and Energy Forum, New Orleans, LA, USA, 24–26 August 2020. [Google Scholar]
- Werling, L.; Hörger, T. Experimental analysis of the heat fluxes during combustion of a N2O/C2H4 premixed green propellant in a research rocket combustor. Acta Astronaut. 2021, 189, 437–451. [Google Scholar] [CrossRef]
- Werling, L.; Lauck, F.; Negri, M.; Goos, E.; Wischek, J.; Besel, Y.; Valencia-Bel, F. High Performance Propellant Development-Overview of Development Activities Regarding Premixed, Green N2O/C2H6 Monopropellants. In Proceedings of the 8th Space Propulsion Conference, Estoril, Portugal, 9–13 May 2022. [Google Scholar]
- Werling, L. Entwicklung und Erprobung von Flammensperren für Einen Vorgemischten, Grünen Raketentreibstoff aus Lachgas (N2O) und Ethen (C2H4). Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2020. [Google Scholar]
- Musker, A.; Rusek, J.; Kappenstein, C.; Roberts, G. Hydrogen peroxide-From bridesmaid to bride. In Proceedings of the 3rd ESA International Conference on Green Propellants for Space Propulsion, Poitiers, France, 17–20 September 2006. [Google Scholar]
- Walter, H. Experience with the Application of Hydrogen Peroxide for production of Power. J. Jet Propuls. 1954, 24, 166–171. [Google Scholar] [CrossRef]
- Scharlemann, C.; Winborg, N.; Roberts, G.; Kappenstein, C.; Musker, A.; Russor, A.; Haidn, O.; Walzer, E.; Muszynski, M.; Delbianco, N.; et al. General Assessment of Green Propellants. GRASP Report. 2009. Available online: https://cordis.europa.eu/project/id/218819/reporting (accessed on 9 September 2025).
- Mezyk, L.; Gut, Z.; Mohan, K.; Kindracki, J.; Rarata, G. Initial research on thermal decomposition of 98% concentrated hydrogen peroxide in thruster-like conditions. Eng. Sci. Technol. Int. J. 2022, 31, 101054. [Google Scholar] [CrossRef]
- McBride, B.J. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications; NASA Lewis Research Center: Cleveland, OH, USA, 1996; Volume 2.
- Dolci, S.; Dell’Amico, D.B.; Pasini, A.; Torre, L.; Pace, G.; Valentini, D. Platinum catalysts development for 98% hydrogen peroxide decomposition in pulsed monopropellant thrusters. J. Propuls. Power 2015, 31, 1204–1216. [Google Scholar] [CrossRef]
- Koopmans, R.J.; Shrimpton, J.S.; Roberts, G.T.; Musker, A.J. A one-dimensional multicomponent two-fluid model of a reacting packed bed including mass, momentum and energy interphase transfer. Int. J. Multiph. Flow 2013, 57, 10–28. [Google Scholar] [CrossRef]
- Surmacz, P.; Gut, Z. The Experimental Investigation of a 98% Hydrogen Peroxide Monopropellant Thruster Comprising the Metal-Foam-Supported Manganese Oxide Catalyst. Aerospace 2023, 10, 215. [Google Scholar] [CrossRef]
- Casu, S.; Kiemel, R.; Casu, S.; Geiger, B.; Kiemel, R.D.; Anthoine, J.; Geiger, B.; Anthoine, J.; Lestrade, J.Y.; Lestrade, J.Y. Development and characterization of a catalyst for the decomposition of hydrogen peroxide. In Proceedings of the AIAA Propulsion and Energy 2019 Forum, Indianapolis, IN, USA, 19–22 August 2019. [Google Scholar] [CrossRef]
- Kosdauletov, A.; Jung, E.; Jin, J.; Kwon, S. Catalytic Decomposition of N2O Using Noble Metals to Develop Monopropellant Thruster. In Proceedings of the International Astronautical Congress 2010, Prague, Czech Republic, 27 September–1 October 2010. [Google Scholar]
- McBride, B.; Gordon, S. Chemical Equilibrium and Applications; NASA: Washington, DC, USA, 2004.
- Gotzig, U. Development and Test of a 3D printed Hydrogen Peroxide Flight Control Thruster. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015; American Institute of Aeronautics and Astronautics: Orlando, FL, USA, 2015. [Google Scholar]
- Thompson, R.L.; Lassaletta, L.; Patra, P.K.; Wilson, C.; Wells, K.C.; Gressent, A.; Koffi, E.N.; Chipperfield, M.P.; Winiwarter, W.; Davidson, E.A.; et al. Acceleration of global N2O emissions seen from two decades of atmospheric inversion. Nat. Clim. Chang. 2019, 9, 993–998. [Google Scholar] [CrossRef]
- Karabeyoglu, A.; Dyer, J.; Stevens, J.; Cantwell, B. Modeling of N2O decomposition events. In Proceedings of the 44th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Hartford, CT, USA, 21–23 July 2008; p. 4933. [Google Scholar]
- Zakirov, V.; Zhang, H.y. A model for the operation of nitrous oxide monopropellant. Aerosp. Sci. Technol. 2008, 12, 318–323. [Google Scholar] [CrossRef]
- Pasini, A.; Pace, G.; Torre, L. Propulsive performance of a 1 N 98% hydrogen peroxide thruster. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015; American Institute of Aeronautics and Astronautics: Orlando, FL, USA, 2015; p. 4059. [Google Scholar]
- Miao, M.; Zhang, M.; Kong, H.; Zhou, T.; Yang, X.; Yang, H. Progress in catalytic decomposition and removal of N2O in fluidized bed. Energies 2021, 14, 6148. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Z.; Huang, L.; Fan, H.; Hou, Q.; Cui, P.; Wang, W. Advances in Catalytic Decomposition of N2O by Noble Metal Catalysts. Catalysts 2023, 13, 943. [Google Scholar] [CrossRef]
- Kondratenko, V.A.; Baerns, M. Effect of different oxygen species originating from the dissociation of O2, N2O and NO on the selectivity of the Pt-catalysed NH3 oxidation. Catal. Today 2007, 121, 210–216. [Google Scholar] [CrossRef]
- Hinshelwood, C.N.; Prichard, C.R. LI.—A comparison between the homogeneous thermal decomposition of nitrous oxide and its heterogeneous catalytic decomposition on the surface of platinum. J. Chem. Soc. Trans. 1925, 127, 327–336. [Google Scholar] [CrossRef]
- Doi, K.; Wu, Y.Y.; Takeda, R.; Matsunami, A.; Arai, N.; Tagawa, T.; Goto, S. Catalytic decomposition of N2O in medical operating rooms over Rh/Al2O3, Pd/Al2O3, and Pt/Al2O3. Appl. Catal. B Environ. 2001, 35, 43–51. [Google Scholar] [CrossRef]
- Pachatouridou, E.; Papista, E.; Iliopoulou, E.F.; Delimitis, A.; Goula, G.; Yentekakis, I.V.; Marnellos, G.E.; Konsolakis, M. Nitrous oxide decomposition over Al2O3 supported noble metals (Pt, Pd, Ir): Effect of metal loading and feed composition. J. Environ. Chem. Eng. 2015, 3, 815–821. [Google Scholar] [CrossRef]
- Hörger, T.; Merz, F.; Steelant, J.; Werling, L.; Kirchberger, C. Results of Esa-Greenraim Test Activities Part 2: Experimental Investigation of a 1 Newton Nitrous Oxide Monopropellant Research Thruster. In Proceedings of the Space Propulsion 2024, Glasgow, UK, 20–23 May 2024. [Google Scholar]
- Merz, F.; Hörger, T.; Steelant, J.; Lauck, F.; Kirchberger, C. Results of Esa-Greenraim Test Activities Part 1: Experimental Investigation of a 1 Newton Hydrogen Peroxide Monopropellant Research Thruster. In Proceedings of the Space Propulsion 2024, Glasgow, UK, 20–23 May 2024. [Google Scholar]
- Fonda-Marsland, E.A.P.; Ryan, C.; Roberts, G.; Lear, A.; Fletcher, E.; Gibbon, D.; Palmer, M. Towards flight qualification of a 1 Newton hydrogen peroxide thruster. In Proceedings of the Space Propulsion 2018, Seville, Spain, 14–18 May 2018. [Google Scholar]
- Pasini, A.; Pace, G.; Valentini, D. Experimental Campaign on a 98% H2O2 Pulsed Thruster. In Proceedings of the ESA Space Propulsion 2018 Conference, Bilbao, Spain, 29–31 October 2018; European Space Agency: Paris, France, 2018; pp. 1–13. [Google Scholar]
- Amri, R.; Gibbon, D.; Rezoug, T. The design, development and test of one newton hydrogen peroxide monopropellant thruster. Aerosp. Sci. Technol. 2013, 25, 266–272. [Google Scholar] [CrossRef]
- Barley, S.; Palmer, P.; Coxhill, I. Evaluating the miniaturisation of a monopropellant thruster. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9–12 July 2006; p. 4549. [Google Scholar]
- Surmacz, P.; Kostecki, M.; Gut, Z.; Olszyna, A. Aluminum Oxide–Supported Manganese Oxide Catalyst for a 98% Hydrogen Peroxide Thruster. J. Propuls. Power 2019, 35, 614–623. [Google Scholar] [CrossRef]
- Sutton, G.P.; Biblarz, O. Rocket Propulsion Elements; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Wilhelm, M.; Werling, L.; Strauss, F.; Lauck, F.; Kirchberger, C.; Ciezki, H.; Schlechtriem, S. Test complex M11: Research on future orbital propulsion systems and scramjet engines. In Proceedings of the 70th International Astronautical Congress, IAC 2019, Washington, DC, USA, 21–25 October 2019. [Google Scholar]
- Deutsches Zentrum für Luft-und Raumfahrt e.V. (DLR), Institute of Space Propulsion, M11.2 Test Bench (2019). Available online: https://www.dlr.de/en/images/institutes-1/institute-of-space-propulsion/m11-2-test-bench (accessed on 9 September 2025).
- Lohner, K.; Dyer, J.; Doran, E.; Dunn, Z.; Krieger, B.; Decker, V.; Wooley, E.; Sadhwani, A.; Cantwell, B.; Kenny, T. Design and development of a sub-scale nitrous oxide monopropellant gas generator. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007; p. 5463. [Google Scholar]
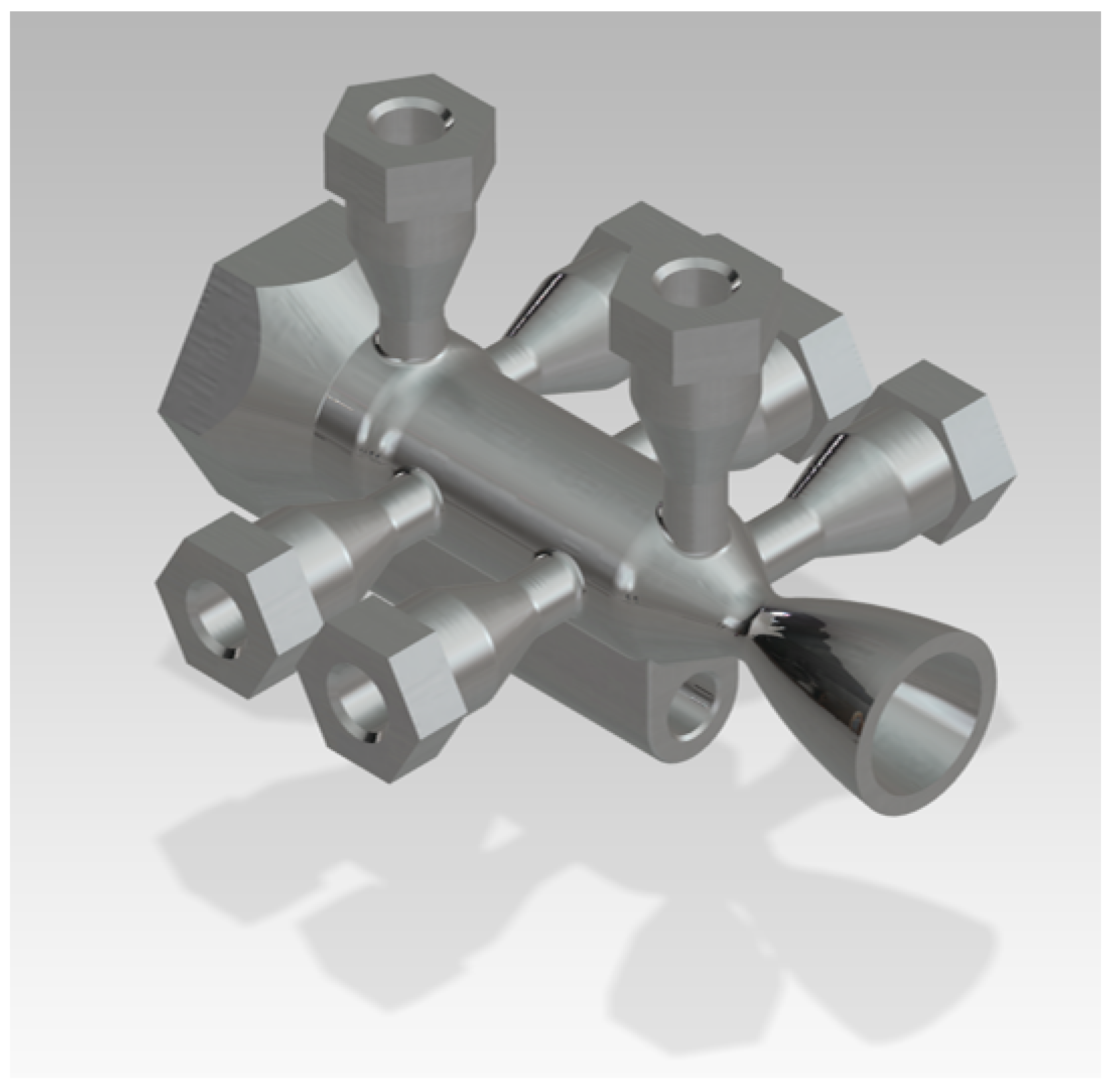
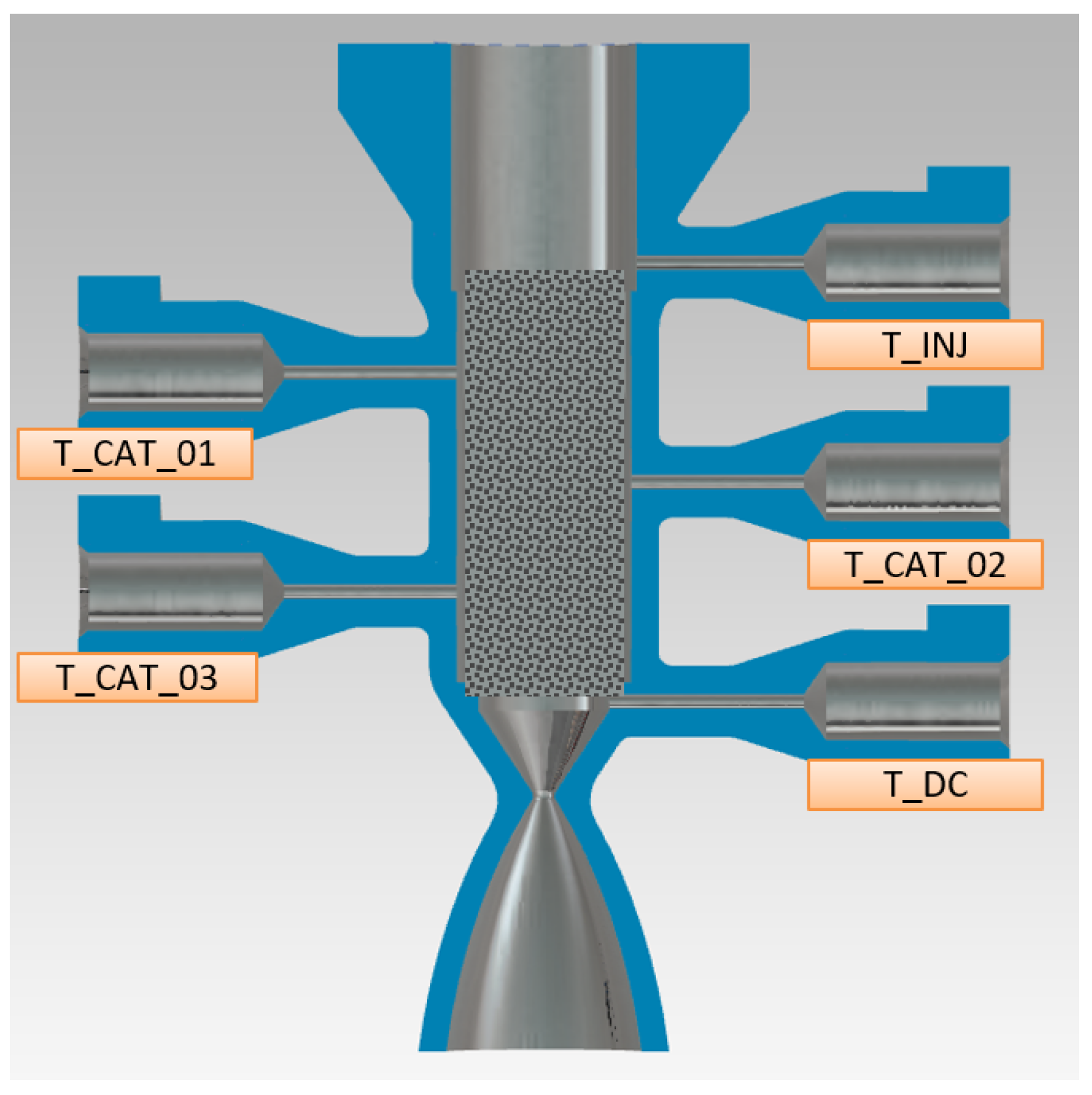
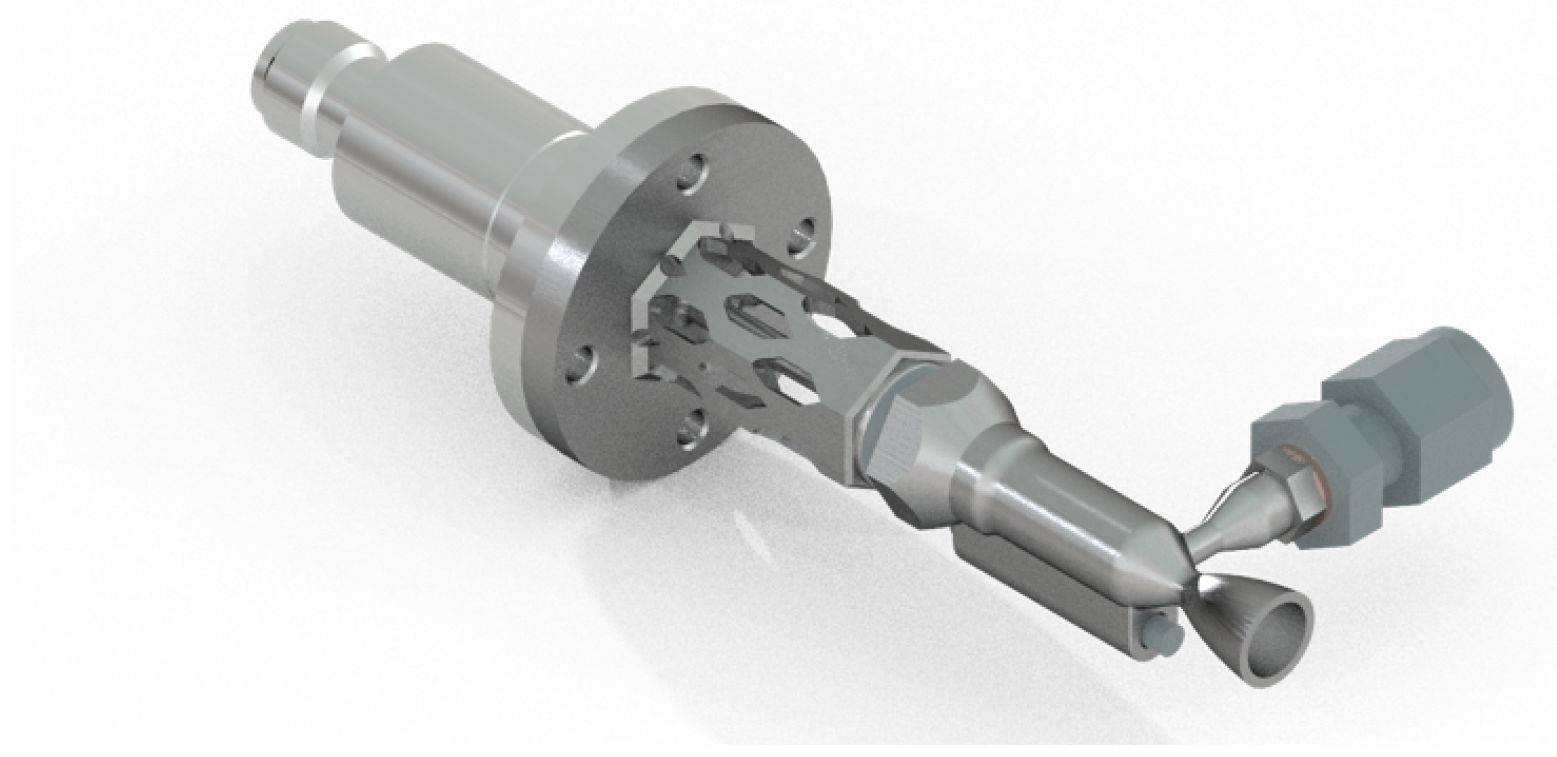
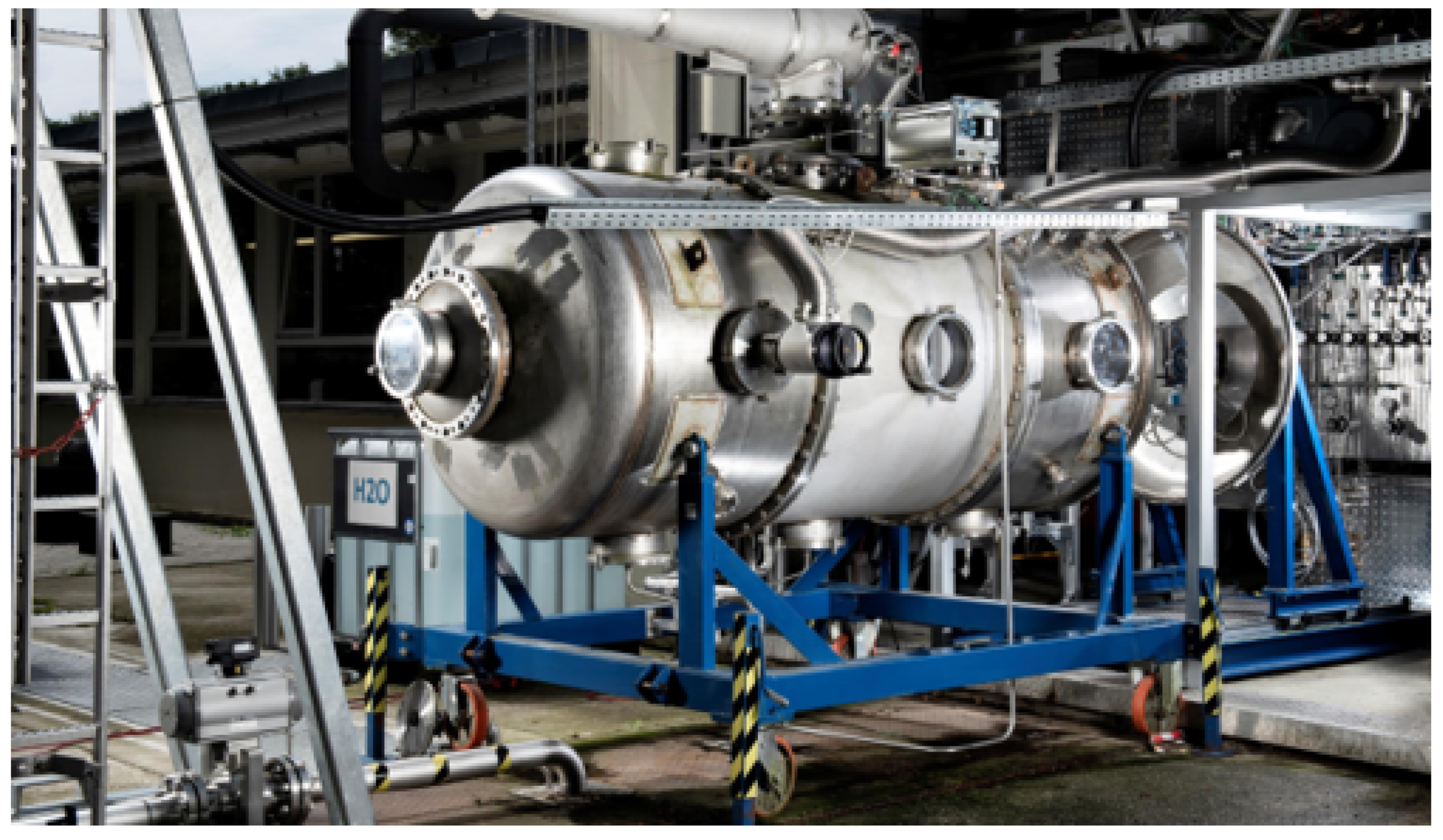
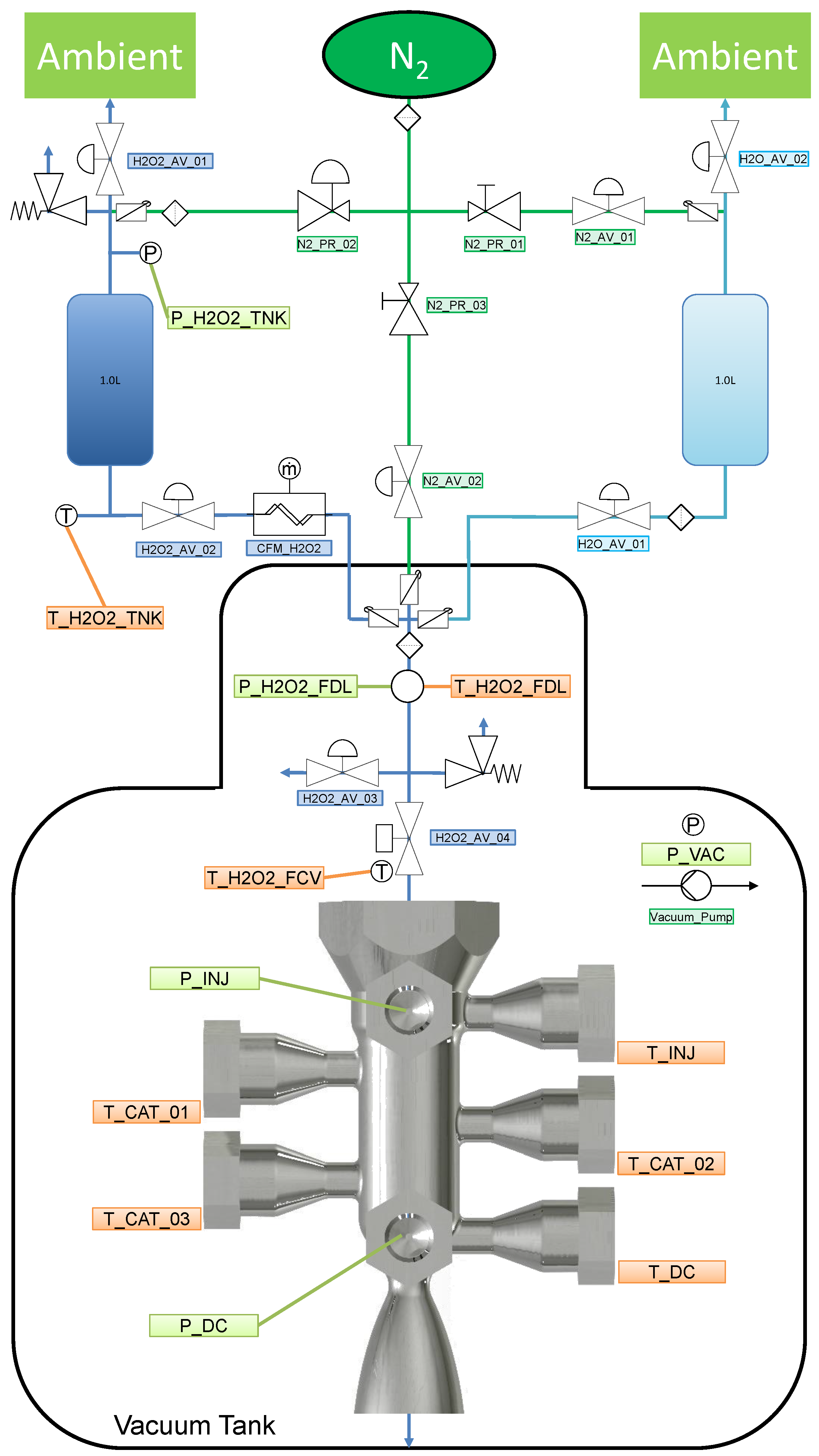


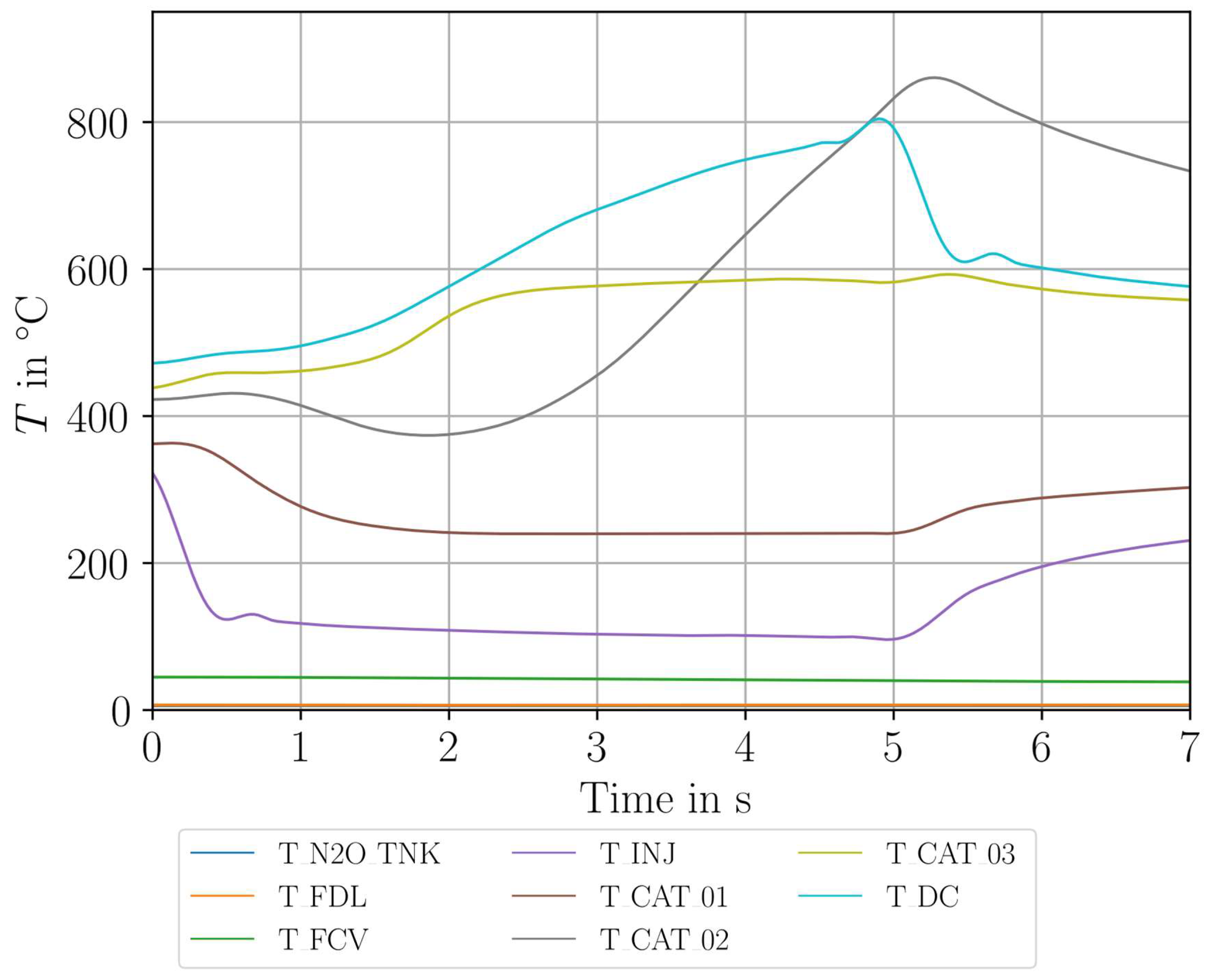
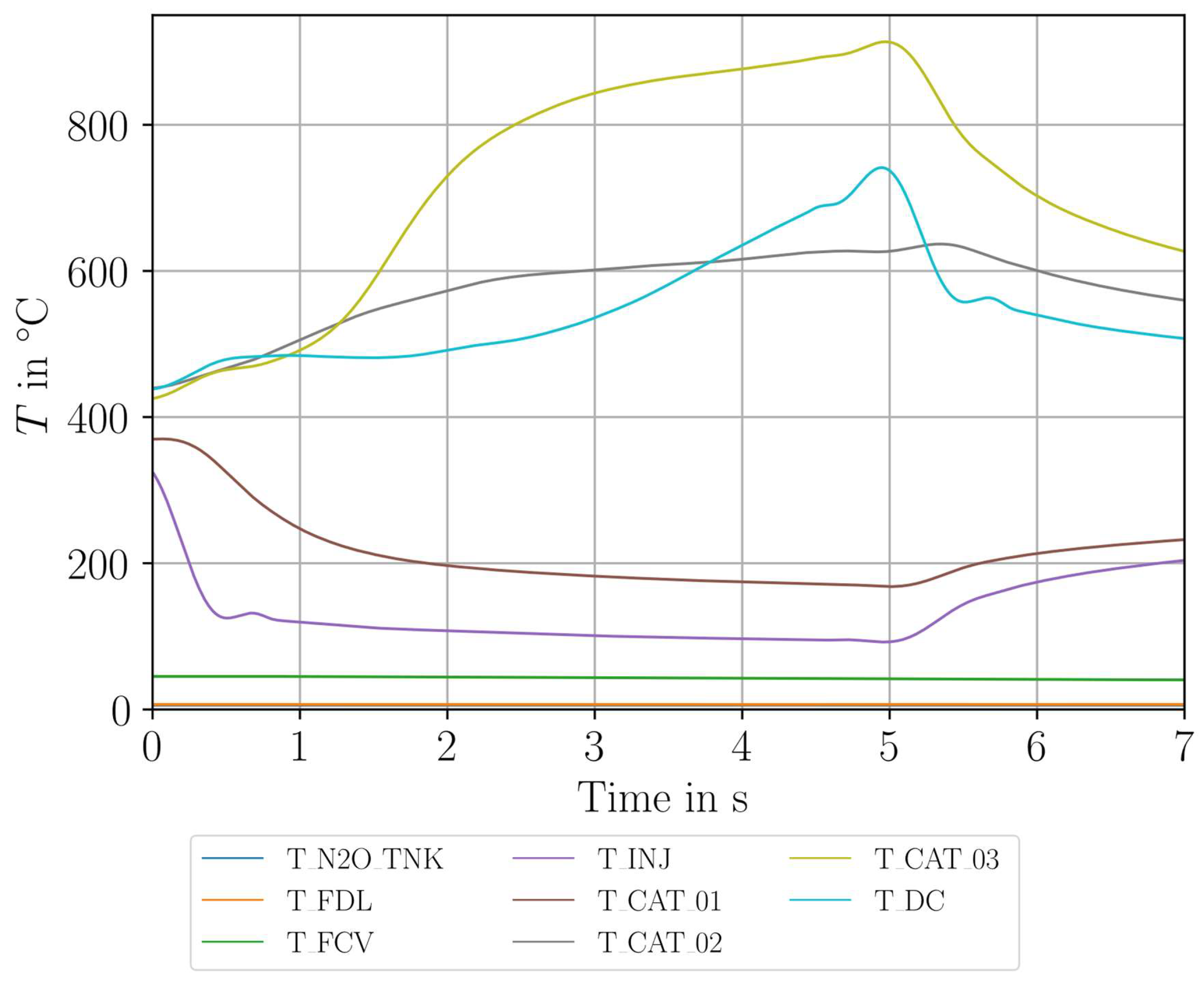
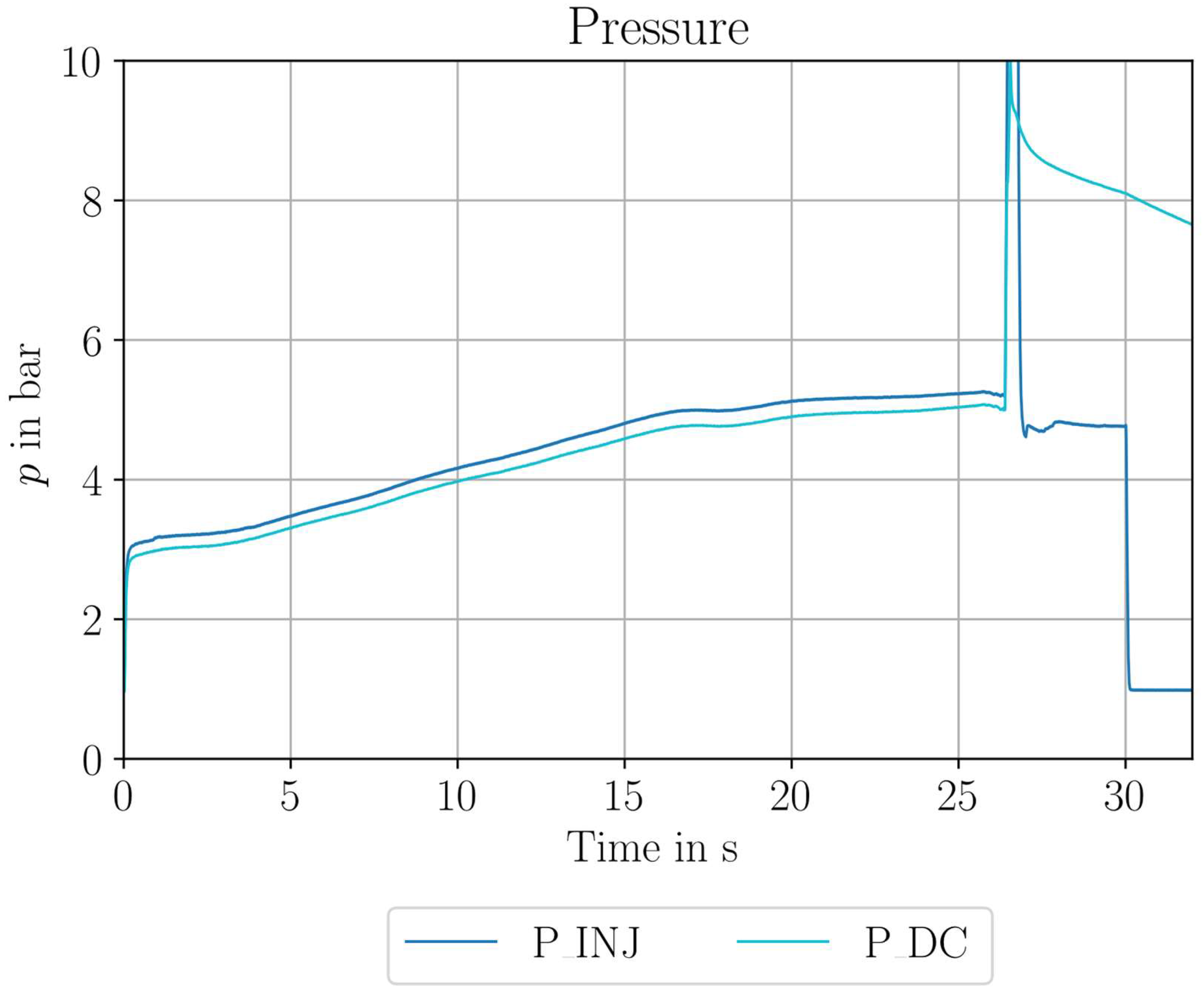
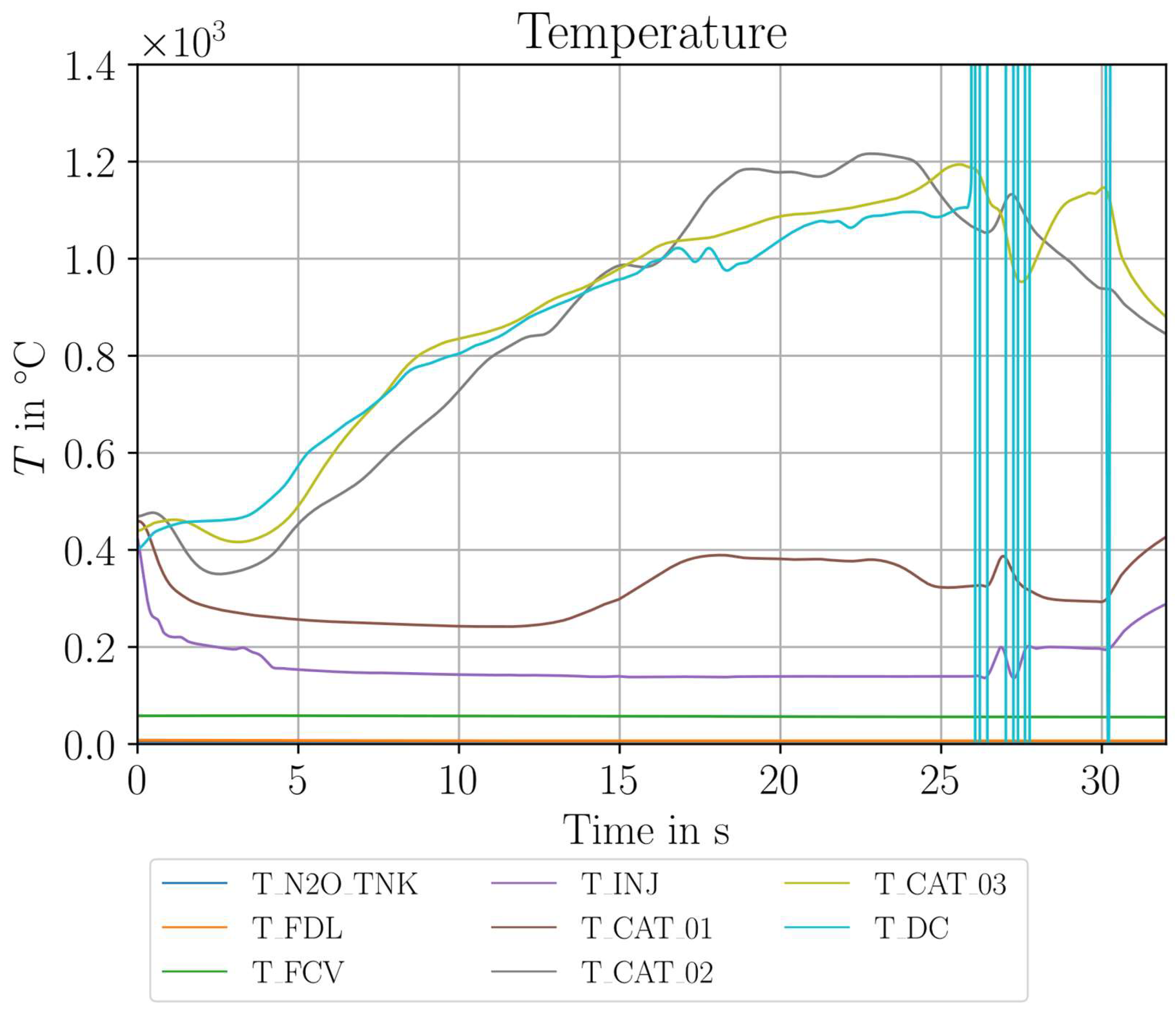
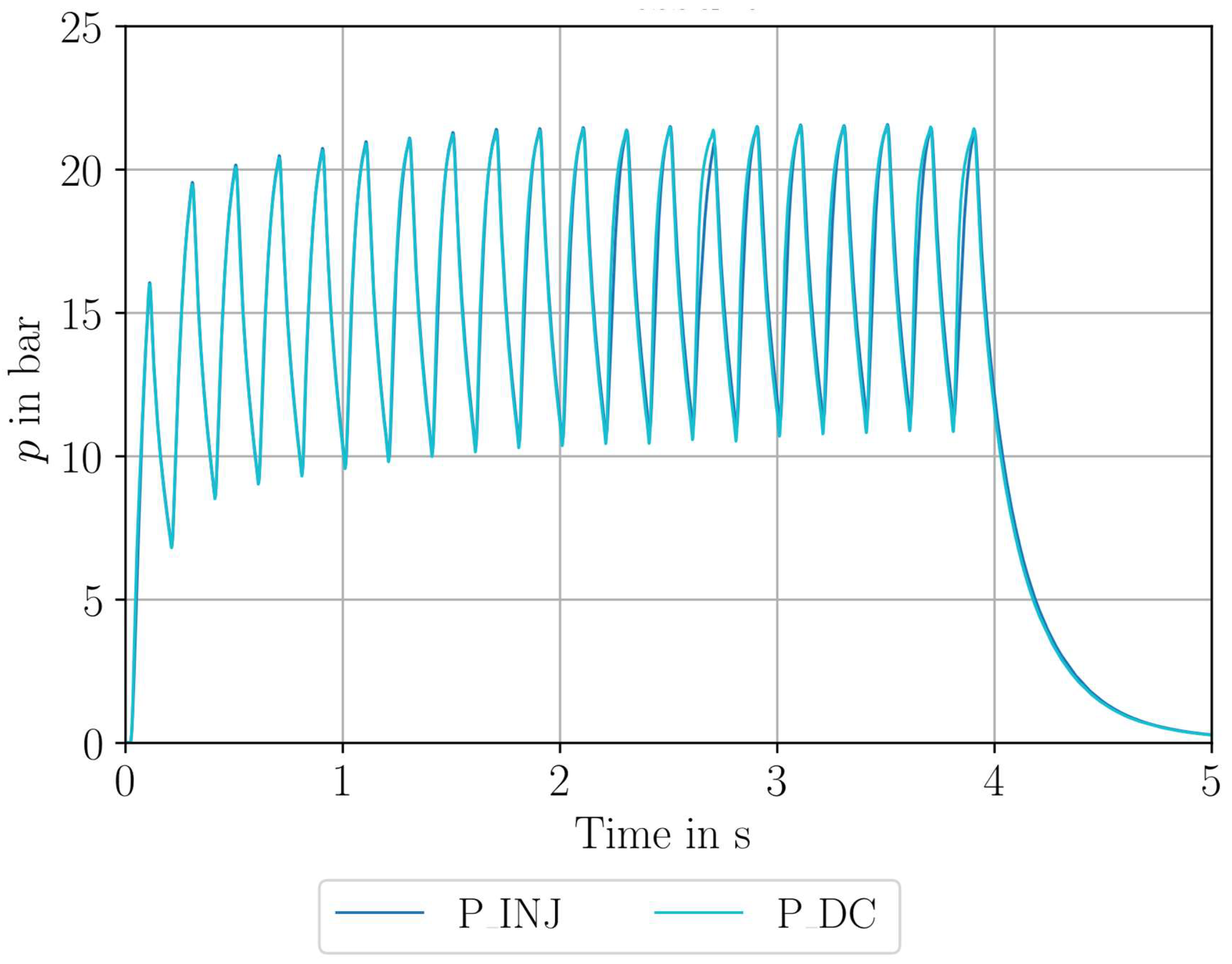
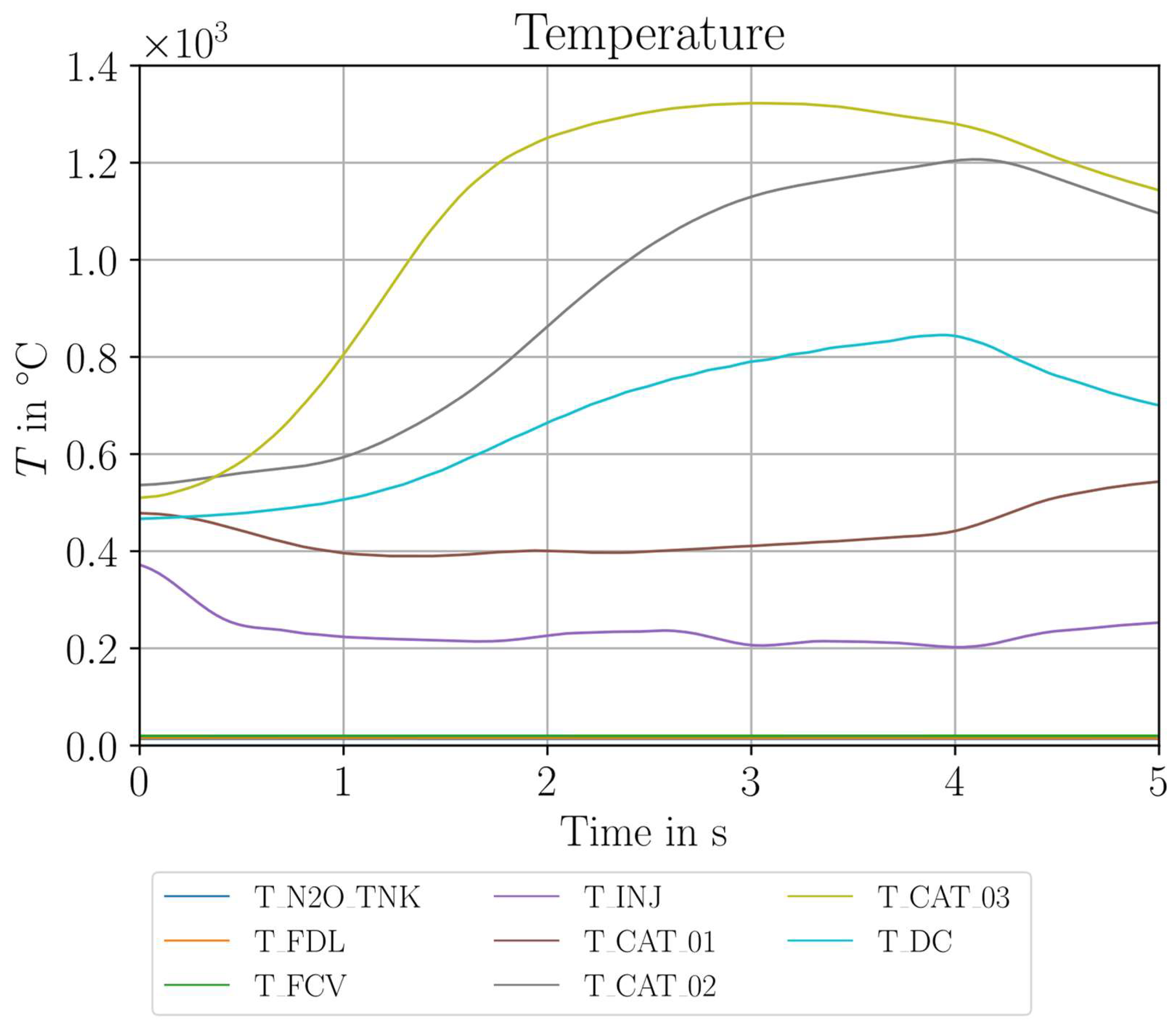
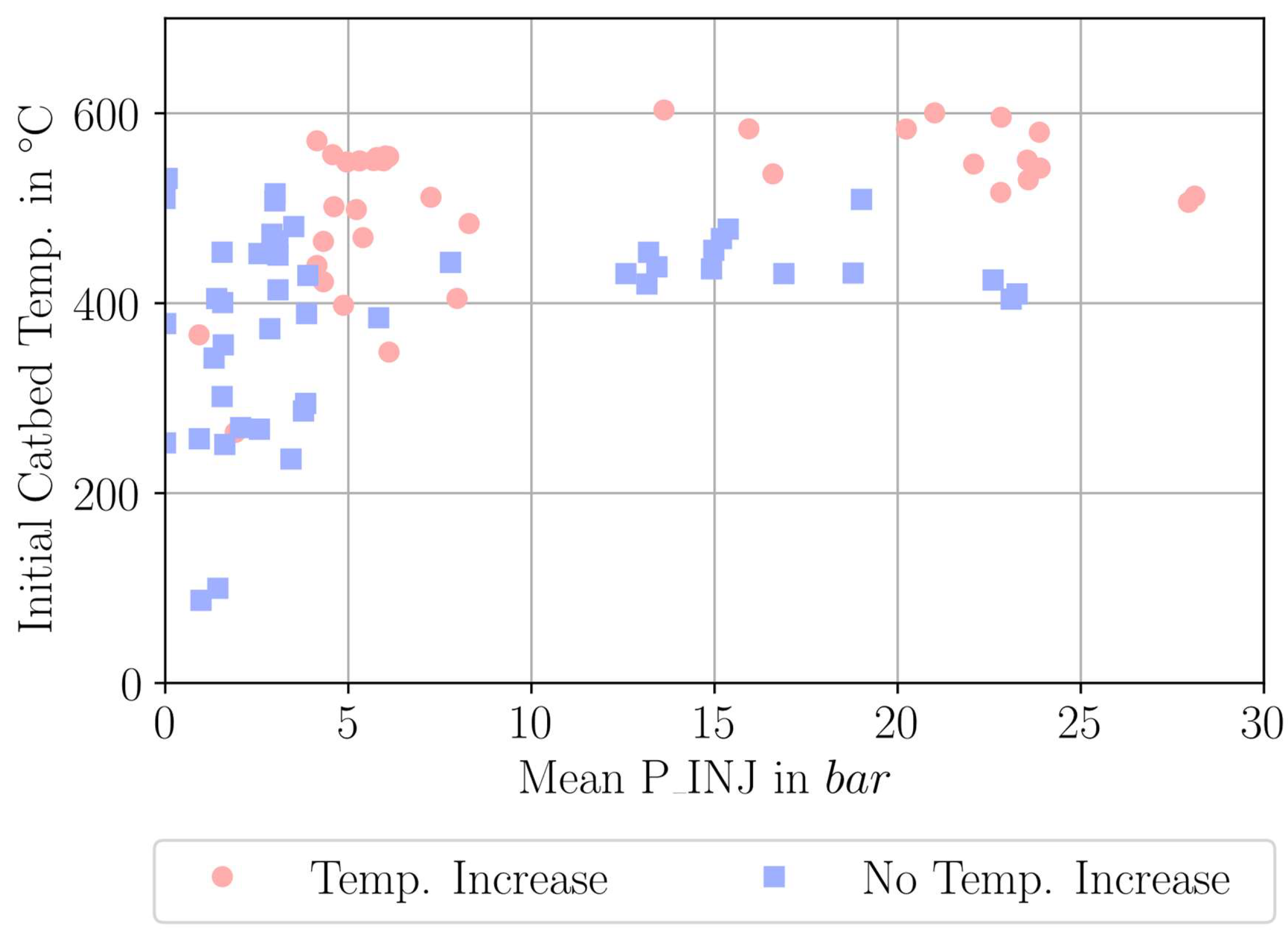
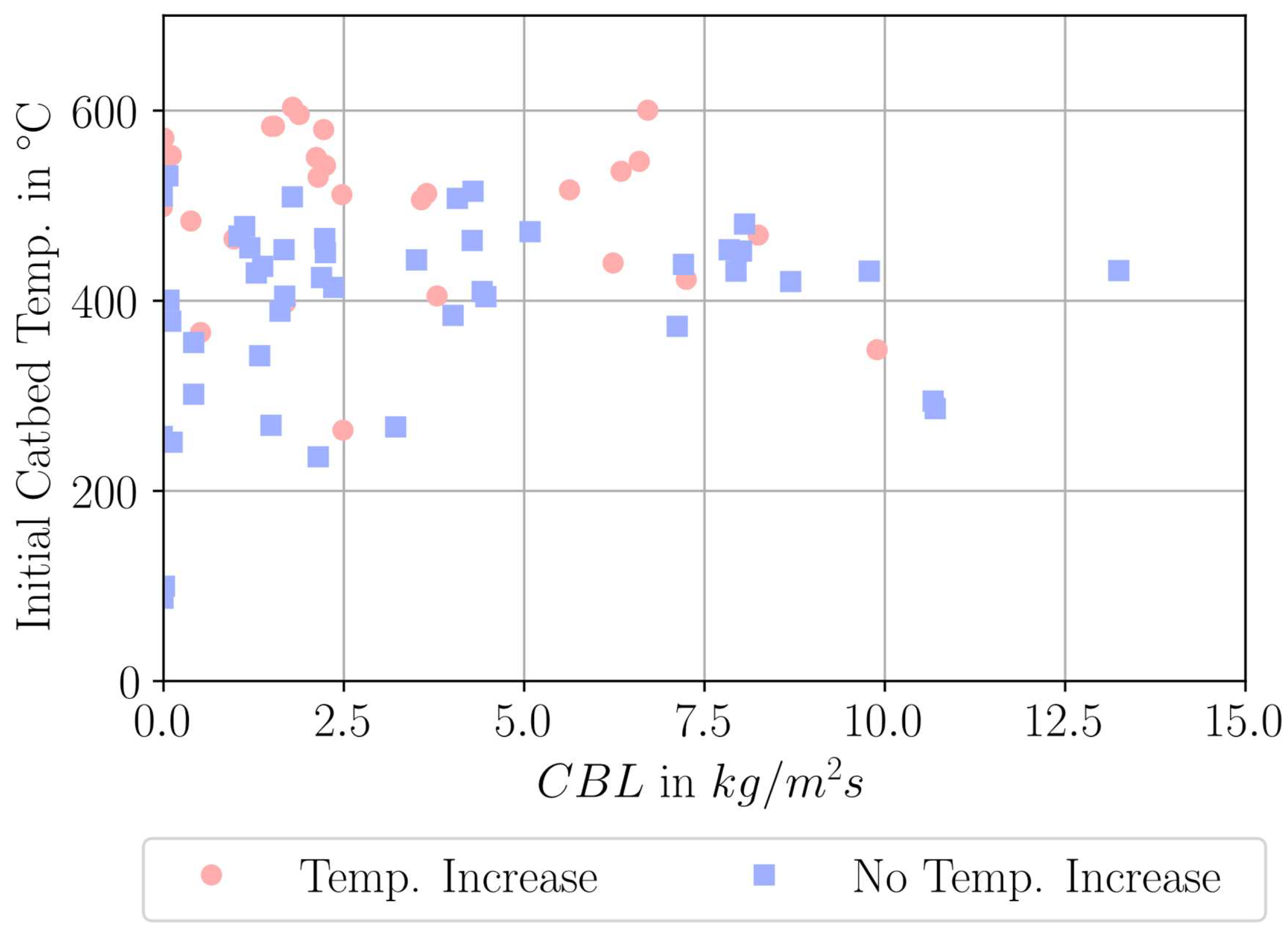
| Parameter Measured | Sensor Type | Sensor Manufacturer and Model | Sampling Rate | Sensor Uncertainty |
|---|---|---|---|---|
| Pressure Chamber | Piezo-Resistive–Passive Transmitter | STS–Type TM (0–30 bar) | 5 | % FS |
| Pressure TNK/FDL | Piezo-Resistive–Passive Transmitter | STS–Type TM (0–100 bar) | 5 | % FS |
| Temperature | Thermocouple | Stainless Steel Sheathed–Type K | 110 | °C |
| Mass Flow Rate | Coriolis | Bronkhorst–M14 (FS: 8.333 g s−1) | 5 | % FS |
| Feeding Pressure | 6 bar | 12 bar nominal | 24 bar |
| Single Pulse | 2 × 60 | 2 × 60 | 2 × 60 |
| Pulse mode 1 Hz ON/OFF in ms | 125/875 | 125/875 | 125/875 |
| 250/750 | 250/750 | 250/750 | |
| 500/500 | 500/500 | 500/500 | |
| 750/250 | 750/250 | 750/250 | |
| 875/125 | 875/125 | 875/125 |
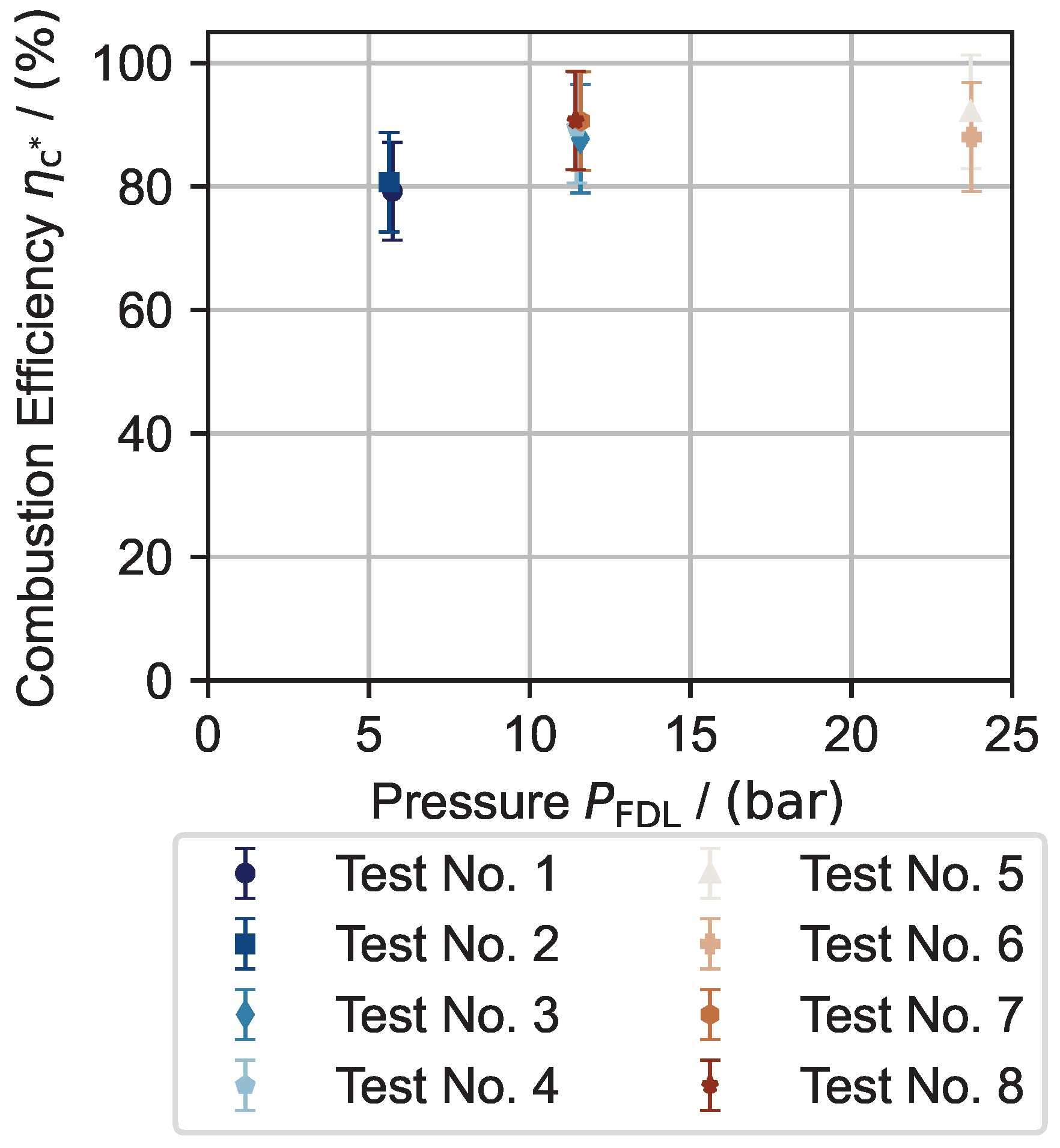
| Test No. | Mean in bar | Mean in bar | Max. in °C | Max. in °C | Mean in g s−1 | Discharge Coefficient | Combustion Efficiency in % | Mean Interval in s |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.73 | 3.89 | 751.33 | 573.37 | 0.248 | 0.68 | 79.20 | 54–58 |
| 2 | 5.63 | 2.39 | 834.79 | 584.15 | 0.136 | 0.47 | 80.69 | 50–55 |
| 3 | 11.58 | 9.98 | 947.16 | 791.95 | 0.536 | 0.80 | 87.72 | 49–59 |
| 4 | 11.46 | 9.66 | 950.44 | 798.20 | 0.506 | 0.74 | 89.51 | 49–59 |
| 5 | 23.72 | 18.46 | 959.26 | 867.86 | 0.938 | 0.70 | 92.06 | 44–54 |
| 6 | 23.74 | 15.73 | 954.68 | 858.56 | 0.835 | 0.80 | 88.02 | 40–50 |
| 7 | 11.92 | 10.55 | - | - | 0.578 | - | 90.62 | 45–55 |
| 8 | 11.92 | 10.39 | - | - | 0.569 | - | 90.44 | 45–55 |
| Test No. | Mean in g/s | Mean in bar | Mean in bar | CBL in kg/(m2s) | Max T in °C | Mean Interval in s |
|---|---|---|---|---|---|---|
| 1 | 0.23 | 4.14 | 23.35 | 7.4 | 860.5 | 3.3–4.9 |
| 2 | 0.20 | 3.98 | 23.10 | 6.2 | 913.7 | 3.3–4.9 |
| 3 | 0.27 | 4.97 | 23.68 | 8.2 | 1216.4 | 20–25 |
| 4 | 0.20 | 16.84 | 23.46 | 6.3 | 1322.7 | 2.6–3.8 |
| Evaluation Interval | |||
|---|---|---|---|
| 3.3–4.9 s | 840.6 m/s | 1105.6 m/s | 76.0% |
| Evaluation Interval | |||
|---|---|---|---|
| 3.3–4.9 s | 938.9 m/s | 1105.6 m/s | 84.9% |
| Evaluation Interval | |||
|---|---|---|---|
| 20–25 s | 976.0 m/s | 1106.4 m/s | 88.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merz, F.; Hörger, T.; Steelant, J.; Lauck, F.; Kirchberger, C. Experimental Investigation of Hydrogen Peroxide and Nitrous Oxide in a 1-Newton Catalyst-Based Monopropellant Research Thruster. Aerospace 2025, 12, 835. https://doi.org/10.3390/aerospace12090835
Merz F, Hörger T, Steelant J, Lauck F, Kirchberger C. Experimental Investigation of Hydrogen Peroxide and Nitrous Oxide in a 1-Newton Catalyst-Based Monopropellant Research Thruster. Aerospace. 2025; 12(9):835. https://doi.org/10.3390/aerospace12090835
Chicago/Turabian StyleMerz, Florian, Till Hörger, Johan Steelant, Felix Lauck, and Christoph Kirchberger. 2025. "Experimental Investigation of Hydrogen Peroxide and Nitrous Oxide in a 1-Newton Catalyst-Based Monopropellant Research Thruster" Aerospace 12, no. 9: 835. https://doi.org/10.3390/aerospace12090835
APA StyleMerz, F., Hörger, T., Steelant, J., Lauck, F., & Kirchberger, C. (2025). Experimental Investigation of Hydrogen Peroxide and Nitrous Oxide in a 1-Newton Catalyst-Based Monopropellant Research Thruster. Aerospace, 12(9), 835. https://doi.org/10.3390/aerospace12090835







