Light Energy Partitioning under Various Environmental Stresses Combined with Elevated CO2 in Three Deciduous Broadleaf Tree Species in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. N Limitation under Elevated CO2
2.2. Drought under Elevated CO2
2.3. Elevated O3 under Elevated CO2
2.4. Measurements of Gas Exchange and Chlorophyll Fluorescence
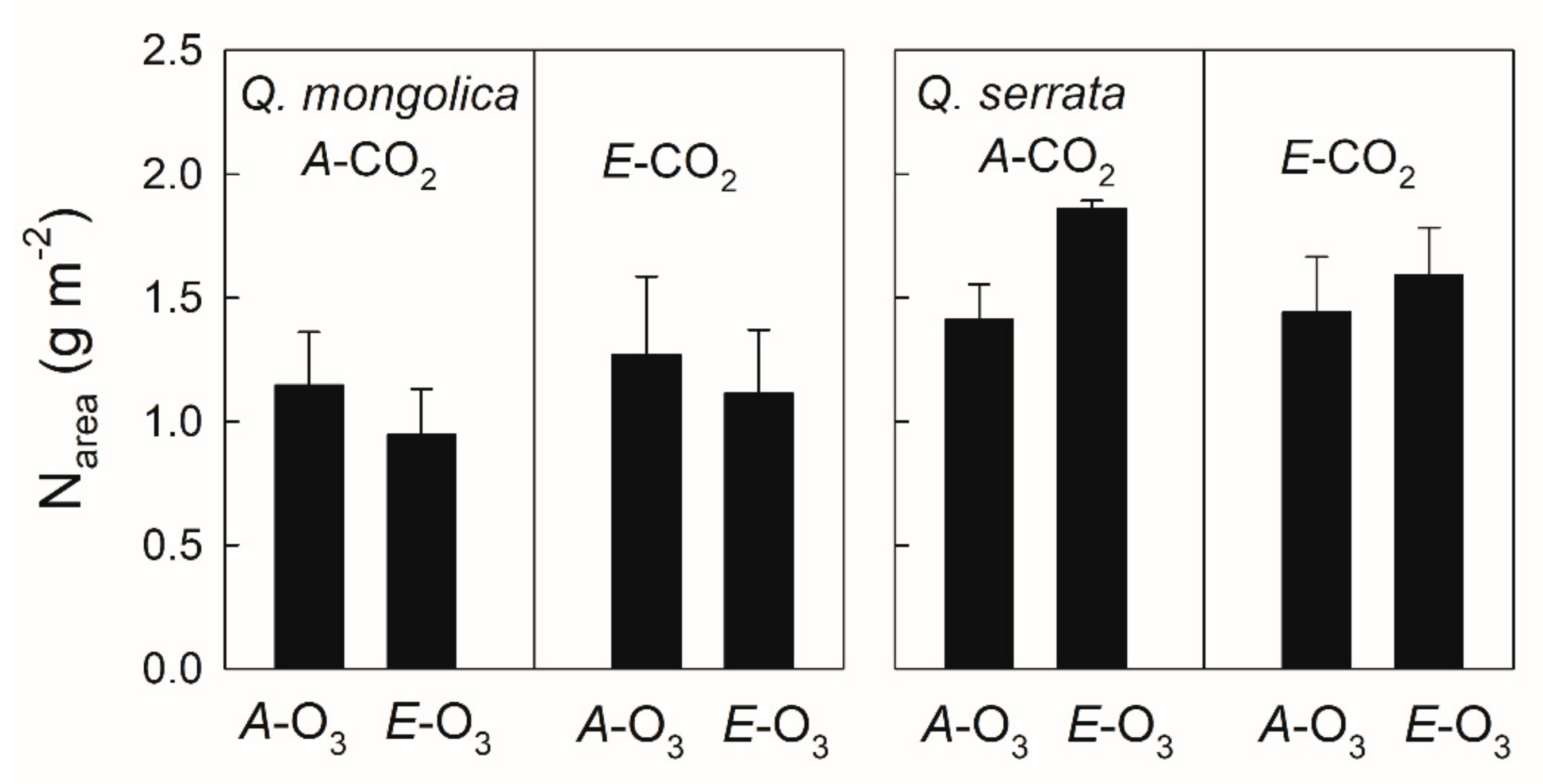
2.5. Leaf N Content
2.6. Statistical Analysis
3. Results
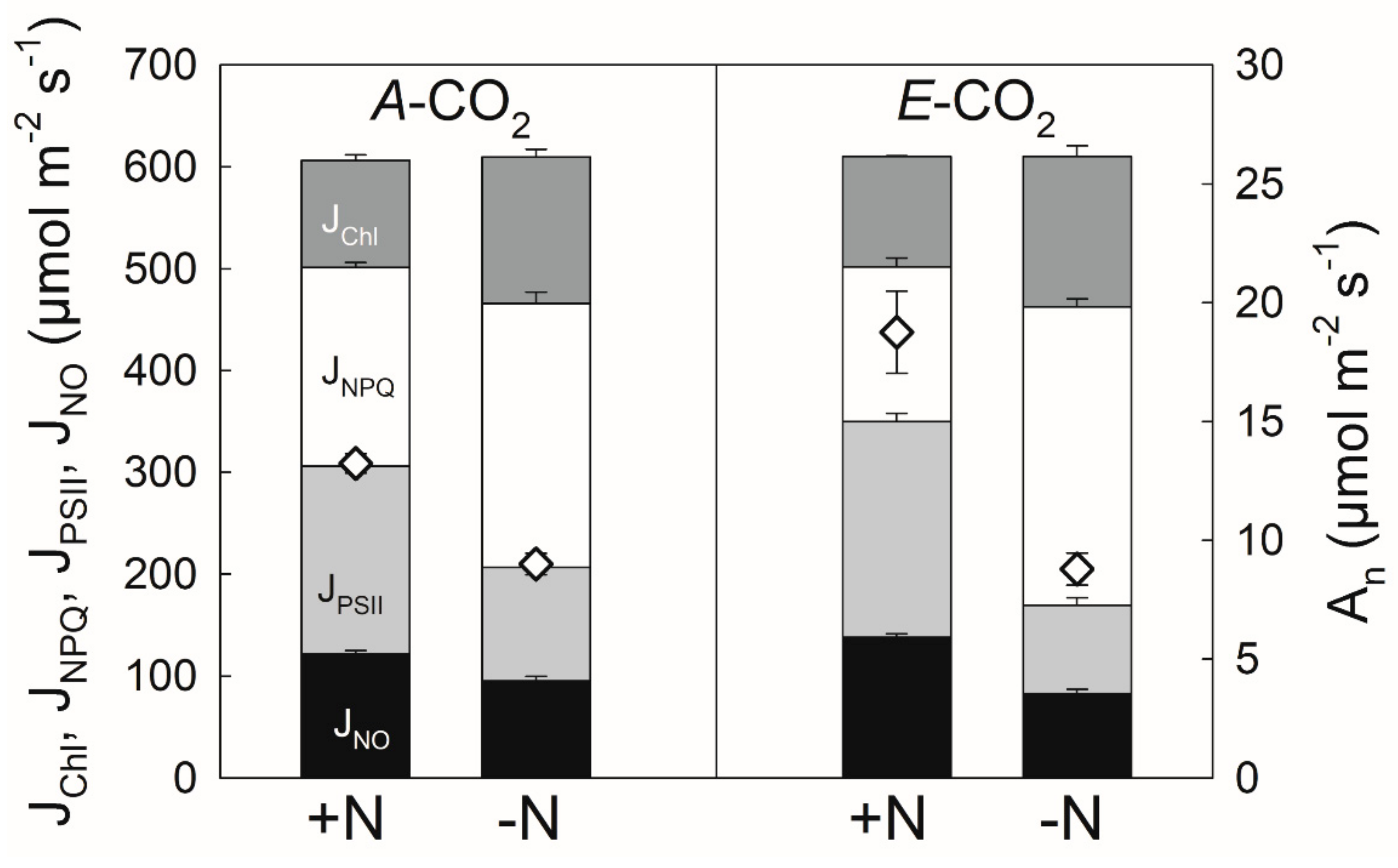
3.1. Nitrogen Limitation under Elevated CO2
3.2. Drought under Elevated CO2
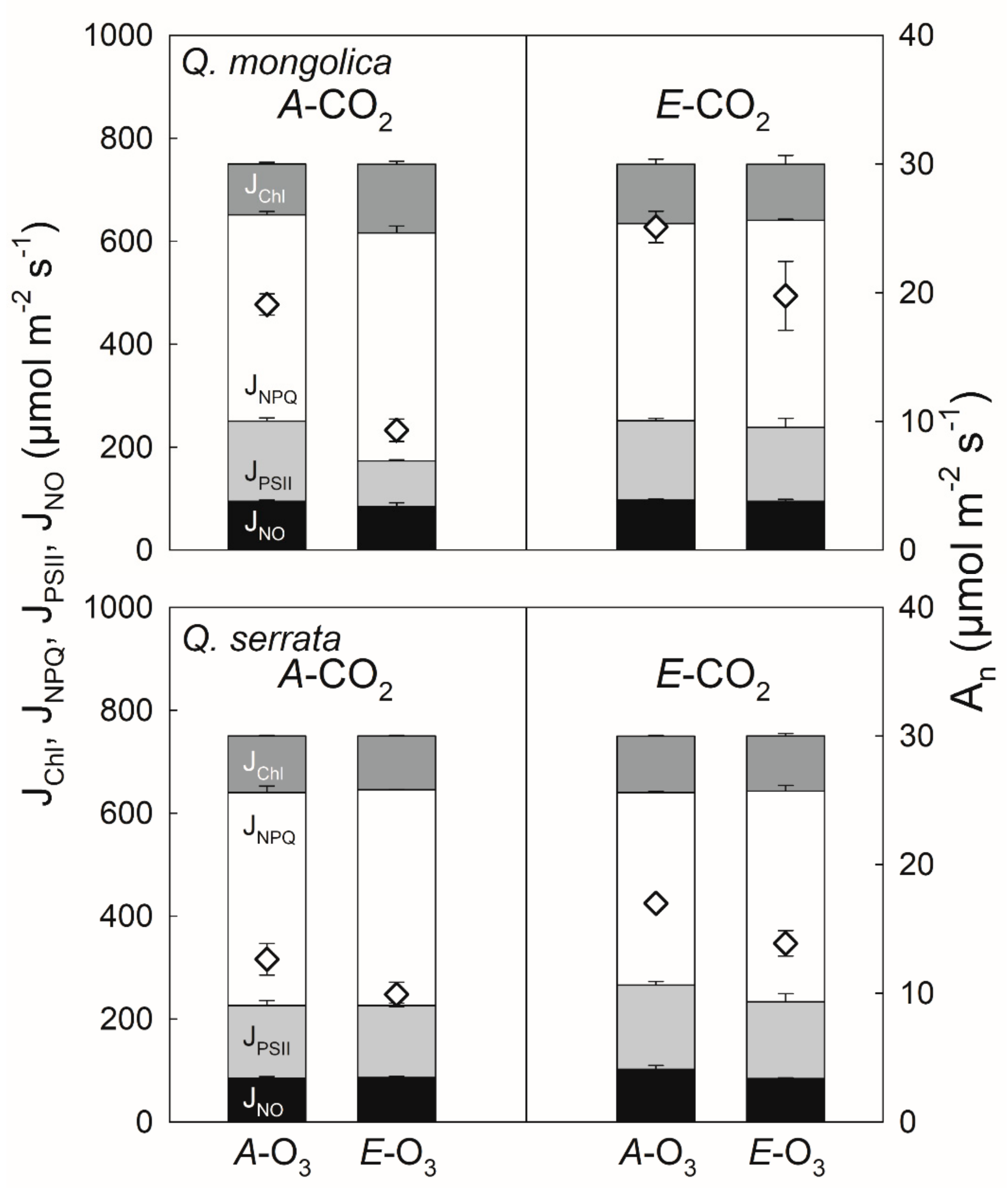
3.3. Elevated O3 under Elevated CO2
4. Discussion
4.1. Nitrogen Limitation under Elevated CO2
4.2. Drought under Elevated CO2
4.3. Elevated O3 under Elevated CO2
4.4. Regulated and Non-regulated Non-photochemical Quenching under Elevated CO2
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cornic, G.; Fresneau, C. Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Ann. Bot. 2002, 89, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Lazár, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, L.; Furbank, R.T.; Chow, W.S. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res. 2004, 82, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Ruban, A.V.; Berera, R.; Ilioaia, C.; van Stokkum, I.H.M.; Kennis, J.T.M.; Pascal, A.A.; van Amerongen, H.; Robert, B.; Horton, P.; van Grondelle, R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 2007, 450, 575–578. [Google Scholar] [CrossRef]
- Wilhelm, C.; Selmar, D. Energy dissipation is an essential mechanism to sustain the viability of plants: The physiological limits of improved photosynthesis. J. Plant Physiol. 2011, 168, 79–87. [Google Scholar] [CrossRef]
- Kornyeyev, D.; Logan, B.A.; Holaday, A.S. Excitation pressure as a measure of the sensitivity of photosystem II to photoinactivation. Funct. Plant Biol. 2010, 37, 943–951. [Google Scholar] [CrossRef]
- Kasajima, I.; Ebana, K.; Yamamoto, T.; Takahara, K.; Yano, M.; Kawai-Yamada, M.; Uchimiya, H. Molecular distinction in genetic regulation of nonphotochemical quenching in rice. Proc. Natl. Acad. Sci. USA 2011, 108, 13835–13840. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age-dependent photoprotective and antioxidative response mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef]
- Moustaka, J.; Ouzounidou, G.; Sperdouli, I.; Moustakas, M. Photosystem II is more sensitive than photosystem I to Al3+ induced phytotoxicity. Materials (Basel) 2018, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Vass, I. Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol. Plant. 2011, 142, 6–16. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. A better energy allocation of absorbed light in photosystem II and less photooxidative damage contribute to acclimation of Arabidopsis thaliana young leaves to water deficit. J. Plant Physiol. 2014, 171, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Moustakas, M. Photoprotective mechanism of the non-target organism Arabidopsis thaliana to paraquat exposure. Pestic. Biochem. Physiol. 2014, 111, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Zhang, G.; Guo, J.; Dong, Z. Effects of soil drought on photosynthetic traits and antioxidant enzyme activities in Hippophae rhamnoides seedlings. J. For. Res. 2017, 28, 255–263. [Google Scholar] [CrossRef]
- Keenan, T.F.; Niinemets, Ü. Global leaf trait estimates biased due to plasticity in the shade. Nat. Plants 2016, 3, 16201. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Anten, N.P.R.; Borjigidai, A.; Kamiyama, C.; Sakai, H.; Hasegawa, T.; Oikawa, S.; Iio, A.; Watanabe, M.; Koike, T.; et al. A meta-analysis of leaf nitrogen distribution within plant canopies. Ann. Bot. 2016, 118, 239–247. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationship in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Bilger, W.; Kull, O.; Tenhunen, J. Responses of foliar photosynthetic electron transport, pigment stoichiometry, and stomatal conductance to interacting environmental factors in a mixed species forest canopy. Tree Physiol. 1999, 19, 839–852. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitao, M.; Kitaoka, S.; Komatsu, M.; Utsugi, H.; Tobita, H.; Koike, T.; Maruyama, Y. Leaves of Japanese oak (Quercus mongolica var. crispula) mitigate photoinhibition by adjusting electron transport capacities and thermal energy dissipation along the intra-canopy light gradient. Physiol. Plant. 2012, 146, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Kitao, M.; Kitaoka, S.; Harayama, H.; Tobita, H.; Agathokleous, E.; Utsugi, H. Canopy nitrogen distribution is optimized to prevent photoinhibition throughout the canopy during sun flecks. Sci. Rep. 2018, 8, 503. [Google Scholar] [CrossRef]
- Von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; CSIRO Pub: Clayton, Australia, 2000; ISBN 064306379X. [Google Scholar]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. Nutrient constraints on terrestrial carbon fixation: The role of nitrogen. J. Plant Physiol. 2016, 203, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2012, 3, 52–58. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef] [PubMed]
- Matyssek, R.; Wieser, G.; Ceulemans, R.; Rennenberg, H.; Pretzsch, H.; Haberer, K.; Löw, M.; Nunn, A.J.J.; Werner, H.; Wipfler, P.; et al. Enhanced ozone strongly reduces carbon sink strength of adult beech (Fagus sylvatica)—Resume from the free-air fumigation study at Kranzberg Forest. Environ. Pollut. 2010, 158, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kontunen-Soppela, S.; Pandey, A.K.; Keski-Saari, S.; Oksanen, E.; Pandey, V. Impacts of increasing ozone on Indian plants. Environ. Pollut. 2013, 177, 189–200. [Google Scholar]
- Kitao, M.; Koike, T.; Tobita, H.; Maruyama, Y. Elevated CO2 and limited nitrogen nutrition can restrict excitation energy dissipation in photosystem II of Japanese white birch (Betula platyphylla var. japonica) leaves. Physiol. Plant. 2005, 125, 64–73. [Google Scholar] [CrossRef]
- Kitao, M.; Lei, T.T.; Koike, T.; Kayama, M.; Tobita, H.; Maruyama, Y. Interaction of drought and elevated CO2 concentration on photosynthetic down-regulation and susceptibility to photoinhibition in Japanese white birch seedlings grown with limited N availability. Tree Physiol. 2007, 27, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Kitao, M.; Komatsu, M.; Yazaki, K.; Kitaoka, S.; Tobita, H. Growth overcompensation against O3 exposure in two Japanese oak species, Quercus mongolica var. crispula and Quercus serrata, grown under elevated CO2. Environ. Pollut. 2015, 206, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vera, U.M.; De Souza, A.P.; Long, S.P.; Ort, D.R. The role of sink strength and nitrogen availability in the down-regulation of photosynthetic capacity in field-grown Nicotiana tabacum L. at elevated CO2 concentration. Front. Plant Sci. 2017, 8, 998. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components – calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, O.; Tenhunen, J.D. Within-canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ. 2004, 27, 293–313. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Kitao, M.; Lei, T.T.T. Circumvention of over-excitation of PSII by maintaining electron transport rate in leaves of four cotton genotypes developed under long-term drought. Plant Biol. 2007, 9, 69–76. [Google Scholar] [CrossRef]
- Kitao, M.; Lei, T.T.; Koike, T.; Tobita, H.; Maruyama, Y. Higher electron transport rate observed at low intercellular CO2 concentration in long-term drought-acclimated leaves of Japanese mountain birch (Betula ermanii). Physiol. Plant. 2003, 118, 406–413. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S. Modelling of Photosynthetic Response to Environmental Conditions. In Physiological Plant Ecology II; Springer: Berlin/Heidelberg, Germany, 1982; pp. 549–587. [Google Scholar]
- Nagashima, T.; Sudo, K.; Akimoto, H.; Kurokawa, J.; Ohara, T. Long-term change in the source contribution to surface ozone over Japan. Atmos. Chem. Phys. 2017, 17, 8231–8246. [Google Scholar] [CrossRef]
- Sicard, P.; Anav, A.; De Marco, A.; Paoletti, E. Projected global ground-level ozone impacts on vegetation under different emission and climate scenarios. Atmos. Chem. Phys. 2017, 17, 12177–12196. [Google Scholar] [CrossRef]
- Karnosky, D.F.; Pregitzer, K.S.; Zak, D.R.; Kubiske, M.E.; Hendrey, G.R.; Weinstein, D.; Nosal, M.; Percy, K.E. Scaling ozone responses of forest trees to the ecosystem level in a changing climate. Plant Cell Environ. 2005, 28, 965–981. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Norby, R.J.; Zak, D.R. Ecological Lessons from Free-Air CO2 Enrichment (FACE) Experiments. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 181–203. [Google Scholar] [CrossRef]
- Agathokleous, E.; Saitanis, C.J.; Wang, X.; Watanabe, M.; Koike, T. A review study on past 40 years of research on effects of tropospheric O3 on belowground structure, functioning and processes of trees: A linkage with potential ecological implications. Water Air Soil Pollut. 2016, 227, 33. [Google Scholar] [CrossRef]
- Watanabe, M.; Hoshika, Y.; Inada, N.; Wang, X.; Mao, Q.; Koike, T. Photosynthetic traits of Siebold’s beech and oak saplings grown under free air ozone exposure in northern Japan. Environ. Pollut. 2013, 174, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Pell, E.J.; Schlagnhaufer, C.D.; Arteca, R.N. Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant. 1997, 100, 264–273. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Environmental hormesis and its fundamental biological basis: Rewriting the history of toxicology. Environ. Res. 2018, 165, 274–278. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 2019, in press. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Building biological shields via hormesis. Trends Pharmacol. Sci. 2019, 40, 8–10. [Google Scholar] [CrossRef]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of Photosynthesis in Nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]

| F-statistics | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Effect | JNO | JPSII | JNPQ | JPSII + JNPQ | JChl | An |
| N limitation | CO2 (F1,13) | 0.69 ns | 0.47 ns | 0.81 ns | 0.11 ns | 0.03 ns | 51.8 *** |
| N (F1,13) | 106 *** | 133 *** | 124 *** | 0.19 ns | 28.6 *** | 322 *** | |
| CO2 × N (F1,13) | 13.3 ** | 9.63 ** | 18.4 *** | 3.08 ns | 0.00 ns | 71.4 *** | |
| Drought | CO2 (F1,4) | 0.01 ns | 25.1 ** | 2.68 ns | 0.00 ns | 0.01 ns | 3.75 ns |
| Drought (F1,4) | 0.71 ns | 0.40 ns | 3.90 ns | 2.23 ns | 1.39 ns | 0.74 ns | |
| CO2 × Drought (F1,4) | 0.02 ns | 0.40 ns | 1.86 ns | 1.28 ns | 2.04 ns | 0.29 ns | |
| Elevated O3 | CO2 (F1,8) | 5.00 ns | 5.46 * | 16.4 ** | 0.50 ns | 0.05 ns | 35.6 *** |
| O3 (F1,8) | 5.54 * | 6.60 * | 14.9 ** | 0.07 ns | 0.72 ns | 25.5 *** | |
| CO2 × O3 (F1,8) | 0.91 ns | 1.35 ns | 0.08 ns | 2.54 ns | 2.46 ns | 0.94 ns | |
| Species (F1,8) | 1.36 ns | 12.7 *** | 0.42 ns | 4.36 ns | 2.13 ns | 39.3 *** | |
| CO2 × Species (F1,8) | 0.08 ns | 2.35 ns | 0.22 ns | 0.50 ns | 0.35 ns | 6.64 * | |
| O3 × Species (F1,8) | 0.10 ns | 18.0 ** | 1.15 ns | 4.79 ns | 4.32 ns | 8.59 * | |
| CO2 × O3 × Species (F1,8) | 4.43 ns | 23.9 ** | 7.58 * | 0.97 ns | 6.32 * | 2.30 ns | |
| F-Statistics | |
|---|---|
| Effect | Narea |
| CO2 | 0.01 ns |
| O3 | 0.25 ns |
| CO2 × O3 | 0.27 ns |
| Species | 7.03 * |
| CO2 × Species | 0.58 ns |
| O3 × Species | 1.94 ns |
| CO2 × O3 × Species | 0.25 ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitao, M.; Tobita, H.; Kitaoka, S.; Harayama, H.; Yazaki, K.; Komatsu, M.; Agathokleous, E.; Koike, T. Light Energy Partitioning under Various Environmental Stresses Combined with Elevated CO2 in Three Deciduous Broadleaf Tree Species in Japan. Climate 2019, 7, 79. https://doi.org/10.3390/cli7060079
Kitao M, Tobita H, Kitaoka S, Harayama H, Yazaki K, Komatsu M, Agathokleous E, Koike T. Light Energy Partitioning under Various Environmental Stresses Combined with Elevated CO2 in Three Deciduous Broadleaf Tree Species in Japan. Climate. 2019; 7(6):79. https://doi.org/10.3390/cli7060079
Chicago/Turabian StyleKitao, Mitsutoshi, Hiroyuki Tobita, Satoshi Kitaoka, Hisanori Harayama, Kenichi Yazaki, Masabumi Komatsu, Evgenios Agathokleous, and Takayoshi Koike. 2019. "Light Energy Partitioning under Various Environmental Stresses Combined with Elevated CO2 in Three Deciduous Broadleaf Tree Species in Japan" Climate 7, no. 6: 79. https://doi.org/10.3390/cli7060079
APA StyleKitao, M., Tobita, H., Kitaoka, S., Harayama, H., Yazaki, K., Komatsu, M., Agathokleous, E., & Koike, T. (2019). Light Energy Partitioning under Various Environmental Stresses Combined with Elevated CO2 in Three Deciduous Broadleaf Tree Species in Japan. Climate, 7(6), 79. https://doi.org/10.3390/cli7060079






