Multivariate Analysis of Amino Acids and Health Beneficial Properties of Cantaloupe Varieties Grown in Six Locations in the United States
Abstract
:1. Introduction
2. Results and Discussion
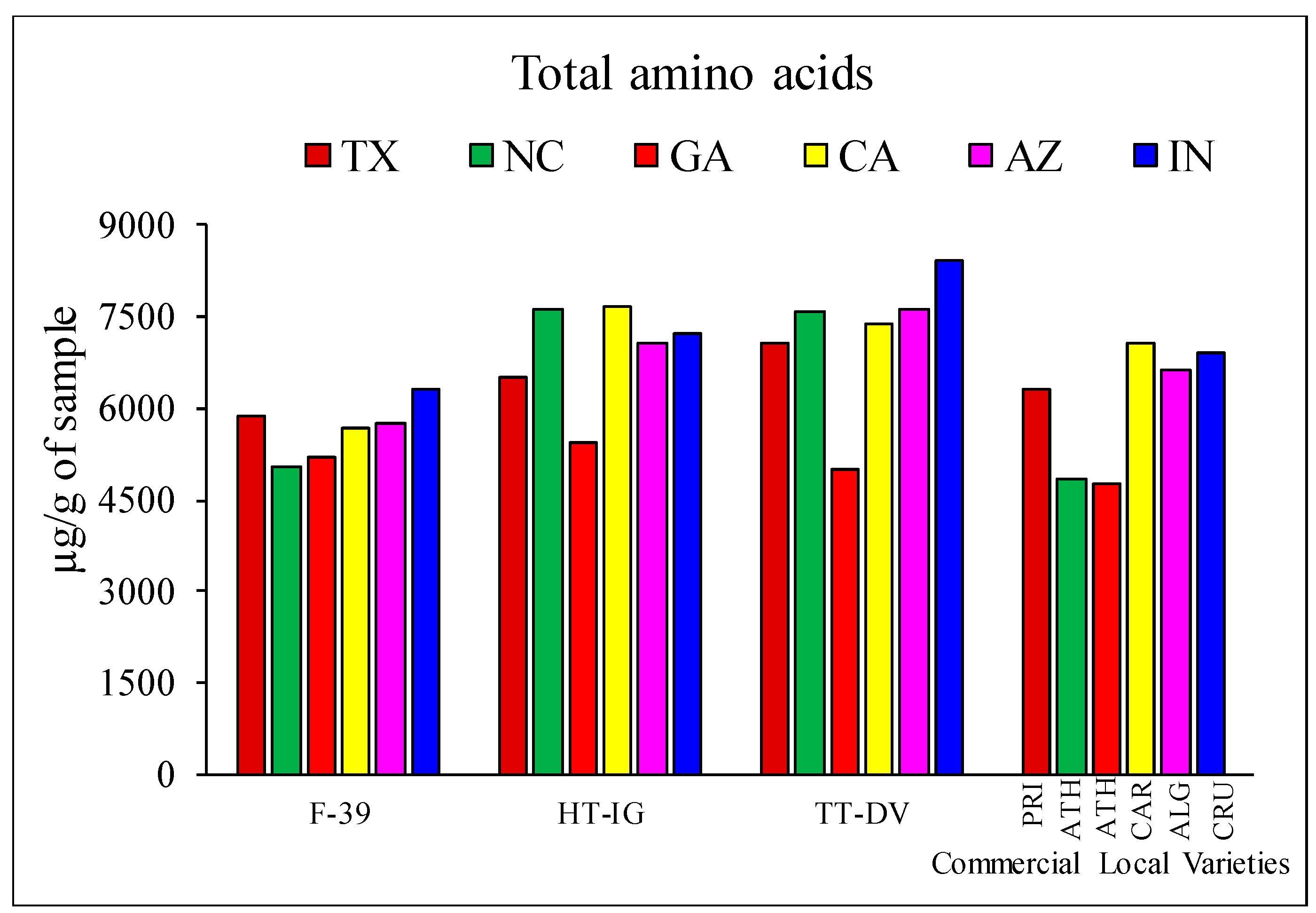
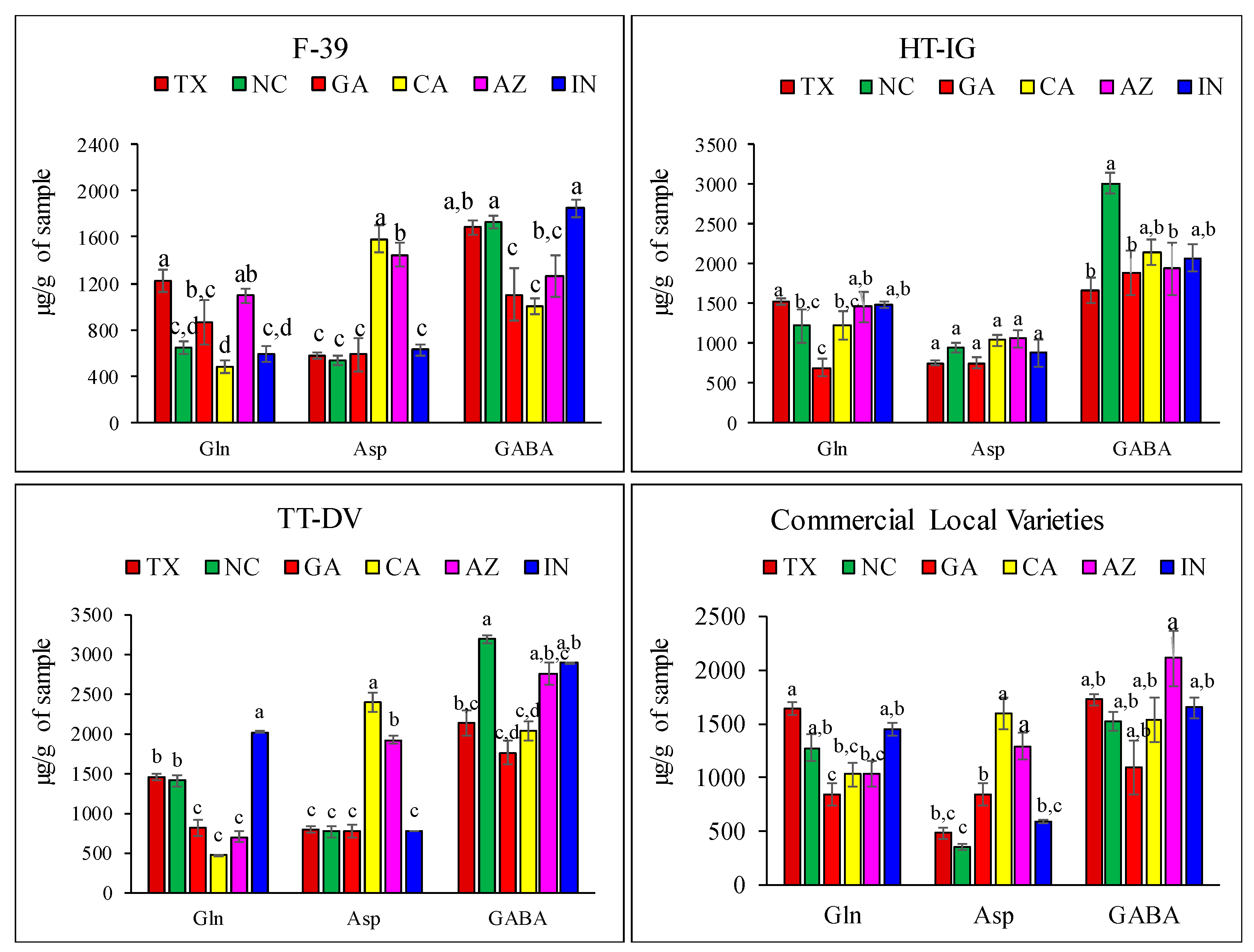
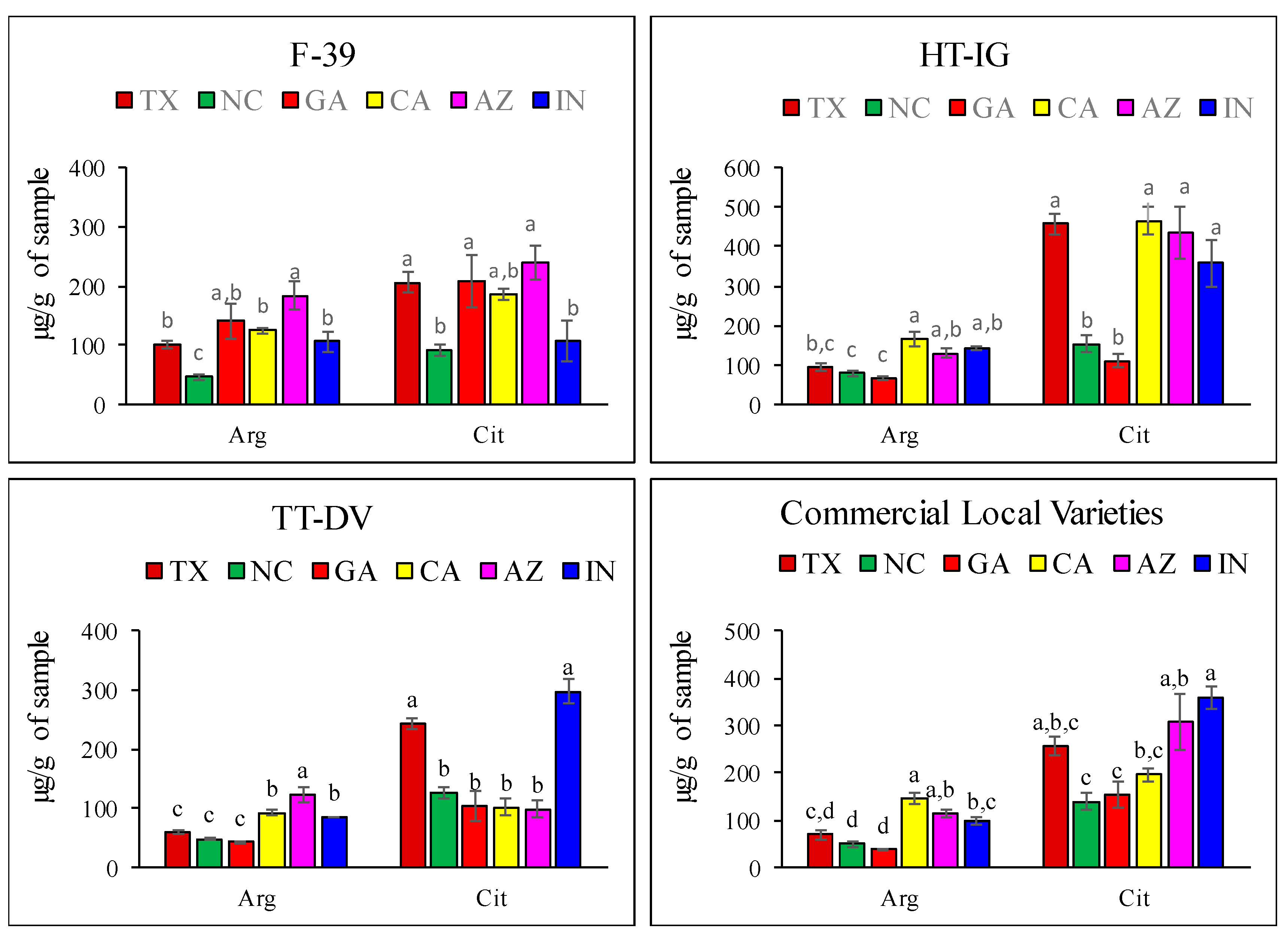
2.1. Quantitative Analysis of Amino Acids
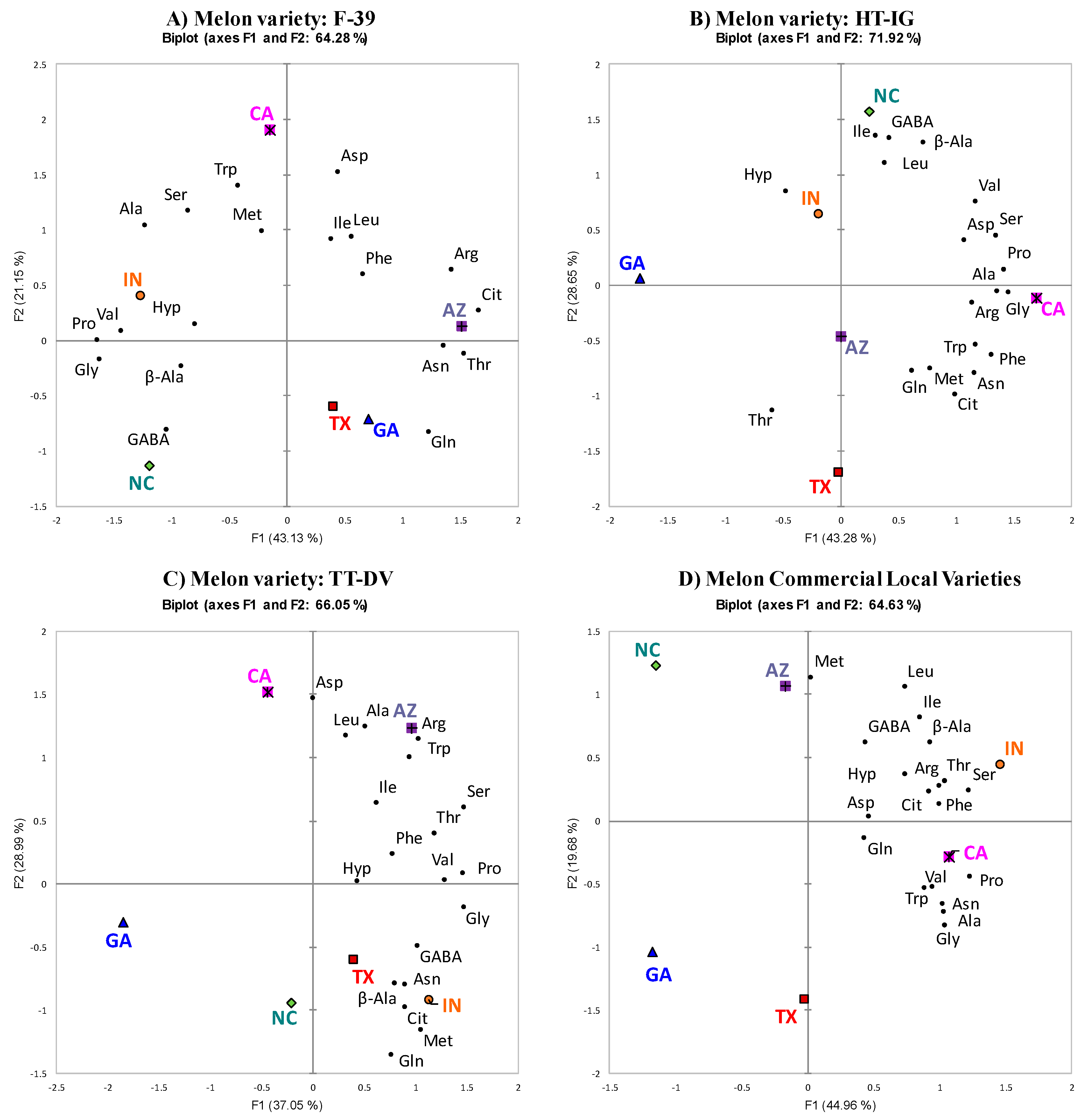
2.2. PCA of Each Variety Grown in Different Locations
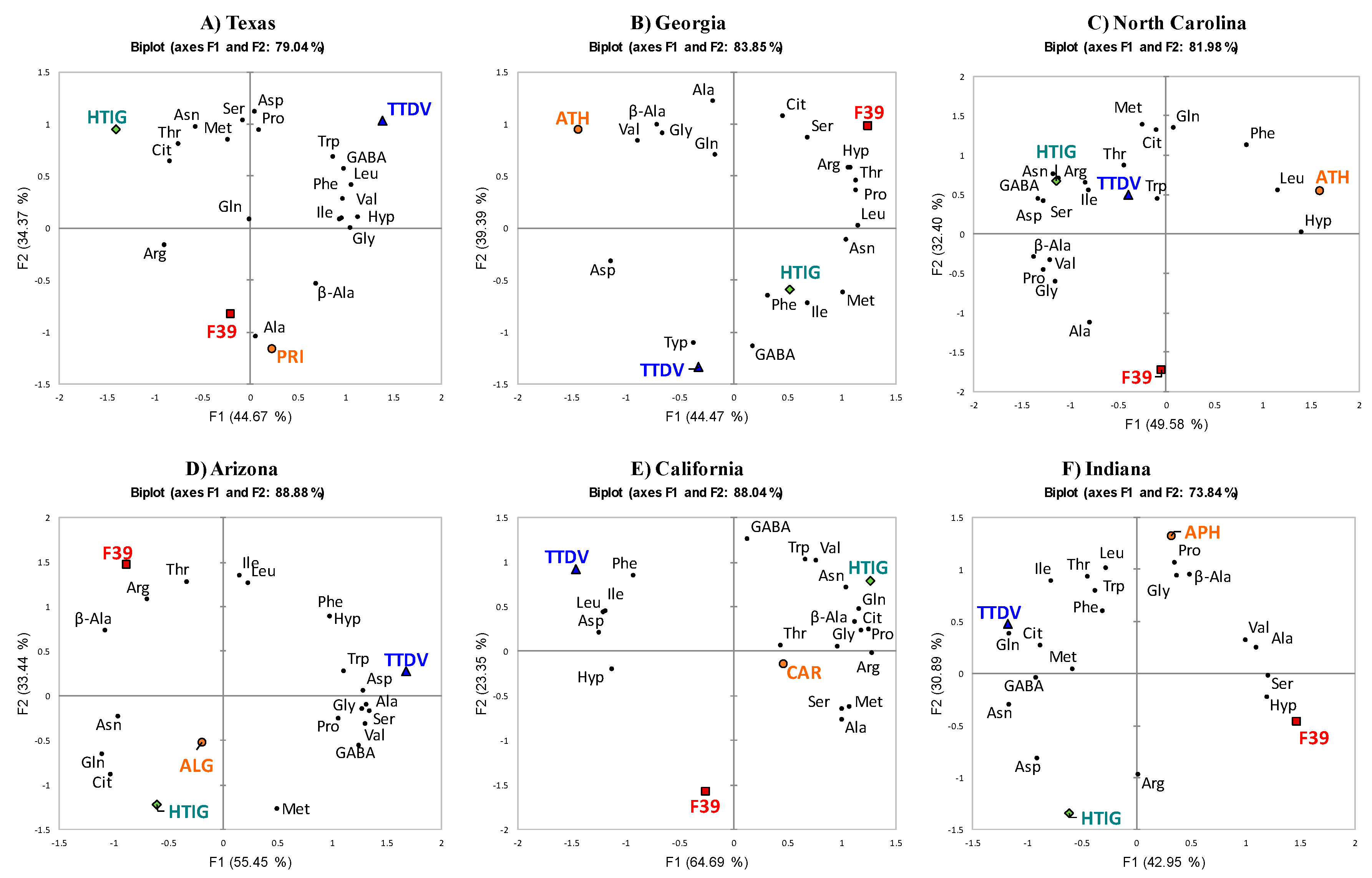
2.3. PCA of Different Varieties with a Single Growing Location
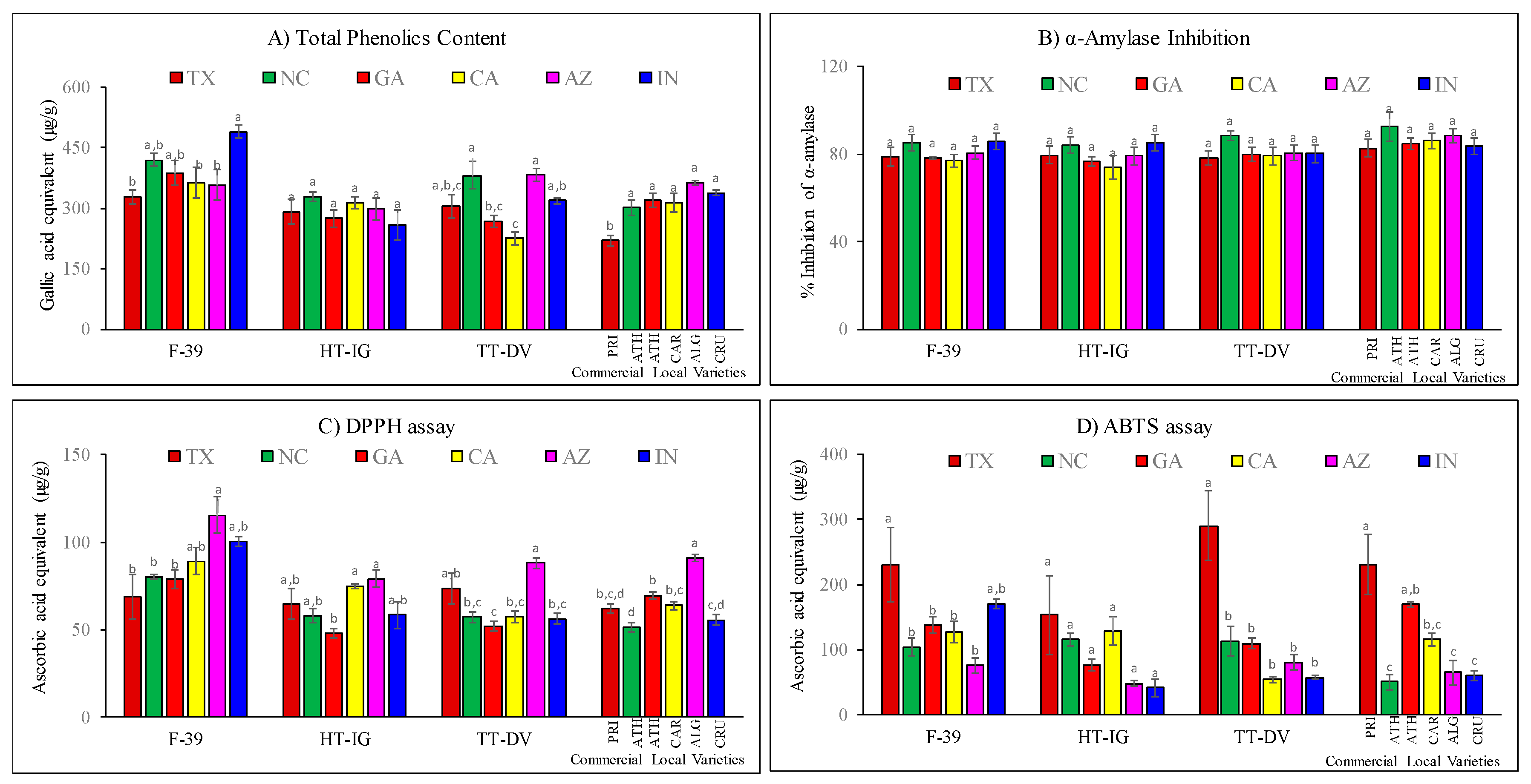
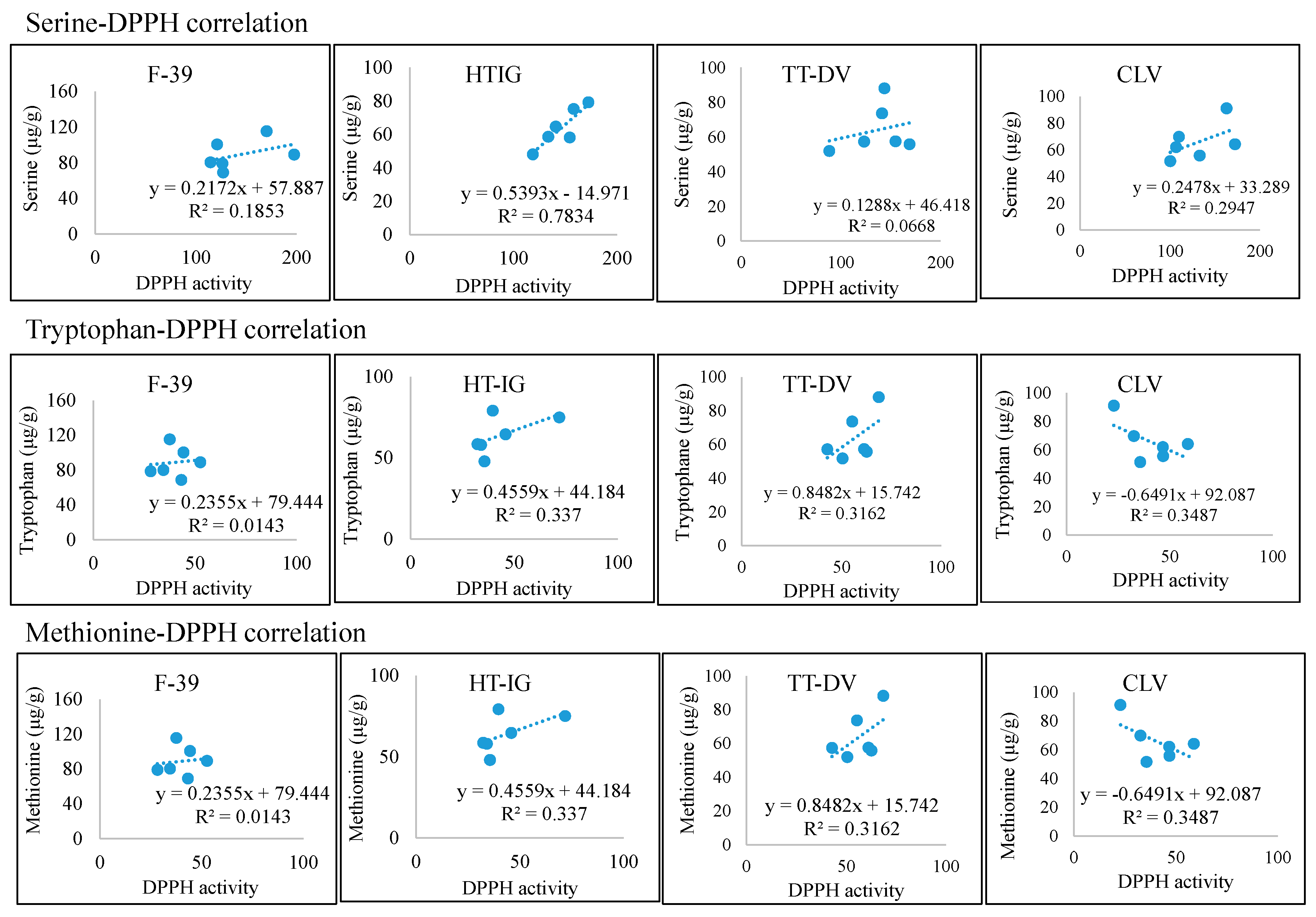
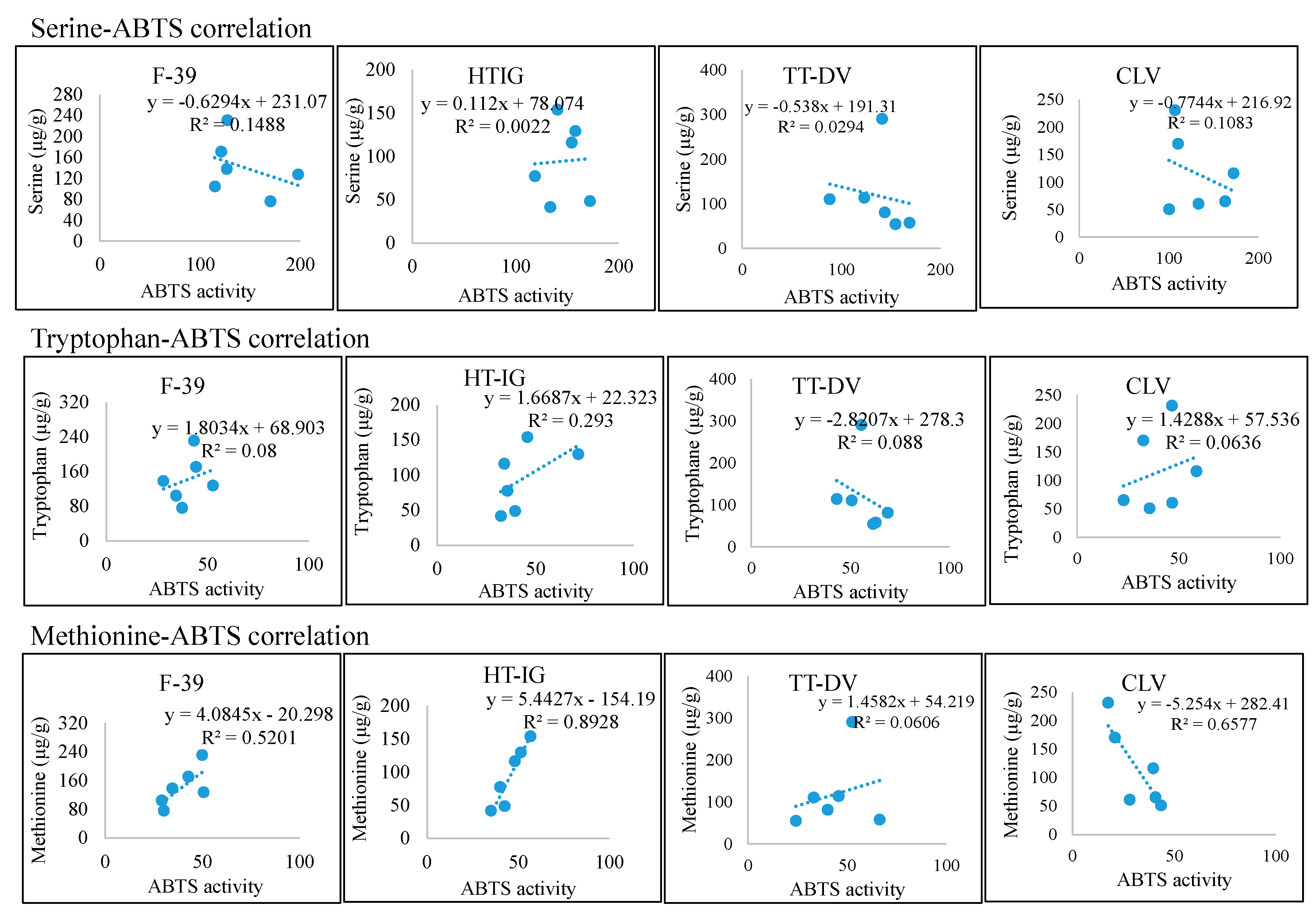
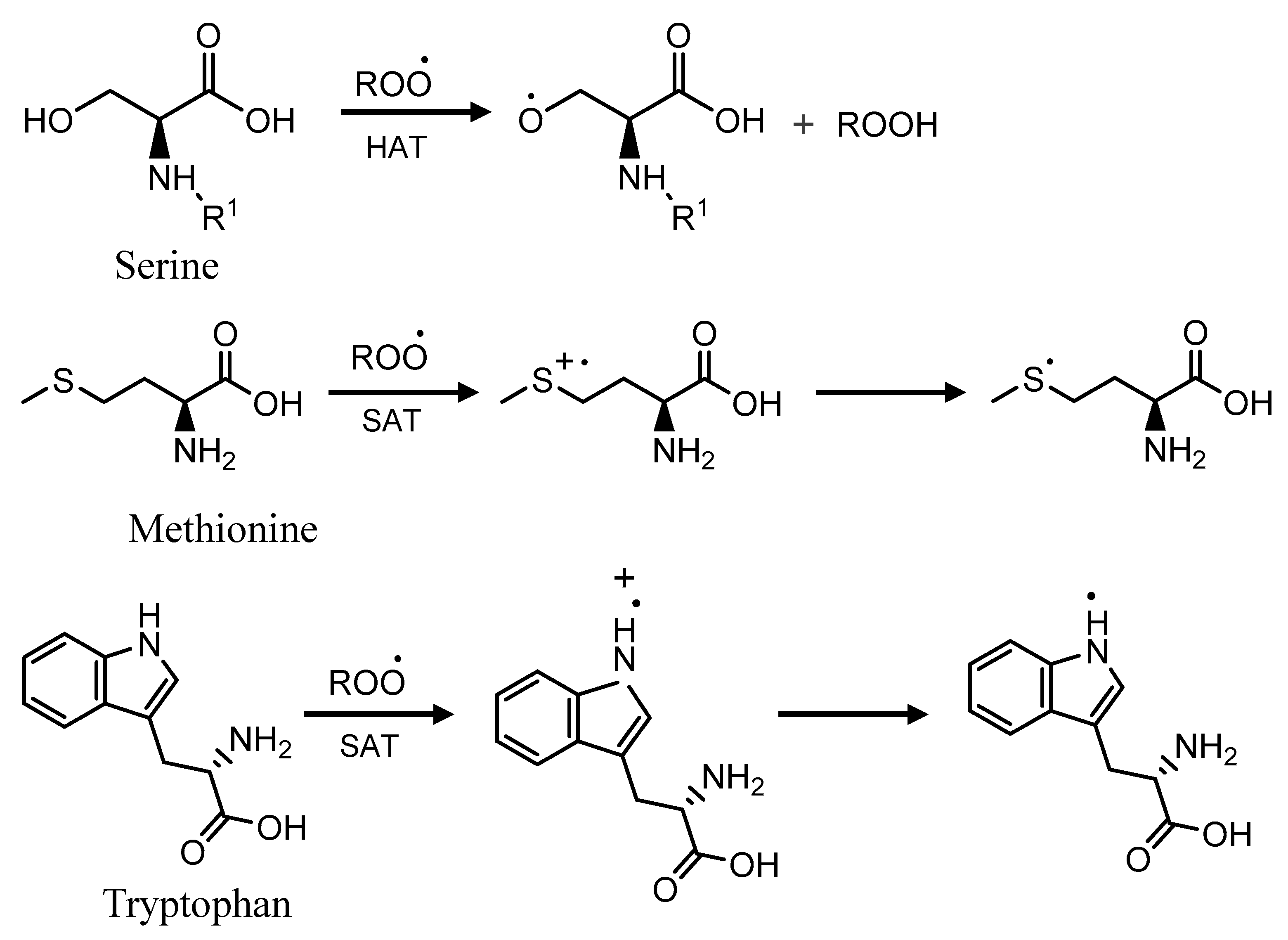
2.4. Total Phenolics, α-Amylase and Antioxidant Activities
3. Material and Methods
3.1. Fruit Growing Locations
3.2. Chemicals
3.3. Sample Preparation
3.4. Amino Acid Derivatization
3.5. HPLC-FLD for Quantitation of Amino Acids
3.6. Total Phenolic Content, α-Amylase, and Antioxidant Activities
3.6.1. Total Phenolic Content
3.6.2. α-Amylase Activity
3.6.3. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
3.6.4. 2,2′-Azino-Bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS·) Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spadafora, N.D.; Cocetta, G.; Cavaiuolo, M.; Bulgari, R.; Dhorajiwala, R.; Ferrante, A.; Spinardi, A.; Rogers, H.J.; Müller, C.T. A complex interaction between pre-harvest and post-harvest factors determines fresh-cut melon quality and aroma. Sci. Rep. 2019, 9, 2745. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Müller, C.T.; Rogers, H.J.; Pintado, M. Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef]
- Toyoizumi, T.; Ohba, S.; Fujii, K.-S.; Ikegaya, A.; Matsuura, H.; Nakajima, T. Differential GABA concentration gradients are present in the edible parts of greenhouse melon (Cucumis melo L.) during all four seasonal croppings. Biosci. Biotechnol. Biochem. 2019, 83, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Zhao, L.; Shen, T.; Sun, J.; Chen, H.; Kong, Q.; Nawaz, M.A.; Bie, Z. Melon fruit sugar and amino acid contents are affected by fruit setting method under protected cultivation. Sci. Hortic. 2017, 214, 288–294. [Google Scholar] [CrossRef]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.; Patil, B.S. A sensitive HPLC-FLD method combined with multivariate analysis for the determination of amino acids in l-citrulline rich vegetables. J. Food Drug Anal. 2019, 27, 717–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaprakasha, G.; Patil, B.S. A metabolomics approach to identify and quantify the phytochemicals in watermelons by quantitative 1HNMR. Talanta 2016, 153, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Katona, Z.F.; Sass, P.; Molnár-Perl, I. Simultaneous determination of sugars, sugar alcohols, acids and amino acids in apricots by gas chromatography-mass spectrometry. J. Chromatogr. A 1999, 847, 91–102. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.; Cacho, J.; Ferreira, V. Amino acid determination in grape juices and wines by HPLC using a modification of the 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) method. Chromatographia 2003, 58, 29–35. [Google Scholar]
- Jámbor, A.; Molnár-Perl, I. Amino acid analysis by high-performance liquid chromatography after derivatization with 9-fluorenylmethyloxycarbonyl chloride: Literature overview and further study. J. Chromatogr. A 2009, 1216, 3064–3077. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Congiu, F.; Serreli, G.; Mameli, S. Determination of dansylated amino acids and biogenic amines in Cannonau and Vermentino wines by HPLC-FLD. Food Chem. 2015, 175, 29–35. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.-P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häusler, R.E.; Ludewig, F.; Krueger, S. Amino acids—A life between metabolism and signaling. Plant Sci. 2014, 229, 225–237. [Google Scholar] [CrossRef]
- Joshi, V.; Joshi, M.; Silwal, D.; Noonan, K.; Rodriguez, S.; Penalosa, A. Systematized biosynthesis and catabolism regulate citrulline accumulation in watermelon. Phytochemistry 2019, 162, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Kumar, G.; Arya, P.; Jaswal, R.; Jain, P.; Singh, K.; Sharma, T.R. Genome-wide analysis and expression profiling of rice hybrid proline-rich proteins in response to biotic and abiotic stresses and hormone treatment. Plants 2019, 8, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.-A.; Im, K.-H.; Park, S.U.; Oh, S.-D.; Choi, J.; Kim, J.K. Dynamics of short-term metabolic profiling in radish sprouts (Raphanus sativus L.) in response to nitrogen deficiency. Plants 2019, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Wang, X.; Dong, F.; Watanabe, N.; Yang, Z. Increasing postharvest high-temperatures lead to increased volatile phenylpropanoids/benzenoids accumulation in cut rose (Rosa hybrida) flowers. Postharvest Biol. Technol. 2019, 148, 68–75. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.; Luo, W.; Zhao, W.; Brecht, J.; Bai, J. Key tomato volatile compounds during postharvest ripening in response to chilling and pre-chilling heat treatments. Postharvest Biol. Technol. 2019, 154, 11–20. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Mahboobi, S.; Tsang, C.; Rezaei, S.; Jafarnejad, S. Effect of L-citrulline supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Human Hypertens. 2019, 33, 10–21. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered connectivity in depression: GABA and Glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Dato, S.; Hoxha, E.; Crocco, P.; Iannone, F.; Passarino, G.; Rose, G. Amino acids and amino acid sensing: Implication for aging and diseases. Biogerontology 2019, 20, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cui, L.; Pen, B. L-Arginine prevents stroke-like episodes but not brain atrophy: A 20-year follow-up of a MELAS patient. Neurol. Sci. 2019, 40, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.T.; Sanacora, G. A new generation of antidepressants: An update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 2019, 24, 606–615. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Singh, J.; Metrani, R.; Shivanagoudra, S.R.; Jayaprakasha, G.K.; Patil, B.S. Review on bile acids: Effects of the gut microbiome, interactions with dietary fiber and alterations in the bioaccessibility of bioactive compounds. J. Agric. Food Chem. 2019, 67, 9124–9138. [Google Scholar] [CrossRef]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [Green Version]
- Davila, A.-M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.-H.; Sanz, Y.; Tomé, D. Re-print of “Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host”. Pharmacol. Res. 2013, 69, 114–126. [Google Scholar] [CrossRef]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaszadeh, H.; Keikhaei, B.; Mottaghi, S. A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother. Res. 2019, 33, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Shukla, R.K.; Painuly, D.; Shukla, A.; Singh, J.; Porval, A.; Vats, S. In vitro biological activity and total phenolic content of Morus nigra seeds. J. Chem. Pharm. Res. 2014, 6, 200–210. [Google Scholar]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Comparative characterization of phenolic and other polar compounds in Spanish melon cultivars by using high-performance liquid chromatography coupled to electrospray ionization quadrupole-time of flight mass spectrometry. Food Res. Int. 2013, 54, 1519–1527. [Google Scholar] [CrossRef] [Green Version]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo L.) methanolic extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Bouzo, C.; Küchen, M. Effect of temperature on melon development rate. Agron. Res. 2012, 10, 283–294. [Google Scholar]
- Pardossi, A.; Giacomet, P.; Malorgio, F.; Albini, F.M.; Murelli, C.; Serra, G.; Vernieri, P. The influence of growing season on fruit yield and quality of greenhouse melon (Cucumis melo L.) grown in nutrient film technique in a Mediterranean climate. J. Hortic. Sci. Biotechnol. 2000, 75, 488–493. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Sun, X.; Li, J.; Chen, N. Inhibition of sucrose and galactosyl-sucrose oligosaccharide metabolism in leaves and fruits of melon (Cucumis melo L.) under low light stress. Sci. Hortic. 2019, 244, 343–351. [Google Scholar] [CrossRef]
- Tassoni, A.; Zappi, A.; Melucci, D.; Reisch, B.I.; Davies, P.J. Seasonal changes in amino acids and phenolic compounds in fruits from hybrid cross populations of American grapes differing in disease resistance. Plant Physiol. Biochem. 2019, 135, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.K.; Singh, J.; Acharya, P.; Jayaprakasha, G.; Patil, B.S. Identification and quantification of phytochemicals, antioxidant activity and bile acid-binding capacity of Garnet Stem dandelion (Taraxacum officinale). J. Food Sci. 2018, 83, 1569–1578. [Google Scholar] [CrossRef]
- Acharya, P.; Crosby, K.M.; Jayaprakasha, G.K.; Jifon, J.L.; Patil, B.S. Nanoparticle-mediated seed priming improves germination, growth, yield and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganji, S.M.; Singh, H.; Friedman, M. Phenolic content and antioxidant activity of extracts of 12 melon (Cucumis melo L.) peel powders prepared from commercial melons. J. Food Sci. 2019, 84, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Olea-Azar, C.; Cavieres, C.; Norambuena, E.; Delgado-Castro, T.; Soto-Delgado, J.; Araya-Maturana, R. Antioxidant properties and free radical-scavenging reactivity of a family of hydroxynaphthalenones and dihydroxyanthracenones. Bioorg. Med. Chem. 2007, 15, 7058–7065. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Y.-H. In vitro inhibitory effect of oriental melon (Cucumis melo L. var. makuwa Makino) seed on key enzyme linked to type 2 diabetes: Assessment of anti-diabetic potential of functional food. J. Funct. Foods 2013, 5, 981–986. [Google Scholar] [CrossRef]
- Hartman, J.L.; Wehner, T.C.; Ma, G.; Perkins-Veazie, P. Citrulline and arginine content of taxa of Cucurbitaceae. Horticulturae 2019, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Young, V.R. Adult amino acid requirements: The case for a major revision in current recommendations. J. Nutr. 1994, 124, 1517S–1523S. [Google Scholar] [CrossRef]
- Colle, R.; Masson, P.; Verstuyft, C.; Fève, B.; Werner, E.; Boursier-Neyret, C.; Walther, B.; David, D.J.; Boniface, B.; Falissard, B. Peripheral tryptophan, serotonin, kynurenine and their metabolites in major depression: A case–control study. Psychiatry Clin. Neurosci. 2020, 74, 112–117. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Bellaloui, N.; McClure, A.M.; Mengistu, A.; Abbas, H.K. The influence of agricultural practices, the environment and cultivar differences on soybean seed protein, oil, sugars and amino acids. Plants 2020, 9, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melandri, G.; AbdElgawad, H.; Riewe, D.; Hageman, J.A.; Asard, H.; Beemster, G.T.; Kadam, N.; Jagadish, K.; Altmann, T.; Ruyter-Spira, C. Biomarkers for grain yield stability in rice under drought stress. J. Exp. Bot. 2020, 71, 669–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akçay, N.; Bor, M.; Karabudak, T.; Özdemir, F.; Türkan, İ. Contribution of Gamma amino butyric acid (GABA) to salt stress responses of Nicotiana sylvestris CMSII mutant and wild type plants. J. Plant Physiol. 2012, 169, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Jayaprakasha, G.K.; Singh, J.; Ong, K.; Crosby, K.M.; Patil, B.S. Metabolomics-based biomarkers of Fusarium wilt disease in watermelon plants. J. Plant Dis. Prot. 2020, 127, 591–596. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ospina, L.; Moison, M.; Yoshimoto, K.; Masclaux-Daubresse, C. Autophagy, plant senescence and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar] [CrossRef] [Green Version]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Yoon, Y.-E.; Kuppusamy, S.; Cho, K.M.; Kim, P.J.; Kwack, Y.-B.; Lee, Y.B. Influence of cold stress on contents of soluble sugars, vitamin C and free amino acids including gamma-aminobutyric acid (GABA) in spinach (Spinacia oleracea). Food Chem. 2017, 215, 185–192. [Google Scholar] [CrossRef]
- Mazzucotelli, E.; Tartari, A.; Cattivelli, L.; Forlani, G. Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J. Exp. Bot. 2006, 57, 3755–3766. [Google Scholar] [CrossRef] [Green Version]
- Bernillon, S.; Biais, B.; Deborde, C.; Maucourt, M.; Cabasson, C.; Gibon, Y.; Hansen, T.H.; Husted, S.; de Vos, R.C.; Mumm, R. Metabolomic and elemental profiling of melon fruit quality as affected by genotype and environment. Metabolomics 2013, 9, 57–77. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.L.; Fundo, J.F.; Oliveira, A.; Beaulieu, J.C.; Fernández-Trujillo, J.P.; Almeida, D.P. 1-Methylcyclopropene effects on temporal changes of aroma volatiles and phytochemicals of fresh-cut cantaloupe. J. Sci. Food Agric. 2013, 93, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Fundo, J.F.; Miller, F.A.; Garcia, E.; Santos, J.R.; Silva, C.L.; Brandão, T.R. Physicochemical characteristics, bioactive compounds and antioxidant activity in juice, pulp, peel and seeds of cantaloupe melon. J. Food Meas. Charact. 2018, 12, 292–300. [Google Scholar] [CrossRef]
- Chethan, S.; Sreerama, Y.; Malleshi, N. Mode of inhibition of finger millet malt amylases by the millet phenolics. Food Chem. 2008, 111, 187–191. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Perez, J.L.; Jayaprakasha, G.; Patil, B.S. Metabolite profiling and in vitro biological activities of two commercial bitter melon (Momordica charantia Linn.) cultivars. Food Chem. 2019, 288, 178–186. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Girennavar, B.; Patil, B.S. Antioxidant capacity of pummelo and navel oranges: Extraction efficiency of solvents in sequence. LWT-Food Sci. Technol. 2008, 41, 376–384. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Vouldoukis, I.; Lacan, D.; Kamate, C.; Coste, P.; Calenda, A.; Mazier, D.; Conti, M.; Dugas, B. Antioxidant and anti-inflammatory properties of a Cucumis melo LC. extract rich in superoxide dismutase activity. J. Ethnopharmacol. 2004, 94, 67–75. [Google Scholar] [CrossRef]
- Triantis, T.; Yannakopoulou, E.; Nikokavoura, A.; Dimotikali, D.; Papadopoulos, K. Chemiluminescent studies on the antioxidant activity of amino acids. Anal. Chimic. Acta 2007, 591, 106–111. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Decker, E.A.; Ivanov, V.; Zhu, B.-Z.; Frei, B. Inhibition of low-density lipoprotein oxidation by carnosine and histidine. J. Agric. Food Chem. 2001, 49, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, E.; Loreau, N.; Lagrost, L.; Blache, D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic. Res. 2005, 39, 15–20. [Google Scholar] [CrossRef]
- Caylak, E.; Aytekin, M.; Halifeoglu, I. Antioxidant effects of methionine, α-lipoic acid, N-acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. Exp. Toxicol. Pathol. 2008, 60, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Winkler-Moser, J.K.; Liu, S.X. Study on antioxidant activity of amino acids at frying temperatures and their interaction with rosemary extract, green tea extract and ascorbic acid. J. Food Sci. 2019, 84, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, L.; Yu, G. The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integr. Plant Biol. 2011, 53, 608–618. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Wang, M.; Sun, M.; Gu, Z.; Yang, R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019, 270, 593–601. [Google Scholar] [CrossRef]
- Çekiç, F.Ö. Exogenous GABA stimulates endogenous GABA and phenolic acid contents in tomato plants under salt stress. Celal Bayar Üniv. Fen Bilim. Derg. 2018, 14, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and tyrosine as exogenous precursors of wheat (triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phongthai, S.; D’Amico, S.; Schoenlechner, R.; Homthawornchoo, W.; Rawdkuen, S. Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem. 2018, 240, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, A.; Selga, A.; Torres, J.L.; Julià, L. Reducing activity of polyphenols with stable radicals of the TTM series. Electron transfer versus H-abstraction reactions in flavan-3-ols. Org. Lett. 2004, 6, 4583–4586. [Google Scholar] [CrossRef]
- Ketnawa, S.; Wickramathilaka, M.; Liceaga, A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chem. 2018, 254, 36–46. [Google Scholar] [CrossRef]
- Barkat, N.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Effect of harvest time on the levels of phytochemicals, free radical-scavenging activity, α-amylase inhibition and bile acid-binding capacity of spinach (Spinacia oleracea). J. Sci. Food Agric. 2018, 98, 3468–3477. [Google Scholar] [CrossRef]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.; Patil, B.S. Storage stability of dietary nitrate and phenolic compounds in beetroot (Beta vulgaris) and arugula (Eruca sativa) juices. J. Food Sci. 2018, 83, 1237–1248. [Google Scholar] [CrossRef]

| A. Amino acid contents in F-39 fruit grown in different locations. | ||||||
| Amino Acid | TX | NC | GA | CA | AZ | IN |
| Asn | 467.24 ± 30.95a | 164.34 ± 31.28c | 343.22 ± 69.86ab | 278.4 ± 57.9bc | 414.8 ± 52.37ab | 270.37 ± 36.04bc |
| Ser | 127.06 ± 8.39b | 114.52 ± 16.43b | 126.35 ± 23.64b | 170.07 ± 12.84ab | 120.86 ± 18.22b | 197.57 ± 4.78a |
| Hyp | 374.62 ± 45.16c | 467.8 ± 41.09c | 783.54 ± 151.41b | 521.89 ± 44.67c | 357.72 ± 30.26c | 960.82 ± 34.29a |
| Thr | 53.99 ± 12.67b | 10.48 ± 4.8c | 106.76 ± 21.28a | 36.02 ± 3.03bc | 132.49 ± 14.01a | 39.38 ± 2.82bc |
| Gly | 80.73 ± 6.47ab | 92.05 ± 2.41a | 62.45 ± 13.2bc | 74.8 ± 8.42abc | 53.58 ± 7.36c | 95.06 ± 4.82a |
| β-Ala | 34.59 ± 4.32b | 74.59 ± 2.77a | 35.73 ± 6.71b | 39.54 ± 3.8bc | 63.86 ± 6.64a | 78.49 ± 4.31a |
| Ala | 253.12 ± 54.99bc | 355.59 ± 28.34abc | 272.67 ± 63.77bc | 494.13 ± 53.3a | 178.64 ± 38.06c | 405.26 ± 38.52ab |
| Pro | 78.42 ± 6.17abc | 99.02 ± 5.73a | 68.13 ± 13.57bc | 85.65 ± 11.66abc | 61.13 ± 7.65c | 90.59 ± 0.92ab |
| Met | 49.84 ± 3.06a | 29.14 ± 1.85b | 34.6 ± 6.91ab | 50.66 ± 8.85a | 30.21 ± 4.07b | 42.78 ± 1.73ab |
| Val | 340.15 ± 14.09c | 455.75 ± 3.54b | 324.42 ± 69.29c | 357.7 ± 33.51bc | 287.86 ± 30.32c | 671.41 ± 23.84a |
| Trp | 43.3 ± 5.2a | 34.52 ± 7.33ab | 28.26 ± 4.49b | 52.64 ± 5.59ab | 37.54 ± 4.62ab | 44.31 ± 1.17a |
| Phe | 111.02 ± 14.66a | 40.83 ± 2.93c | 66.1 ± 13.6bc | 82.65 ± 11.36ab | 87.83 ± 6.81ab | 88.64 ± 4.51ab |
| Ile | 20.11 ± 1.79b | 21.35 ± 0.63ab | 19.13 ± 3.8b | 23.13 ± 2.08ab | 25.46 ± 0.78a | 22.23 ± 0.59ab |
| Leu | 19.85 ± 1.28c | 25.78 ± 1.79bc | 28.01 ± 5.42b | 31.78 ± 1.96ab | 34.8 ± 1.28a | 27.98 ± 0.98bc |
| B. Amino acid contents in HT-IG fruit grown in different locations. | ||||||
| Amino Acid | TX | NC | GA | CA | AZ | IN |
| Asn | 572.79 ± 22.58a | 468.04 ± 20.24ab | 395.28 ± 23.86b | 553.82 ± 11.97a | 493.44 ± 75.74ab | 505.65 ± 14.18ab |
| Ser | 140.68 ± 3.02ab | 154.28 ± 5.55ab | 118.71 ± 4.02b | 172.17 ± 5.99a | 133.58 ± 20.71ab | 157.97 ± 15.53ab |
| Hyp | 288.94 ± 31.88c | 414.74 ± 46.25bc | 543.95 ± 65.91ab | 436.05 ± 21.48bc | 282.21 ± 40.8c | 628.38 ± 17.4a |
| Thr | 81.19 ± 30.44ab | 32 ± 2.4b | 72.59 ± 16.47ab | 45.59 ± 2.41ab | 100.28 ± 5.35a | 52.92 ± 2.18ab |
| Gly | 76.88 ± 10.4ab | 80.64 ± 5.66ab | 47.7 ± 4.73b | 89.97 ± 2.72a | 71.38 ± 12.13ab | 63.53 ± 6.05ab |
| β-Ala | 20.41 ± 5.42c | 81.39 ± 7.5a | 33.14 ± 2.88bc | 66.56 ± 4.41a | 55.03 ± 9.36ab | 74.49 ± 10.28a |
| Ala | 206.13 ± 4.98b | 231.39 ± 25.96b | 158.38 ± 18.4b | 477.01 ± 52.75a | 248.04 ± 61b | 196.59 ± 34.59b |
| Pro | 99.2 ± 7.53ab | 110.5 ± 13.11a | 65.04 ± 6.13b | 122.27 ± 4.19a | 83.63 ± 11.75ab | 84.52 ± 10.38ab |
| Met | 56.67 ± 0.99a | 48.17 ± 4.4a | 40.11 ± 5.26a | 51.48 ± 3.77a | 42.59 ± 7.1a | 35.04 ± 6.08a |
| Val | 345.22 ± 9.67a | 445.42 ± 22.07a | 318.36 ± 25.52a | 424.48 ± 22.62a | 383.35 ± 67.48a | 350.47 ± 39.31a |
| Trp | 45.84 ± 0.78b | 34.07 ± 2.8b | 35.63 ± 2.44b | 71.82 ± 1.73a | 39.61 ± 6.99b | 32.34 ± 1.7b |
| Phe | 68.85 ± 7.73ab | 53.61 ± 5.64b | 50.36 ± 6.17b | 88.96 ± 4.54a | 66.77 ± 7.5ab | 62.48 ± 1.26b |
| Ile | 15.58 ± 0.92a | 39.52 ± 20.28a | 17.83 ± 0.33a | 21.09 ± 1.83a | 19.87 ± 2.2a | 25.82 ± 0.29a |
| Leu | 18.63 ± 1.66b | 27.35 ± 1.84ab | 23.43 ± 3.05ab | 27.12 ± 2.97ab | 27.63 ± 3.29ab | 31 ± 0.65a |
| C. Amino acid contents in TT-DV fruit grown in different locations. | ||||||
| Amino Acid | TX | NC | GA | CA | AZ | IN |
| Asn | 509.47 ± 13.85a | 336.83 ± 19.67b | 244.81 ± 19.47b | 331.7 ± 18.49b | 267.46 ± 47.15b | 482.57 ± 7.08a |
| Ser | 141.19 ± 5.92ab | 123.19 ± 6.39ab | 88.39 ± 5.65c | 143.7 ± 6.13ab | 168.77 ± 10.23a | 154.69 ± 1.39a |
| Hyp | 537.19 ± 254.53a | 446.47 ± 25.48a | 409.15 ± 27.68a | 565.07 ± 18.86a | 401.96 ± 23.01a | 531.02 ± 18.26a |
| Thr | 60.6 ± 6.93ab | 64.2 ± 33.45ab | 29.57 ± 2.61b | 41.03 ± 3.71ab | 109.29 ± 11.08a | 59.22 ± 1.34ab |
| Gly | 99.96 ± 8.47a | 91 ± 3.98ab | 56.12 ± 7.06c | 68.89 ± 1.9bc | 108.16 ± 9.78a | 97.31 ± 1.69a |
| β-Ala | 30.33 ± 5.69b | 72.08 ± 4.65a | 30.74 ± 3.68b | 32.34 ± 2.88b | 47.37 ± 2.41b | 75.52 ± 3.05a |
| Ala | 201.64 ± 32.17bc | 149.02 ± 10.53bcd | 98.16 ± 47.04cd | 252.98 ± 34.31b | 443.81 ± 57.3a | 50.11 ± 10.05d |
| Pro | 99.63 ± 6.66a | 78.37 ± 3.15abc | 56.65 ± 3.71c | 72.93 ± 5.81bc | 102.81 ± 10.77a | 89.82 ± 3.78ab |
| Met | 52.48 ± 2.77b | 45.68 ± 2.45b | 33.01 ± 2.39cd | 24.03 ± 2.66d | 40.01 ± 4.91bc | 66.28 ± 1.25a |
| Val | 409.28 ± 20.74b | 509.08 ± 27.11a | 303.99 ± 24.47c | 388.01 ± 11.56bc | 541.5 ± 41.1a | 475.65 ± 6.22ab |
| Trp | 55.38 ± 3.14ab | 42.92 ± 4.2b | 50.52 ± 4.11ab | 61.27 ± 5.82ab | 68.67 ± 10.75a | 62.67 ± 1.66ab |
| Phe | 145.48 ± 11.3a | 59.44 ± 8.7c | 81.61 ± 8.82bc | 111.88 ± 7.03ab | 104.82 ± 17.52ab | 117.68 ± 1.87ab |
| Ile | 22 ± 1.16c | 20.17 ± 1.35c | 20.31 ± 1.15c | 30.55 ± 1.66a | 23.53 ± 2.11bc | 29.67 ± 0.68ab |
| Leu | 24.71 ± 1.41c | 25.4 ± 1.44bc | 23.8 ± 1.91c | 47.01 ± 2.57a | 32.97 ± 3.63bc | 33.78 ± 1.23b |
| D. Amino acid contents in commercial local varieties grown in different locations. | ||||||
| Amino Acid | TX | NC | GA | CA | AZ | IN |
| Asn | 454.28 ± 17.55a | 147.21 ± 24.61d | 206.74 ± 30.48cd | 464.6 ± 21.43a | 277.92 ± 30.72bc | 361.34 ± 24.49ab |
| Ser | 106.71 ± 5.77c | 100.28 ± 10.76c | 110.13 ± 7.98c | 162.78 ± 8ab | 133.04 ± 12.3bc | 172.27 ± 4.18a |
| Hyp | 420.85 ± 19.55bcd | 557.59 ± 37.21ab | 406.19 ± 23.23cd | 533.1 ± 50.55bc | 343.34 ± 42d | 692.04 ± 13.03a |
| Thr | 55.6 ± 9.35bc | 23.66 ± 4.12c | 26.98 ± 1.97c | 90.79 ± 11.25a | 99.01 ± 5.55a | 76.74 ± 10.64ab |
| Gly | 94.66 ± 4.96a | 40.03 ± 4.75c | 71.49 ± 4.84b | 103.56 ± 4.25a | 57.86 ± 5.5bc | 95.06 ± 6.72a |
| β-Ala | 27.64 ± 3.83d | 51.4 ± 6.95bc | 43.67 ± 2.57cd | 65.81 ± 2.58b | 50.37 ± 4.71bc | 89.45 ± 0.79a |
| Ala | 319.32 ± 37.81abc | 58.75 ± 16d | 307.72 ± 18.44bc | 429.43 ± 46.75a | 211.62 ± 7.18c | 416.52 ± 16.56ab |
| Pro | 89.63 ± 5.96a | 46.75 ± 4.55b | 56.35 ± 5.16b | 104.32 ± 9.16a | 56.32 ± 4.93b | 108.06 ± 9.85a |
| Met | 17.51 ± 1.62c | 43.55 ± 4.87a | 20.82 ± 1.33c | 39.68 ± 2.23ab | 40.77 ± 5.28ab | 28.17 ± 1.12bc |
| Val | 398.58 ± 8.98b | 243.75 ± 18.72c | 407.27 ± 28.43b | 395.03 ± 15.7b | 362.61 ± 41.12b | 510.73 ± 4.5a |
| Trp | 46.75 ± 3.38ab | 35.68 ± 9.11bc | 32.68 ± 1.05bc | 58.82 ± 2.04a | 22.92 ± 2.61c | 46.85 ± 2.09ab |
| Phe | 81.56 ± 8.13a | 70.53 ± 15.59a | 47.13 ± 3.65a | 82.21 ± 6.88a | 67.84 ± 7.51a | 81.41 ± 3.92a |
| Ile | 17.38 ± 2.04b | 22.69 ± 2.49ab | 15.78 ± 1.27b | 21.09 ± 0.81b | 21.17 ± 2.01b | 30.31 ± 1.48a |
| Leu | 20.31 ± 3.06b | 33.51 ± 3.04a | 19.41 ± 1.78b | 30.72 ± 1.43ab | 31.9 ± 3.05a | 40.98 ± 2.81a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.; Metrani, R.; Jayaprakasha, G.K.; Crosby, K.M.; Ravishankar, S.; Patil, B.S. Multivariate Analysis of Amino Acids and Health Beneficial Properties of Cantaloupe Varieties Grown in Six Locations in the United States. Plants 2020, 9, 1058. https://doi.org/10.3390/plants9091058
Singh J, Metrani R, Jayaprakasha GK, Crosby KM, Ravishankar S, Patil BS. Multivariate Analysis of Amino Acids and Health Beneficial Properties of Cantaloupe Varieties Grown in Six Locations in the United States. Plants. 2020; 9(9):1058. https://doi.org/10.3390/plants9091058
Chicago/Turabian StyleSingh, Jashbir, Rita Metrani, Guddadarangavvanahally K. Jayaprakasha, Kevin M. Crosby, Sadhana Ravishankar, and Bhimanagouda S. Patil. 2020. "Multivariate Analysis of Amino Acids and Health Beneficial Properties of Cantaloupe Varieties Grown in Six Locations in the United States" Plants 9, no. 9: 1058. https://doi.org/10.3390/plants9091058
APA StyleSingh, J., Metrani, R., Jayaprakasha, G. K., Crosby, K. M., Ravishankar, S., & Patil, B. S. (2020). Multivariate Analysis of Amino Acids and Health Beneficial Properties of Cantaloupe Varieties Grown in Six Locations in the United States. Plants, 9(9), 1058. https://doi.org/10.3390/plants9091058








