Monitoring the Efficiency of Rhazya stricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil
Abstract
:1. Introduction
2. Results
2.1. Growth Rate and Resource Allocation
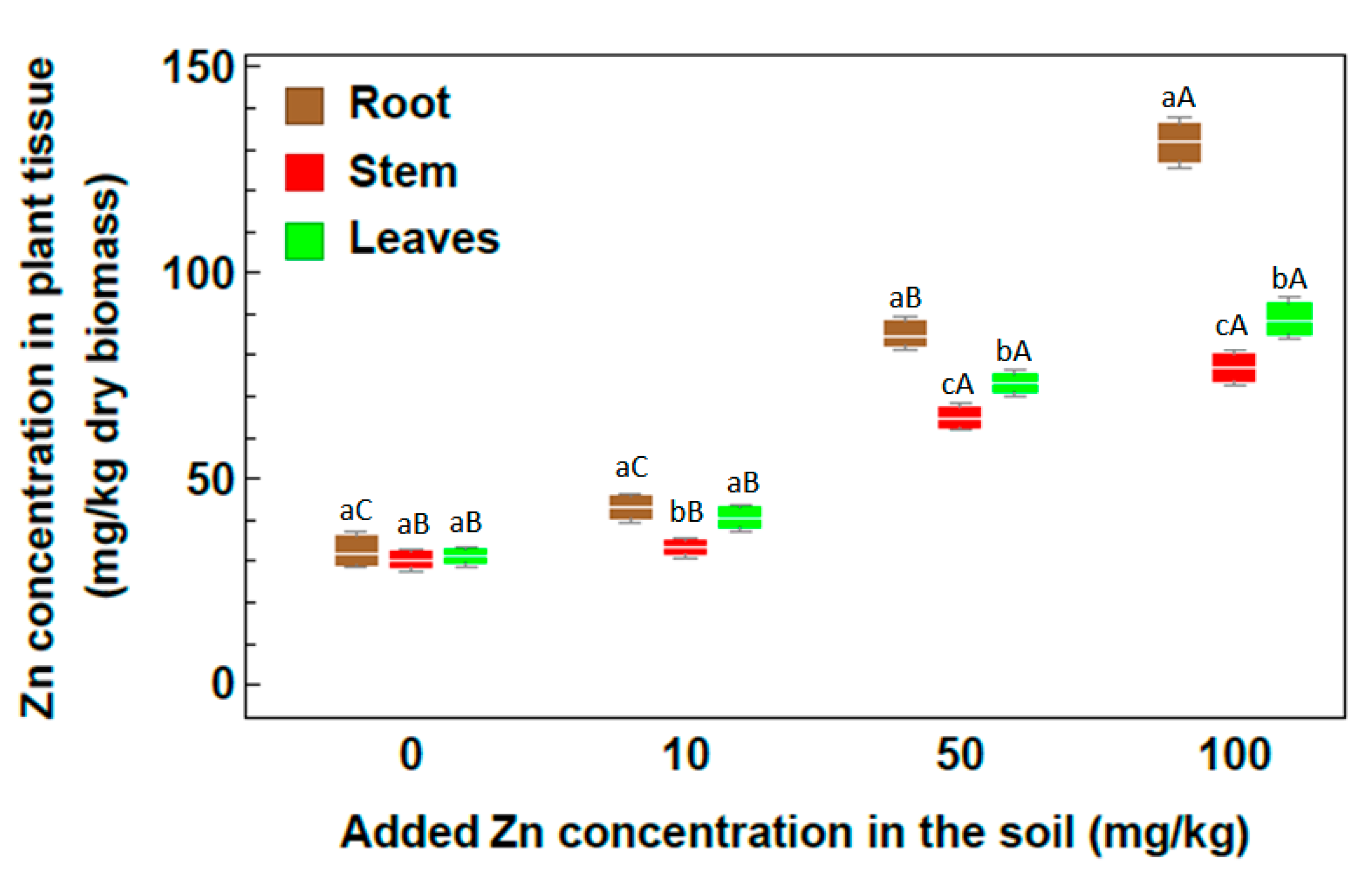
2.2. Concentration of Heavy Metals
2.3. Correlation Analysis
2.4. Bioconcentration and Translocation Factors
3. Discussion
4. Materials and Methods
4.1. Study Species
4.2. Pot Experiment
4.3. Growth Rate
4.4. Heavy Metal Analysis
4.5. Calculation of Bioconcentration and Translocation Factors
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Liu, C.; Hsu, P.-C.; Zhao, J.; Wu, T.; Tang, J.; Liu, K.; Cui, Y. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun. 2019, 10, 2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Ying, G.-G. Chapter 14 remediation and mitigation strategies. In Integrated Analytical Approaches for Pesticide Management; Maestroni, B., Cannavan, A., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Roy, M.; McDonald, L.M. Metal uptake in plants and health risk assessments in metal-contaminated smelter soils. Land Degrad. Dev. 2013, 26, 785–792. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Gohre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.J.; Luo, C.L.; Chen, Y.H.; Wang, G.P.; Li, X.D.; Shen, Z.G. Cadmium and other metal uptake by Lobelia chinensis and Solanum nigrum from contaminated soils. Bull. Environ. Contam. Toxicol. 2009, 83, 260–264. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Emam, M.H.; Lovett-Doust, L.; Azab, E.; El-Khatib, A.A. Response of duckweed to lead exposure: Phytomining, bioindicators and bioremediation. Desalin. Water Treat. 2017, 70, 227–234. [Google Scholar] [CrossRef]
- Gratão, P.L.; Prasad, M.N.V.; Cardoso, P.F.; Lea, P.J.; Azevedo, R.A. Phytoremediation: Green technology for the clean up of toxic metals in the environment. Braz. J. Plant Physiol. 2005, 17, 53–64. [Google Scholar] [CrossRef]
- Azab, E.; Kebeish, R.; Hegazy, A.K. Expression of the human gene CYP1A2 enhances tolerance and detoxification of the phenylurea herbicide linuron in Arabidopsis thaliana plants and Escherichia coli. Environ. Pollut. 2018, 238, 281–290. [Google Scholar] [CrossRef]
- Azab, E.; Hegazy, A.K.; El-Sharnouby, M.E.; Abd Elsalam, H.E. Phytoremediation of the organic Xenobiotic simazine by p450-1a2 transgenic Arabidopsis thaliana plants. Int. J. Phytoremediat. 2016, 18, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Moubasher, H.A.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.M.; Kabiel, H.F.; Hamad, A.A. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Chehregani, A.; Noori, M.; Yazdi, H.L. Phytoremediation of heavy-metal-polluted soils: Screening for new accumulator plants in Angouran mine (Iran) and evaluation of removal ability. Ecotoxicol. Environ. Saf. 2009, 72, 1349–1353. [Google Scholar] [CrossRef]
- Willscher, S.; Mirgorodsky, D.; Jablonski, L.; Ollivier, D.; Merten, D.; Büchel, G.; Wittig, J.; Werner, P. Field scale phytoremediation experiments on a heavy metal and uranium contaminated site, and further utilization of the plant residues. Hydrometallurgy 2013, 131–132, 46–53. [Google Scholar] [CrossRef]
- Li, C.; Ji, X.; Luo, X. Phytoremediation of heavy metal pollution: A bibliometric and scientometric analysis from 1989 to 2018. Int. J. Environ. Res. Public Health 2019, 16, 4755. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, A.K.; Emam, M.H. Accumulation and Soil-to-Plant Transfer of Radionuclides in the Nile Delta Coastal Black Sand Habitats. Int. J. Phytoremediat. 2010, 13, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Antonkiewicz, J.; Jasiewicz, C. The use of plants accumulating heavy metals for detoxification of chemically polluted soils. Electron. J. Pol. Agric. Univ. 2002, 5, 121–143. [Google Scholar]
- Hegazy, A.K. Phytomonitoring and management of tar piles on the Qatari coastal marshes, Arabian Gulf. Environ. Pollut. 1995, 90, 187–190. [Google Scholar] [CrossRef]
- Maiti, S.K.; Jaiswal, S. Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: A field study from Santaldih thermal power plant, West Bengal, India. Environ. Monit. Assess. 2008, 136, 355–370. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.; Yavaş, İ.; Ünay, A.; Abdel Daim, M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of Floating Aquatic Plants in Phytoremediation of Heavy Metals Polluted Water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef] [Green Version]
- Oyuela Leguizamo, M.A.; Fernández Gómez, W.D.; Sarmiento, M.C.G. Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—A review. Chemosphere 2017, 168, 1230–1247. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.; Lovett-Doust, J. Plant Ecology in the Middle East; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Humphries, M.M.; Studd, E.K.; Menzies, A.K.; Boutin, S. To everything there is a season: Summer-to-winter food webs and the functional traits of keystone species. Integr. Comp. Biol. 2017, 57, 961–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeshen, M.N.; Al-Attas, S.G.; Ahmed, M.M.; Hanafy, A.A.-E.L.M.E.L.; Anwar, Y.; Alotibi, I.A.; Baeshen, N.A. The effect of Rhazya stricta aqueous leaves extract on MRSA genotypes in Jeddah province. Biotechnol. Biotechnol. Equip. 2016, 30, 368–374. [Google Scholar] [CrossRef]
- Al-Farraj, A.S.; Al-Wabel, M.I.; Al-Shahrani, T.S.; El-Maghraby, S.E.; Al-Sewailem, M.A.S. Accumulation coefficient and translocation factor of heavy metals through Rhazya strictagrown in the mining area of Mahad AD’Dahab, Saudi Arabia. Waste Manag. Environ. V 2010, 140, 325–336. [Google Scholar]
- Ghoneim, A.M.; Al-Zahrani, S.S.; El-Maghraby, S.E.; Al-Farraj, A.S. Heavy Metals Accumulation in Rhazya stricta L. Plant Growing on Industrial Wastewater of Riyadh City, Saudi Arabia. J. Appl. Sci. 2014, 14, 2007–2010. [Google Scholar] [CrossRef] [Green Version]
- Badr, N.; Al-Qahtani, K.M. Treatment of wastewater containing arsenic using Rhazya stricta as a new adsorbent. Environ. Monit. Assess. 2013, 185, 9669–9681. [Google Scholar] [CrossRef] [Green Version]
- Badr, N.; Fawzy, M.; Al-Qahtani, K.M. Phytoremediation: An Ecological Solution to Heavy-Metal-Polluted Soil and Evaluation of Plant Removal Ability. World Appl. Sci. J. 2012, 16, 1292–1301. [Google Scholar]
- Al-Qahtani, K. Assessment of heavy metals accumulation in native plant species from soils contaminated in riyadh city, saudi arabia. Life Sci. J. 2012, 9, 384–392. [Google Scholar]
- Ibrahim, M.M.; Alsahli, A.A.; El-Gaaly, G. Evaluation of phytoremediation potential of six wild plants for metal in a site polluted by industrial wastes: A field study in Riyadh, Sudi Arabia. Pak. J. Bot. 2013, 42, 571–576. [Google Scholar]
- Khattak, I.M.; Jabeen, R.; Hameed, M.; Arfat, Y. A study of some heavy metals found in medicinal plants (Euphorbia cornigera, Rhazya stricta and Citrullus colocynthis) in Turbat region of Balochistan with reference to prevention of environmental pollution. Pak. J. Bot. 2015, 47, 1511–1516. [Google Scholar]
- Shah, A.; Niaz, A.; Ullah, N.; Rehman, A.; Akhlaq, M.; Zakir, M.; Khan, M.S. Comparative Study of Heavy Metals in Soil and Selected Medicinal Plants. J. Chem. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Salemaa, M.; Derome, J.; Kiikkila, O.; Uhlig, C.; Nieminen, T.M. Remediation of heavy metal-contaminated forest soil using recycled organic matter and native woody plants. J. Environ. Qual. 2007, 36, 1145–1153. [Google Scholar] [CrossRef]
- Hameed, M.A.; Reid, J.B.; Rowe, R.N. Root confinement and its effects on the water relations, growth and assimilate partitioning of tomato (lycopersicon esculentum mill). Ann. Bot. 1987, 59, 685–692. [Google Scholar] [CrossRef]
- Smical, A.-I.; Hotea, V.; Oros, V.; Juhasz, J.; Pop, E. Studies on transfer and bioaccumulation of heavy metals from soil into lettuce. Environ. Eng. Manag. J. 2008, 7, 609–615. [Google Scholar] [CrossRef]
- Kimbrough, R.D.; Krouskas, C.A. Contribution of lead in soil to children’s lead burden, an update. Chem. Speciat. Bioavailab. 2012, 24, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, M.; Hajabbasi, M.A.; Afyuni, M.; Charkhabi, A.H.; Shariatmadari, H. Bioaccumulation of nickel and lead by Bermuda grass (Cynodon dactylon) and tall fescue (Festuca arundinacea) from two contaminated soils. Casp. J. Environ. Sci. 2009, 7, 59–70. [Google Scholar]
- Ji-Tao, S.; Bao-guo, T.; Hong-tao, W.; Basta, N.; Schroder, J.; Casillas, M. Assessing availability, phytotoxicity and bioaccumulation of lead to ryegrass and millet based on 0.1 mol/L ca(NO3)2 extraction. J. Environ. Sci. 2006, 18, 958–963. [Google Scholar] [CrossRef]
- Midrar-ul-Haq; Khattak, R.A.; Puno, H.K.; Saif, M.S.; Memon, K.S.; Sial, N.B. Bioaccumulation of trace elements by different plant species grown on potentially contaminated soils of nwfp, pakistan. Asian J. Plant Sci. 2005, 4, 383–387. [Google Scholar]
- Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; Montes, M.; de la Rosa, G.; Corral-Diaz, B. Bioaccumulation of cadmium, chromium and copper by Convolvulus arvensis L.: Impact on plant growth and uptake of nutritional elements. Bioresour. Technol. 2004, 92, 229–235. [Google Scholar] [CrossRef]
- Rudani, K.; Patel, V.; Kalavati, P. The importance of zinc in plant growth—A review. Int. Res. J. Nat. Appl. Sci. 2018, 5, 38–48. [Google Scholar]
- Mertens, J.; Smolders, E. Zinc. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 465–493. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements a review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Bert, V.; Macnair, M.; De Laguerie, P.; Saumitou-Laprade, P.; Petit, D. Zinc tolerance and accumulation in metallicolous and nonmetallicolous populations of Arabidopsis halleri (Brassicaceae). New Phytol. 2000, 146, 225–233. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.J.; Kim, H.; Seo, S.H.; Hwang, B.G.; Chang, Y.S.; Lee, J.; Lee, D.W.; Sohn, E.J.; Lee, S.J.; Lee, Y.; et al. Cytochrome b5 Reductase 1 Triggers Serial Reactions that Lead to Iron Uptake in Plants. Mol. Plant 2016, 9, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals: Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Bini, C.; Wahsha, M.; Fontana, S.; Maleci, L. Effects of heavy metals on morphological characteristics of Taraxacum officinale Web growing on mine soils in NE Italy. J. Geochem. Explor. 2012, 123, 101–108. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci Rep. 2019, 9, 5658. [Google Scholar] [CrossRef] [Green Version]
- Wuana, R.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Netw. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Jadia, C.D.; Fulekar, M. Phytoremediation of heavy metals: Recent techniques. Afr. J. Biotechnol. 2009, 8, 921–928. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, T.; Huang, H.; Zou, T.; Zhang, X.; Yu, H.; Zheng, Z.; Wang, Y. Cd accumulation and phytostabilization potential of dominant plants surrounding mining tailings. Environ. Sci. Pollut. Res. 2012, 19, 3879–3888. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.K.; Abdel-Ghani, N.T.; El-Chaghaby, G.A. Adsorption of phenol onto activated carbon from Rhazya stricta: Determination of the optimal experimental parameters using factorial design. Appl. Water Sci. 2014, 4, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, M.S.; Amin, F. Accumulation and distribution of lead (Pb) in plant tissues of guar (Cyamopsis tetragonoloba L.) and sesame (Sesamum indicum L.): Profitable phytoremediation with biofuel crops. Geol. Ecol. Landsc. 2018, 2, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Aragón, L.; Lasso, E. How do young cacti (seeds and seedlings) from tropical xeric environments cope with extended drought periods? J. Arid Environ. 2018, 154, 1–7. [Google Scholar]
- Ruiz, M.A.; Golberg, A.; Molas, M.L. From seed to seedling: An ecophysiological point of view. In From Seed Germination to Young Plants: Ecology, Growth and Environmental Influences; Busso, C.A., Ed.; Nova Biomedical: New York, NY, USA, 2013; pp. 3–26. [Google Scholar]
- Güleryüz, G.; Kırmızı, S.; Arslan, H.; Derya, S. The effects of heavy metals on the seed germination and seedling growth of two endemic verbascum species. Fresenius Environ. Bull. 2016, 25, 1134–1142. [Google Scholar]
- Sethy, S.K.; Ghosh, S. Effect of heavy metals on germination of seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhong, G.; Shi, G.; Pan, F. Toxicity of Cu, Pb, and Zn on Seed Germination and Young Seedlings of Wheat (Triticum aestivum L.). In Proceedings of the Computer and Computing Technologies in Agriculture IV, Nanchang, China, 22–25 October 2010; pp. 231–240. [Google Scholar]
- Wang, J.; Li, W.; Zhang, C.; Ke, S. Physiological responses and detoxific mechanisms to Pb, Zn, Cu and Cd in young seedlings of Paulownia fortunei. J. Environ. Sci. 2010, 22, 1916–1922. [Google Scholar] [CrossRef]
- Bae, J.; Benoit, D.L.; Watson, A.K. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef]
- Abraham, K.; Sridevi, R.; Suresh, B.; Damodharam, T. Effect of heavy metals (Cd, Pb, Cu) on seed germination of Arachis hypogeae L. Asian J. Plant Sci. Res. 2013, 3, 10–12. [Google Scholar]
- Awofolu, O.R. A survey of trace metals in vegetation, soil and lower animal along some selected major roads in metropolitan city of Lagos. Environ. Monit. Assess. 2005, 105, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation technology: Hyper-accumulation metals in plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]

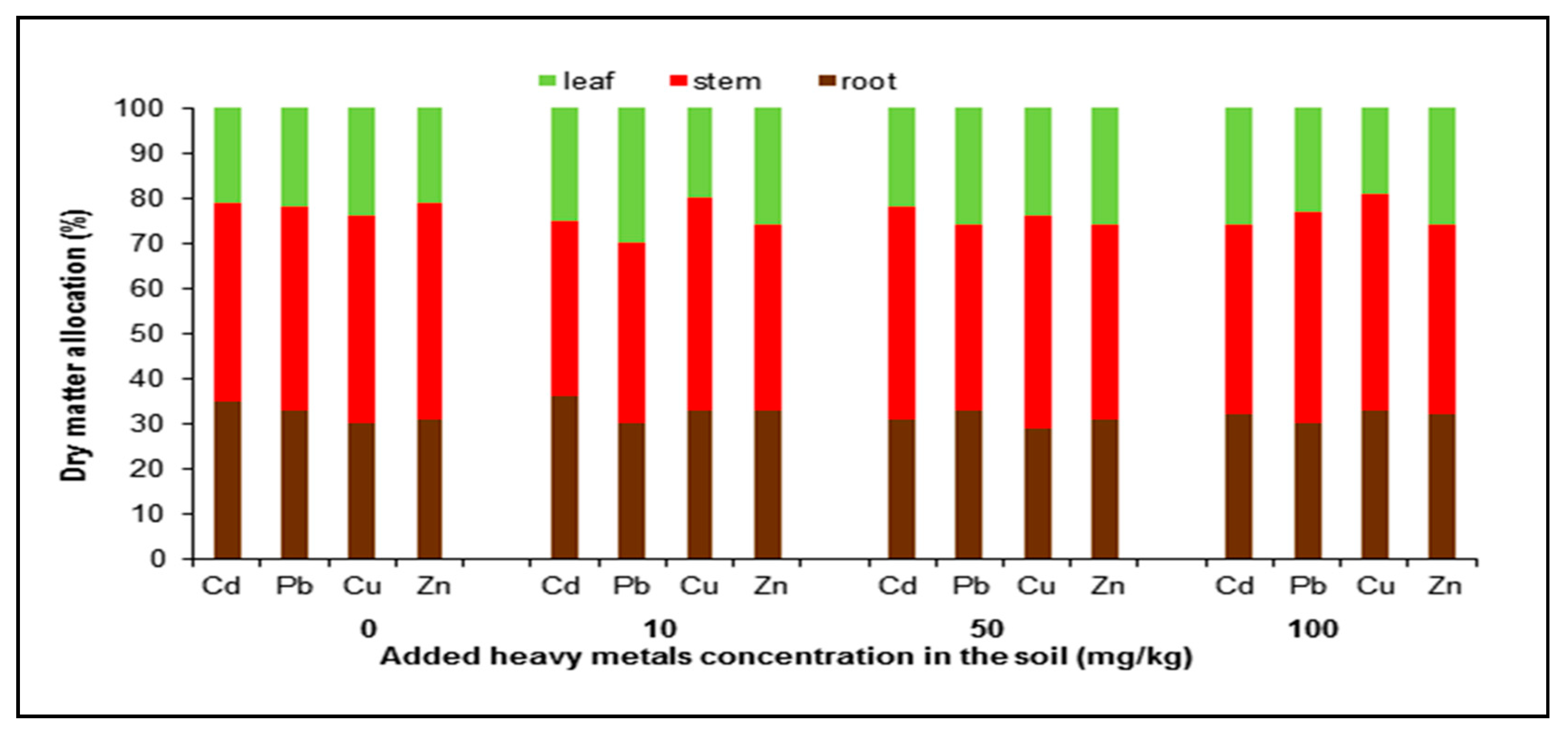
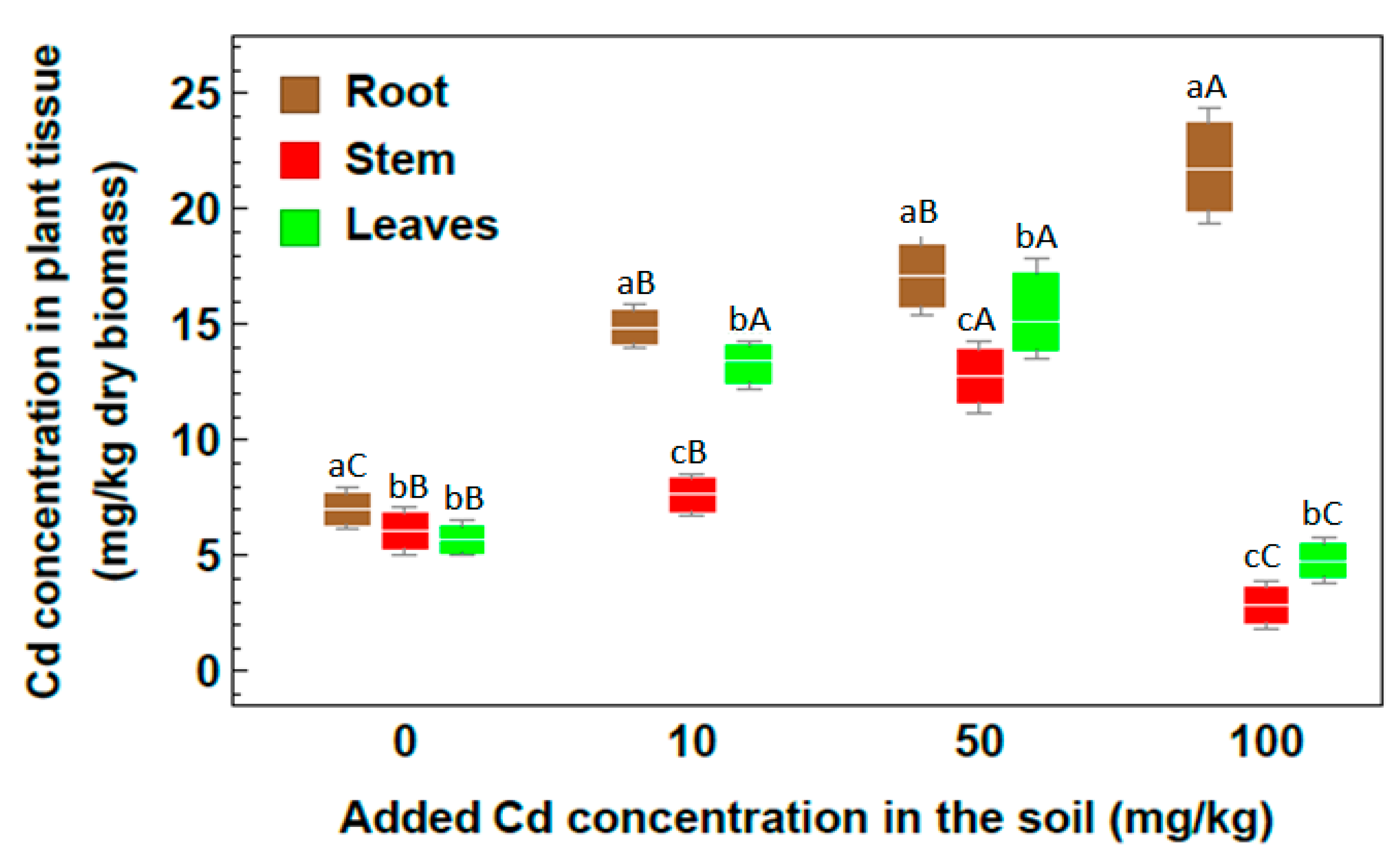
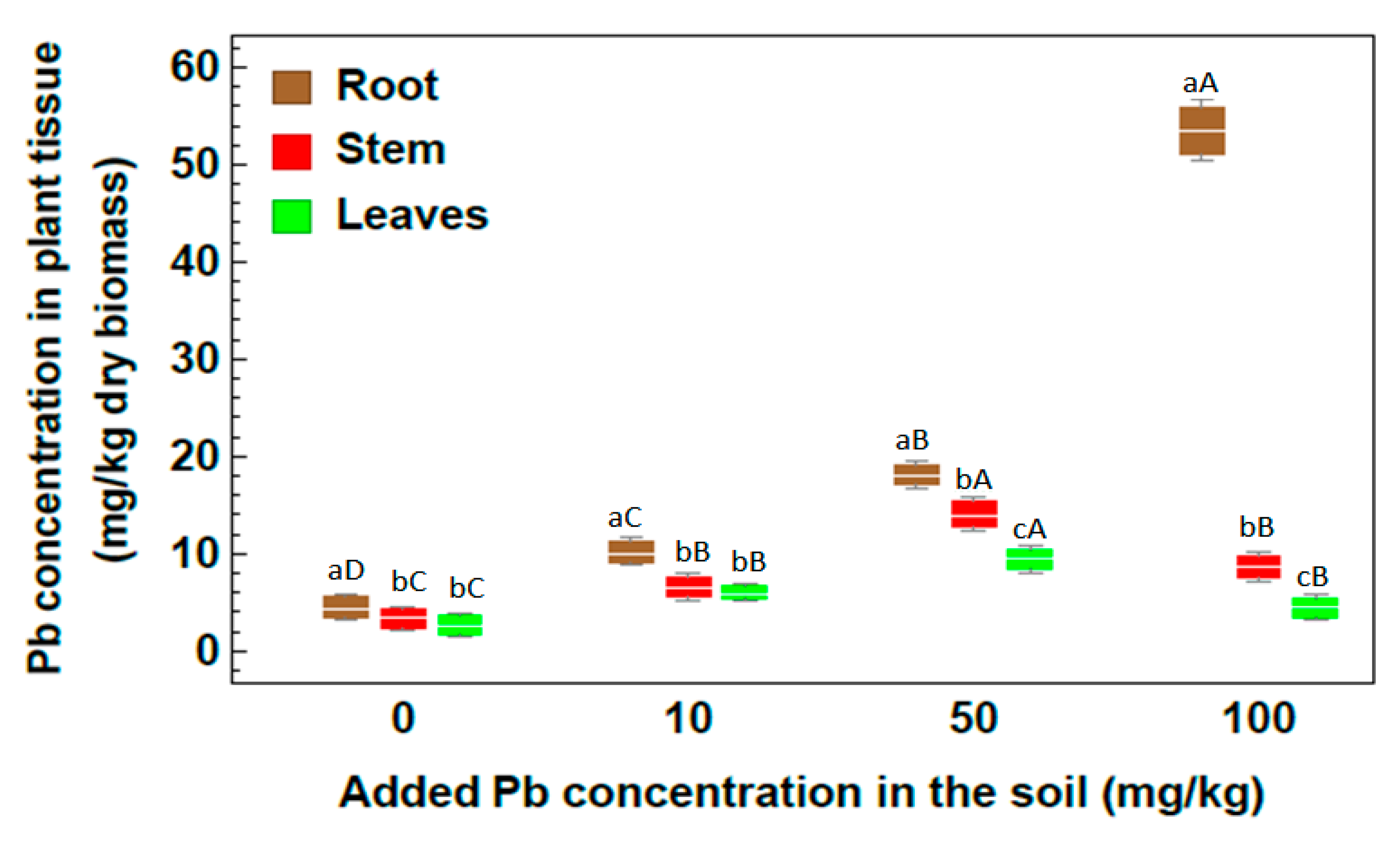
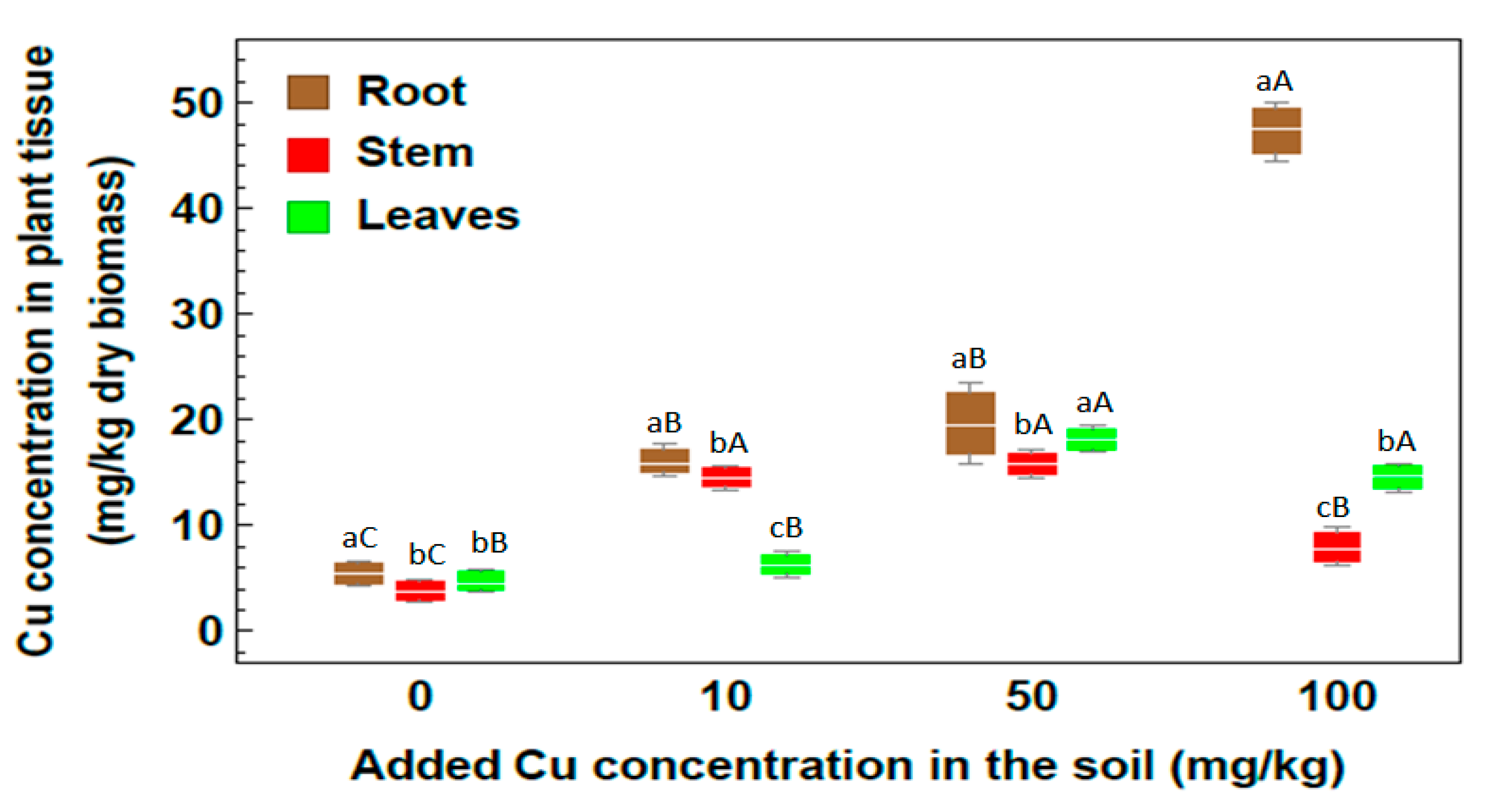

| Metal | Root | Stem | Leaf |
|---|---|---|---|
| Cd | 0.856 | −0.250 | −0.201 |
| Pb | 0.988 * | 0.478 | 0.210 |
| Cu | 0.95 * | 0.576 | 0.117 |
| Zn | 0.996 * | 0.968 * | 0.964 * |
| Metal Conc. in Soil | Translocation Factor in Plant Shoots | |||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Cu | Zn | |||||
| TF Stem | TF Leaves | TF Stem | TF Leaves | TF Stem | TF Leaves | TF Stem | TF Leaves | |
| 0 | 0.92 a | 0.81 a | 0.69 a | 0.56 a | 0.74 a | 0.95 a | 0.90 a | 0.96 a |
| 10 | 0.51 b | 0.88 a | 0.61 a | 0.57 a | 0.81 a | 0.35 b | 0.79 b | 0.92 a |
| 50 | 0.79 a | 0.91 a | 0.74 a | 0.53 b | 0.83 a | 0.92 a | 0.76 b | 0.88 a |
| 100 | 0.13 c | 0.23 b | 0.15 b | 0.08 c | 0.16 b | 0.29 b | 0.59 c | 0.68 b |
| L.S.D | 0.15 | 0.21 | 0.12 | 0.02 | 0.27 | 0.12 | 0.01 | 0.12 |
| Heavy Metal | Lamp Current (mA) | Fuel | Support | Wavelength (nm) |
|---|---|---|---|---|
| Cadmium | 5 | Acetylene | Air | 228.8 |
| Lead | 10 | Acetylene | Air | 217 |
| Copper | 5 | Acetylene | Air | 324.8 |
| Zinc | 10 | Acetylene | Air | 213.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azab, E.; Hegazy, A.K. Monitoring the Efficiency of Rhazya stricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil. Plants 2020, 9, 1057. https://doi.org/10.3390/plants9091057
Azab E, Hegazy AK. Monitoring the Efficiency of Rhazya stricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil. Plants. 2020; 9(9):1057. https://doi.org/10.3390/plants9091057
Chicago/Turabian StyleAzab, Ehab, and Ahmad K. Hegazy. 2020. "Monitoring the Efficiency of Rhazya stricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil" Plants 9, no. 9: 1057. https://doi.org/10.3390/plants9091057
APA StyleAzab, E., & Hegazy, A. K. (2020). Monitoring the Efficiency of Rhazya stricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil. Plants, 9(9), 1057. https://doi.org/10.3390/plants9091057






