Secondary Bioactive Metabolites from Plant-Derived Food Byproducts through Ecopharmacognostic Approaches: A Bound Phenolic Case Study
Abstract
:1. Introduction and Historical Background
2. Food Losses and Wastes
2.1. Definitions
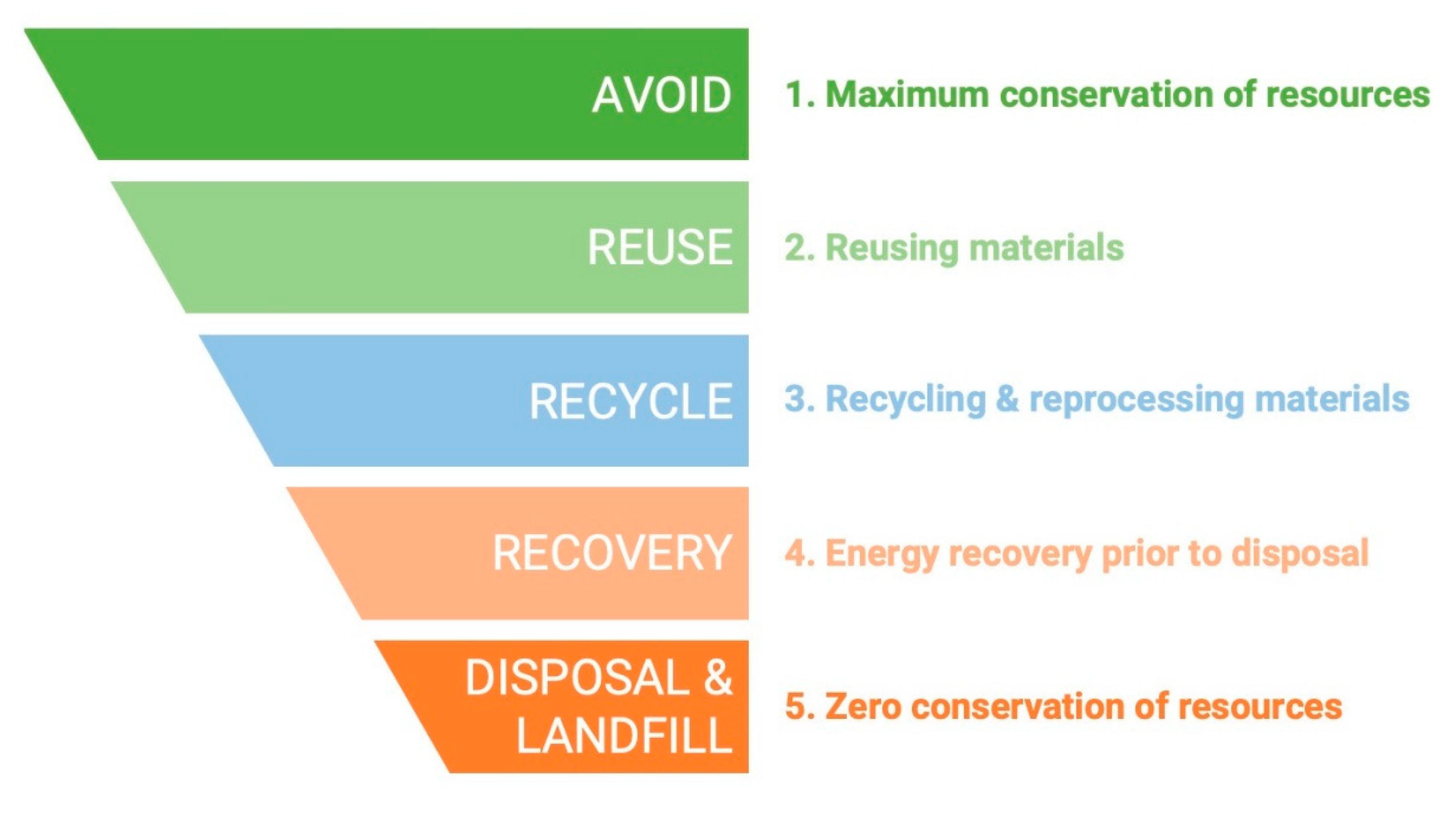
2.2. Types of Food and Waste (or ByProducts) Management
2.3. Extend, Costs and Global Concern
2.4. The EU Agenda against Food Wastes: The “Circular Economy Action Plan”
3. Natural Products Research
3.1. The Role of Natural Products Research
3.2. The Biorefinery Concept in Ecopharmacognosy: Recovery of Bioactive Compounds
4. Emerging Technologies in Ecopharmacognosy: Green Extractions
- Innovation through selection and use of varieties of renewable plant sources;
- Using alternative green solvents, mainly water or agri-solvents;
- Reducing energy consumption by energy recovery and use of innovative technologies;
- Favoring the production of coproducts instead of wastes in the bio- and agri-refining industries;
- Reducing producing operations favoring short, safe, robust and controlled processes;
- Aiming for obtaining non-denatured, biodegradable and contaminants-free extracts (i.e., without heavy metals, mycotoxins, etc.).
- Coming from renewable feedstock;
- Recyclable with eco-efficient treatments;
- Exhibit similar solvating properties of commonly used solvents;
- Have a high boiling point and low vapor pressure;
- Be biodegradable under environmental conditions.
4.1. Ultrasound-Assisted Extraction (UAE)
4.2. Microwave-Assisted Extraction (MAE)
4.3. Pressurized Solvent Extraction (Naviglio® Extractor)
4.4. Supercritical Fluids Extraction (SFE)
4.5. Subcritical Water Extraction (SWE)
4.6. Natural Deep Eutectic Solvents (NADES)
4.7. Enzyme-Assisted Extraction
4.8. Hybrid Techniques
5. Bound Phenolics from Waste Biomass: A Case Study
6. Conclusions: Up-Scaling and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clemént, G. Giardini, Paesaggio e Genio Naturale, 1st ed.; Quodlibet srl: Macerata, Italy, 2013; pp. 19–21. [Google Scholar]
- Stahel, W.R.; Reday-Mulvey, G. Jobs for tomorrow: The potential for substituting manpower for energy. In Circular Economy and Sustainability—Two Faces of the Same Coin; Vantage Press: New York, NY, USA, 1981; Volume 116. [Google Scholar]
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and social Committee and the Committee of the Regions. In Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Saavedra, Y.M.B.; Iritani, D.R.; Pavan, A.L.R.; Ometto, A.R. Theoretical contribution of industrial ecology to circular economy. J. Clean. Prod. 2018, 170, 1514–1522. [Google Scholar] [CrossRef]
- Zitkowski, H.E. The Recovery of Potash from Beet-Sugar House Waste Liquors. J. Ind. Eng. Chem. 1917, 9, 692–694. [Google Scholar] [CrossRef]
- Pruthi, J.S.; Parekh, C.M.; Girdhari, L. An integrated process for the recovery of essential oil and pectin from mandarin orange waste. J. Food Sci. 1961, 10, 372–378. [Google Scholar]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [Green Version]
- Stenmarck, Å.; Jensen, C.; Quested, T. Fusions: Estimates of European Food Waste Levels; Fusions-IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- FAO (Food and Agricultural Organization of the United Nations). Global Food Losses and Food Waste-Extend, Causes and Prevention; FAO: Düsseldorf, Germany, 2011. [Google Scholar]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Vision 2020. Achieving Zero Waste to Landfill. Available online: www.vision2020.info (accessed on 28 January 2020).
- Ki Lin, C.S.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.K.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, N.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2016, 6, 426–464. [Google Scholar] [CrossRef]
- Laufenberg, G.; Kunz, B.; Nystroem, M. Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- FAO (Food and Agricultural Organization of the United Nations). Food Wastage Foodprint-Full-Cost Accounting 79; FAO: Düsseldorf, Germany, 2014. [Google Scholar]
- Fattibene, D.; Bianchi, M. Fighting against Food Losses and Waste: An EU Agenda; IAI Working Papers 17; IAI: Rome, Italy, 2017. [Google Scholar]
- Ministero dell’Ambiente e della Tutela del Territorio e del Mare. Available online: https://www.minambiente.it/comunicati/litalia-avra-un-piano-nazionale-di-prevenzione-dello-spreco-alimentare (accessed on 16 July 2019).
- FAO (Food and Agricultural Organization of the United Nations). Food Wastage Footprint. Impacts on Natural Resources; Summary Report; FAO: Düsseldorf, Germany, 2013. [Google Scholar]
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and social Committee and the Committee of the Regions. In On Implementation to the Circular Economy Action Plan; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and social Committee and the Committee of the Regions. In On implementation to the Circular Economy Action Plan; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. Available online: https://ec.europa.eu/international-partnerships/news/european-union-presents-its-progress-towards-sustainable-development_en (accessed on 18 December 2019).
- Cordell, G.A. Cognate and cognitive ecopharmacognosy-in an anthropogenic era. Phytochem. Lett. 2017, 20, 540–549. [Google Scholar] [CrossRef]
- Cordell, G.A. Ecopharmacognosy and the responsibilities of natural product research to sustainability. Phytochem. Lett. 2015, 11, 332–346. [Google Scholar] [CrossRef]
- Devappa, R.K.; Rakshit, S.K.; Dekker, R.F.H. Forest biorefinery: Potential of poplar phytochemicals as value-added co-products. Biotechnol. Adv. 2015, 33, 681–716. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.Y.; Emami, S.; Ratanapariyanuch, K.; Reaney, M.J.T. Biorefinery of Plant-Based Products. In Plant Bioproducts; Chen, G., Weselake, R., Singer, S., Eds.; Springer: New York, NY, USA, 2018; pp. 201–218. [Google Scholar]
- Yang, X.; Lee, S.J.; Yoo, H.Y.; Choi, H.S.; Park, C.; Kim, S.W. Biorefinery of instant noodle waste to biofuels. Bioresour. Technol. 2014, 159, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Seong, P.J.; Jeon, B.W.; Lee, M.; Cho, D.H.; Kim, D.K.; Jung, K.S.; Kim, S.W.; Han, S.O.; Kim, Y.H.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil and dimethyl carbonate. Enzym. Microb. Technol. 2011, 48, 505–509. [Google Scholar] [CrossRef]
- Lee, M.; Lee, D.; Cho, J.; Park, C. Enzymatic Biodiesel Synthesis in Semi-Pilot Continuous Process in Near-Critical Carbon Dioxide. Appl. Biochem. Biotechnol. 2013, 171, 1118–1127. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. 2018, 17, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Tacchini, M.; Burlini, I.; Bernardi, T.; De Risi, C.; Massi, A.; Guerrini, A.; Sacchetti, G. Chemical characterisation, antioxidant and antimicrobial screening for the revaluation of wine supply chain by-products oriented to circular economy. Plant Biosyst. 2018, 153, 809–816. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Hromadkova, Z.; Ebringerova, A. Ultrasonic extraction of plant materials—Investigation of hemicellulose release from buckwheat hulls. Ultrason. Sonochem. 2003, 10, 127–133. [Google Scholar] [CrossRef]
- Caili, F.; Haijun, T.; Quanhong, L.; Tongyi, C.; Wenjuan, D. Ultrasound-assisted extraction of xyloglucan from apple pomace. Ultrason. Sonochem. 2006, 13, 511–516. [Google Scholar]
- Rabelo, R.S.; Machado, M.T.C.; Martínez, J.; Hubinger, M.D. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J. Food Eng. 2016, 178, 170–180. [Google Scholar] [CrossRef]
- da Silva Lima, R.; Nunes, I.L.; Block, J.M. Ultrasound-Assisted Extraction for the Recovery of Carotenoids from Guava’s Pulp and Waste Powders. Plant Foods Hum. Nutr. 2020, 75, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, N.; Elhami Rad, A.H.; Javanmard, M.; Azarpazhouh, E.; Armin, M. Optimization of conditions of ultrasound-assisted extraction of phenolic compounds from orange pomace (Citrus sinensis). Int. J. Biol. Chem. 2020, 12, 10–19. [Google Scholar] [CrossRef]
- Tacchini, M.; Burlini, I.; Maresca, I.; Grandini, A.; Bernardi, T.; Guerrini, A.; Lerin, L.; Sacchetti, G. Polyphenols from Vitis vinifera Lambrusco By-Products (Leaves from Pruning): Extraction Parameters Evaluation Through Design of Experiment. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Périno-Issartier, S.; Huma, Z.-e.; Abert-Vian, M.; Chemat, F. Solvent Free Microwave-Assisted Extraction of Antioxidants from Sea Buckthorn (Hippophae rhamnoides) Food by-Products. Food Bioprocess Technol. 2010, 4, 1020–1028. [Google Scholar] [CrossRef]
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of microwave-assisted extraction of ergosterol from Agaricus bisporus L. by-products using response surface methodology. Food Bioprod. Process. 2016, 100, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Zeković, Z.; Pintać, D.; Majkić, T.; Vidović, S.; Mimica-Dukić, N.; Teslić, N.; Pavlić, B. Utilization of sage by-products as raw material for antioxidants recovery—Ultrasound versus microwave-assisted extraction. Ind. Crop. Prod. 2017, 99, 49–59. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydr. Polym. 2014, 101, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D.; Pizzolongo, F.; Ferrara, L.; Naviglio, B.; Aragòn, A.; Santini, A. Extraction of pure lycopene from industrial tomato waste in water using the extractor Naviglio®. Afr. J. Food Sci. 2008, 2, 37–44. [Google Scholar]
- Manna, L.; Bugnone, C.A.; Banchero, M. Valorization of hazelnut, coffee and grape wastes through supercritical fluid extraction of triglycerides and polyphenols. J. Supercrit. Fluids 2015, 104, 204–211. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Ormazabal, M.; Vallejo, A.; Olivares, M.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Optimization of Supercritical Fluid Consecutive Extractions of Fatty Acids and Polyphenols from Vitis Vinifera Grape Wastes. J. Food Sci. 2015, 80, E101–E107. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.T.; Uddin, M.d.S.; Jung, G.-W.; Sim, J.E.; Chun, B.S. Supercritical Carbon Dioxide Extraction of Phenolics and Tocopherols Enriched Oil from Wheat Bran. Int. J. Nutr. Food Eng. 2010, 4, 188–193. [Google Scholar]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gu, W. Study on supercritical fluid extraction of solanesol from industrial tobacco waste. J. Supercrit. Fluids 2018, 138, 228–237. [Google Scholar] [CrossRef]
- Fabian, C.; Tran-Thi, N.Y.; Kasim, N.S.; Ju, Y.-H. Release of phenolic acids from defatted rice bran by subcritical water treatment. J. Sci. Food Agric. 2010, 90, 2576–2581. [Google Scholar] [CrossRef]
- García-Marino, M.; Rivas-Gonzalo, J.C.; Ibáñez, E.; García-Moreno, C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal. Chim. Acta 2006, 563, 44–50. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Kim, S.-W.; Ko, M.-J.; Chung, M.-S. Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J. Clean. Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]
- Li, W.; Fan, Z.; Wu, Y.; Jiang, Z.; Shi, R. Eco-friendly extraction and physicochemical properties of pectin from jackfruit peel waste by subcritical water. J. Sci. Food Agric. 2019, 7, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Klinchongkon, K.; Khuwijitjaru, P.; Wiboonsirikul, J.; Adachi, S. Extraction of Oligosaccharides from Passion Fruit Peel by Subcritical Water Treatment. J. Food Process. Eng. 2015, 40, e12269. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Xia, H.; Matharu, A.S. Unavoidable food supply chain waste: Acid-free pectin extraction from mango peel via subcritical water. Faraday Discuss. 2017, 202, 31–42. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić, I.; Ković, R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Methyl β-cyclodextrin as a booster for the extraction for Olea europaea leaf polyphenols with a bio-based deep eutectic solvent. Biomass Convers. Biorefinery 2017, 8, 345–355. [Google Scholar] [CrossRef]
- Bogaars, R.A. Exploring Commercial Applications of Natural Deep Eutectic Solvents. Master’s Thesis, TU Delft, Delft, The Netherlands, 2015; p. 78. [Google Scholar]
- Pal, C.B.T.; Jadeja, G.C. Deep Eutectic Solvents-based Extraction of Polyphenolic Antioxidants from Onion (Allium cepa L.) Peel. J. Sci. Food Agric. 2018, 99, 1969–1979. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Karabelas, A.J. Licopene recovery from tomato peel under mild conditions assisted by enzymatic pre-treatment and non-ionic surfactants. Acta Biochim. Pol. 2012, 59, 71–74. [Google Scholar] [CrossRef]
- Laroze, L.; Soto, C.; Zúñiga, M.E. Phenolic antioxidants extraction from raspberry wastes assisted by-enzymes. Electron. J. Biotechnol. 2010, 13, 11–12. [Google Scholar] [CrossRef]
- Faulds, C.B.; Sancho, A.I.; Bartolomè, B. Mono- and dimeric ferulic acid release from brewer’s spent grain by fungal feruloyl esterases. Appl. Microbiol. Biotechnol. 2002, 60, 489–494. [Google Scholar] [PubMed]
- Yu, P.; Maenz, D.D.; McKinnon, J.J.; Racz, V.J.; Christensen, D.A. Release of Ferulic Acid from Oat Hulls by Aspergillus Ferulic Acid Esterase and Trichoderma Xylanase. J. Agric. Food Chem. 2002, 50, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.T.; Duy, N.Q.; Minh, N.P.; Dao, D.T.A. Optimization of beta-glucans extraction from waste brewer’s yeast saccharomyces cerevisiae using autolysis, enzyme, ultrasonic and combined enzyme-ultrasonic treatment. Am. J. Res. Commun. 2013, 1, 149–158. [Google Scholar]
- Luque-Garcıa, J.L.; Luque de Castro, M.D. Ultrasound-assisted soxhlet extraction: An expeditive approach for solid sample treatment: Application to the extraction of total fat from oleaginous seeds. J. Chromatogr. A 2004, 1034, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Natolino, A.; Decorti, D. The combined extraction of polyphenols from grape marc: Ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT -Food Sci. Technol. 2015, 61, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, B.; Liu, Y.; Zhang, H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014, 150, 482–488. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Wang, W.; Zou, M.; Ding, T.; Ye, X.; Liu, D. Synergistic effect and Mechanism of combining ultrasound and pectinase on pectin hydrolysis. Food Bioprocess Technol. 2016, 9, 1249–1257. [Google Scholar] [CrossRef]
- Ladole, M.R.; Nair, R.R.; Bhutada, Y.D.; Amritkar, V.D.; Pandit, A.B. Synergistic effect of ultrasonication and co-immobilized enzymes on tomato peels for lycopene extraction. Ultrason. Sonochem. 2018, 48, 453–462. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Zhang, C.; Li, W. Ultrasound-assisted enzyme catalyzed hydrolysis of olive waste and recovery of antioxidant phenolic compounds. Innov. Food Sci. Emerg. Technol. 2017, 44, 224–234. [Google Scholar] [CrossRef]
- Mulinari, J.; Venturin, B.; Sbardelotto, M.; Dall Agnol, A.; Scapini, T.; Camargo, A.F.; Baldissarelli, D.P.; Modkovski, T.A.; Rossetto, V.; Dalla Rosa, C.; et al. Ultrasound-assisted hydrolysis of waste cooking oil catalyzed by homemade lipases. Ultrason. Sonochem. 2017, 35, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.L.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valoriz. 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Raes, K.; Van Camp, J. Combined alkaline hydrolysis and ultrasound-assisted extraction for the release of nonextractable phenolics from cauliflower (Brassica oleracea var. botrytis) waste. J. Agric. Food Chem. 2014, 62, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Burlini, I.; Grandini, A.; Tacchini, M.; Maresca, I.; Guerrini, A.; Sacchetti, G. Different strategies to obtain corn (Zea mays L.) germ extracts with enhanced antioxidant properties. Nat. Prod. Commun. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Wu, D.; Gao, T.; Yang, H.; Du, Y.; Li, C.; Wei, L.; Zhou, T.; Lu, J.; Bi, H. Simultaneous microwave/ultrasonic-assisted enzymatic extraction of antioxidant ingredients from Nitraria tangutorun Bobr. juice by-products. Ind. Crop. Prod. 2015, 66, 229–238. [Google Scholar] [CrossRef]
- Xu, M.H.; Yang, X.Y.; Fu, M.R. Combined Ultrasonic and Microwave Method for Juglone Extraction from Walnut Green Husk (Juglone Nigra). Waste Biomass Valoriz. 2016, 7, 1159–1166. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef]
- Liu, Z.; Qiao, L.; Yang, F.; Gu, H.; Yang, L. Brönsted acidic ionic liquid based ultrasound-microwave synergistic extraction of pectin from pomelo peels. Int. J. Biol. Macromol. 2017, 94, 309–318. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidovic, S.; Radojčić Redovnikovic, I.; Jokic, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Kerton, F.M.; Mariotte, R. Alternative Solvents for Green Chemistry, 2nd ed.; Royal Society of Chemistry: Croydon, UK, 2013; pp. 1–325. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent Extraction Techniques for Natural Products: Microwave-assisted Extraction and Pressurised Solvent Extraction. Phytochem. Anal. 2002, 13, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D. Naviglio’s Principle and Presentation of an Innovative Solid–Liquid Extraction Technology: Extractor Naviglio. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Sapkale, G.N.; Patil, S.M.; Surwasee, U.S.; Bhatbhage, P.K. Supercritical fluid extraction—A Review. Int. J. Chem. Sci. 2010, 8, 729–743. [Google Scholar]
- Nastic, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Barroso, M.S.; Soares, C.; Moreira, M.M.; Morais, S.; Maškovic, P.; Srček, V.G.; Slivac, I.; et al. Subcritical water extraction as an environmentally-friendly technique to recover bioactive compounds from traditional Serbian medicinal plants. Ind. Crop. Prod. 2018, 111, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Aroso, I.M.; Craveiro, R.; Rocha, Â.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES—Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, M.; Emilia, A. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar]
- Balachandran, S.; Kentish, S.E.; Mawson, R.; Ashokkumar, M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. The distribution of phenolic acids in rice. Food Chem. 2004, 87, 401–406. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Barberousse, H.; Roiseux, O.; Robert, C.; Paquot, M.; Deroanne, C.; Blecker, C. Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 2008, 88, 1494–1511. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. A review of distribution, extraction methods and health benefits of bound phenolics in food plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Geetha Balasubramaniam, V.; Ayyappan, P.; Sathvika, S.; Antony, U. Effect of enzyme preatrement in the ultrasound assisted extraction of finger millet polyphenols. J. Food Sci. Technol. 2019, 56, 1583–1594. [Google Scholar] [CrossRef]
- Đurović, S.; Nikolić, B.; Luković, N.; Jovanović, J.; Stefanović, A.; Šekuljica, N.; Mijin, D.; Knežević-Jugović, Z. The impact of high-power ultrasound and microwave on the phenolic acid profile and antioxidant activity of the extract from yellow soybean seeds. Ind. Crop. Prod. 2018, 122, 223–231. [Google Scholar] [CrossRef]
- Prado, J.M.; Veggy, P.C.; Maireles, M.A.A. Scale-Up Issues and Cost of Manufacturing Bioactive Compounds by Supercritical Fluid Extraction and Ultrasound Assisted Extraction; Global Food Security and Wellness; Springer: New York, NY, USA, 2017; pp. 377–433. [Google Scholar]
| Vegetable Commodities and Products | |
|---|---|
| Agricultural Production | Losses for mechanical damage and/or spillage during harvest operations, crops separated postharvest, etc. |
| Postharvest Handling and Storage | Handling spillage and/or degradation, storage and transport during distribution |
| Processing | Spillage and degradation during processing, separated crops not suitable to process or during washing, peeling, boiling or accidental spillage |
| Distribution | Market system |
| Consumption | Household level |
| World Food Losses and Wastes (%) by Manufacturing | |
|---|---|
| Cereals | 4.1 |
| Roots and tubers | 10.2 |
| Oil crops and pulses | 5.9 |
| Fruits and vegetables | 8.5 |
| Meat | 4.8 |
| Fish | 6.3 |
| Diary | 1.4 |
| Extraction Method | Food Waste Biomass | Bioactives of Interest | References | |
|---|---|---|---|---|
| Ultrasound-assisted extraction (UAE) | Coffee waste (Coffea arabica; C. canephora) | Phenolic compounds | [31] | |
| Pomegranate waste (Punica granatum) | Carotenoids | [32] | ||
| Tomato waste (Solanum lycopersicum) | Pectins | [33] | ||
| Apple pomace (Malus domestica) | Xyloglucan | [34] | ||
| Grapeseed (Vitis spp.) | Anthocyanins, Protantocianidins | [30] | ||
| Grape pomace | Flavonoids | [30] | ||
| Grape leaves | Phenolic compounds | [35] | ||
| Artichoke solid waste (Cynara scolymus) | Phenolic compounds | [36] | ||
| Guava’s pulp (Psidium guajava) | Carotenoids | [37] | ||
| Orange peel (Citrus spp.) | Phenolic compounds | [38] | ||
| Microwave-assisted extraction (MAE) | Tomato waste (S. lycopersicum) | Carotenoids | [39] | |
| Sea Buckthorn byproducts (Hippophae rhamnoides) | Flavonoids | [40] | ||
| Agaricus bisporus L. byproducts | Erogosterol | [41] | ||
| Sage byproducts (Salvia officinalis) | Phenolic compounds | [42] | ||
| Broccoli by-products (Brassica oleracea var. italica) | Phenolics, sugars, glucosinolates | [43] | ||
| Citrullus lanatus fruit rinds | Pectins | [44] | ||
| Pressurized solvent extraction (Naviglio® extractor) | Tomato waste (S. lycopersicum) | Lycopene | [45] | |
| Vine-shoots (Vitis spp.) | Phenolic, volatile, mineral compounds | [46] | ||
| Grape pomace (Vitis spp.) | Polyphenols | [30] | ||
| Supercritical fluids extraction (SFE) | Hazelnut wastes (Corylus avellana) | Tryglycerides | [47] | |
| Grape wastes (Vitis spp.) | Polyphenols and Fatty Acids | [48] | ||
| Wheat bran (Triticum aestivum) | Phenolic compounds and tocopherol | [49] | ||
| Sweet Potato (Ipomoea batatas) | Carotenoids | [50] | ||
| Tobacco waste (Nicotiana tabacum) | Solanesol | [51] | ||
| Subcritical water extraction (SWE) | Rice bran (Oryza sativa) | Phenolic acids | [52] | |
| Winery byproducts (Vitis spp.—from wine supply chain production) | Catechins and proanthocyanidins | [53] | ||
| Onion waste (Allium cepa) | Flavonoids | [54,55] | ||
| Jackfruit peel waste (Arctocarpus heterophyllus) | Pectins | [56] | ||
| Passion fruit peel (Passiflora edulis) | Oligosaccharides | [57] | ||
| Grape skin (Vitis spp.) | Polyphenols | [58] | ||
| Mango peel (Mangifera indica) | Pectins | [59] | ||
| Deep eutectic solvents (DESs and NADES) | Wine lees (Vitis spp.—from wine supply chain production) | Anthocyanins | [60] | |
| Olive pomace (Olea europaea) | Phenolic compounds | [61] | ||
| Olive leaves (O. europaea) | Polyphenols | [62] | ||
| Orange waste (Citrus spp.) | d-Limonene, Pectin and Hesperidin. | [63] | ||
| Onion peel (A. cepa) | Polyphenols | [64] | ||
| Enzyme-assisted extraction | Tomato peels (S. lycopersicum) | Lycopene | [65] | |
| Raspberry wastes (Rubus spp.) | Phenolic compounds | [66] | ||
| Brewer’s spent grain (Triticum aestivum) | Mono and dimeric ferulic acid | [67] | ||
| Oat hulls (Avena sativa) | Ferulic acid | [68] | ||
| Beer wastes | Beta-glucans | [69] | ||
| Hybrid techniques | UAE- sohxlet | Oleaginous seeds | Fixed oils | [70] |
| UAE-SFE | Grape pomace (Vitis spp.) | Polyphenols | [71] | |
| UAE- enzymatic | Wheat bran (T. aestivum) | Arabinoxylans | [72] | |
| Citrus peel (Citrus spp.) | Pectins | [73] | ||
| Tomato peel (S. lycopersicum) | Lycopene | [74] | ||
| Olive waste (O. europaea) | Phenolic compounds | [75] | ||
| Waste cooking oil | [76] | |||
| UAE-NADESs | Olive cake (O. europaea) | Phenolic compounds | [77] | |
| Onion seed (A. cepa) | Phenolic compounds | [77] | ||
| Tomato byproducts (S. lycopersicum) | Phenolic compounds | [77] | ||
| Pear canning byproducts (Pyrus communis) | Phenolic compounds | [77] | ||
| Tartary buckwheat hull (Fagopyrum esculentum) | Rutin | [78] | ||
| Red grape pomace (Vitis spp.) | Phenolic compounds | [79] | ||
| Olive leaves (O. europaea) | Phenolic compounds | [79] | ||
| Wheat bran (T. aestivum) | Phenolic compounds | [79] | ||
| Lemon waste peels (Citrus limon) | Phenolic compounds | [79] | ||
| UAE-hydrolysis | Cauliflowers byproducts (Brassica oleracea) | Bound phenolics | [80] | |
| Maize germ (Zea mays) | Bound phenolics | [81] | ||
| UAE-MAE | Nitratia tangutorum Bobr. byproduct | Pectins | [82] | |
| Walnut green husk (Juglans regia) | Juglone | [83] | ||
| Pomelo peel (Citrus maxima) | Pectins | [84,85] | ||
| Bound Phenolics in Food Sources | ||
|---|---|---|
| Source | Species | Insoluble Bound Phenolics in Total Phenolic % |
| Apple | Malus domestica | 6.50 |
| Banana | Musa acuminate | 33.10 |
| Cranberry pomace | Vaccinium macrocarpon | 76.27 |
| Orange | Citrus sinensis | 24.30 |
| Carrot | Daucus carota | 37.6 |
| Onion | Allium cepa | 9.70 |
| Potato | Solanum tubersum | 39.9 |
| Barley | Hordeum vulgare | 70.08 |
| Maize | Zea mays | 85.00 |
| Rice | Oryza sativa | 62 |
| Brown rice | Oryza sativa | 88 |
| Wheat | Triticum spp. | 75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlini, I.; Sacchetti, G. Secondary Bioactive Metabolites from Plant-Derived Food Byproducts through Ecopharmacognostic Approaches: A Bound Phenolic Case Study. Plants 2020, 9, 1060. https://doi.org/10.3390/plants9091060
Burlini I, Sacchetti G. Secondary Bioactive Metabolites from Plant-Derived Food Byproducts through Ecopharmacognostic Approaches: A Bound Phenolic Case Study. Plants. 2020; 9(9):1060. https://doi.org/10.3390/plants9091060
Chicago/Turabian StyleBurlini, Ilaria, and Gianni Sacchetti. 2020. "Secondary Bioactive Metabolites from Plant-Derived Food Byproducts through Ecopharmacognostic Approaches: A Bound Phenolic Case Study" Plants 9, no. 9: 1060. https://doi.org/10.3390/plants9091060
APA StyleBurlini, I., & Sacchetti, G. (2020). Secondary Bioactive Metabolites from Plant-Derived Food Byproducts through Ecopharmacognostic Approaches: A Bound Phenolic Case Study. Plants, 9(9), 1060. https://doi.org/10.3390/plants9091060






